Effects of Nitrogen and Phosphorus Supplementation on Responses of Trembling Aspen and White Spruce Seedlings in Reclamation Soils Amended by Non-Segregating Oil Sands Tailings
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Preparation
2.2. Plant Material and Experimental Set-Up
2.3. Growth Parameters
2.4. Leaf Chlorophyll Concentrations
2.5. Net Photosynthesis (Pn) and Transpiration (E) Rates
2.6. Leaf Elemental Concentrations
2.7. Statistical Analysis
3. Results
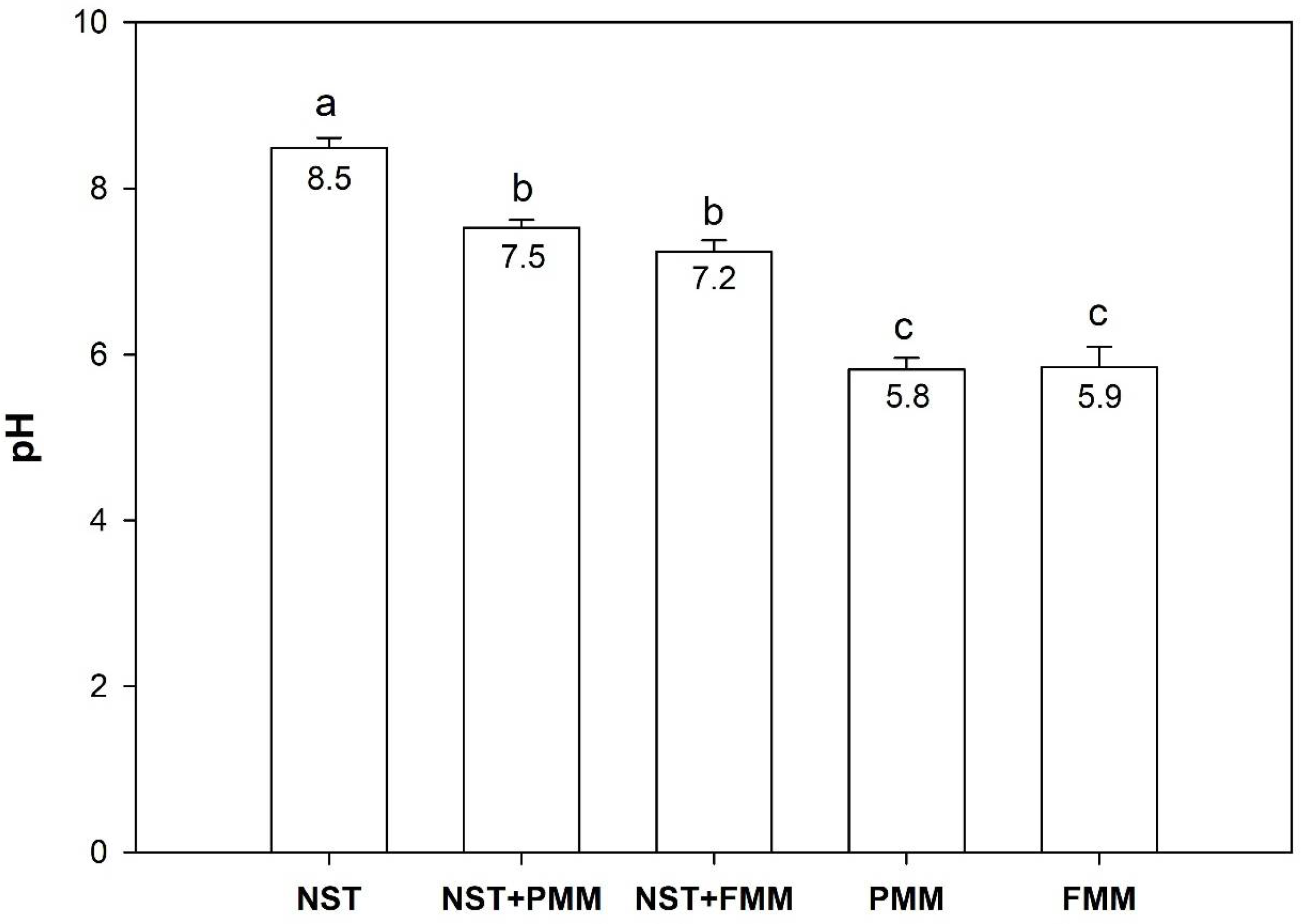
3.1. Growth Substrate pH
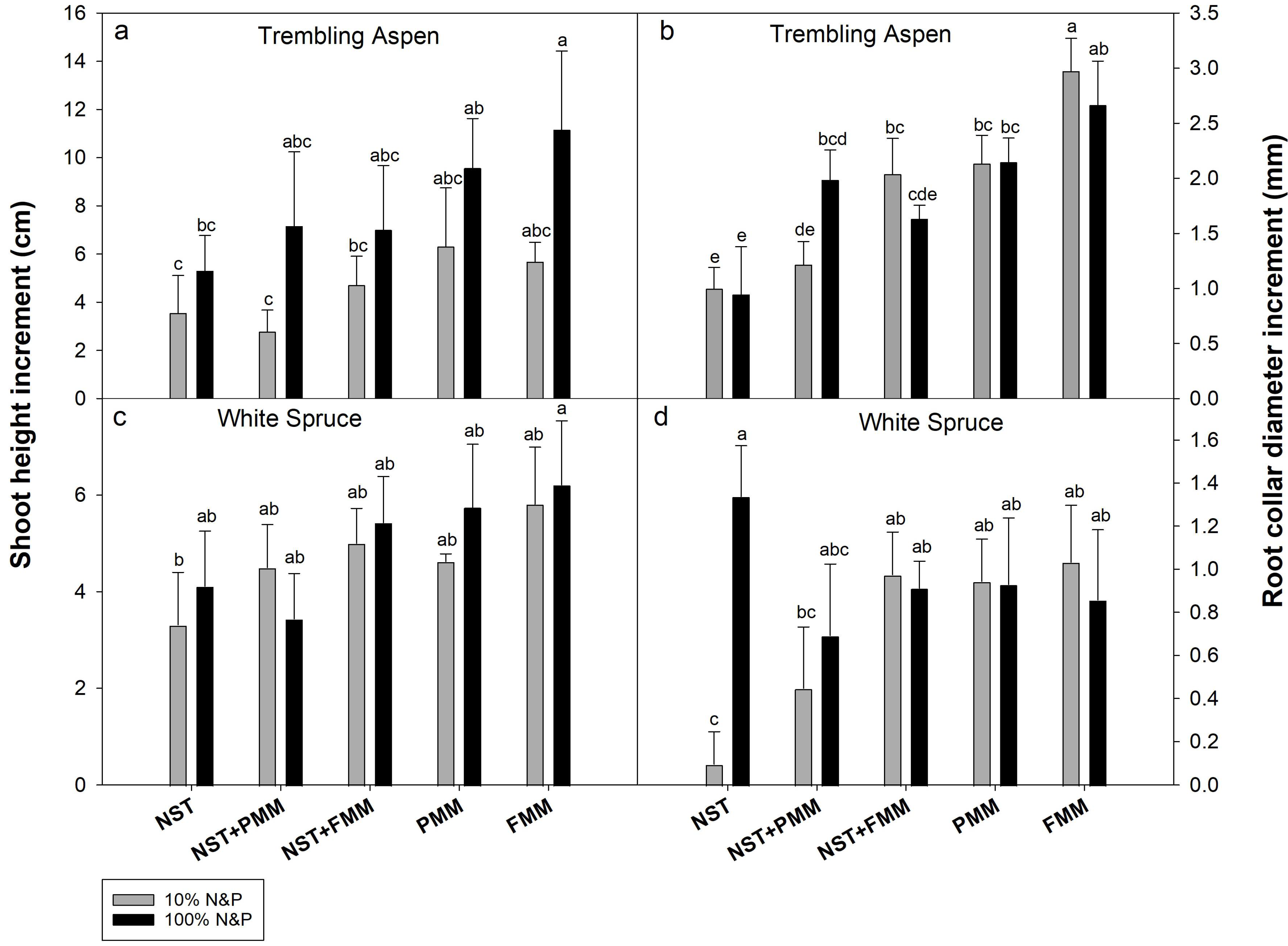
3.2. Plant Shoot Heights and Root Collar Diameters
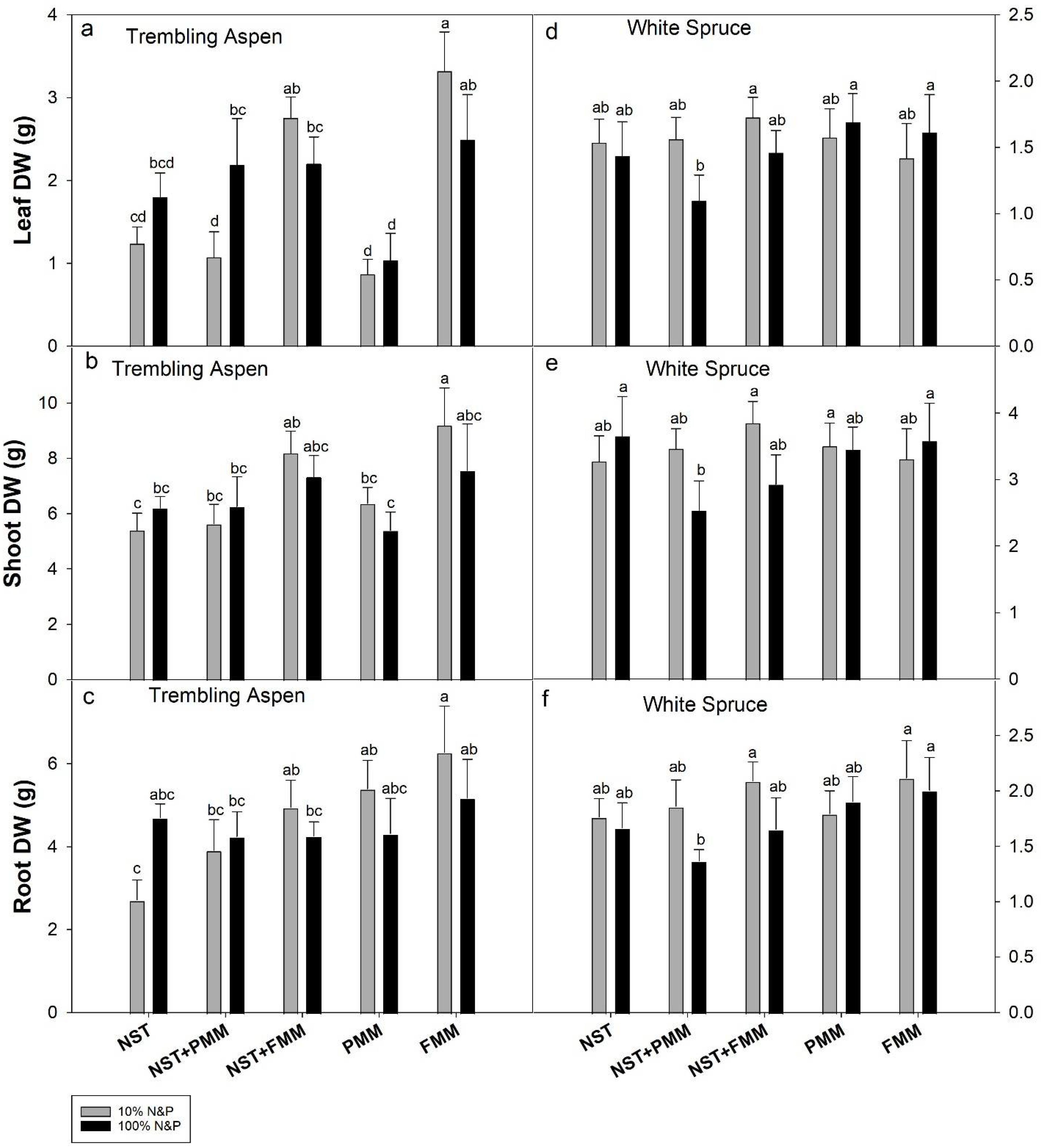
3.3. Dry Weights
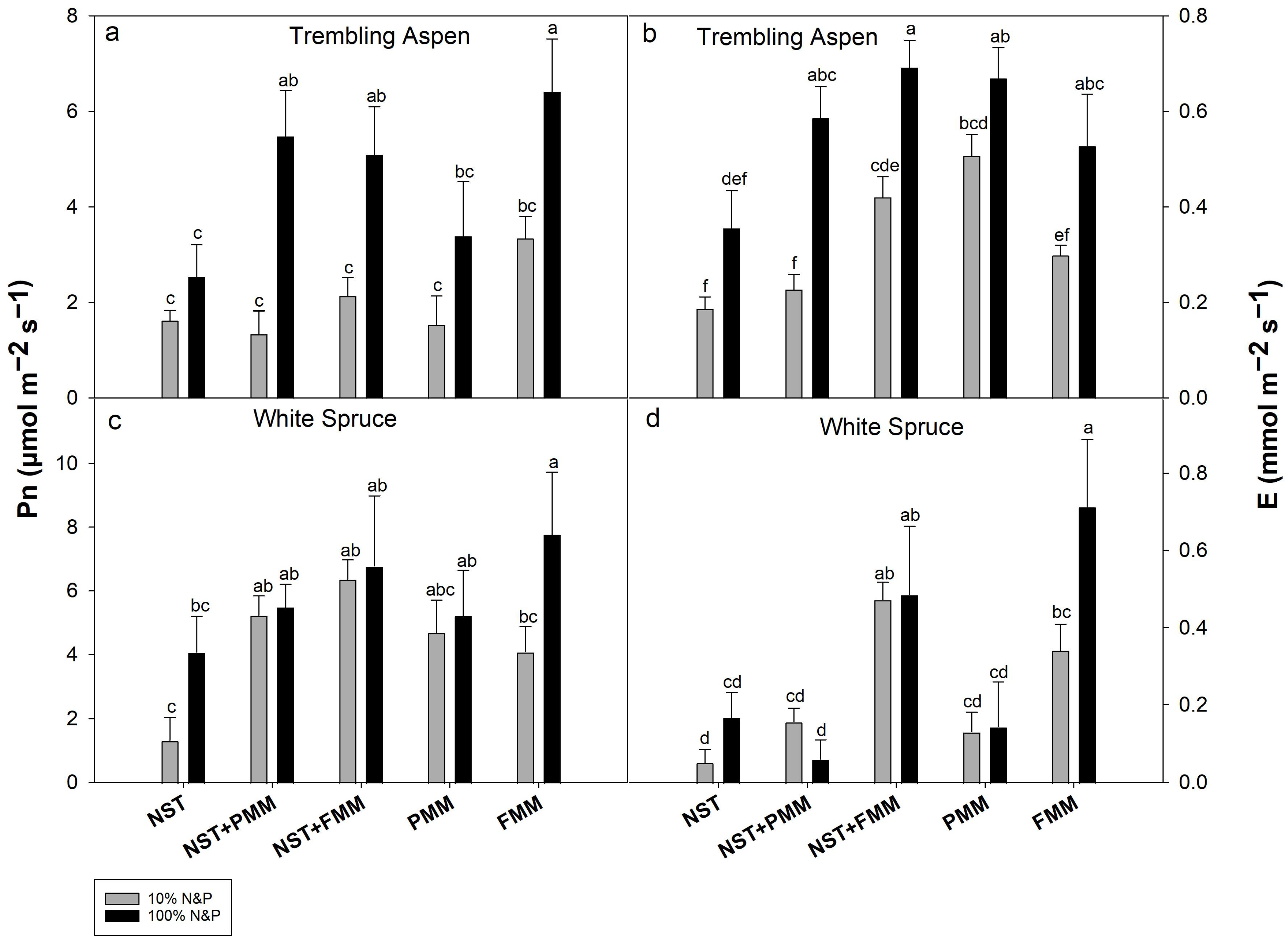
3.4. Net Photosynthesis (Pn) and Transpiration (E) Rates
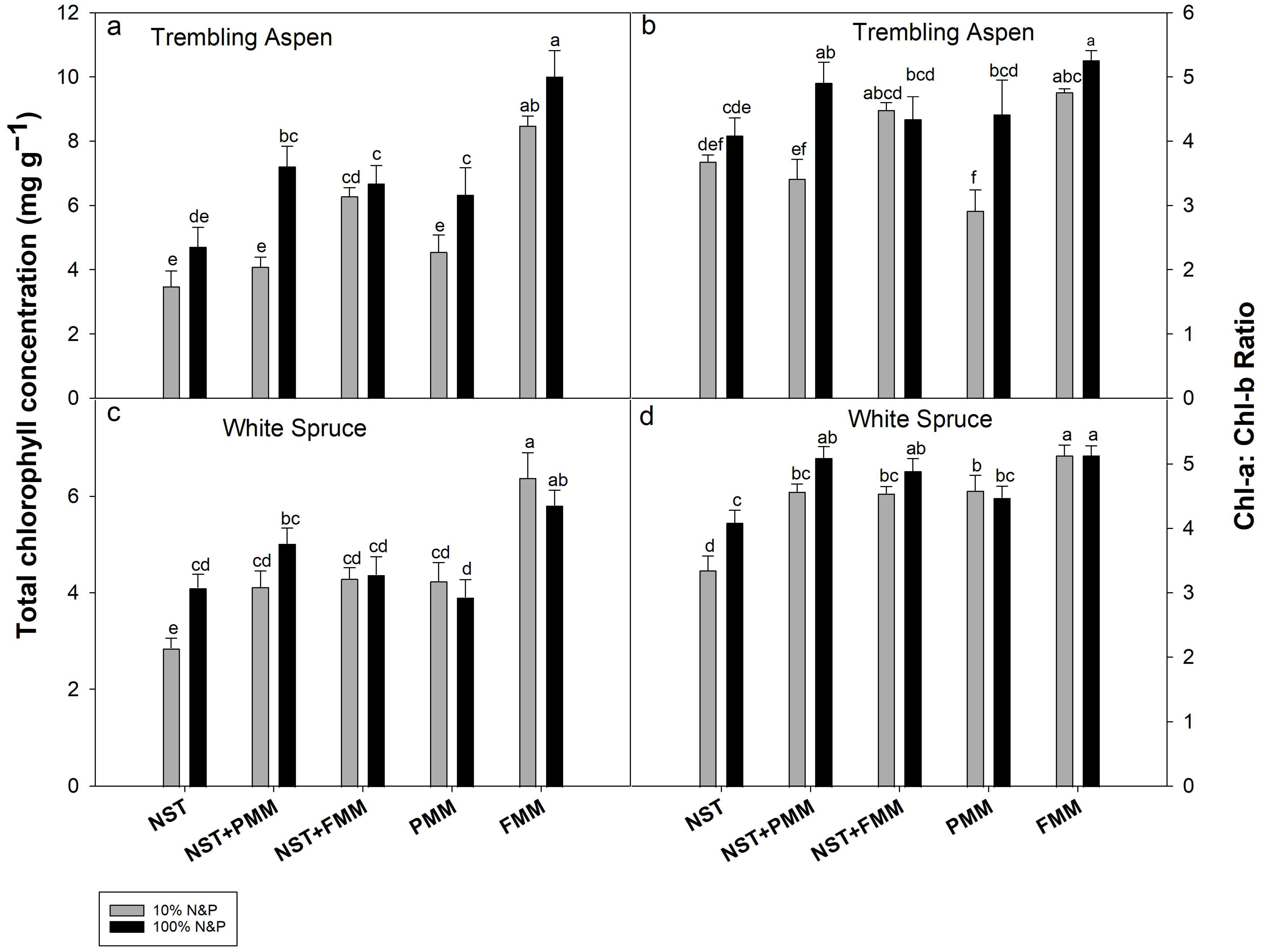
3.5. Chlorophyll Concentrations
3.6. Leaf Elemental Concentrations
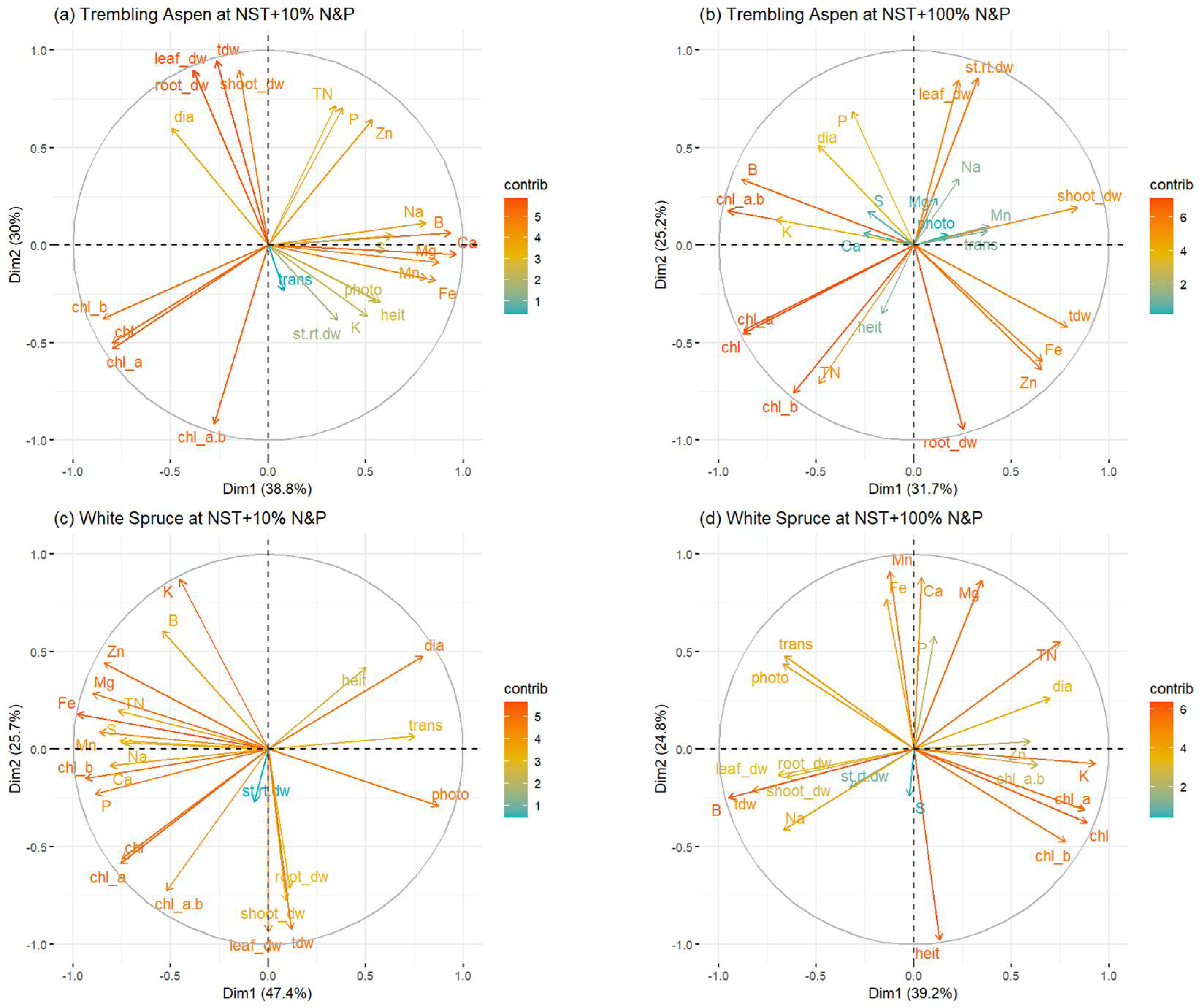
3.7. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberta Environment and Parks. Oil Sands Mine Reclamation and Disturbance Tracking by Year. 2023. Available online: http://osip.alberta.ca/library/Dataset/Details/27 (accessed on 7 March 2025).
- Oil Sands Vegetation Reclamation Committee. Guidelines for Reclamation to Forest Vegetation in the Athabasca Oil Sands Region; Alberta Environmental Protection. Report No. ESD/LM/99-1; Provincial Government of Alberta: Edmonton, AB, Canada, 1998.
- Howat, D. Acceptable Salinity, Sodicity and pH Values for Boreal Forest Reclamation; Alberta Environment; Environmental Sciences Division: Edmonton, AB, Canada, 2000. [Google Scholar]
- Valentine, D.W.; Kielland, K.; Chapin, F.S., III; McCuire, A.D.; Van Cleve, K. Patterns of biogeochemistry in Alaskan boreal forests. In Alaska’s Changing Boreal Forest; Oxford University Press: New York, NY, USA, 2006; pp. 241–269. [Google Scholar]
- Leatherdale, J.; Chanasyk, D.S.; Quideau, S. Soil water regimes of reclaimed upland slopes in the oil sands region of Alberta. Can. J. Soil Sci. 2012, 92, 117–129. [Google Scholar] [CrossRef]
- Jamro, G.M.; Chang, S.X.; Naeth, M.A. Organic capping type affected nitrogen availability and associated enzyme activities in reconstructed oil sands soils in Alberta, Canada. Ecol. Eng. 2014, 73, 92–101. [Google Scholar] [CrossRef]
- Kamaluddin, M.; Zwiazek, J.J. Naphthenic acids inhibit root water transport, gas exchange and leaf growth in aspen (Populus tremuloides) seedlings. Tree Physiol. 2002, 22, 1265–1270. [Google Scholar] [CrossRef]
- MacKenzie, M.D.; Quideau, S.A. Microbial community structure and nutrient availability in oil sands reclaimed boreal soils. Appl. Soil Ecol. 2010, 44, 32–41. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Zwiazek, J.J.; Jones, M.D.; MacKinnon, M.D. Effects of NaCl on responses of ectomycorrhizal black spruce (Picea mariana), white spruce (Picea glauca) and jack pine (Pinus banksiana) to fluoride. Physiol. Plant. 2009, 135, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Apostol, K.G.; Zwiazek, J.J.; MacKinnon, M.D. NaCl and Na2SO4 alter responses of jack pine (Pinus banksiana) seedlings to boron. Plant Soil 2002, 240, 321–329. [Google Scholar] [CrossRef]
- Alberta Environment and Water. Best Management Practices for Conservation of Reclamation Materials in the Mineable Oil Sands Region of Alberta; Prepared by MacKenzie, D. for the Terrestrial Subgroup; Best Management Practices Task Group of the Reclamation Working Group of the Cumulative Environmental Management Association: Fort McMurray, AB, Canada, 2011.
- Naeth, M.A.; Wilkinson, S.R.; Mackenzie, D.D.; Archibald, H.A.; Powter, C.B. Potential of LFH Mineral Soil Mixes for Reclamation of Forested Lands in Alberta; OSRIN Report No. TR-35. Oil Sands Research and Information Network; University of Alberta, School of Energy and the Environment: Edmonton, AB, Canada, 2013. [Google Scholar]
- Mackenzie, D.D.; Naeth, M.A. The role of the forest soil propagule bank in assisted natural recovery after oil sands mining. Restor. Ecol. 2010, 18, 418–427. [Google Scholar] [CrossRef]
- MacKenzie, D.D. Oil Sands Mine Reclamation Using Boreal Forest Surface Soil (LFH) in Northern ALBERTA. Ph.D. Thesis, University of Alberta, Department of Renewable Resources, Edmonton, AB, Canada, 2013. [Google Scholar]
- Hahn, A.S.; Quideau, S.A. Long-term effects of organic amendments on the recovery of plant and soil microbial communities following disturbance in the Canadian boreal forest. Plant Soil 2013, 363, 331–344. [Google Scholar] [CrossRef]
- Fung, M.Y.; Macyk, T.M. Reclamation of oil sands mining areas. In Reclamation of Drastically Disturbed Lands; Barnhisel, R.I., Darmody, R.G., Daniels, W.L., Eds.; American Society of Agronomy: Madison, WI, USA, 2000; Volume 41, pp. 755–774. [Google Scholar]
- CNRL (Canadian Natural Resources Limited). Stewardship Report to Stakeholders; CNRL (Canadian Natural Resources Limited): Calgary, AB, Canada, 2022. [Google Scholar]
- Zhang, W.Q.; Fleurial, K.; Moawad, M.; Vassov, R.; Macdonald, S.E.; Zwiazek, J.J. Growth responses of 20 boreal forest species to oil sands non-segregating tailings: Significance for reclamation. Restor. Ecol. 2023, 31, e13874. [Google Scholar] [CrossRef]
- Gundersen, P.; Sevel, L.; Christiansen, J.R.; Vesterdal, L.; Hansen, K.; Bastrup-Birk, A. Do indicators of nitrogen retention and leaching differ between coniferous and broadleaved forests in Denmark? For. Ecol. Manag. 2009, 258, 1137–1146. [Google Scholar] [CrossRef]
- Yan, E.R.; Hu, Y.L.; Salifu, F.; Tan, X.; Chen, Z.C.; Chang, S.X. Effectiveness of soil N availability indices in predicting site productivity in the oil sands region of Alberta. Plant Soil 2012, 359, 215–231. [Google Scholar] [CrossRef]
- Duan, M.; House, J.; Chang, S.X. Limiting factors for lodgepole pine (Pinus contorta) and white spruce (Picea glauca) growth differ in some reconstructed sites in the Athabasca oil sands region. Ecol. Eng. 2015, 75, 323–331. [Google Scholar] [CrossRef]
- Rowe, E.C.; Healey, J.R.; Edwards-Jones, G.; Hills, J.; Howells, M.; Jones, D.L. Fertilizer application during primary succession changes the structure of plant and herbivore communities. Biol. Conserv. 2006, 131, 510–522. [Google Scholar] [CrossRef]
- Walker, L.R.; Moral, R.D. Lessons from primary succession for restoration of severely damaged habitats. Appl. Veg. Sci. 2009, 12, 55–67. [Google Scholar] [CrossRef]
- Shrestha, R.K.; Lal, R. Changes in physical and chemical properties of soil after surface mining and reclamation. Geoderma 2011, 161, 168–176. [Google Scholar] [CrossRef]
- Duan, M.; Chang, S.X. Nitrogen fertilization improves the growth of lodgepole pine and white spruce seedlings under low salt stress through enhancing photosynthesis and plant nutrition. For. Ecol. Manag. 2017, 404, 197–204. [Google Scholar] [CrossRef]
- Dluzniewska, P.; Gessler, A.; Dietrich, H.; Schnitzler, J.P.; Teuber, M.; Rennenberg, H. Nitrogen uptake and metabolism in Populus x canescens as affected by salinity. New Phytol. 2007, 173, 279–293. [Google Scholar] [CrossRef]
- Fredeen, A.L.; Rao, I.M.; Terry, N. Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol. 1989, 89, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.; Lauchli, A.; Epstein, E. Vegetative growth of the common bean in response to phosphorus nutrition. Crop Sci. 1991, 31, 380–387. [Google Scholar] [CrossRef]
- George, E.; Horst, W.J.; Neumann, E. Adaptation of plants to adverse chemical soil conditions. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 409–472. [Google Scholar]
- Clarkson, D.T.; Carvajal, M.; Henzler, T.; Waterhouse, R.N.; Smyth, A.J.; Cooke, D.T.; Steudle, E. Root hydraulic conductance: Diurnal aquaporin expression and the effects of nutrient stress. J. Exp. Bot. 2000, 51, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zwiazek, J.J. Responses of Reclamation Plants to High Root Zone pH: Effects of Phosphorus and Calcium Availability. J. Environ. Qual. 2016, 45, 1652–1662. [Google Scholar] [CrossRef]
- Bois, G.; Piche, Y.; Fung, M.Y.P.; Khasa, D.P. Mycorrhizal inoculum potentials of pure reclamation materials and revegetated tailing sands from the Canadian oil sand industry. Mycorrhiza 2005, 15, 149–158. [Google Scholar] [CrossRef]
- Dhar, A.; Comeau, P.G.; Karst, J.; Pinno, B.D.; Chang, S.X.; Naeth, A.M.; Vassov, R.; Bampfylde, C. Plant community development following reclamation of oil sands mine sites in the boreal forest: A review. Environ. Rev. 2018, 26, 286–298. [Google Scholar] [CrossRef]
- Bolan, N.S. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 1991, 134, 189–207. [Google Scholar] [CrossRef]
- Perala, D.A. Populus tremuloides Michx. In Silvics of North America: Volume 2. Hardwoods; Burns, R.M., Honkala, B.H., Eds.; Agriculture Handbook 654; USDA, Forest Service: Washington, DC, USA, 1990. [Google Scholar]
- Nienstaedt, H.; Zasada, J.C. Picea glauca (Moench) Voss. In Silvics of North America: Volume 1. Conifers; Burns, R.M., Honkala, B.H., Eds.; Agriculture Handbook 654; USDA, Forest Service: Washington, DC, USA, 1990. [Google Scholar]
- Renström, A.; Choudhary, S.; Gandla, M.L.; Jönsson, L.J.; Hedenström, M.; Jämtgård, S.; Tuominen, H. The effect of nitrogen source and levels on hybrid aspen tree physiology and wood formation. Physiol. Plant. 2024, 176, e14219. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Glass, A.D.; Yaeesh Siddiqi, M. Nitrate induction in spruce: An approach using compartmental analysis. Planta 1995, 196, 683–690. [Google Scholar] [CrossRef]
- Forsch, K.B.; Dhar, A.; Naeth, M.A. Effects of woody debris and cover soil types on soil properties and vegetation 4–5 years after oil sands reclamation. Restor. Ecol. 2021, 29, e13420. [Google Scholar] [CrossRef]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; Wiley: New York, NY, USA, 1972. [Google Scholar]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Sestak, Z.; Catský, J.; Jarvis, P.G. Plant Photosynthetic Production. Manual of Methods; Dr. W. Junk Publishers: The Hague, The Netherlands, 1971. [Google Scholar]
- Zhang, W.; Calvo-Polanco, M.; Chen, Z.C.; Zwiazek, J.J. Growth and physiological responses of trembling aspen (Populus tremuloides), white spruce (Picea glauca) and tamarack (Larix laricina) seedlings to root zone pH. Plant Soil 2013, 373, 775–786. [Google Scholar] [CrossRef]
- Zhang, W.; Fleurial, K.; Sherr, I.; Vassov, R.; Zwiazek, J.J. Growth and physiological responses of tree seedlings to oil sands non-segregated tailings. Environ. Pollut. 2020, 259, 113945. [Google Scholar] [CrossRef]
- Tang, C.; Cobley, B.T.; Mokhtara, S.; Wilson, C.E.; Greenway, H. High pH in the Nutrient Solution Impairs Water Uptake in Lupinus Angustifolius L. Plant Soil 1993, 155, 517–519. [Google Scholar] [CrossRef]
- Fischer, M.; Kaldenhoff, R. On the pH regulation of plant aquaporins. J. Biol. Chem. 2008, 283, 33889–33892. [Google Scholar] [CrossRef]
- Koca, N.; Karadeniz, F.; Burdurlu, H.S. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chem. 2007, 100, 609–615. [Google Scholar] [CrossRef]
- Voitsekhovskaja, O.V.; Tyutereva, E.V. Chlorophyll b in angiosperms: Functions in photosynthesis, signaling and ontogenetic regulation. J. Plant Physiol. 2015, 189, 51–64. [Google Scholar] [CrossRef]
- Munson, A.D.; Margolis, H.A.; Brand, D.G. Seasonal nutrient dynamics in white pine and white spruce in response to environmental manipulation. Tree Physiol. 1995, 15, 141–149. [Google Scholar] [CrossRef]
- Robinson, B.H.; Green, S.R.; Chancerel, B.; Mills, T.M.; Clothier, B.E. Poplar for the phytomanagement of boron contaminated sites. Environ. Pollut. 2007, 150, 225–233. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.; Quideau, S.A.; MacKenzie, M.D.; Biryukova, O. Nitrogen mineralization and microbial activity in oil sands reclaimed boreal forest soils. J. Environ. Qual. 2007, 36, 1470–1478. [Google Scholar] [CrossRef]
- Jing, J.; Rui, Y.; Zhang, F.; Rengel, Z.; Shen, J. Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Res. 2010, 119, 355–364. [Google Scholar] [CrossRef]
- Duan, M.; House, J.; Liu, Y.; Chang, S.X. Contrasting responses of gross and net nitrogen transformations to salinity in a reclaimed boreal forest soil. Biol. Fertil. Soils 2018, 54, 385–395. [Google Scholar] [CrossRef]
- DeByle, N.V.; Winokur, R.P. Aspen: Ecology and Management in the Western United States; USDA Forest Service General Technical Report RM-119; Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1985. [Google Scholar]
- Peterson, E.B.; Peterson, N.M. Ecology and silviculture of trembling aspen. For. Chron. 1996, 5, 28–44. [Google Scholar]
- Shaviv, A.; Mikkelsen, R.L. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation—A review. Fert. Res. 1993, 35, 1–12. [Google Scholar] [CrossRef]
- Hangs, R.D.; Knight, J.D.; Van Rees, K.C.J. Nitrogen accumulation by conifer seedlings and competitor species from 15Nitrogen-labeled controlled-release fertilizer. Soil Sci. Soc. Am. J. 2003, 67, 300–308. [Google Scholar] [CrossRef]
- Sloan, J.L.; Uscola, M.; Jacobs, D.F. Nitrogen recovery in planted seedlings, competing vegetation, and soil in response to fertilization on a boreal mine reclamation site. For. Ecol. Manag. 2016, 360, 60–68. [Google Scholar] [CrossRef]
- Dietrich, S.T.; MacKenzie, M.D. Biochar affects aspen seedling growth and reclaimed soil properties in the Athabasca oil sands region. Can. J. Soil Sci. 2018, 98, 519–530. [Google Scholar] [CrossRef]
- Martinkosky, L.; Barkley, J.; Sabadell, G.; Gough, H.; Davidson, S. Earthworms (Eisenia fetida) demonstrate potential for use in soil bioremediation by increasing the degradation rates of heavy crude oil hydrocarbons. Sci. Total Environ. 2017, 580, 734–743. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Zhang, W.-Q.; Zwiazek, J.J. Effects of Nitrogen and Phosphorus Supplementation on Responses of Trembling Aspen and White Spruce Seedlings in Reclamation Soils Amended by Non-Segregating Oil Sands Tailings. Soil Syst. 2025, 9, 90. https://doi.org/10.3390/soilsystems9030090
Sun X, Zhang W-Q, Zwiazek JJ. Effects of Nitrogen and Phosphorus Supplementation on Responses of Trembling Aspen and White Spruce Seedlings in Reclamation Soils Amended by Non-Segregating Oil Sands Tailings. Soil Systems. 2025; 9(3):90. https://doi.org/10.3390/soilsystems9030090
Chicago/Turabian StyleSun, Xuehui, Wen-Qing Zhang, and Janusz J. Zwiazek. 2025. "Effects of Nitrogen and Phosphorus Supplementation on Responses of Trembling Aspen and White Spruce Seedlings in Reclamation Soils Amended by Non-Segregating Oil Sands Tailings" Soil Systems 9, no. 3: 90. https://doi.org/10.3390/soilsystems9030090
APA StyleSun, X., Zhang, W.-Q., & Zwiazek, J. J. (2025). Effects of Nitrogen and Phosphorus Supplementation on Responses of Trembling Aspen and White Spruce Seedlings in Reclamation Soils Amended by Non-Segregating Oil Sands Tailings. Soil Systems, 9(3), 90. https://doi.org/10.3390/soilsystems9030090







