1. Introduction
As several governments have already committed to reducing the use of gasoline and diesel-powered vehicles by 2030, it is expected that electric vehicles (or EVs) will replace a large portion of today’s fleet. Several industries have planned towards achieving the goal and one such instance is the electric battery plant near Nissan’s Sunderland plant in Northeast England, where the automaker has committed to increasing production of electric vehicles. In Ellesmere port facility, Stellantis (the owner of Vauxhall) has announced an investment of £100,000 million (about $139 million). Most electric vehicles today use lithium-ion batteries, but they have several drawbacks. The scientists and engineers are working on several solutions to address these issues, which could expedite the transition to more effective Li-ion battery use in electric vehicles, as well.
Since their introduction in 1991, Sony’s Li-ion batteries have been widely used in automobiles, smartphones, and laptop computers [
1]. Compared to standard lead–acid batteries, they are three times more efficient and last up to 20 years. If a vehicle is equipped with a lithium-ion battery, it provides an advantage of extra capacity for storing a lot of power and is not cumbersome in facilitating the vehicle to move with less energy. In general, global sales of electric vehicles in 2020 exceeded expectations and are constantly on the rise. In addition, Europe announced an extra 200 GWh of projected capacity in 2020, a number that will rapidly rise in 2021 up to 600 GWh for electric vehicles and lithium-ion batteries. There has been a surge in the electric car market’s pace in recent years and the demand has resulted in competition among several manufacturers of EVs such as Tesla, Volkswagen, GM, SK Innovation, and LG Chem. Thus, understanding Li-ion batteries, as well as multiple design and implementation trends, is even more important.
Li-ion batteries include four essential components: the anode, cathode, electrolyte, and separator. A Li-ion battery is powered by a chemical process involving lithium. The market for Li-ion batteries is booming, as they are the most effective way to power a wide range of electric vehicles. In recent years, there has been a massive investment in technology development. As a result, battery makers as well as original equipment manufacturers have started revealing their plans to electrify their vehicles [
2,
3]. Electric car sales bolstered the industry in 2020, despite the ongoing COVID-19 pandemic. For electric vehicles using Li-ion battery cells, the market is anticipated to be worth over
$70 billion by 2026 based on a comprehensive analysis of the drivers and restraints in the industry [
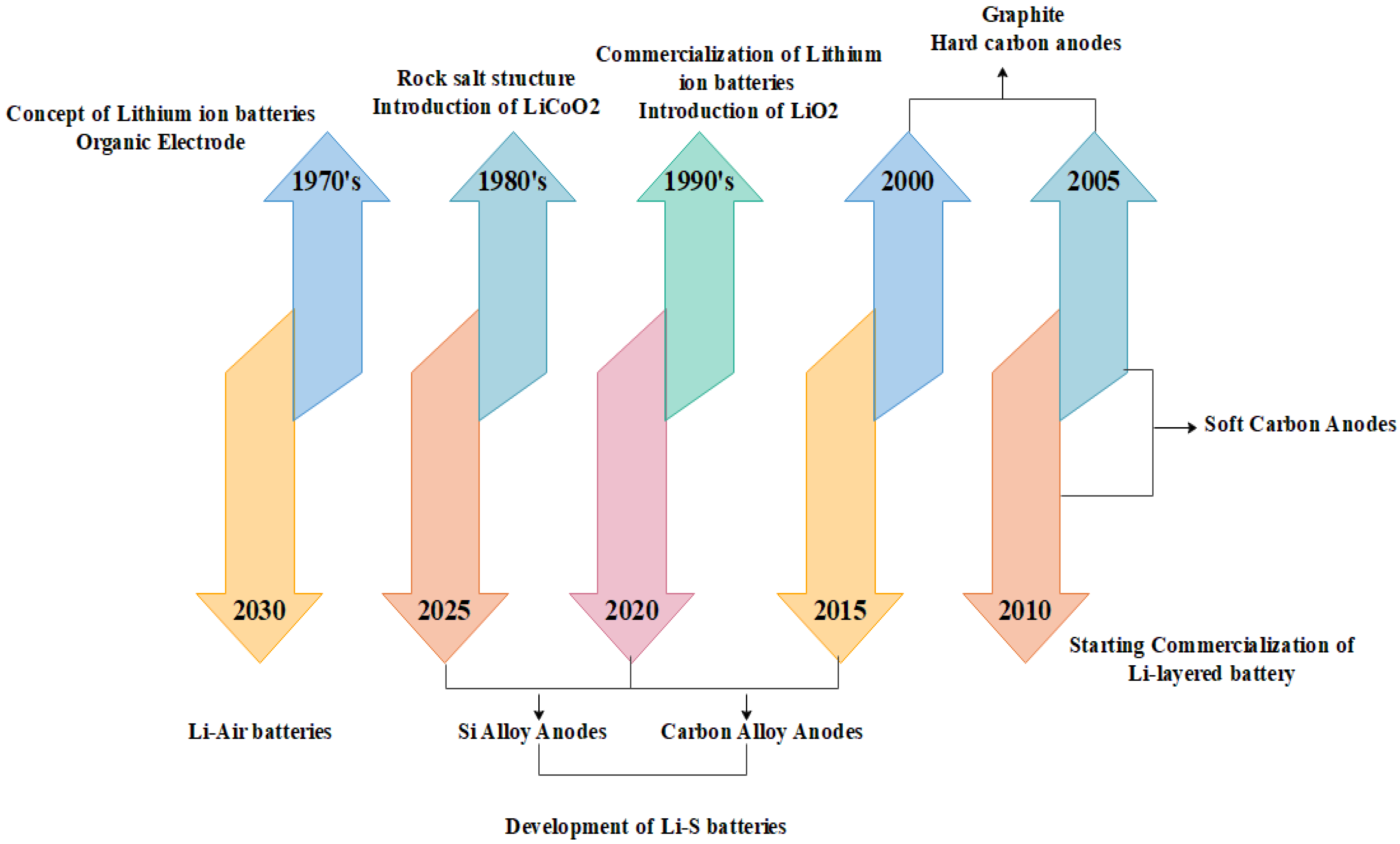
4]. A schematic of the evolution of lithium-ion battery technology is shown in
Figure 1.
In a regular Li-ion cell, the negative electrode is constructed of carbon (C), whereas the positive electrode is often built of metal oxide (MnO) (MO). Lithium ions can be stored on both the anode and the cathode. The electrolyte between these electrodes is traversed by 24 lithium ions, which store and release energy. There is a problem with typical lithium salts when they are utilized in organic solvents as an electrolyte (standard), although the anode’s life is diminished when a high voltage is applied to the anode materials. An entirely new lithium-ion battery is being created for electric vehicles with three times the thickness of typical batteries to address this issue. Increasing the volume ratio of active electrodes, for example by increasing the thickness of electrode materials, is an effective technique for enabling the growth of lithium-ion batteries with thicker electrode layers. LIPO batteries benefit from a high electrode-to-current ratio (E/I ratio). A smaller cell with a lower collector-to-volume ratio is more energy efficient. Production costs can be decreased by eliminating the need for additional cutting and staple steps, and this battery has been used in its products at Hitachi Ltd. (TSE: 6501) in Tokyo, Japan. Electric vehicles can now drive twice as far as they could with the advances in this new technology. Regular electrodes now double the thickness provide a greater flexibility for the batteries to hold more lithium-ions [
5].
To better comprehend lithium-ion mobility, a recently developed approach for three-dimensional imaging of the electrode structure was used to accomplish this task. More lithium ions are charged and discharged in this battery than in other types. Because they are so little, they can readily be separated from the electrode. As a result of Hitachi’s research, a method for attaching silicon materials together has been developed. The intended outcome was achieved by using an electrode to suppress the separation. Carbon-based materials have a comparable service life. Anodes made of standard materials are subject to corrosion. Electrolytes decompose when a high voltage is applied, resulting in a reduced lifespan. A simple solution was devised to cover the cathode with a piece of aluminum foil. These tactics can help them achieve their aims. As the lifespan of the batteries is enhanced, the range of electric vehicles becomes extremely vast. This paper presents the challenges and opportunities associated with lithium-ion batteries for electric vehicles in the form of a brief review.
2. Market Opportunities for Lithium-Ion Batteries in Electric Vehicles
Currently, rules and subsidies are supporting the sale of electric vehicles, and this trend is expected to continue presently in the United States as well. Li-ion battery advances are needed for a variety of vehicle types if mass-market adoption is to be achieved through consumer-driven innovation. Electric vehicles including cars, buses, lorries, and boats can benefit from Li-ion chemistry and battery design alternatives due to their wide range of performance needs [
6]. To fully appreciate this, it is necessary to examine the possibilities for electric vehicles like these.
Commonly used automotive units include watt-hours (Wh) and kilowatt-hours (kWh) (kWh). Batteries like these are used in everything from cell phones to electric vehicles. High storage density is part of lithium-ion technology’s success. However, even though solid-state battery technology is in its infancy, lithium-ion technology now provides the best potential balance of energy storage capacity, volume, and mass when it comes to use in electric vehicles. As a component of the circular economy concept, it has a high voltage, is simple to recharge, and has a long lifespan, all of which allow for a variety of complimentary use scenarios throughout its life cycle. Different facets of Li-ion cell technology are ever-changing cell form factors and chemistries [
7]. Higher nickel-layered oxides such as nickel manganese cobalt, such as NMC 622 through NMC 811-based batteries for BEVs, are obviously on the rise, although these higher nickel cathodes are not suited for all applications. Lithium iron phosphate (LFP) has become popular in low-cost cars, whereas Chinese e-buses rely only on LFP for their power supply. Car segment-specific cathode materials and OEM/pack manufacturer strategies are specifically given importance. Supply and demand for cathodes are anticipated till 2031. Li-ion may have to contend with less-capable technologies that are still in their infancy as competition in the future. Batteries for electric vehicles can be improved by developing and improving modern Li-ion cells and chemistry. With a specific focus on solid-state/Li-metal and silicon anode cell technologies for usage in electric vehicles, these breakthroughs have always been vital [
8,
9,
10].
Gains in energy density at the pack level are just as significant as those at the cell level. It is becoming increasingly common for battery packs to use temperature management techniques, modular construction methods, and lightweight materials in their construction. Considering the safety and critical aspects of Li-ion batteries, the temperature must be regulated. Effective heat management is becoming increasingly critical considering previous fires and the Chinese government’s desire to improve the safety of battery pack operation, especially in public transportation. All the cooling methods, whether they use air, liquid, or refrigerant, have pros and cons.
Batteries for non-car vehicle types, such as heavy-duty trucks, buses, and logistical vehicles, are examined in Europe and the United States. For example, product form factors, chemical composition, and performance are all considered while comparing turnkey items. There will be an increasing need for better and more environmentally friendly batteries as Li-ion batteries reach the limits of their performance and concerns about their availability and the environment are raised. In the future, the utilization of lithium metal anodes, sodium-ion batteries, lithium-ion batteries, and redox flow batteries will be witnessed more [
11]. Solid electrolytes, high-Ni cathodes, silicon and lithium metal anodes, and a range of cell design components are a few examples. Battery electric vehicles, particularly those powered by lithium-ion batteries, are expected to keep Li-ion at the top of the battery market for years to come. In the field of lithium-ion batteries, silicon anodes and lithium metal anodes are two of the most exciting material developments (the latter of which is frequently but not always used in conjunction with solid electrolytes). In addition to their potential to improve energy density, these anode materials are being investigated for their potential to increase rate capability, safety, and even cost. In the past, silicon and lithium metal were both hampered by the fact that their practical implementation has been delayed and constrained [
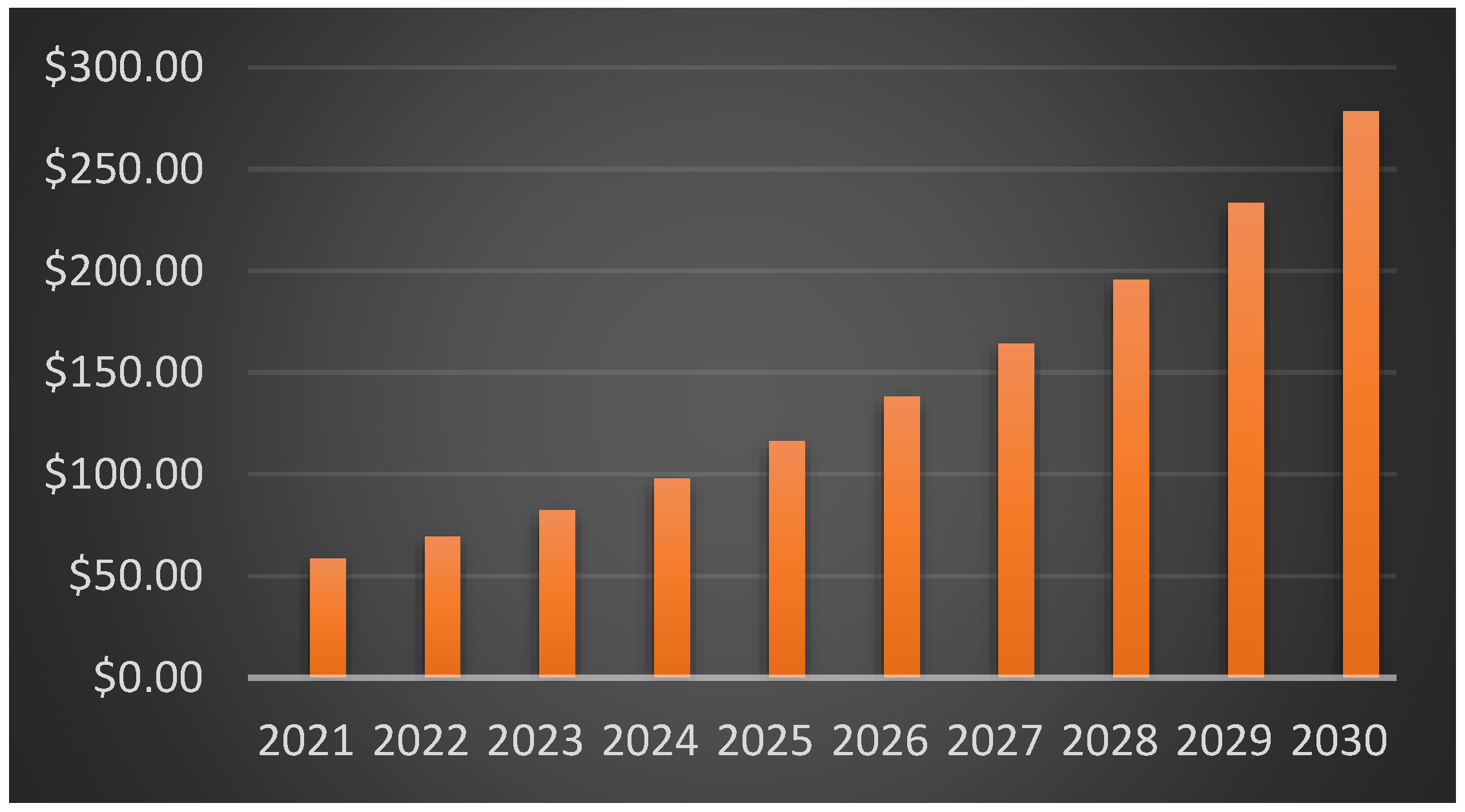
12]. To improve performance, Li-S batteries use a conversion-type sulfur cathode instead of the intercalation cathodes found in Li-ion batteries. This results in a longer lifespan for the battery. The market opportunities for Li-ion batteries have been expanding. By 2024, the market for Lithium-ion batteries is expected to grow to a value of USD 90.01 billion. Over the projection period of 2020–2026, the market is anticipated to develop at a Compound Annual Growth Rate (CAGR) of 20% due to the booming electric car industry, strong consumer electronics demand, the transition to smart electronics, rising shipments of smart wearables, and falling lithium-ion battery prices. The need for energy in the form of solar, wind, fossil fuels, etc., has caused the current society significant concern. Batteries play a crucial part in illustrating the successful use of renewable energy in this setting, where the demand for efficient storage devices is likely to have existed for a long time. The global lithium-ion battery market size was estimated at USD 58.61 billion in 2021 and is expected to surpass around USD 278.27 billion by 2030 with a registered CAGR of 18.9% from 2022 to 2030, as shown in
Figure 2.
3. Past and Present Global Market for Electric Vehicles
The worldwide EV industry is dominated by passenger vehicles, with EVs making up about 1% of the global passenger vehicle market. There were more than 5 million electric vehicles on the road worldwide in 2018, up from just 2 million in 2017. More than half of the world’s electric vehicle fleet (2.3 million vehicles) was located in China in 2018, up from 37% in 2017. While Europe had 24% (1.2 million), the United States had 22% (1.1 million). Electric vehicles can be used for commercial purposes, with buses now dominating the global industry. China has 421,000 electric buses (accounting for around 18% of its total bus fleet), Europe has 2250, and the United States has 300. Most commercial vehicles, including trucks, remain ICE vehicles [
13,
14]. There were around 2000 medium-sized trucks in the fleet of electric vehicles for freight transport in 2018 (250,000). There is an increasing demand for transportation services, including taxis, ridesharing, and car-sharing fleets; EVs account for 1.8% of the shared mobility fleet. With 10 billion rides in 2018, China is the largest ride-hailing market, whereas the United States had just over 3 billion rides. Automobile manufacturers and mobility service providers in China have formed joint ventures (such as Didi Chuxing) or other forms of cooperation (such as BAIC Motor, SAIC Motor, GAC Motor, and Geely Auto). Profits have been difficult to come by in this market area. As a result of Daimler’s decision to withdraw its Smart mini-cars from China, it has formed a joint venture with Geely to take advantage of the premium ride-hailing market [
15].
More than half (60%) of all electric vehicle (EV) sales in 2018 occurred in China. The number of light electric vehicles sold in China increased from 220,000 in 2015 to 1,120,000 in 2018 (48% CAGR). Light passenger vehicle sales increased from 0.9% to 3.9% in the same time frame. More than 90% of China’s extremely small “city” automobiles were electric. Sport utility vehicles (SUVs) make up nearly a third of China’s electric automobiles. Small BEVs can be bought for the same price as an ICE with the help of government incentives.
China’s EV market is three times greater than the markets in Europe and the United States, on a per capita basis. A total of 385,000 electric vehicles (EVs) were sold in Europe in 2018 (320,000 of these were sold in EU countries). The European Union’s light passenger vehicle sales penetration rate climbed from 1% in 2015 to 1.8% in 2018. Despite Europe’s leading position in electric vehicle sales, the continent’s 2018 growth rate (31%) is lower than the worldwide average. Norwegian new car sales in 2018 accounted for 46% of the country’s total new vehicle sales, making it the world’s leading EV market. Germany, the United Kingdom, and France all sell more merchandise than Norway. A growing number of analysts believe that the European electric vehicle market will thrive in the future due to the promises of domestic automakers and more stringent fuel economy standards [
16]. Between 2015 and 2018, sales of light electric vehicles in the United States increased from 115,000 to 361,000 units (33% CAGR). From a 2015 penetrating rate of 0.7%, its 2018 penetrating rate was 2.1%. US EV sales nearly doubled to 361,000 units in 2018, which was higher than the global market’s growth pace of 160,000 units per year. In 2018, 134,000 BEVs were sold, mostly due to the success of Tesla’s Model 3, which had a substantial backlog of orders and was eligible for EV tax credits.
There are a number of leading electric vehicle battery manufacturers, including Panasonic, Automotive Energy Supply Corporation, Robert Bosch, SAMSUNG SDI, Beijing Pride New Energy Battery Technology Co. Ltd., BYD Co. Ltd., Daimler, Mitsubishi, and Tianneng Power International Co., Ltd. Other notable electric vehicle battery manufacturers include Tesla, Nissan, and Toyota. The market for electric car batteries is expected to expand from $23.74 billion in 2021 to $25.43 billion in 2022, at a compound annual growth rate (CAGR) of 7.1%.
Since COVID-19, which had previously resulted in strict containment measures such as the stoppage of economic activity and social separation, firms have been returning to work and adjusting to the new normal. The market is predicted to increase at a compound annual growth rate of 6.9% to reach
$33.26 billion by 2026. The sale of battery packs for electric cars dominates this sector. Electric vehicles use rechargeable batteries to power their electric motors. This battery series is a proponent of clean energy because it does not emit any dangerous gases when it is working. Electric vehicles routinely make use of a variety of battery kinds. In addition to lithium-ion and lead–acid batteries, nickel metal hydride and sodium ion are also good examples. In electric vehicles, lithium-ion batteries are safer and more stable than liquid lithium-ion batteries because of their higher energy density. Plug-in hybrid and battery-powered electric vehicles are two options that are available for personal and commercial use [
17]. In 2021, Asia Pacific electric vehicle battery sales were expected to hit a record high. European battery sales in 2017 topped
$2 billion, making it the second largest market worldwide. Many countries and regions in Asia, the Middle East, and Africa are included in this investigation. This will lead to an increase in the demand for electric vehicle batteries over the next few years. This is a good indicator for business. Battery improvements and faster charging times have made electric vehicles more accessible to the public.
The average electric vehicle’s range is expected to rise from 73 to 400 miles between 2011 and 2021, according to the US Department of Energy. Furthermore, the cost of electronic batteries is decreasing because of technological advances. According to Bloomberg, a kilowatt-hour of battery electricity, which in 2010 cost more than
$1100 USD, is currently merely
$156 USD and is predicted to be about
$100 USD by the end of 2023. Because of technological improvements in electronic batteries and their accompanying technologies, electronic batteries have become increasingly popular. For environmental reasons, countries prohibit the extraction of lithium cobalt, a basic material used in electric vehicle batteries. Lithium mining requires 50,000 gallons of water to extract one metric ton of the metal. Over half of Chile’s Salar de Atacama region was depleted by lithium mining, and hazardous leaks resulted in severe water shortages across the country and South America. Governments will implement stronger rules on mining to protect the environment, which will lead to an increase in production costs. Electric car batteries are predicted to have a negative impact on the market for electric vehicle batteries soon because of their environmental impact. Demand for grid-connected charging is predicted to rise soon on the market for electric vehicle batteries. Batteries, plug-in hybrid electric vehicles, and hydrogen fuel cell electric vehicles (FCEVs) all interface with the power grid via bi-directional charging systems, allowing them all to send and receive electricity during peak hours while also boosting their charging rate. A vehicle-to-grid (V2G) pilot project began in Turin, Italy in January of 2021 by FCA, Engie EPS, and Terna to test the viability of integrating the company’s vehicles into the grid. The European Union is providing financial support for this endeavor. As a result, the electric battery market is expected to be dominated by vehicle-to-grid technologies in the next years [
18,
19,
20].
Tesla paid
$235.0 million for battery and ultracapacitor maker Maxwell Technologies, situated in San Diego, California. Tesla is a clean-energy and electric vehicle manufacturer. Tesla will be able to create its own high-density and long-lasting battery cell system using Maxwell’s dry cathode technology, which the two firms collaboratively developed. By using dry battery electrodes, the energy capacity of batteries can be increased by 50%, resulting in a two-fold increase in battery life. Australia, France, Germany, India, Indonesia, Brazil, China, Japan, Russia, South Korea, the United Kingdom, and the United States are major players in the electric car battery market [
21].
4. Type of Lithium-Ion Batteries
The classifications of Li-ion batteries are based on the chemical compositions [
22,
23,
24,
25,
26].
4.1. Lithium Cobalt Oxide
Lithium cobalt oxide batteries and lithium-ion cobalt batteries are other names for these batteries. These batteries are made from lithium carbonate and cobalt, which are both heavy metals. Because of their high specific energy density, it is not commonplace to see these batteries in mobile devices such as cellphones, laptops, and digital cameras (SED). The anode is made of cobalt oxide, and the cathode is made of graphite, with lithium ions passing between them when the electrodes conduct electricity. It is not the most energy-efficient option in terms of battery life. In addition, we would like to point out that these batteries are not as safe as other types of batteries. Although this is the case, they continue to be a popular choice for mobile phones and other portable electronic gadgets.
4.2. Lithium Manganese Oxide
Lithium manganese oxide is stored in lithium manganese oxide batteries, which are usually called manganese spinel batteries or Li-manganese cells (or lithium-ion manganese). The original battery technology was initially created in the 1980s and published in 1983 for the first time in the Materials Research Bulletin. Moli Energy’s first commercial lithium-ion cells, produced in 1996, used lithium manganese oxide as the cathode material. Lithium-ion manganese oxide batteries have higher thermal stability than other types of lithium-ion batteries, making them safer to use. Power tools, electric motorbikes, and a wide range of other electronic devices are all examples of devices that use them. Lithium manganese oxide batteries are employed in a variety of various applications, including laptop computers and electric vehicles.
4.3. Lithium Iron Phosphate (LFP)
These batteries are also known as “li-phosphate batteries” because of the cathode’s phosphorus content. As a result of their low resistance, they are more dependable. They can be recharged for an extended amount of time without deterioration due to batteries with long lifecycles. Because of their long battery life, lithium phosphate batteries are a good bargain in many applications. Lithium phosphate batteries are less powerful than other batteries due to their lower voltage. This type of battery is commonly used in electric bikes and other gadgets that require long-lasting batteries and high levels of security. These batteries are also used in electric automobiles, which are becoming increasingly popular.
4.4. Lithium Nickel Cobalt Aluminum Oxide
In electric vehicles and grid storage, nickel cobalt aluminium oxide (NCA) batteries are being used. NCA batteries have a lot of potential in the automotive industry, despite their lack of use in consumer devices. Despite their better energy density and longer lifespan, NCA batteries are less secure and more expensive than other types of lithium-ion batteries. To ensure the safety of drivers, NCA batteries must be supported by monitoring devices. As the number of electric vehicles increases, so will the need for NCA batteries, due to their extensive application in those vehicles.
4.5. Lithium Titanate
Lithium titanate, or Li-titanate, is a fast-growing battery that can be used in a variety of ways. The lithium titanate battery may be recharged in as little as a few minutes due to its advanced nanotechnology. Li-titanate batteries, which are already used in autos and bicycles, could be employed in electric buses. Batteries with higher intrinsic voltage or energy density are available, but there are several downsides to this lithium-ion battery. The increased density of lithium titanate batteries compared to non-lithium-ion batteries is an advantage. With the utilization of these batteries, smart grids and the storage of renewable energy could both benefit. According to Battery Space, these batteries could also be employed as system-critical backups in power systems.
4.6. Lithium Nickel Manganese Cobalt Oxide (NMC)
It is a combination of nickel and manganese. Lithium-ion batteries and cobalt oxide batteries, both of which go by the name of “NMC batteries,” use many of the same raw components. A nickel, manganese, and cobalt cathode is one option. The power density of NMC batteries is higher than that of other lithium-ion battery types. Even if they could, neither of these things would be possible. In power tools and car powertrains, this battery type is the most used. There are three key ingredients in cathode mixture: nickel, manganese, and cobalt. Cobalt is more expensive than other lithium-ion battery options. This lowers the cost of raw materials. The price of lithium-ion batteries could fall even more if battery producers opt to convert to a chemistry with a larger percentage of nickel and so require less cobalt. This battery type is often utilized in electric vehicles because of its low self-heating rate.
The cathodes used in the most successful Li-ion systems are constructed of nickel, manganese, or cobalt. Depending on the application, a variety of systems can be utilized as power cells or energy cells, respectively. It has been discovered that using this optimization, the NMC in an 18,650 cell may operate at 4 to 5 A in output voltage, depending on the cell. It is possible to always discharge a battery with a capacity of 2000 mAh at a rate of 20 A. However, while it is possible to boost the anode’s capacity up to 4000 mAh, this comes at the sacrifice of loading capacity and cycle life. During the charging and discharging processes, the anode’s shape changes, resulting in the cell being unstable. Adding silicon to graphite can help to increase the stability of a cell. A significant component of NMC’s success has been the use of nickel and manganese. Sodium and chloride, which are the two most essential elements of table salt, are combined to form seasoning salt and food preservative, respectively. Manganese has a low specific energy because of its spinel structure, which lowers internal resistance and results in a low specific energy. Nickel’s disadvantage is that it has high specific energy. The total power of the metals has been boosted. When it comes to electric vehicles, NMC batteries are the finest available option. Cathode 1-1-1 is the most prevalent type of cathode, which has equal amounts of nickel, manganese, and copper. Because of the decreased cobalt percentage, this results in a more unique mix, while also lowering the cost of raw materials. The NCM alloy, on the other hand, is composed of 5 nickel, 3 cobalt, and 2 manganese components (5-3-2). Different designs can be created by varying the amount of cathode material used. New electrolytes and additives have been developed, allowing cells to be charged up to 4.4 V per cell and at higher voltages. Because NMC-blended Li-ion is both cost-efficient and effective, it is becoming increasingly popular among battery users. A variety of automotive and energy storage system (EES) applications requiring frequent cycling can benefit from the use of nickel, manganese, and cobalt, which are three active materials that are easily mixed. Some of the properties of lithium-ion batteries are presented in
Table 1 along with their prices.
5. Lithium-Ion Batteries’ Life Expectancy
Batteries for electric vehicles that are based on lithium undergo four separate stages in their lifecycles: initial raw materials, battery manufacturing, operation, and end-of-life management. After these materials have been improved by pre-processing factories, the battery manufacturing companies take over and begin manufacturing batteries and assembling them into packs. They are then sent to automobile manufacturers for use in electric vehicle integration. A lack of management could result in the waste of important materials in batteries at the end of the manufacturing process. The purpose of a successful end-of-life care phase is to bring the cycle full circle. Depending on the condition of the battery, it will either be recycled or repurposed for another purpose. To ensure that batteries are durable, companies and governments must collaborate closely to achieve this goal. By now, the raw materials phase, together with the battery manufacturing and operating phases, have all been thoroughly tested and proven. Because of economic constraints, the recycling industry has been unable to expand [
27]. It is predicted that only 6% of lithium-ion batteries were collected for recycling in Australia during the 2017–2018 fiscal year. The value of wrapping up loose ends, on the other hand, cannot be stressed. Recycling electric vehicle batteries has the potential to maximize the environmental benefit for a variety of reasons, not the least of which is a forecasted shortage of nickel, cobalt, and lithium in the future. Recycling has proven to be one effective strategy.
Li-ion batteries are the most common form of electric car battery. We may already be familiar with this battery due to its widespread use in mobile devices such as smartphones and laptop computers. Scale is the primary factor separating the two. The physical capacity and size of the traction battery pack in electric cars are significantly larger. In terms of power to weight, lithium-ion batteries are the best. These batteries are extremely efficient at storing energy. High-temperature performance is also good. The battery has a higher energy-to-weight ratio, which is critical for electric car battery performance. The longer the automobile can go on a single charge, the lighter the battery weight (for the same kWh capacity). Additionally, this battery has a low “self-discharge” level, which means that the battery is superior to any other battery in terms of its capacity to maintain its full charge. Li-ion batteries can also be recycled, making them a good option for those who care about the environment while purchasing electric vehicles. Lithium batteries are used in most BEV and PHEV vehicles [
28].
There is one advantage currently associated with Li-ion batteries. Currently, a vehicle’s HV battery must be changed as a whole, even if only one cell is damaged. This is neither resource-saving nor cost-efficient. An external cell balancer, on the other hand, provides for a targeted repair of the truly faulty sections and so leads to substantially better resource and cost efficiency. An additional environmental consequence derives from the removal of hazardous goods transfers that are now incurred by the central maintenance system. If a section of the cells or a module in a lithium-ion HV battery of a vehicle is bad and needs to be replaced, the defective cell must first be located and removed [
29,
30]. The freshly inserted cell must first be brought to the same state of charge as the remaining intact cells of the battery. If this were not considered, the weakest cells would be destroyed, and in severe situations, this may spark a fire. A cell balancer enables the ability of conditioning individual modules in the workshop and thus mending the HV battery on site.
6. Challenges and Chances Associated with the Energy of Lithium-Ion Battery
As the specific energy and energy density are higher in LIB, this has encouraged the displacement of competing battery chemistries in nearly every industry and application. NiMH and NiCad can only maintain cell voltages three times higher than those found in LIBs, which are currently the most used rechargeable chemistries (NiCd). Even though the electrochemical pair involving Co
3+/
4+ and Ni
3+/4+ as a part of high energy design has remained unaltered since their origin, LIB nominal voltages have improved slightly over time (against graphite). Scientific advancements in the active material capacity and cell/electrode optimization are responsible for nearly all the gains in specific energy. This means that future efforts to electrify a wide range of vehicles will confront a significant challenge. In the future, higher specific energy batteries will require the addition of new materials. Vehicle-level goals were translated into material-level ones by the USA aims [
31,
32,
33]. For the cathode and anode voltage connections, we assumed that they were conventional LIB cells. The materials chemistry sector can take comfort from the partnership of two automotive manufacturing consortia. No mature cathode materials can meet or exceed the performance parameters for future automotive materials according to the results of an examination of their performance as a cathode. For LIB cathodes, stacked lithium TMOs have become the most common cathode material since their introduction. It has been possible to enhance the capacitance of lithium cobalt oxide over time by adding dopants and coatings, and this has allowed the material to handle higher charge voltages. As a result, the price of the metal has been reduced while still maintaining a balance between energy consumption and production costs. However, structural stability and voltage fade concerns must be solved before EUCAR cathode objectives can be met with lithium-rich-layered oxide (LLO). Because future LIB cathode materials are currently unavailable, further progress is being hampered.
Carbon anodes are being phased out in favor of lithium-ion battery anodes, which can satisfy future energy needs but are less efficient. Lithium and silicon are the two anode materials that have drawn the most interest due to their design versatility. Technical hurdles must be solved before lithium metal may be used in automotive applications. When commercial carbon/silicon mixes were employed, silicon was a more practical advanced anode material because of its higher melting point. However, this was no longer the case. When compared to carbon, silicon has a much higher gravitational potential energy. There are practical constraints to pure silicon because of lithium loss and particle isolation owing to cycling volume changes. Even though this material only has a capacity of 2000 mAh/g compared to pure silicon, it is still suitable for industrial use. As a result, silicon is capable of withstanding changes in weight and volume over time [
34,
35].
Customers who frequently exceed the battery’s maximum capacity should be especially worried. A car can be charged to 80% SOC in less than 40 min, giving it a few hundred miles of range. At night or while at work, drivers of electric vehicles are out of luck when it comes to finding a quick charge. To reach 200 miles of range in 7.5 min with ultra-fast charging is the long-term goal of the Department of Energy. A target of 80% SOC in 15 min has been set for 2023 by the USABC. Because lithium-ion implantation in carbon has a low electrochemical potential, LIBs offer great energy density. At or around the negative electrode potential, lithium metal has the most potential. Short-circuiting and thermal runaway are just some of the possible consequences of a loss in capacity. Because of this, faster charging is not becoming more widespread [
36]. As a result of these new methodologies, cell and battery charging can be sped up significantly. The thickness and porosity of electrodes can be optimized to reduce voltage polarization. Consequently, lithium intercalation is now achievable. The P/E of a cell can be altered by altering the electrode chemistry. High power can be achieved with the same active material and modifications to the electrode structure. Instead of focusing on cell and electrode engineering, scientists should focus on generating high-energy materials. Customers have the option of purchasing a vehicle with a much higher starting price, with any possibility of 600–700 Wh/L charging rates being attainable. Fast charging speeds of up to 50 kW result in a decrease in cell capacity of 1.5% to 4%, according to previous cell designs. When designing future cell designs with the same versatility as today’s cell designs, this trade-off correlation can be applied to intercalation materials combined into agglomeration coatings rather than solid-state electrolytes with metal electrodes. Furthermore, there are higher thermal management requirements for charging at a faster rate, which are not considered.
Controlling the temperature of a system is critical. To keep up with the increasing charge rates, cooling systems must be modified on a regular basis. A device’s cooling capacity is currently limited by the amount of surface area it can utilize. Heat removal pathways are limited in the surface area because of the need to maximize battery pack density. An increase in material density means that a smaller amount of storage space may be used to increase heat removal capabilities. It is possible to attain incredibly rapid charging with existing materials using high temperatures and greater room in the pack for thermal management with more advanced technologies [
37,
38]. If high-temperature charging is to be more than just a one-time event, it needs to be made more durable at high temperatures.
7. Access to Resources, Threats of Depletion, and Long-Term Sustainability of Lithium
It is important to note that LIB extraction/mining and refining/processing are directly linked to resource availability, depletion, and the sustainable use of resources. The supply of LIB materials is limited, as with all natural resources. LIB material resource and reserve volume assessments are challenging to calculate, since estimates of economically recoverable reserves have been rising over time and lack consensus [
39,
40,
41]. According to the US Geological Survey, worldwide lithium reserves climbed 241.5% between 2009 and 2018 to 14.0 million metric tons. The demand for LIB materials now outstrips supply, which generally indicates adequate resource and reserve quantities, as well as stable or dropping costs. However, if attempts to cut transportation-related CO
2 emissions intensify, demand will increase significantly. Concerns concerning scarcity, resource depletion, sustainability, and increased pricing of necessary components will only grow as the demand for automotive LIB surges. Peak lithium or peak cobalt, as with the concept of peak oil, might be a source of concern [
22]. In spite of the optimism of many analysts, the various assumptions that must be made, as well as the lack of knowledge, are said to create poor forecasts [
42]. There is a lot of ambiguity about LIB growth projections [
43] arguing that solid governance and effective laws are essential at both the national and international levels to ensure the long-term sustainability of LIB materials.
LIB materials can be stored in a “metal bank” to protect against supply shocks and variable costs. Stockpiling petroleum with strategic oil reserves has served as a precedent for this strategy, which tries to stabilize prices during periods of high volatility. Stockpiling of cobalt is the focus of the Canadian corporation Cobalt 27 Capital Corp [
44]. Recycling spent batteries could be another way to mitigate the depletion of LIB resources and raw materials supply problems. Because so few EV LIBs have reached the end of their useful lives, research estimates that nearly 95% of LIBs are now being landfilled based on data relating to LIBs used in portable devices. There will soon be many EV LIBs that need to be disposed of or recycled. Collection, technology, and economics all play a role in recycling LIBs. Prior to being disposed of in landfills, automobile LIBs should be collected. However, LIBs can be recovered through a process known as “landfill mining” [
45]. It is also difficult, expensive, and environmentally damaging to separate the various elements from a LIB.
Depending on their availability, cost, and simplicity of removal from the battery, decisions must be made on which materials should be prioritized for removal and re-use. Since raw materials are much more expensive than recycling, governments will likely need to offer the necessary incentives. All throughout the world, the LIB sector is paying greater attention to vertical integration, which includes everything from mining and refining raw materials to producing battery cells and whole batteries and then recycling the finished products. Even though the United States has some lithium reserves, it lacks upstream capacity in mining and processing any of the essential raw materials needed to produce lithium ion. Recent Chinese efforts to vertically integrate their capacity have yielded promising results. Another 21% of the market is in the remainder of Asia, particularly South Korea and Japan. Most of the main components, including anodes, electrolytes, separators, and cathodes, are manufactured in China. A Strategic Action Plan for Batteries issued by the European Union (EU) in May 2018 includes initiatives aimed at promoting synergies between the government and industry to expand the European LIB value chain.
8. Factors Influencing Fire Hazard in Li-Ion Battery-Based Electric Vehicles
It is possible for fires to start in cars, which is posing as a danger to electric vehicles. As the number of EVs increases, so does the number of EVs on the road. In BEV and PHEV battery systems, self-ignition is a typical occurrence. When it comes to moving a vehicle, the capacity of a battery is comparable to that of an ICEV’s gas tank. To put it another way, the size and capacity of an electric vehicle’s battery pack is directly related to its risk of a battery cell fire. Increasing the batteries eventually enhances the storage of energy as well further leading to the risk of an electric vehicle’s batteries catching fire.
In electric vehicles, there are a lot of batteries. A higher battery capacity is necessary since an electric vehicle consumes thousands of times more electricity and does so much more quickly than a regular smartphone. Batteries for electric vehicles must have high energy capacity and output power (up to 100 kW) (up to tens of kWh). In addition, they must be able to overcome significant weight and space constraints while still being competitively priced. An electric vehicle’s battery is composed of three main parts: a pack consisting of three cells, and the first two are known as cells. To make a LIB, the battery cells are connected in series/parallel to form the basic building block: a battery module. A suitable frame is employed to safeguard the cells from damage caused by external factors such as shock, heat, and vibration. The modules are assembled by the battery pack. Basically, it is just one piece of gear. Power electronics and cooling loops are also part of the system’s infrastructure. Modules for handling electricity, charging and discharging, and temperature control are also included. These systems are referred to as the Battery Management System (BMS) for short (BMS). Electric vehicles (EV) have considerable energy storage capacity because of their tiny size [
46,
47].
When it comes to temperature regulation, it can be problematic because of this. Battery cell characteristics have a major impact on the overall performance and range of an electric vehicle. Lead acid, nickel cadmium, nickel metal hydride, and nickel metal hydride batteries have all been utilized in electric cars (NiMh). These devices have a lower energy density, capacity, and charging and discharging speeds than LIBs. As a result, today’s EVs are unable to use them. Since Dr. Goodenough’s LIB was commercialized by Sony in 1991, it has been used in a variety of electrical devices, including electric automobiles. Currently, the LIB leads the electric vehicle market and is anticipated to remain so for some years to come [
48]. Because of their high energy density and long cycle lives, LIBs have grown in appeal as an alternative to regular battery technologies. Because of its lighter weight, the LIB can be moved by car more simply.
Cylindrical, prismatic, and pouch cells all have separate designs and manufacturing processes. The capacity of EV LIB cells ranges from 3 to 300 Ah, depending on the kind and manufacturer of the battery pack. Vehicle energy density is typically above 100 Wh/kg. The LIB cell’s chemistry and design generate this power. As a result, Tesla’s cylindrical 18,650 battery has a specific energy of 3.4 Ah per cell or 248 Wh/kg weight per kilogram, which is made up of nickel, cobalt, and aluminum oxide (NCA). The Tesla Model S, with its 100 kWh battery, is currently the most potent passenger electric car on the road. On a single charge, it can travel over 380 km (240 miles). The risk of an EV fire due to overloaded batteries grows if the potential fuel load increases (or fuel) [
49,
50].
There has been an increase in the risk of fire and LIB since the widespread use of Li-ion batteries in electric vehicles over a decade ago. Battery packs’ high energy density and large deployment scale account for this. To say that lithium has many potential dangers would be an understatement. Lithium-ion batteries can release hazardous smoke and flammable gases when they are damaged by an external force and exposed to hard working conditions. It is therefore feasible to have continuous combustion, flame jets, and even a gas explosion. Battery systems are unlikely to self-ignite under normal operating settings, but they are subject to heat, mechanical, and electrical assaults from the outside. Even though they are rare, electrical shocks and other hazardous working conditions are considered the standard for most computers and smartphones. Because of their inability to handle sudden acceleration and deceleration, EV batteries confront more severe working conditions than those of conventional batteries. EVs have hundreds of times more battery capacity (or fuel load) than portable electrical devices, which increases the fire hazard in the event of a battery failure. Advanced safety systems are built into electric vehicles and their batteries, reducing the chance of (spontaneous) breakdowns. In the context of electric cars, the concept of portable electronic gadget battery fire danger should not be used as a basis for evaluation [
51].
The first impact may be due to the thermal aspect. For cold- and hot-temperature driving, electric cars have the same user demand as traditional internal combustion vehicles. It is possible that electric cars (EVs) might be used in areas where temperatures can reach 45 degrees Fahrenheit in the summer and in Canada, where daily lows can fall to −5 degrees Fahrenheit and occasionally even −15 degrees Fahrenheit, soon. The battery performs best when kept at a temperature of 20 to 30 degrees Fahrenheit. Batteries’ lifespans are shortened when they are subjected to high temperatures [
52]. Chemical processes occurring at high temperatures can cause batteries to overheat. An EV can cause a thermal runaway, which can lead to an explosion, if the thermal dispassion is insufficient. Batteries lose efficiency because of an increase in internal resistance produced by low temperatures. Through the stimulation of metallic dendrite growth and a consequent increase in battery heat generation, this resistance escalates the danger of a fire.
Most commercial LIB cells are sensitive to mechanical damage without the protection of an electric vehicle frame or a battery module and pack container. It is possible for an electric vehicle to be involved in a traffic collision at any time during its lifetime. Battery degradation is a major concern for most current LIBs and EVs. It is common practice to place LIB packs in heavily reinforced areas of a vehicle to make them less vulnerable in the case of a collision. Even the most rigorous safeguards fall short when it comes to preventing fires from occurring frequently at high speeds, as some electric vehicles may do in a short period of time.
Faster charging and discharging speeds and better driving performance raise the risk of a fire in electric vehicles. Long-term energy buffers (LIBs) accept and store energy for a predetermined period. Overloading or charging the battery too quickly can lead to early failure, which can happen in some situations. Misuse of electricity frequently results in the generation of heat and the generation of joules. While one may result in a short circuit, the other generates heat within the gadget. An EV fire can be caused by a wide variety of factors. Short circuits, overcharging, and a heated environment are just a few of the possible causes. Battery management systems (BMSs) prevent most sorts of electrical abuse in electric vehicles if they are correctly built and functioning [
53]. Batteries that spontaneously ignite, or “self-ignition,” are not usually the result of damaged cells, but rather of poor manufacturing and design practices, electrical control systems, and battery management software.
When it comes to electrical vehicle fires, using a LIB is the most common culprit. The quantity of heat that can be emitted from an EV in the event of a fire is rising along with the number of LIBs and EVs. A battery fire is more likely if its mass and capacity are increased (or the fuel). During the combustion of LIBs, toxic fumes, and combustible gases such as hydrogen (H
2), methane (CH
4), and carbon monoxide (CO) may emerge. Individuals who encounter these gases are at risk. When it comes to fire safety, electric vehicles (EVs) provide a unique set of challenges that must be addressed. When it comes to making an informed decision about an electrical vehicle (EV) fire, having a better understanding of five essential fire characteristics will help. Thermal runaway, the most common type of battery failure, can cause an electric vehicle to catch fire if the batteries fail [
54,
55]. Thermal runaway events occur when exothermic chain reactions overwhelm cooling. In chemical and combustion processes, this is a common phenomenon.
Thermal, thermochemical, and electrochemical reactions in the LIB can be detected by increasing temperatures by more than 10 degrees Celsius per minute or by activating the safety vent. There is a great deal of smoke and high-intensity sparks and jet flames produced when a battery goes into a thermal runaway state. A thermal runaway or fire could develop across the battery’s cells if this process is allowed to continue in each cell. After it has begun, thermal runaway causes the safety valve to emit smoke or the battery shell to shatter. In the smoke that results from this procedure, you can find these flammable and deadly gases. Combustible gases can be ignited by sparks, flames, electrical arcs, and other heat and light sources. Self-ignition can also occur if there is not enough cooling in place. If the flame is extinguished, it is likely that the battery’s temperature would rise even further [
56]. If the pace at which gas is expelled from the battery shell exceeds the rate at which gas is generated within the battery, the battery cell could explode. The pre-ignition thermal runaway phase may be alleviated somewhat by a safety valve, but it may not prevent the cell from being heated externally, for as by radiation from a nearby flame or a nearby burning battery.
Both internal combustion engines (ICEs) and electric vehicles (EVs) have power systems, liquid petroleum fuel (LPF), and flammable plastic components (EVs). Cars today weigh between 100 and 200 kg, making plastic a considerable portion of their total weight, which is significantly heavier than petrol (less than 50 kg). Polyethylene’s heat output (47 MJ/kg) is comparable to that of gasoline, which indicates that burning plastic components in automobiles may contribute significantly to vehicle fires, especially when fuel tanks are not completely full. Comparing electric vehicles (EVs) to ICEVs (internal combustion vehicles) (ICEVs) (internal combustion engines) (ICEVs) (internal combustion engines) (gasoline vs. battery), a direct correlation exists between LIB’s heat of combustion and the chemistry, packing, capacity, and state of charge of its combustible components (SOC). A total of 18,650 cylindrical batteries have been discovered to have a combustion heat of 2 MJ/kg, which is much higher than the combustion heat of a commercial pouch-type LIB (2.9 Ah, 11 Wh). Most of the time, the heat created by LIB is orders of magnitude less than that generated by gasoline in the same circumstances. Even though batteries are heavier than gasoline tanks due to their lower energy density, this does not make them any more difficult to move around than fuel tanks (chemical or electrical) [
57]. It has been shown that both internal and external heat from the battery’s flame and flammable gases contributed significantly to the release of thermochemical energy, which included both internal and external heat. In a single second, a fire in an electrical battery can dissipate 510 times its own weight in power (or kinetic energy).
9. Fast Charging of Lithium-Ion Batteries
Battery development has always sought to advance by providing more energy in less time, at a cheaper cost, and with greater safety. Since the commercialization of lithium-ion batteries (LIBs) in 1991, significant advancements have been made in the industry [
58,
59]. By employing other cathode materials, LIBs’ specific energy might be greatly boosted. For instance, using LiNi
xCo
yMn
1−x−yO
2 (NCM), it could achieve 421 W h kg
−1 as compared to 279 W h kg
−1 of original LiCoO
2 (LCO).
The usage of batteries that charge too slowly in comparison to vehicles powered by traditional internal combustion engines is a significant obstacle to the broad adoption of electric vehicles. Customers anticipate that recharging an electric vehicle’s range (500–800 km) at a gas station will take no more than five minutes based on their previous experience.
It is necessary to raise the power density PV of used battery cells at the expense of a lower energy density WV to achieve fast-charging capabilities.
Thus, there are always compromises to be made between long-range and quick charging. According to kinetic models of such cells, overpotentials can be found in every area of battery cells. Li metal plating, insufficient use of the active material, and an increase in temperature result from the movement of lithium ions and electrons in the electrodes, charge transfer across phase boundaries to transit via the electrolyte, and polarization processes that limit the charging rate [
60].
The diffusion of lithium ions in the anode active material (AAM), cathode active material (CAM), transport of lithium ions in the electrolyte phase (liquid or solid), and charge-transfer kinetics at the phase boundaries have all been identified as rate-limiting processes.
12. Conclusions
The Li-ion battery has advanced to its current state of high energy density, high cycle life, and high efficiency through high levels of research and has clear fundamental benefits. However, research on new electrode materials continues in an effort to push the boundaries of cost, energy density, power density, cycle life, and safety. Many potential anode and cathode materials have poor electrical conductivity, slow Li transport, dissolution or other negative interactions with the electrolyte, poor thermal stability, rapid volume expansion, and mechanical brittleness, despite the fact that there are many different potential anode and cathode materials. Numerous new products have entered the intercalation cathode market, and conversion material technology is steadily moving toward widespread commercialization. Over the past two decades, research on Li-ion battery electrode materials has exploded. As new materials and processes are developed, Li-ion batteries will unquestionably have a bigger and bigger impact on our lives.