Removal of Odors (Mainly H2S and NH3) Using Biological Treatment Methods
Abstract
1. Introduction
2. Gases Emitted in the Municipal Sector
- sulfur compounds, i.e., hydrogen sulfide, thiols, sulfides, and alkyl disulfides,
- nitrogen compounds, i.e., ammonia, and aliphatic amines,
- organic compounds, including aldehydes, ketones, and fatty acids (phenol, cresol, butyric acid, acetic acid, and valeric acid)
3. Commonly Used Odor Removal Technologies
3.1. Physicochemical Methods of Odor Removal
3.2. Biological Methods of Odor Removal
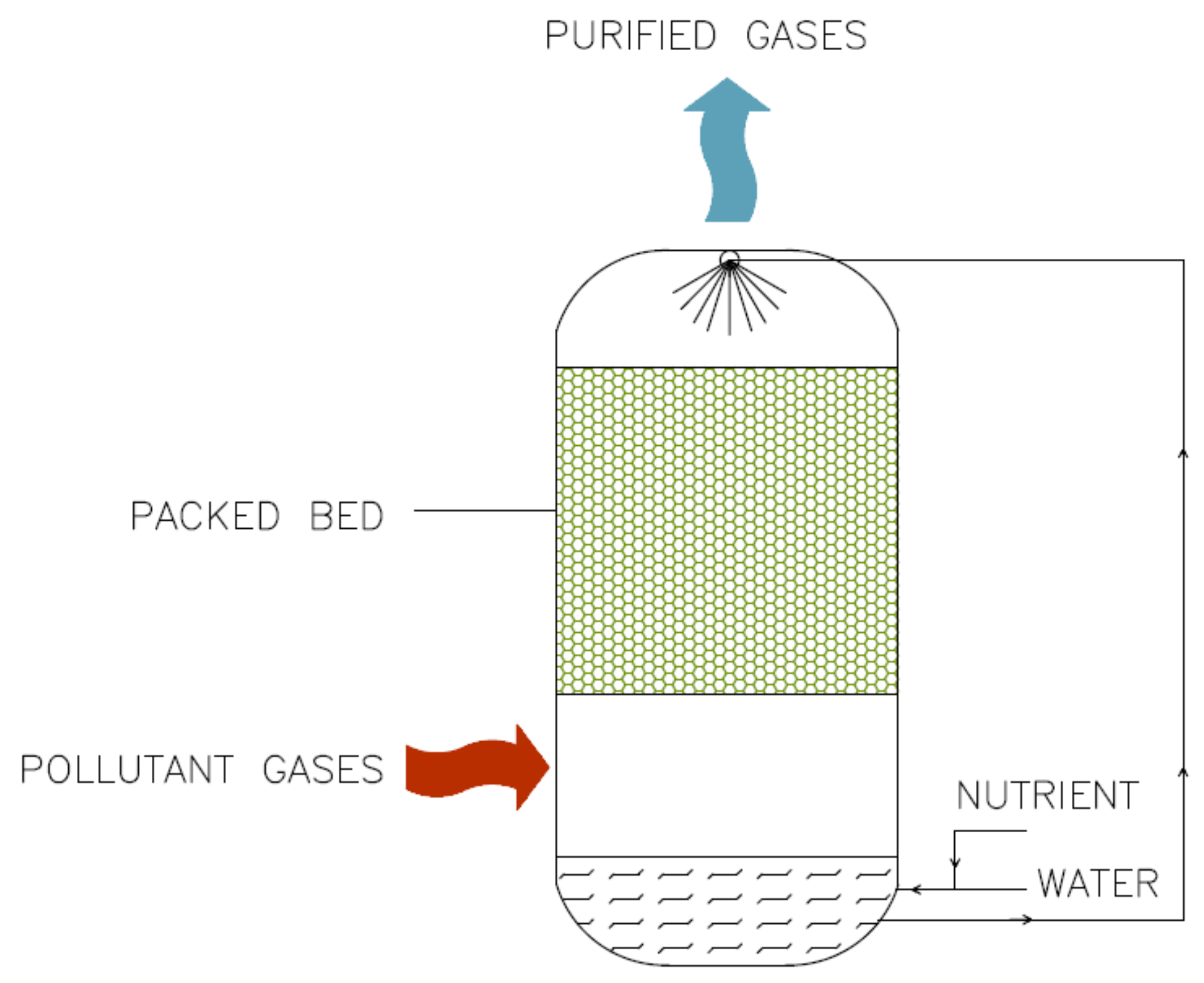
3.2.1. Biofilters
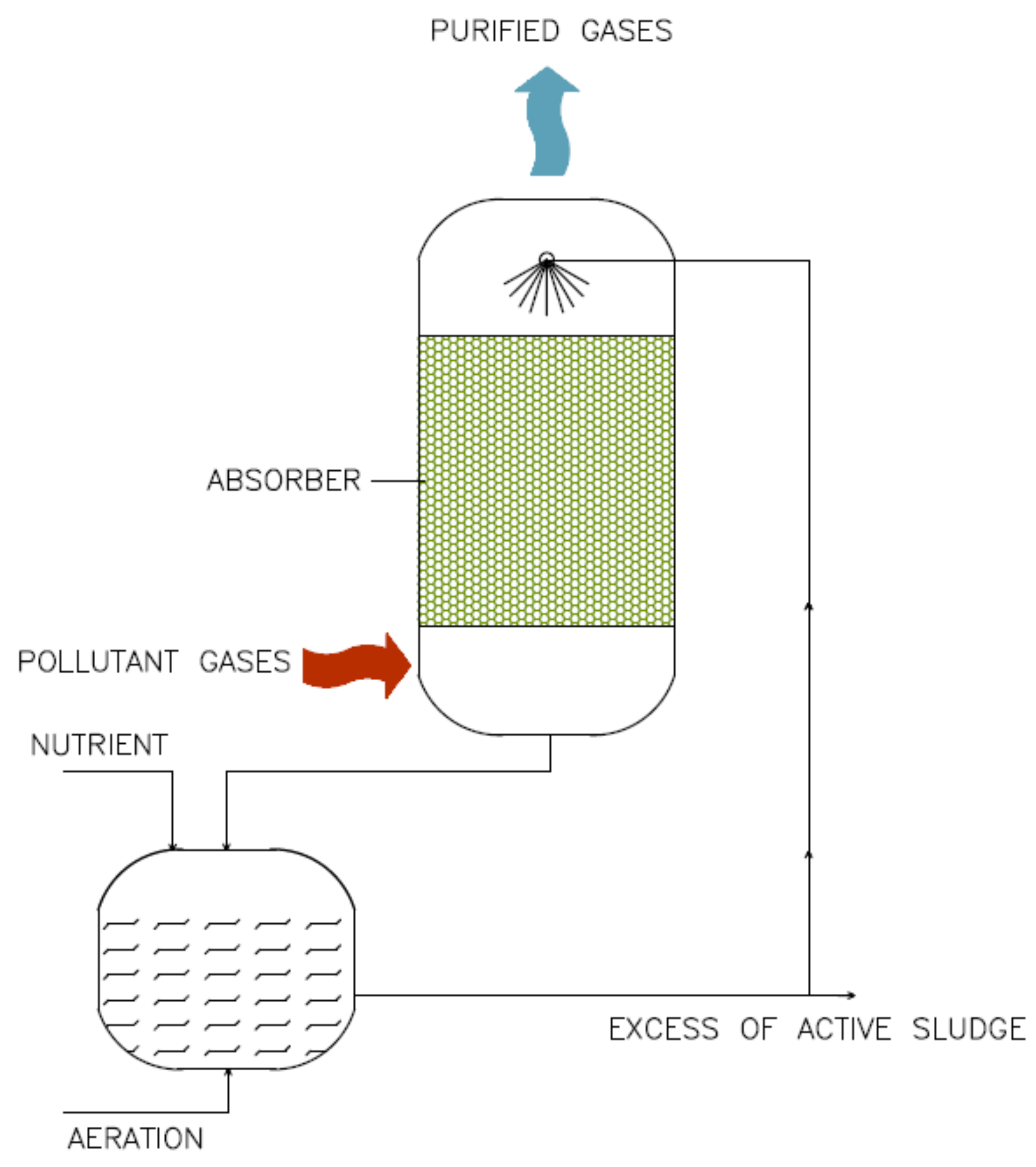
3.2.2. Bioscrubbers
3.2.3. Biotrickling Filters
4. Effectiveness of H2S and NH3 Removal Using Biological Methods of Odor Degradation
4.1. Application of Biofilters to Remove H2S and NH3
4.2. Application of Biotrickling Filters to Remove H2S and NH3
| Type of Odor | Method of Biological Biodegradation | Type of Microorganism/Bacterial Strain | Parameters (Type of Filling, T, pH) | Efficiency | References |
|---|---|---|---|---|---|
| H2S | Biofilters | Thiobacillus thioparus(H2S), Nitrosomonas europaea(NH3) | 30 °C Ca-alginate beads | 95% | [37] |
| Sulfur Oxidizing Bacteria and microorganisms from compost | Compost pH = 7.5 | 95% | [41] | ||
| Bacillus sp., Pseudomonas sp., Xanthomonoadacea sp. | Peat | 99% | [42] | ||
| Acidithiobacillus thiooxidans | Wood chips | 75 ± 13% to 97% | [44] | ||
| Sulfur oxidizing bacteria | Acid resistant polyethylene packing material—AMB BiomediaBioballs | 91.96% | [45] | ||
| - | Pine bark | 94% | [46] | ||
| Activated sludge | Pieces of Poly Vinyl Chloride with compost | 84–99% | [6] | ||
| Biotrickling filters | Raw influent water from plant (Hyperion treatment plant) | 7 layers of a PVC COOLdektmtmMunsters | 98% | [48] | |
| Heterotrophs, yeast, fungi, autotrophic sulfur-oxidizers | Pall rings, I biotrickling filter pH = 4.5, II biotrickling filter pH = 7 | ~100% | [49] | ||
| Primary and secondary sludge from Orange County Sanitation District | Polyurethane foam, T = 18–24 °C | ~98% | [50] | ||
| Thiobacillus thioparus, Acidithiobacillus thiooxidans | Volcanic stones, polypropylene rings, polyvinilclorure, pH = 5.5–7 | 100% | [51] | ||
| Acidithiobacillus thiooxidans | Polyurethane foam | 98–99% | [52] | ||
| Pseudomonas fluorescens, Thiobacillus sp. | Polyethylene rings, T = ~30 °C, pH = 5.5–7.5 | 97% | [20] | ||
| Bacterial strains | Polyethylene rings, pH = 5–7.5, T = ~30 °C | 80–99%; 60–80% | [55] | ||
| Pseudomonas sp., Thiobacillus sp. | Polypropylene rings | 91.8% | [56] | ||
| Acidithiobacillus sp., Metallibacterium sp. | Polypropylene rings | 86.1% | [57] | ||
| Activated sludge from Wastewater Treatment Plant (WWTP) | Bamboo charcoal | 99% | [58] | ||
| Dokdonella sp., Ferruginibacter sp., Nitrosomonas sp. and Thiobacillus sp. | Polyhedral spheres | 98.25% | [58] | ||
| Acidithiobacillus sp., Thiobacillus sp. | Raschig rings and ceramsite | 99% | [62] | ||
| NH3 | Biofilters | Thiobacillus thioparus(H2S), Nitrosomonas europaea(NH3) | 30 °C Ca-alginate beads | 95% | [37] |
| Activated sludge from Wastewater Treatment Plant (WWTP) | Organic: compost, bark, peat Inorganic: pearlite | 100% | [38] | ||
| - | Fibrous peat, coarse peat, wheat straw, composts, horse manure | 89% | [39] | ||
| Compost | ~96% | [40] | |||
| Nitrate oxidizing bacteria (Nitrosomonas sp., Nitrobacter sp.)—from cattle manure | Cattle manure, rice husk, gravel as a supporting media, 32–39 °C | 61.5%—for a bed 20 cm deep, 71.45%—for a bed 40 cm deep | [43] | ||
| - | Acid resistant polyethylene packing material—AMB BiomediaBioballs | 100% | [45] | ||
| - | Pine bark | 91% | [46] | ||
| Activated sludge | Pieces of Poly Vinyl Chloride with compost | 88–99.6% | [6] | ||
| Micromycetes: Acremoniumstrictum, Aspergillus versicolor, Aureobasidium pullulans, Cladosporium sp., Penicillium sp., Gliocladiumviride, Stachybotrys sp., Cladosporiumherbarum; Yeast: Exophiala sp., Aureobasidiumpullulans; Bacteria: Rhodococcus sp., Bacillus subtilis | Straight and wavy lamellar plates (hydrophilic synthetic texture), pH = 7, T = 24–32 °C | 84.2%–87% | [47] | ||
| Biotricklingfilters | Acivated sludge from Wastewater Treatment Plant (WWTP) | Composite balls made of ceramics and bovine bones | 92–100% | [59] | |
| Autotrophic and heterotrophic bacteria | Polyurethane foam | 98.4% | [60] | ||
| Dokdonella sp., Ferruginibacter sp., Nitrosomonas sp. Thiobacillus sp. | Polyhedral spheres | 88.55% | [61] | ||
| Acidithiobacillus sp., | Raschig rings and ceramsite | 99% | [62] | ||
| Thiobacillus sp., Ammonia Oxidizing Bacteria, Nitrite Oxidizing Bacteria | Activated carbon fiber | 98.5% | [63] |
5. Directions of Future Research
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seo, Y.-K.; Suvarapu, L.N.; Baek, S.-O. Characterization of Odorous Compounds (VOC and Carbonyl Compounds) in the Ambient Air of Yeosu and Gwangyang, Large Industrial Areas of South Korea. Sci. World J. 2014, 2014, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.; Krzeszowiak, J.; Pawlas, K. Does exposure to unpleasant odors harm human health? Environ. Med. 2014, 17, 76–81. [Google Scholar]
- Abraham, M.H.; Gola, J.M.; Cometto-Muñiz, J.E.; Cain, W.S. The Correlation and Prediction of VOC Thresholds for Nasal Pungency, Eye Irritation and Odour in Humans. Indoor Built Environ. 2001, 10, 252–257. [Google Scholar] [CrossRef]
- Mudliar, S.N.; Giri, B.; Padoley, K.; Satpute, D.; Dixit, R.; Bhatt, P.; Pandey, R.; Juwarkar, A.; Vaidya, A. Bioreactors for treatment of VOCs and odours—A review. J. Environ. Manag. 2010, 91, 1039–1054. [Google Scholar] [CrossRef]
- Kośmider, J.; Mazur-Chrzanowska, B.; Wyszyński, B. Odors; Polish Scientific Publishers PWN: Odory, Poland, 2012. [Google Scholar]
- Alinezhad, E.; Haghighi, M.; Rahmani, F.; Keshizadeh, H.; Abdi, M.; Naddafi, K. Technical and economic investigation of chemical scrubber and bio-filtration in removal of H2S and NH3 from wastewater treatment plant. J. Environ. Manag. 2019, 241, 32–43. [Google Scholar] [CrossRef]
- Liang, J.; Cheng, G.; Feng, H. Engineering practices of dezodorization for odor in urban sewage treatment plant in China. Adv. Econ. Bus. Manag. Res. 2016, 30, 86–91. [Google Scholar] [CrossRef][Green Version]
- National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals; The National Academies Press: Washington, DC, USA, 2008; Volume 6. [Google Scholar]
- United States Department of Labor. Available online: https://www.osha.gov/SLTC/hydrogensulfide/hazards.html (accessed on 18 July 2020).
- Nagaraj, A.; Sattler, M.L. Correlating Emissions with Time and Temperature to Predict Worst-Case Emissions from Open Liquid Area Sources. J. Air Waste Manag. Assoc. 2005, 55, 1077–1084. [Google Scholar] [CrossRef]
- Sówka, I.; Sobczyński, P.; Miller, U. Impact of Seasonal Variation of Odour Emission from Passive Area Sources on Odour Impact Range of Selected WWTP. Rocz. Ochr. Sr. 2015, 17, 1339–1349. [Google Scholar]
- Sobczyński, P.; Sówka, I.; Bezyk, Y. Characteristics of primary clarifiers odour emission variability and its influence on wastewater treatment plant odour impact range. Interdiscip. IssuesEng. Environ. Prot. 2015, 6, 356–363. (In Polish) [Google Scholar]
- Adebayo, A.; Jekayinfa, S.; Linke, B. Effects of Organic Loading Rate on Biogas Yield in a Continuously Stirred Tank Reactor Experiment at Mesophilic Temperature. Br. J. Appl. Sci. Technol. 2015, 11, 1–9. [Google Scholar] [CrossRef]
- Lewkowska, P.; Cieślik, B.M.; Dymerski, T.; Konieczka, P.; Namieśnik, J. Characteristics of odors emitted from municipal wastewater treatment plant and methods for their identification and deodorization techniques. Environ. Res. 2016, 151, 573–586. [Google Scholar] [CrossRef]
- Barbusiński, K.; Kalemba, K. Use of Biological Methods for Removal of H2s from Biogas in Wastewater Treatment Plants—A Review. Arch. Civ. Eng. Environ. 2016, 9, 103–112. [Google Scholar] [CrossRef]
- Zabava, B.S.; Voicu, G.; Ungureanu, N.; Dinca, M.; Paraschiv, G.; Munteanu, M.; Ferdes, M. Methods of biogas purification—A review. In Proceedings of the Acta Technica Corviniensis—Bulletin of Engineering, Hunedoara, Romania, 27 January 2019; Volume 12, pp. 65–68. [Google Scholar]
- Okoro, O.V.; Sun, Z. Desulphurisation of Biogas: A Systematic Qualitative and Economic-Based Quantitative Review of Alternative Strategies. Chem. Eng. 2019, 3, 76. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valorization 2017, 8, 267–283. [Google Scholar] [CrossRef]
- Kwarciak-Kozłowska, A.; Bańka, B. Biofiltracja jako metoda unieszkodliwiania odorów powstających podczas kompostowania frakcji biodegradowalnej odpadów komunalnych i przemysłowych. Inżynieria Ochr. Sr. 2014, 17, 631–645. [Google Scholar]
- Kasperczyk, D.; Urbaniec, K.; Barbusinski, K.; Rene, E.R.; Colmenares-Quintero, R.F. Application of a compact trickle-bed bioreactor for the removal of odor and volatile organic compounds emitted from a wastewater treatment plant. J. Environ. Manag. 2019, 236, 413–419. [Google Scholar] [CrossRef]
- Alfonsín, C.; Lebrero, R.; Estrada, J.M.; Muñoz, R.; Kraakman, N.J.R.; Feijoo, G.; Feijoo, G. Selection of odour removal technologies in wastewater treatment plants: A guideline based on Life Cycle Assessment. J. Environ. Manag. 2015, 149, 77–84. [Google Scholar] [CrossRef]
- Sarbak, Z. Bezpieczeństwoodoroweśrodowiska. PrzeglądNaukowo-Metodyczny. Eduk. Bezpieczeństwa 2014, 7, 151–171. [Google Scholar]
- Wysocka, I.; Gębicki, J.; Namieśnik, J. Technologies for deodorization of malodorous gases. Environ. Sci. Pollut. Res. 2019, 26, 9409–9434. [Google Scholar] [CrossRef]
- Kullavanijaya, E.; Trimm, D.L.; Cant, N. Adsocat: Adsorption/catalytic combustion for VOC and odour control. Stud. Surf. Sci. Catal. 2000, 130, 569–574. [Google Scholar] [CrossRef]
- Gospodarek, M.; Rybarczyk, P.; Szulczyński, B.; Gębicki, J. Comparative Evaluation of Selected Biological Methods for the Removal of Hydrophilic and Hydrophobic Odorous VOCs from Air. Processes 2019, 7, 187. [Google Scholar] [CrossRef]
- Barbusinski, K.; Kalemba, K.; Kasperczyk, D.; Urbaniec, K.; Kozik, V. Biological methods for odor treatment—A review. J. Clean. Prod. 2017, 152, 223–241. [Google Scholar] [CrossRef]
- Rene, E.R.; Montes, M.; Veiga, M.C.; Kennes, C. Novel Bioreactors for Waste Gas Treatment. In Environmental Chemistry for a Sustainable World; Springer: Dordrecht, The Netherlands, 2012; Volume 2, pp. 121–164. [Google Scholar]
- Chang, S.; Lu, C.; Huang, H.; Hsu, S. Removal of VOCs emitted from p-xylene liquid storage tanks by a full-scale compost biofilter. Process. Saf. Environ. Prot. 2015, 93, 218–226. [Google Scholar] [CrossRef]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef]
- Kala, R.; Chauhan, H.; Rajput, A.; Kutty, R. Biofilm characterization and quorum quenching in pathogenic strains Staphylococcus aureus and Pseudomonas aeruginosa. Int. J. Adv. Biotechnol. Res. 2012, 3, 515–522. [Google Scholar]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial Extracellular Polysaccharides Involved in Biofilm Formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Vergara-Fernández, A.; Quiroz, E.F.; Aroca, G.; Pulido, N.A.A. Biological treatment of contaminated air with toluene in an airlift reactor. Electron. J. Biotechnol. 2008, 11. [Google Scholar] [CrossRef]
- Alonso, C.; Zhu, X.; Suidan, M.T.; Kim, B.R.; Kim, B.J. Mathematical Model of Biofiltration of VOCs: Effect of Nitrate Concentration and Backwashing. J. Environ. Eng. 2001, 127, 655–664. [Google Scholar] [CrossRef]
- Smith, F.L.; Sorial, G.A.; Suidan, M.T.; Breen, A.W.; Biswas, P.; Brenner, R.C. Development of Two Biomass Control Strategies for Extended, Stable Operation of Highly Efficient Biofilters with High Toluene Loadings. Environ. Sci. Technol. 1996, 30, 1744–1751. [Google Scholar] [CrossRef]
- Cox, H.H.; Deshusses, M.A. Biological waste air treatment in biotrickling filters. Curr. Opin. Biotechnol. 1998, 9, 256–262. [Google Scholar] [CrossRef]
- Schiavon, M.; Ragazzi, M.; Rada, E.C.; Torretta, V. Air pollution control through biotrickling filters: A review considering operational aspects and expected performance. Crit. Rev. Biotechnol. 2016, 36, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Huang, C.; Tseng, C.-P.; Pan, J.R. Biotreatment of H2S- and NH3-containing waste gases by co-immobilized cells biofilter. Chemosphere 2000, 41, 329–336. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, Y.H.; Joo, D.J.; Choi, S.-J.; Ha, T.W.; Lee, D.H.; Park, I.H.; Jeong, Y.S. Removal of Ammonia by Biofilters: A Study with Flow-Modified System and Kinetics. J. Air Waste Manag. Assoc. 2003, 53, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Tymczyna, L.; Chmielowiec-Korzeniowska, A.; Saba, L. Biological treatment of laying house air with open biofilter use. Pol. J. Environ. Stud. 2004, 13, 425–428. [Google Scholar]
- Pagans, E.; Font, X.; Sánchez, A. Biofiltration for ammonia removal from composting exhaust gases. Chem. Eng. J. 2005, 113, 105–110. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Farooqi, I.H.; Ayub, S. Performance of Biofilter for the Removal of Hydrogen SulphideOdour. Int. J. Environ. Res. 2009, 3, 537–544. [Google Scholar] [CrossRef]
- Omri, I.; Aouidi, F.; Bouallagui, H.; Godon, J.-J.; Hamdi, M. Performance study of biofilter developed to treat H2S from wastewater odour. Saudi J. Biol. Sci. 2013, 20, 169–176. [Google Scholar] [CrossRef]
- Kavyashree, B.A.; Ramya, N.; Sanjay Urs, A.M.; Chandan, K.B.; Shilpa, B.S.; Rashmi, M.B. Ammonia gas removal using biofilter. Int. Adv. Res. J. Sci. Eng. Technol. 2015, 2, 110–114. [Google Scholar] [CrossRef]
- Aita, B.C.; Mayer, F.D.; Muratt, D.T.; Brondani, M.; Pujol, S.B.; Denardi, L.B.; Hoffmann, R.; Da Silveira, D.D. Biofiltration of H2S-rich biogas using Acidithiobacillusthiooxidans. Clean Technol. Environ. Policy 2015, 18, 689–703. [Google Scholar] [CrossRef]
- Rabbani, K.A.; Charles, W.; Kayaalp, A.; Cord-Ruwisch, R.; Ho, G. Pilot-scale biofilter for the simultaneous removal of hydrogen sulphide and ammonia at a wastewater treatment plant. Biochem. Eng. J. 2016, 107, 1–10. [Google Scholar] [CrossRef]
- Janas, M.; Zawadzka, A. Biofiltration as an effective method for reduction of pollutant emissions. Acta Innov. 2017, 23, 43–50. [Google Scholar]
- Baltrėnas, P.; Januševičius, T.; Zagorskis, A.; Baltrėnaitė-Gedienė, E. Removal of ammonia by biofilters with straight and wavy lamellar plates. Int. J. Environ. Sci. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Cox, H.H.J.; Deshusses, M.A.; Converse, B.M.; Schroeder, E.D.; Iranpour, R. Odor and volatile organic compound treatment by biotrickling filters: Pilot-scale studies at hyperion treatment plant. Water Environ. Res. 2002, 74, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Cox, H. Co-treatment of H2S and toluene in a biotrickling filter. Chem. Eng. J. 2002, 87, 101–110. [Google Scholar] [CrossRef]
- Gabriel, D.; Cox, H.H.J.; Deshusses, M.A. Conversion of Full-Scale Wet Scrubbers to Biotrickling Filters for H2S Control at Publicly Owned Treatment Works. J. Environ. Eng. 2004, 130, 1110–1117. [Google Scholar] [CrossRef]
- Aroca, G.; Urrutia, H.; Núñez, D.; Oyarzún, P.; Arancibia, A.; Guerrero, K.; Oyarzún, P. Comparison on the removal of hydrogen sulfide in biotrickling filters inoculated with Thiobacillusthioparus and Acidithiobacillusthiooxidans. Electron. J. Biotechnol. 2007, 10, 514–520. [Google Scholar] [CrossRef]
- Ramírez, M.; Gómez, J.M.; Cantero, D.; Paca, J.; Halecky, M.; Kozliak, E.I.; Sobotka, M. Hydrogen sulfide removal from air by Acidithiobacillusthiooxidans in a trickle bed reactor. Folia Microbiol. 2009, 54, 409–414. [Google Scholar] [CrossRef]
- Kasperczyk, D.; Barbusiński, K.; Kozik, V. Use of Compact Trickle Bed Bioreactor for the Purification of Air from a Voc’s Mixture—Preliminary Research. Arch. Civ. Eng. Environ. 2016, 9, 137–143. [Google Scholar] [CrossRef]
- Kasperczyk, D.; Parzentna-Gabor, A.; Barbusiński, K.; Urbaniec, K. Ecoinnovationnot Only for the paint industry. Industrialvarnishing 2020, 1, 38–40. (In Polish) [Google Scholar]
- Kasperczyk, D.; Urbaniec, K.; Barbusinski, K. Removal of Pollutants from the Air in a Copper-Ore Mine Using a Compact Trickle-Bed Bioreactor. Chem. Eng. Trans. 2014, 39, 1309–1314. [Google Scholar] [CrossRef]
- Sun, S.H.; Jia, T.P.; Chen, K.Q.; Peng, Y.Z.; Zhang, L. Removal of hydrogen sulfide produced in a municipal WWTP using a biotrickling filter with polypropylene rings as the packing material and microbial community analysis. Huan Jing KeXue 2019, 40, 4585–4593. [Google Scholar] [CrossRef]
- Sun, S.; Jia, T.; Chen, K.; Peng, Y.; Zhang, L. Simultaneous removal of hydrogen sulfide and volatile organic sulfur compounds in off-gas mixture from a wastewater treatment plant using a two-stage bio-trickling filter system. Front. Environ. Sci. Eng. 2019, 13, 60. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, L.; Cai, W.; Wu, J. Pilot-scale study using biotrickling filter to remove H2S from sewage lift station: Experiment and CFD simulation. Biochem. Eng. J. 2019, 144, 177–184. [Google Scholar] [CrossRef]
- Sakuma, T.; Jinsiriwanit, S.; Hattori, T.; Deshusses, M.A. Removal of ammonia from contaminated air in a biotrickling filter—Denitrifying bioreactor combination system. Water Res. 2008, 42, 4507–4513. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Khavanin, A.; Sharifi, A. Ammonia removal from a waste air stream using a biotrickling filter packed with polyurethane foam through the SND process. Bioresour. Technol. 2011, 102, 2517–2522. [Google Scholar] [CrossRef]
- Huan, C.; Fang, J.; Tong, X.; Zeng, Y.; Liu, Y.; Jiang, X.; Ji, G.; Xu, L.; Lyu, Q.; Yan, Z. Simultaneous elimination of H2S and NH3 in a biotrickling filter packed with polyhedral spheres and best efficiency in compost deodorization. J. Clean. Prod. 2021, 284, 124708. [Google Scholar] [CrossRef]
- Ying, S.; Kong, X.; Cai, Z.; Man, Z.; Xin, Y.; Liu, D. Interactions and microbial variations in a biotrickling filter treating low concentrations of hydrogen sulfide and ammonia. Chemosphere 2020, 255, 126931. [Google Scholar] [CrossRef]
- Liu, J.; Kang, X.; Liu, X.; Yue, P.; Sun, J.; Lu, C. Simultaneous removal of bioaerosols, odors and volatile organic compounds from a wastewater treatment plant by a full-scale integrated reactor. Process. Saf. Environ. Prot. 2020, 144, 2–14. [Google Scholar] [CrossRef]
- Holub, M.; Brandenburg, R.; Grosch, H.; Weinmann, S.; Hansel, B. Plasma Supported Odour Removal from Waste Air in Water Treatment Plants: An Industrial Case Study. Aerosol Air Qual. Res. 2014, 14, 697–707. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, Y.; Lyczko, N.; Nzihou, A. Current Status and Outlook of Odor Removal Technologies in Wastewater Treatment Plant. Waste Biomass Valorization 2018, 10, 1443–1458. [Google Scholar] [CrossRef]


| Odor Compounds | Substance | Concentration [ppm] | Detection Threshold [ppm] |
|---|---|---|---|
| Compounds with Nitrogen | Ammonia | 0.019–5.2 | 5 |
| Trimethylamine | 1.7 | 0.00044 | |
| Methylamine | 3.3 | 0.02 | |
| Pyridine | 0.013–0.82 | 0.084 | |
| Compounds with Sulfur | Hydrogen sulfide | 0.001–0.78 | 0.008 |
| Dimethyl sulfide | 0.0015–0.02 | 0.0023 | |
| Diethyl sulfide | 0.00025–0.0006 | 0.004 | |
| Diethyl disulfide | 0.000054 | 0.00043 | |
| Methyl mercaptan | 0.0001–0.55 | 0.001 | |
| Ethyl mercaptan | 0.000016–0.074 | 0.00076 | |
| Volatile Organic Compounds | Phenol | 0.047–0.65 | 0.040 |
| Cresol | 0.00047 | 0.0018 | |
| Butter acid | 0.00028–0.00056 | 0.004 | |
| Valeric acid | 0.0006 | 0.005 |
| Concentration NH3 [ppm] | Symptoms |
|---|---|
| <5–53 | Odor threshold |
| 30 | Slight irritation after 10 min. |
| 50 | Prolonged exposure may cause nausea, tearing of the eyes, Moderate irritation to the eyes, nose, throat and chest after 10 min to 2h |
| 80 | Moderate to highly irritation after 30 min to 2 h |
| 110 | Highly intense irritation after 30 min to 2hof exposure |
| 140 | Unbearable irritation after 30 min to 2h |
| 500 | Excessive lacrimation and irritation |
| 570 (21–30 years old) | Reflex glottis closure—a protective response to inhaling irritant vapors |
| 1000 (60 years old) | |
| 1790 (86–90 years old) |
| Concentration H2S [ppm] | Symptoms |
|---|---|
| 0.00011–0.00033 | Typical background concentrations |
| 0.01–1.5 | Odor threshold (rotten egg) |
| 2–5 | Prolonged exposure may cause nausea, tearing of the eyes, headaches or loss of sleep |
| 20 | Possible fatigue, loss of appetite, headache, irritability, poor memory, dizziness |
| 50–100 | Slight conjunctivitis (“gas eye”) and respiratory tract irritation after 1 h. May cause digestive upset and loss of appetite |
| 100 | Coughing, eye irritation, loss of smell after 2–15 min (olfactory fatigue). Altered breathing, drowsiness after 15–30 min. Throat irritation after 1 h. Gradual increase in severity of symptoms over several hours. Death may occur after 48 h |
| 100–150 | Loss of smell (olfactory fatigue or paralysis) |
| 200–300 | Marked conjunctivitis and respiratory tract irritation after 1 h. Pulmonary edema may occur from prolonged exposure |
| 500–700 | Staggering, collapse in 5 min. Serious damage to the eyes in 30 min. Death after 30–60min |
| 700–1000 | Rapid unconsciousness, “knockdown” or immediate collapse within 1 to 2 breaths, breathing stops, death within minutes |
| 1000–2000 | Nearly instant death |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbusiński, K.; Parzentna-Gabor, A.; Kasperczyk, D. Removal of Odors (Mainly H2S and NH3) Using Biological Treatment Methods. Clean Technol. 2021, 3, 138-155. https://doi.org/10.3390/cleantechnol3010009
Barbusiński K, Parzentna-Gabor A, Kasperczyk D. Removal of Odors (Mainly H2S and NH3) Using Biological Treatment Methods. Clean Technologies. 2021; 3(1):138-155. https://doi.org/10.3390/cleantechnol3010009
Chicago/Turabian StyleBarbusiński, Krzysztof, Anita Parzentna-Gabor, and Damian Kasperczyk. 2021. "Removal of Odors (Mainly H2S and NH3) Using Biological Treatment Methods" Clean Technologies 3, no. 1: 138-155. https://doi.org/10.3390/cleantechnol3010009
APA StyleBarbusiński, K., Parzentna-Gabor, A., & Kasperczyk, D. (2021). Removal of Odors (Mainly H2S and NH3) Using Biological Treatment Methods. Clean Technologies, 3(1), 138-155. https://doi.org/10.3390/cleantechnol3010009