Removal of COD and Ammonia Nitrogen by a Sawdust/Bentonite-Augmented SBR Process
Abstract
:1. Introduction
Paper Contribution and Organization
2. Materials and Methods
2.1. Adsorbents
2.1.1. Na-Bentonite
2.1.2. Sawdust
2.1.3. Alkaline and Acidic Treatment of Sawdust
2.2. Leachate Sampling and Characterization
2.3. Operation of the Reactors
2.4. Experimental Design
2.5. Experimental Method
3. Results and Discussion
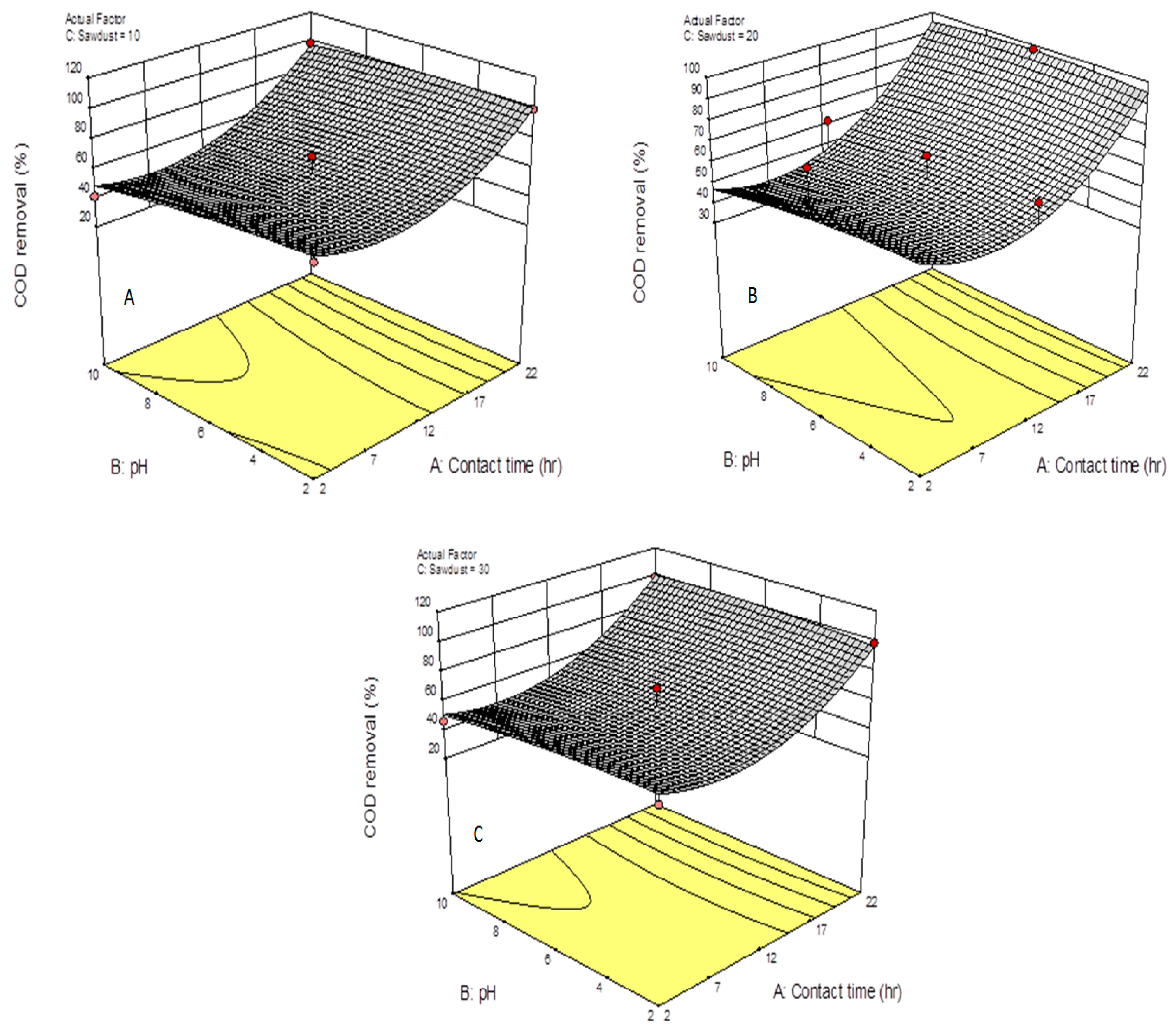
3.1. COD Removal
3.1.1. Adsorption by Clay Mineral
- Water bridging involves the linking of a polar organic molecule to an exchangeable metal cation through a water molecule in the primary hydration shell. One or both protons of the water molecule can participate in the bonding.
- Organic–organic hydrogen bonding occurs when the exchangeable cation on the clay is an organic one. The organic–clay hydrogen bonding mechanism acts when the hydroxyls of the exposed siloxane (Si–O) and aluminol (Al–OH) planes of clay minerals interact with a polar organic molecule [49].
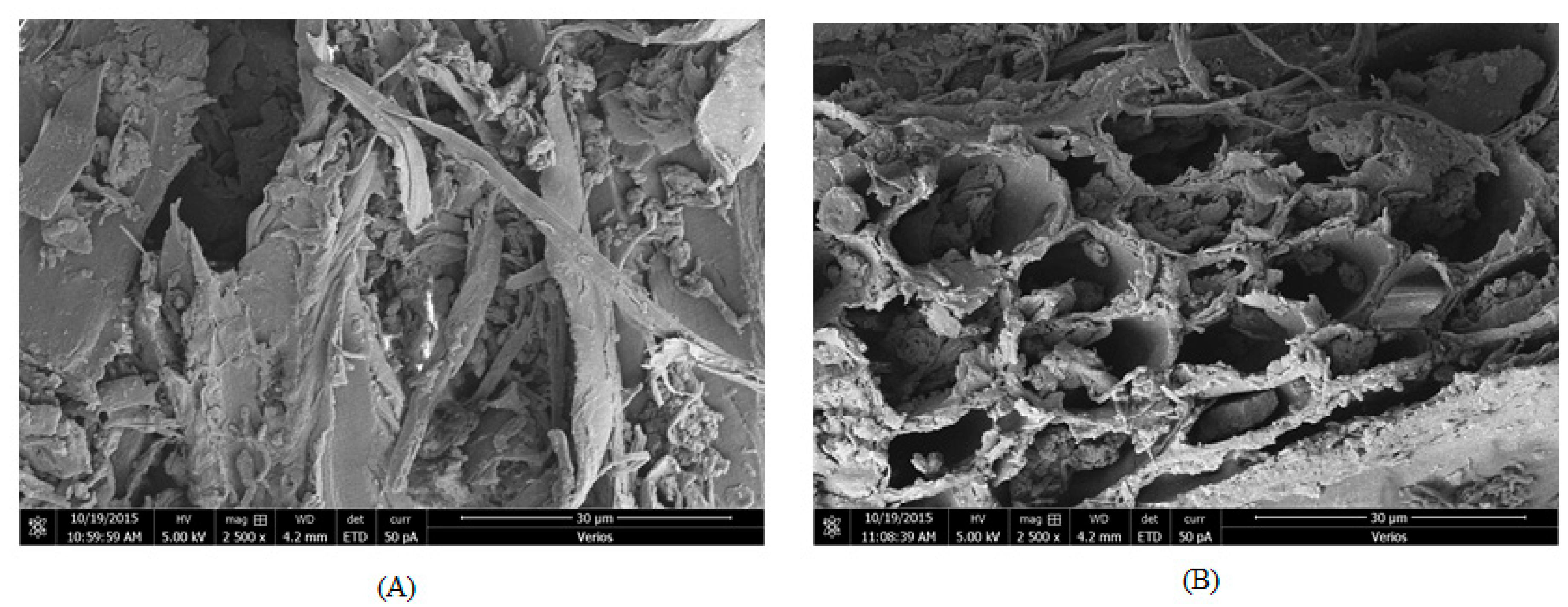
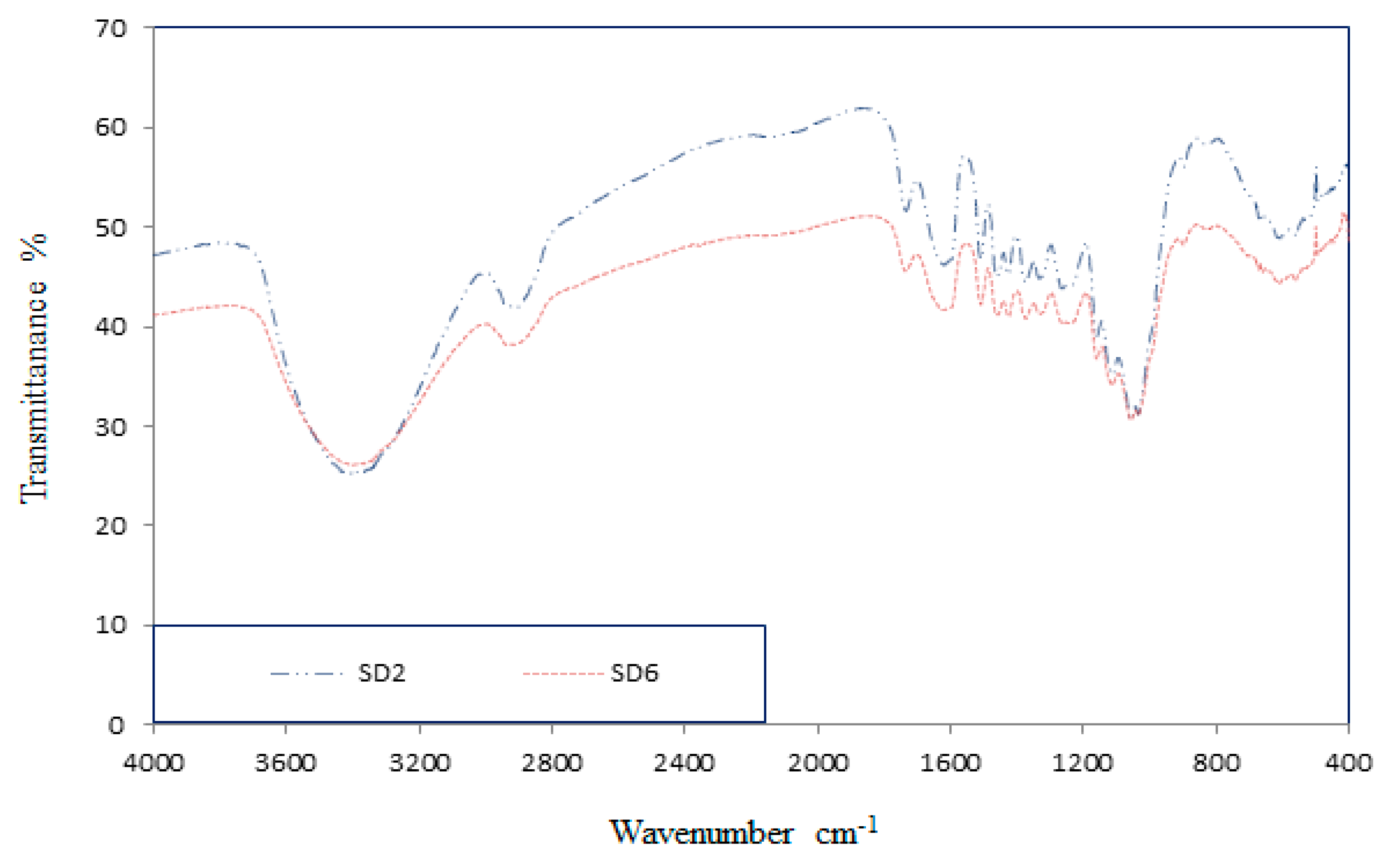
3.1.2. Sawdust Adsorption Mechanism
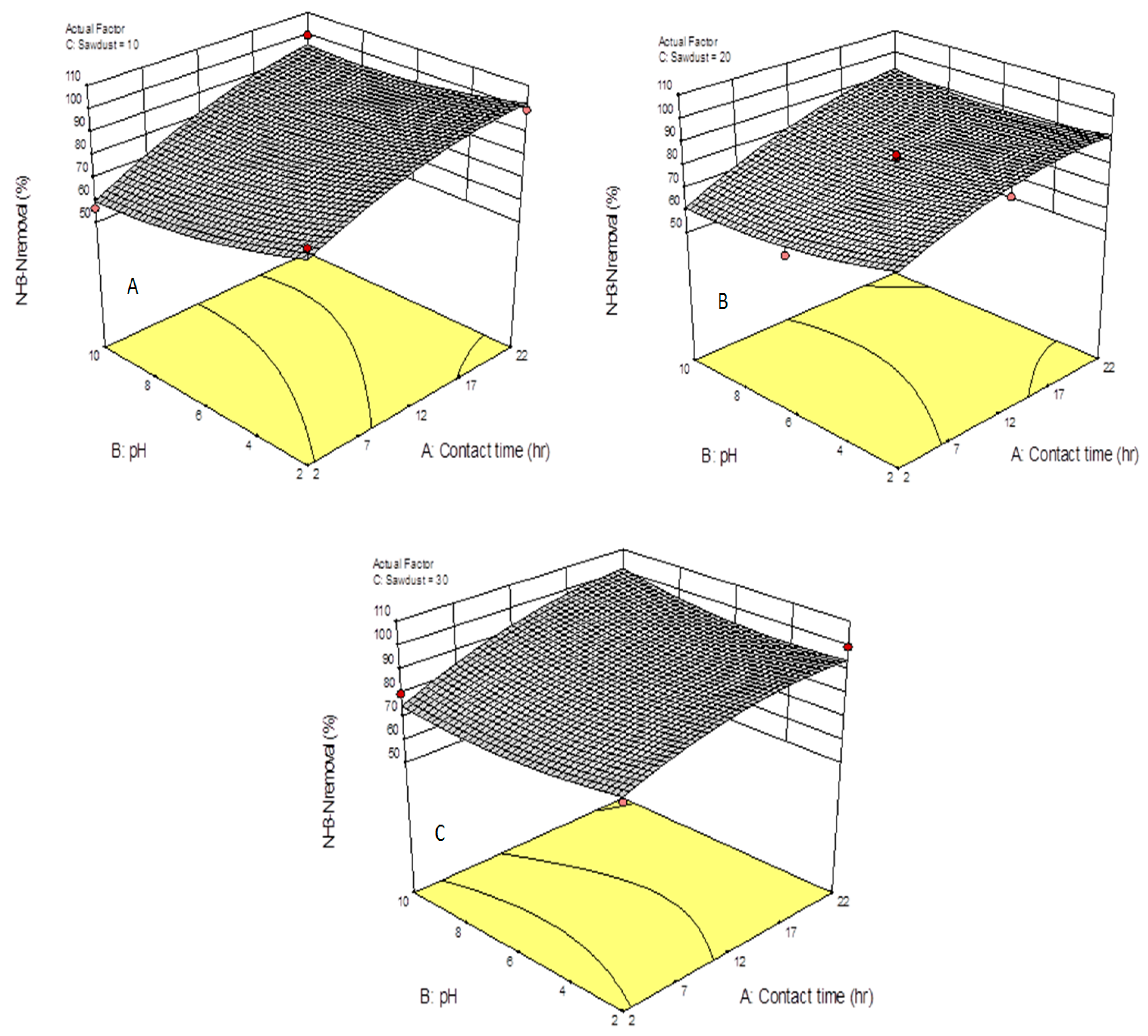
3.2. Ammonia Nitrogen Removal Mechanism
3.2.1. Clay Mineral Adsorption Mechanisms
3.2.2. Sawdust Adsorption Mechanism
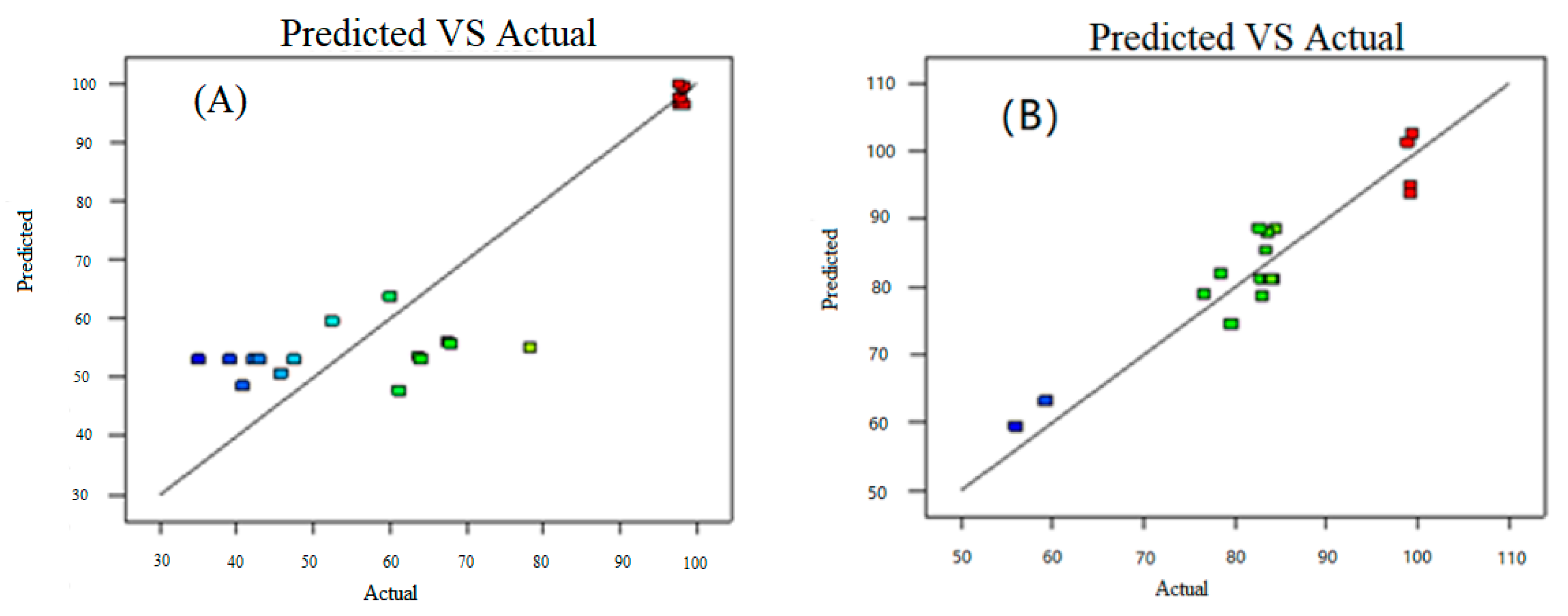
3.3. Statistical Analysis
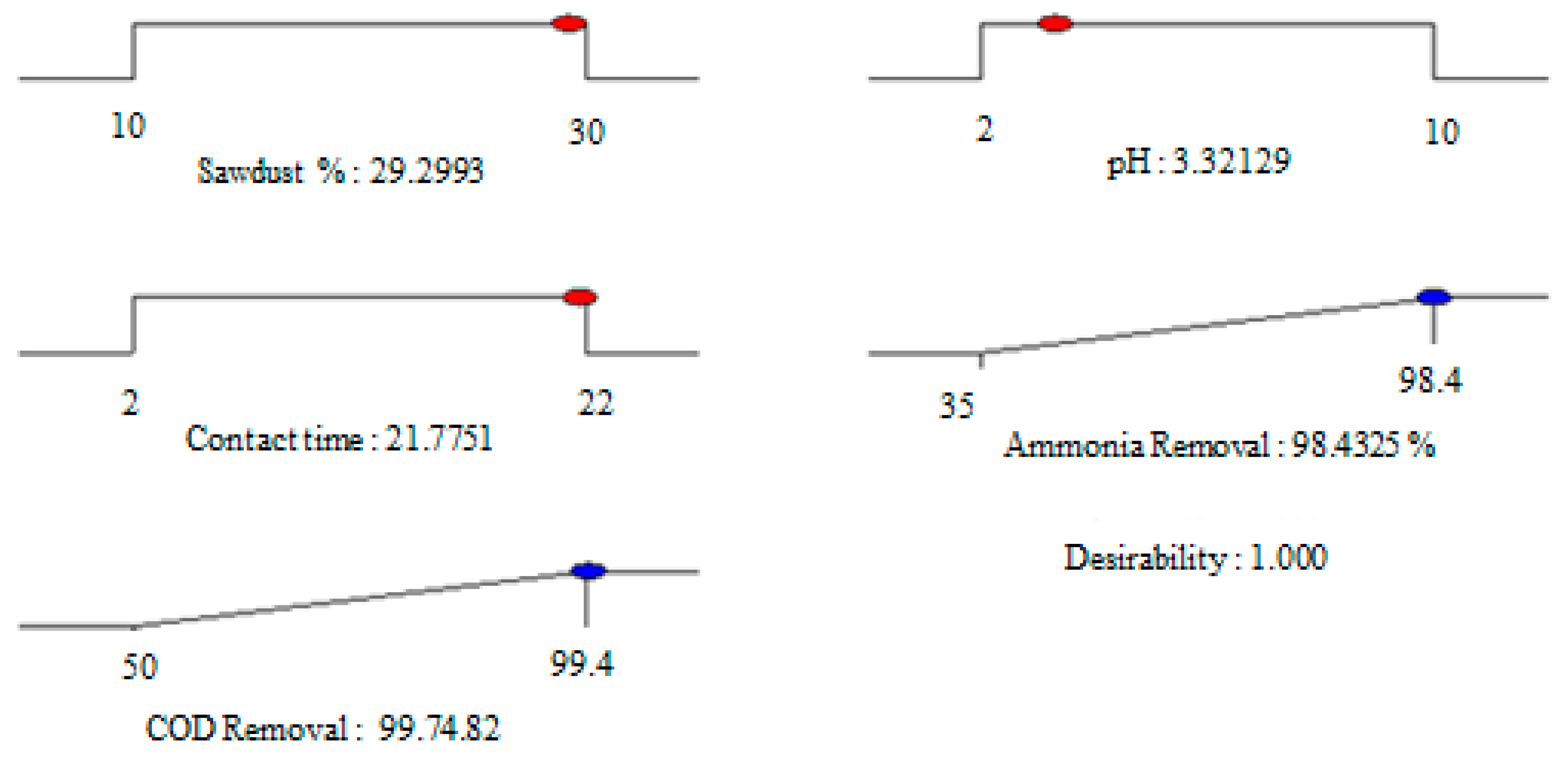
3.4. Optimization of the System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aziz, S.Q.; Aziz, H.A.; Yusoff, M.S.; Bashir, M.J.; Umar, M. Leachate characterization in semi-aerobic and anaerobic sanitary landfills: A comparative study. J. Environ. Manag. 2010, 91, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas, F.J.; Beltrán, F.; Gimeno, O.; Acedo, B.; Carvalho, F. Stabilized leachates: Ozone-activated carbon treatment and kinetics. Water Res. 2003, 37, 4823–4834. [Google Scholar] [CrossRef] [PubMed]
- Turan, M.; Gulsen, H.; Çelik, M.S. Treatment of landfill leachate by a combined anaerobic fluidized bed and zeolite column system. J. Environ. Eng. 2005, 131, 815–819. [Google Scholar] [CrossRef]
- Loukidou, M.X.; Zouboulis, A.I. Comparison of two biological treatment processes using attached-growth biomass for sanitary landfill leachate treatment. Environ. Pollut. 2001, 111, 273–281. [Google Scholar] [CrossRef]
- Kargi, F.; Pamukoglu, M.Y. Simultaneous adsorption and biological treatment of pre-treated landfill leachate by fed-batch operation. Process Biochem. 2003, 38, 1413–1420. [Google Scholar] [CrossRef]
- Seka, A.M.; Verstraete, W. Feasibility of a multi-component additive for efficient control of activated sludge filamentous bulking. Water Res. 2001, 35, 2995–3003. [Google Scholar] [CrossRef]
- Hosterman, J.W.; Patterson, S.H. Bentonite and fuller’s earth resources of the United States. U.S. Geol. Surv. 1992. [Google Scholar] [CrossRef]
- European Bentonite Producers Association. Available online: https://uia.org/s/or/en/1100029366 (accessed on 12 January 1999).
- Koch, D. Bentonites as a basic material for technical base liners and site encapsulation cut-off walls. Appl. Clay Sci. 2002, 21, 1–11. [Google Scholar] [CrossRef]
- Bahmanpour, H.; Awhadi, S.; Enjili, J.; Eslamian, S.; Ostad-Ali-Askari, K. Optimizing absorbent bentonite and evaluation of contaminants removal from petrochemical industries wastewater. Int. J. Constr. Res. Civ. Eng. 2017, 3, 34–42. [Google Scholar]
- Moradi, N.; Salem, S.; Salem, A. Optimizing adsorption of blue pigment from wastewater by nano-porous modified Na-bentonite using spectrophotometry based on response surface method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 193, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kang, Y.; Mu, B.; Wang, A. Attapulgite/bentonite interactions for methylene blue adsorption characteristics from aqueous solution. Chem. Eng. J. 2014, 237, 403–410. [Google Scholar] [CrossRef]
- Mahvi, A.H. Sequencing batch reactor: A promising technology in wastewatertreatment. Iran. J. Environ. Health Sci. Eng. 2008, 5, 79–90. [Google Scholar]
- Zhong, Q.; Li, D.; Tao, Y.; Wang, X.; He, X.; Zhang, J.; Wang, L. Nitrogen removal from landfill leachate via ex situ nitrification and sequential in situ denitrification. Waste Manag. 2009, 29, 1347–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Xie, Y.; Cao, H. Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation. Chemosphere 2015, 121, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.H.; Guo, W.; Zhang, J.; Liang, S.; Ton-That, C.; Zhang, X. Typical low cost biosorbents for adsorptive removal of specific organic pollutants from water. Bioresour. Technol. 2015, 182, 353–363. [Google Scholar] [Green Version]
- Mota, J.P.; Lyubchik, S. Recent Advances in Adsorption Processes for Environmental Protection and Security; Springer: New York, NY, USA, 2008. [Google Scholar]
- Koros, W.J. Membranes: Learning a lesson from nature. Chem. Eng. Prog. 1995, 91, 68. [Google Scholar]
- Yu, F.; Ma, J. Easy and Large-Scale Synthesis of Carbon Nanotube-Based Adsorbents for the Removal of Arsenic and Organic Pollutants from Aqueous Solutions. Smart Mat. Waste Water Appl. 2016, 1. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Cheremisinoff, P.N.; Ellerbusch, F. Carbon Adsorption Handbook; Ann Arbor Science Publishers: Ann Arbor, MI, USA, 1978. [Google Scholar]
- Ali, I. The quest for active carbon adsorbent substitutes: Inexpensive adsorbents for toxic metal ions removal from wastewater. Sep. Purif. Rev. 2010, 39, 95–171. [Google Scholar] [CrossRef]
- Kaur, K.; Mor, S.; Ravindra, K. Removal of Chemical Oxygen Demand from Landfill Leachate using Cow-dung Ash as a Low-cost Adsorbent. J. Colloid Interface Sci. 2016, 469, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Verbinnen, B.; Block, C.; Van Caneghem, J.; Vandecasteele, C. Recycling of spent adsorbents for oxyanions and heavy metal ions in the production of ceramics. Waste Manag. 2015, 45, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Pant, N.; Pal, N. Studies on the efficiency of a local fertilizer waste as a low cost adsorbent. Water Res. 1987, 21, 1389–1394. [Google Scholar] [CrossRef]
- Patteson, J.W. Industrial wastes reduction. Environ. Sci. Technol. 1989, 23, 1032–1038. [Google Scholar] [CrossRef]
- McKay, G.; Poots, V.J. Kinetics and diffusion processes in colour removal from effluent using wood as an adsorbent. J. Chem. Technol. Biotechnol. 1980, 30, 279–292. [Google Scholar] [CrossRef]
- Asfour, H.M.; Fadali, O.A.; Nassar, M.M.; El-Geundi, M.S. Equilibrium studies on adsorption of basic dyes on hardwood. J. Chem. Technol. Biotechnol. Chem. Technol. 1985, 35, 21–27. [Google Scholar] [CrossRef]
- Djilani, C.; Zaghdoudi, R.; Modarressi, A.; Rogalski, M.; Djazi, F.; Lallam, A. Elimination of organic micropollutants by adsorption on activated carbon prepared from agricultural waste. Chem. Eng. J. 2012, 189, 203–212. [Google Scholar] [CrossRef]
- Jeffries, T.W. Biodegradation of lignin and hemicelluloses. In Biochemistry of Microbial Degradation; Springer: New York, NY, USA, 1994; pp. 233–277. [Google Scholar]
- American Society for Testing and Materials; Annual Book of ASTM Standards: Philadelphia, PA, USA, 1992.
- Hendershot, W.H.; Duquette, M. A simple barium chloride method for determining cation exchange capacity and exchangeable cations. Soil Sci. Soc. Am. J. 1986, 50, 605–608. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1989; p. 378. [Google Scholar]
- Hesse, P.R. A Textbook of Soil Chemical Analysis; William Clowes and Sons: London, UK, 1971. [Google Scholar]
- Fagerlund, G. Determination of specific surface by the BET method. Matériauxet Constr. 1973, 6, 239–245. [Google Scholar] [CrossRef]
- Standard Test Method for Performing the Sieve Analysis of Coal and Designating Coal Size; American Society for Testing and Materials: New York, NY, USA, 1994.
- Brown, P.; Jefcoat, I.A.; Parrish, D.; Gill, S.; Graham, E. Evaluation of the adsorptive capacity of peanut hull pellets for heavy metals in solution. Adv. Environ. Res. 2000, 4, 19–29. [Google Scholar] [CrossRef]
- Aghamohammadi, N.; Hamidi, A.A.; Hasnain, I.M.; Zinatizadeh, A.A.; NasrollahzadehSaravi, H.; Ghafari, S. Performance of a powdered activated carbon (PAC) augmented activated sludge process treating semi-aerobic leachate. Int. J. Environ. Res. 2007, 1, 96–103. [Google Scholar]
- Aziz, H.A.; Adlan, M.N.; Zahari, M.S.M.; Alias, S. Removal of ammoniacal nitrogen (N-NH3) from municipal solid waste leachate by using activated carbon and limestone. Waste Manag. Res. 2004, 22, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.A.; Alias, S.; Adlan, M.N.; Asaari, A.H.; Zahari, M.S. Colour removal from landfill leachate by coagulation and flocculation processes. Bioresour. Technol. 2007, 98, 218–220. [Google Scholar] [CrossRef] [PubMed]
- FAOLEX. Environmental Quality (Control of Pollution from Solid Waste Transfer Stationand Landfill) Regulations 2009. Available online: http://www.fao.org/faolex/results/details/en/c/LEX-FAOC099122/ (accessed on 10 December 2009).
- Farraji, H.; Zaman, N.Q.; Abdul Aziz, H.; Ashraf, M.A.; Mojiri, A.; Mohajeri, P. Landfill Leachate Treatment by Bentonite Augmented Sequencing Batch Reactor (SBR) System. Appl. Mech. Mater. 2015, 802, 466–471. [Google Scholar] [CrossRef]
- APHA Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005.
- Rodrıguez, J.; Castrillon, L.; Maranon, E.; Sastre, H.; Fernandez, E. Removal of non-biodegradable organic matter from landfill leachates by adsorption. Water Res. 2004, 38, 3297–3303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortland, M.M. Clay-organic complexes and interactions. Adv. Agron. 1970, 22, 117. [Google Scholar]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Sposito, G. The Chemistry of Soils; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Greenland, D.J.; Quirk, J.P. Adsorption of 1-n-alkyl pyridinium bromides by montmorillonite. Clays Clay Miner. 1962, 9, 484–499. [Google Scholar] [CrossRef]
- Walker, G.F. Interactions of n-alkylammonium ions with mica-type layer lattices. Clay Miner. 1967, 7, 129–143. [Google Scholar] [CrossRef]
- Newman, A.C.D. Chemistry of Clays and Clay Minerals; Longman Scientific and Technical: London, UK, 1987. [Google Scholar]
- Mortland, M.M. Urea complexes with montmorillonite: An infrared absorption study. Clay Miner. 1966, 6, 143–156. [Google Scholar] [CrossRef]
- Tahoun, S.A.; Mortland, M.M. Complexes of Montmorillonite with Primary, Secondary, and Tertiary Amides: II. Coordination of Amides on the Surface of Montmorillonite. Soil Sci. 1966, 102, 314–321. [Google Scholar] [CrossRef]
- Doner, H.E.; Mortland, M.M. Benzene complexes with copper (II) montmorillonite. Science 1969, 166, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; D’Silva, A.P.; Easton, I.A. A general treatment and classification of the solute adsorption isotherm part. II. Experimental interpretation. J. Colloid Interface Sci. 1974, 47, 766–778. [Google Scholar] [CrossRef]
- Rožić, M.; Cerjan-Stefanović, Š.; Kurajica, S.; Vančina, V.; Hodžić, E. Ammoniacal nitrogen removal from water by treatment with clays and zeolites. Water Res. 2000, 34, 3675–3681. [Google Scholar] [CrossRef]
- Hashem, A.; Akasha, R.A.; Ghith, A.; Hussein, D.A. Adsorbent based on agricultural wastes for heavy metal and dye removal: A review. Energy Edu. Sci. Technol. 2007, 19, 69–86. [Google Scholar]
- Adapa, P.K.; Karunakaran, C.; Tabil, L.G.; Schoenau, G.J. Potential applications of infrared and Raman spectromicroscopy for agricultural biomass. Agric. Eng. Int. CIGR J. 2009, XI, 1081. [Google Scholar]
- Aziz, S.Q.; Aziz, H.A.; Yusoff, M.S.; Bashir, M.J. Landfill leachate treatment using powdered activated carbon augmented sequencing batch reactor (SBR) process: Optimization by response surface methodology. J. Hazard. Mater. 2011, 189, 404–413. [Google Scholar] [CrossRef] [PubMed]
| Physical Characteristics | Quantity Measured | Geo-Environmental Characteristics | Quantity Measured |
|---|---|---|---|
| Clay (%) | 76 | Mineral composition in decreasing abundance | Montmorilonite, Quartz, Calsit |
| Silt (%) | 23 | Carbonite content (%) | 8 |
| Sand (%) | 1 | Organic content (%) | 1.4 |
| Liquid limit (%) | 423 | CEC (cmol/kg soil) | 80 |
| Plastic limit (%) | 32 | Specific surface area (10−3 m2/kg) | 425 |
| PI (%) | 391 | pH (1:10, soil/water ratio) | 9.9 |
| Activity (%) | 3.73 | ||
| Soil classification (%) | CH | ||
| Water content (air-dried) (%) | 5.9 | ||
| Water content (oven-dried) (%) | 7.1 | ||
| Gs (%) | 2.45 |
| Parameter | Unit | Standard | Sampling Average |
|---|---|---|---|
| Temperature | °C | 40 | 33 |
| pH value | - | 6.0–9.0 | 9 |
| COD | mg L−1 | 400 | 2955 |
| BOD | mg L−1 | 20 | 269 |
| Ammonia nitrogen | mg L−1 | 5 | 120 |
| Arsenic | mg L−1 | 0.05 | 0.04 |
| Manganese | mg L−1 | 0.20 | 0.12 |
| Nickel | mg L−1 | 0.20 | 0.017 |
| Iron | mg L−1 | 5.0 | 0.48 |
| Color | ADMI | 100 | 1220 |
| Suspended solids | mg L−1 | 50 | 710 |
| TDS | % | - | 5.72 |
| Run | Variable | Responses | |||
|---|---|---|---|---|---|
| A: Contact Time (h) | B: pH | C: Sawdust (%) | Ammonia Removal (%) | COD Removal (%) | |
| 1 | 2 | 2 | 30 | 52.5 | 76.58 |
| 2 | 2 | 10 | 30 | 45.8 | 79.6 |
| 3 | 22 | 10 | 10 | 97.7 | 99.2 |
| 4 | 12 | 10 | 20 | 61.2 | 78.5 |
| 5 | 22 | 6 | 20 | 97.9 | 84.4 |
| 6 | 12 | 6 | 20 | 35 | 82.9 |
| 7 | 2 | 2 | 10 | 60 | 83 |
| 8 | 22 | 2 | 10 | 98.3 | 99.4 |
| 9 | 22 | 10 | 30 | 97.7 | 98.9 |
| 10 | 22 | 2 | 30 | 98.3 | 99.2 |
| 11 | 2 | 10 | 10 | 40.8 | 56 |
| 12 | 12 | 6 | 30 | 67.9 | 82.7 |
| 13 | 12 | 6 | 20 | 39.1 | 84 |
| 14 | 12 | 2 | 20 | 63.7 | 83.6 |
| 15 | 12 | 6 | 10 | 67.5 | 83.4 |
| 16 | 12 | 6 | 20 | 64.1 | 83.8 |
| 17 | 2 | 6 | 20 | 78.3 | 59.3 |
| 18 | 12 | 6 | 20 | 42.3 | 83.6 |
| 19 | 12 | 6 | 20 | 47.5 | 84.2 |
| 20 | 12 | 6 | 20 | 42.9 | 84 |
| Blank1 | 2 | - | - | 37.5 | 12.13 |
| Blank2 | 12 | - | - | 68.75 | 83.55 |
| Blank3 | 22 | - | - | 95.41 | 99.28 |
| Response | Final Equations in Terms of Actual Factors | prob | R2 | Adj. R2 | Adec. P. | SD | CV | Press |
|---|---|---|---|---|---|---|---|---|
| NH3-N removal | +102.79375 + 2.54999 × A − 6.38364 × B − 2.47927 × C + 0.073375 × A × B − 0.022100 × A × C + 0.093500 × B × C − 0.053418 × A2 + 0.24114 × B2 + 0.058582 × C2 | 0.0010 | 0.8897 | 0.7905 | 11.822 | 5.16 | 6.19 | 3322.24 |
| COD removal | +83.16323 − 3.99044 × A − 0.67818 × B − 1.44924 × C + 0.077188 × A × B + 3.125 × 10−3 × A × C + 0.039063 × B × C + 0.23291 × A2 − 0.14744 × B2 + 0.028909 × C2 | 0.0228 | 0.7777 | 0.5777 | 5.036 | 14.66 | 22.57 | 9050.76 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohajeri, P.; Selamat, M.R.; Abdul Aziz, H.; Smith, C. Removal of COD and Ammonia Nitrogen by a Sawdust/Bentonite-Augmented SBR Process. Clean Technol. 2019, 1, 125-140. https://doi.org/10.3390/cleantechnol1010009
Mohajeri P, Selamat MR, Abdul Aziz H, Smith C. Removal of COD and Ammonia Nitrogen by a Sawdust/Bentonite-Augmented SBR Process. Clean Technologies. 2019; 1(1):125-140. https://doi.org/10.3390/cleantechnol1010009
Chicago/Turabian StyleMohajeri, Parsa, Mohammad Razip Selamat, Hamidi Abdul Aziz, and Carol Smith. 2019. "Removal of COD and Ammonia Nitrogen by a Sawdust/Bentonite-Augmented SBR Process" Clean Technologies 1, no. 1: 125-140. https://doi.org/10.3390/cleantechnol1010009
APA StyleMohajeri, P., Selamat, M. R., Abdul Aziz, H., & Smith, C. (2019). Removal of COD and Ammonia Nitrogen by a Sawdust/Bentonite-Augmented SBR Process. Clean Technologies, 1(1), 125-140. https://doi.org/10.3390/cleantechnol1010009





