Assessing the Effect of Undirected Forest Restoration and Flooding on the Soil Quality in an Agricultural Floodplain
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Analytical Methods
2.3. Statistical Analysis
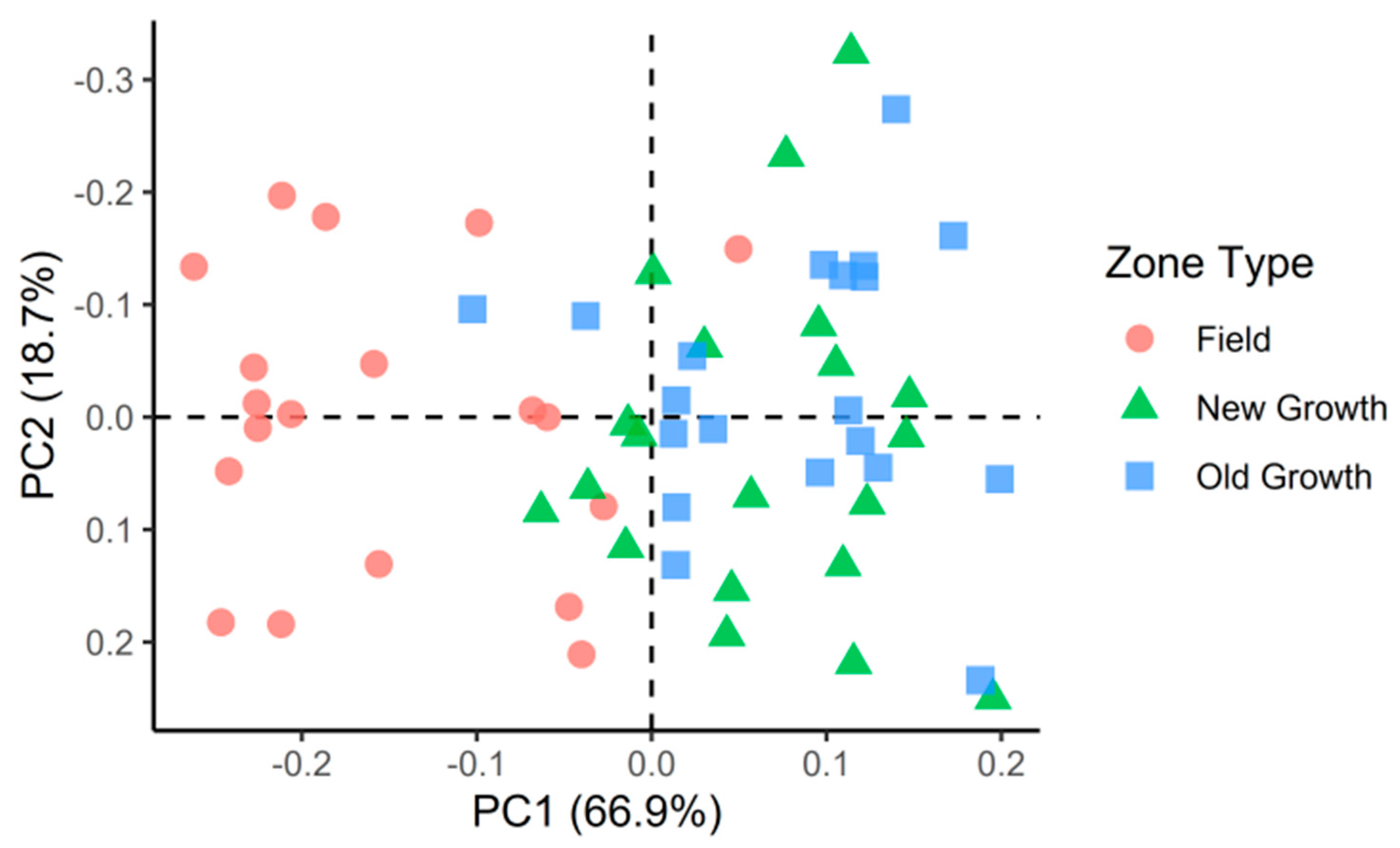
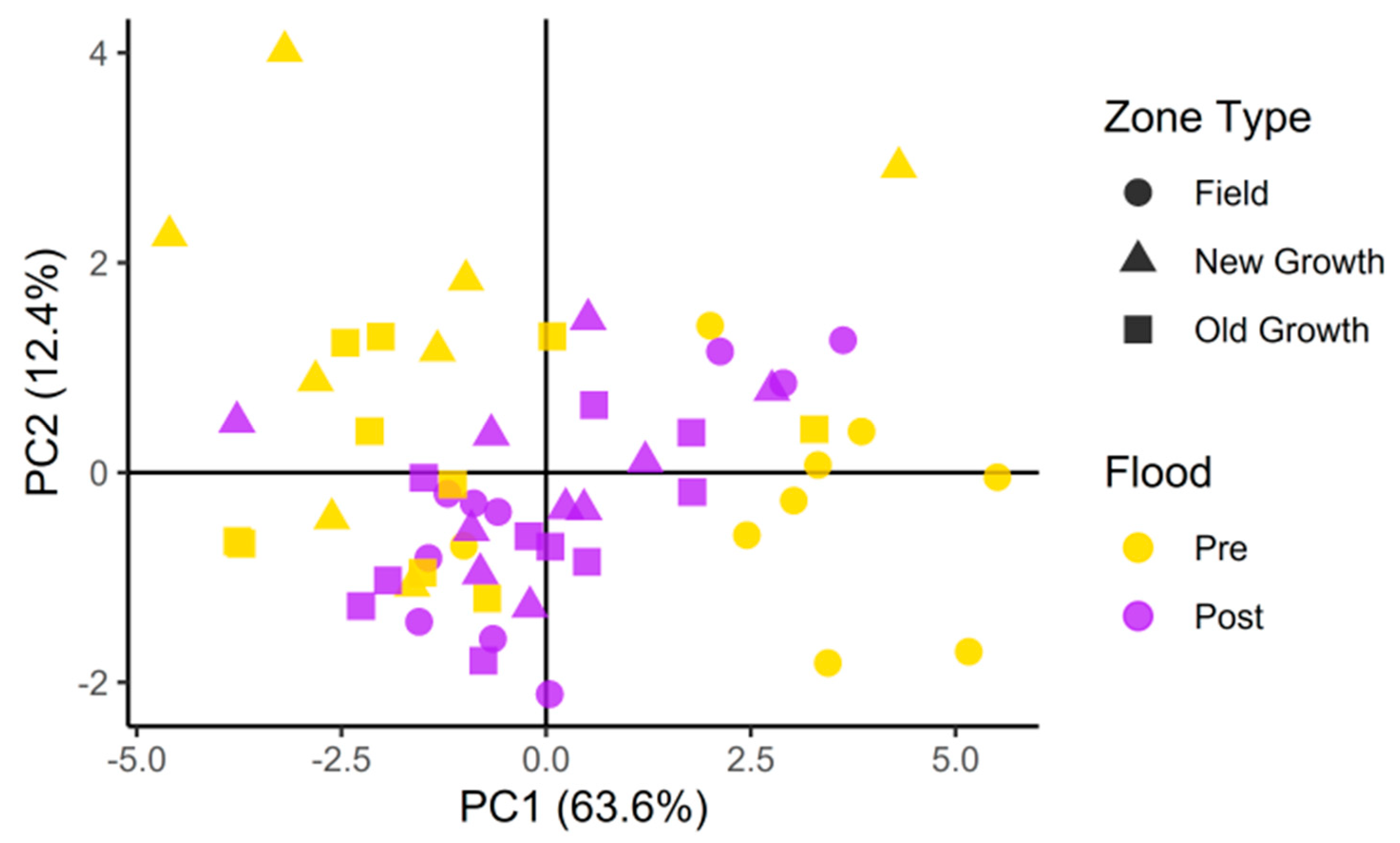
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xenopoulos, M.A.; Barnes, R.T.; Boodoo, K.S.; Butman, D.; Catalán, N.; D’Amario, S.C.; Fasching, C.; Kothawala, D.N.; Pisani, O.; Solomon, C.T.; et al. How Humans Alter Dissolved Organic Matter Composition in Freshwater: Relevance for the Earth’s Biogeochemistry. Biogeochemistry 2021, 154, 323–348. [Google Scholar] [CrossRef]
- Bhaduri, D.; Sihi, D.; Bhowmik, A.; Verma, B.C.; Munda, S.; Dari, B. A Review on Effective Soil Health Bio-Indicators for Ecosystem Restoration and Sustainability. Front. Microbiol. 2022, 13, 938481. [Google Scholar] [CrossRef] [PubMed]
- Raupp, P.; Carrillo, Y.; Nielsen, U.N. Soil Health to Enhance Ecological Restoration and Conservation. J. Sustain. Agric. Environ. 2024, 3, e70022. [Google Scholar] [CrossRef]
- Ashwood, F.; Watts, K.; Park, K.; Fuentes-Montemayor, E.; Benham, S.; Vanguelova, E.I. Woodland Restoration on Agricultural Land: Long-term Impacts on Soil Quality. Restor. Ecol. 2019, 27, 1381–1392. [Google Scholar] [CrossRef]
- Inamdar, S.P.; Kaushal, S.S.; Tetrick, R.B.; Trout, L.; Rowland, R.; Genito, D.; Bais, H. More Than Dirt: Soil Health Needs to Be Emphasized in Stream and Floodplain Restorations. Soil Syst. 2023, 7, 36. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; De Vries, W.; De Wit, C.A.; et al. Planetary Boundaries: Guiding Human Development on a Changing Planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- Perry, L.G.; Reynolds, L.V.; Beechie, T.J.; Collins, M.J.; Shafroth, P.B. Incorporating Climate Change Projections into Riparian Restoration Planning and Design. Ecohydrology 2015, 8, 863–879. [Google Scholar] [CrossRef]
- Lemke, M.J.; Hagy, H.M.; Dungey, K.; Casper, A.F.; Lemke, A.M.; VanMiddlesworth, T.D.; Kent, A. Echoes of a Flood Pulse: Short-Term Effects of Record Flooding of the Illinois River on Floodplain Lakes under Ecological Restoration. Hydrobiologia 2017, 804, 151–175. [Google Scholar] [CrossRef]
- De, M.; Riopel, J.A.; Cihacek, L.J.; Lawrinenko, M.; Baldwin-Kordick, R.; Hall, S.J.; McDaniel, M.D. Soil Health Recovery after Grassland Reestablishment on Cropland: The Effects of Time and Topographic Position. Soil Sci. Soc. Am. J. 2020, 84, 568–586. [Google Scholar] [CrossRef]
- Steiner, M.; Pingel, M.; Falquet, L.; Giffard, B.; Griesser, M.; Leyer, I.; Preda, C.; Uzman, D.; Bacher, S.; Reineke, A. Local Conditions Matter: Minimal and Variable Effects of Soil Disturbance on Microbial Communities and Functions in European Vineyards. PLoS ONE 2023, 18, e0280516. [Google Scholar] [CrossRef] [PubMed]
- Schoonover, J.E.; Crim, J.F. An Introduction to Soil Concepts and the Role of Soils in Watershed Management. J. Contemp. Water Res. Educ. 2015, 154, 21–47. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Kooistra, L. The Role of Soils in Habitat Creation, Maintenance and Restoration. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200170. [Google Scholar] [CrossRef]
- Kooch, Y.; Ghorbanzadeh, N.; Haghverdi, K.; Francaviglia, R. Soil Quality Cannot Be Improved after Thirty Years of Land Use Change from Forest to Rangeland. Sci. Total Environ. 2023, 856, 159132. [Google Scholar] [CrossRef]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Wohl, E. An Integrative Conceptualization of Floodplain Storage. Rev. Geophys. 2021, 59, e2020RG000724. [Google Scholar] [CrossRef]
- Parkhurst, T.; Standish, R.J.; Prober, S.M. P Is for Persistence: Soil Phosphorus Remains Elevated for More than a Decade after Old Field Restoration. Ecol. Appl. 2022, 32, e2547. [Google Scholar] [CrossRef]
- Goossens, E.P.; De Schrijver, A.; Schelfhout, S.; Vanhellemont, M.; Verheyen, K.; Mertens, J. Phosphorus Puts a Mortgage on Restoration of Species-rich Grasslands on Former Agricultural Land. Restor. Ecol. 2022, 30, e13523. [Google Scholar] [CrossRef]
- Maloney, K.O.; Feminella, J.W.; Mitchell, R.M.; Miller, S.A.; Mulholland, P.J.; Houser, J.N. Landuse Legacies and Small Streams: Identifying Relationships between Historical Land Use and Contemporary Stream Conditions. J. N. Am. Benthol. Soc. 2008, 27, 280–294. [Google Scholar] [CrossRef]
- Bieluczyk, W.; Merloti, L.F.; Cherubin, M.R.; Mendes, L.W.; Bendassolli, J.A.; Rodrigues, R.R.; Camargo, P.B.D.; Van Der Putten, W.H.; Tsai, S.M. Forest Restoration Rehabilitates Soil Multifunctionality in Riparian Zones of Sugarcane Production Landscapes. Sci. Total Environ. 2023, 888, 164175. [Google Scholar] [CrossRef] [PubMed]
- Saint-Laurent, D.; Gervais-Beaulac, V.; Paradis, R.; Arsenault-Boucher, L.; Demers, S. Distribution of Soil Organic Carbon in Riparian Forest Soils Affected by Frequent Floods (Southern Québec, Canada). Forests 2017, 8, 124. [Google Scholar] [CrossRef]
- Qin, Y.; Xin, Z.; Wang, D. Comparison of Topsoil Organic Carbon and Total Nitrogen in Different Flood-Risk Riparian Zones in a Chinese Karst Area. Environ. Earth Sci. 2016, 75, 1038. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Y.; Zhu, Y.; Feng, X.; Ou, J.; Li, G.; Tong, Z.; Yan, Q. Mapping Soil Organic Carbon in Floodplain Farmland: Implications of Effective Range of Environmental Variables. Land 2023, 12, 1198. [Google Scholar] [CrossRef]
- Tarnacki, M. Easement Boosts Vibrant Natural Area and Its Ecology. Saint Michael’s College, 14 January 2021. Available online: https://www.smcvt.edu/about-smc/news/2021/january/easement-boosts-vibrant-natural-area-and-its-ecology/ (accessed on 29 July 2025).
- VCGI. Vermont Imagery Finder. Available online: https://vcgi.vermont.gov/ (accessed on 15 May 2025).
- NOAA. The Great Vermont Flood of 10–11 July 2023: Preliminary Meteorological Summary. Available online: https://www.weather.gov/btv/The-Great-Vermont-Flood-of-10-11-July-2023-Preliminary-Meteorological-Summary (accessed on 10 May 2025).
- Tarnacki, M. Natural Area Plays Predictable, Useful Role during Epic Vermont Flooding. Saint Michael’s College, 11 July 2023. Available online: https://www.smcvt.edu/about-smc/news/2023/july/natural-area-plays-predictable-useful-role-during-epic-vermont-flooding/ (accessed on 29 July 2025).
- Post, W.M.; Izaurralde, R.C.; Mann, L.K.; Bliss, N. Monitoring and Verifying Changes of Organic Carbon in Soil. In Storing Carbon in Agricultural Soils: A Multi-Purpose Environmental Strategy; Rosenberg, N.J., Izaurralde, R.C., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 73–99. ISBN 978-90-481-5759-4. [Google Scholar]
- Nelson, N.S. An Acid-persulfate Digestion Procedure for Determination of Phosphorus in Sediments. Commun. Soil. Sci. Plant Anal. 1987, 18, 359–369. [Google Scholar] [CrossRef]
- Seal Analytical Phosphorus-P, Total, in Surface and Saline Waters, Domestic and Industrial Wastes. 2020. Available online: https://seal-analytical.com/en/methods/phosphate/ (accessed on 29 July 2025).
- Hansen, A.; Fleck, J.; Kraus, T.; Downing, B.; von Dessonneck, T.; Bergamaschi, B. Procedures for Using the Horiba Scientific Aqualog® Fluorometer to Measure Absorbance and Fluorescence from Dissolved Organic Matter; Open-File Report; U.S. Geological Survey: Reston, VA, USA, 2018; p. 42. [Google Scholar]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption Spectral Slopes and Slope Ratios as Indicators of Molecular Weight, Source, and Photobleaching of Chromophoric Dissolved Organic Matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Wilson, H.F.; Xenopoulos, M.A. Effects of Agricultural Land Use on the Composition of Fluvial Dissolved Organic Matter. Nat. Geosci. 2009, 2, 37–41. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence Inner-Filtering Correction for Determining the Humification Index of Dissolved Organic Matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Posit team. RStudio: Integrated Development Environment for R, Posit Software, PBC, Boston, MA, USA. 2023. Available online: http://www.posit.co/ (accessed on 29 July 2025).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-10. 2025. Available online: https://CRAN.R-project.org/package=vegan (accessed on 29 July 2025).
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 30 May 2025).
- Perillo, V.L.; Ross, D.S.; Wemple, B.C.; Balling, C.; Lemieux, L.E. Stream Corridor Soil Phosphorus Availability in a Forested–Agricultural Mixed Land Use Watershed. J. Environ. Qual. 2019, 48, 185–192. [Google Scholar] [CrossRef]
- Deng, Q.; McMahon, D.E.; Xiang, Y.; Yu, C.; Jackson, R.B.; Hui, D. A Global Meta-analysis of Soil Phosphorus Dynamics after Afforestation. New Phytol. 2017, 213, 181–192. [Google Scholar] [CrossRef]
- Xu, C.; Xiang, W.; Gou, M.; Chen, L.; Lei, P.; Fang, X.; Deng, X.; Ouyang, S. Effects of Forest Restoration on Soil Carbon, Nitrogen, Phosphorus, and Their Stoichiometry in Hunan, Southern China. Sustainability 2018, 10, 1874. [Google Scholar] [CrossRef]
- Banach, A.M.; Banach, K.; Visser, E.J.W.; Stępniewska, Z.; Smits, A.J.M.; Roelofs, J.G.M.; Lamers, L.P.M. Effects of Summer Flooding on Floodplain Biogeochemistry in Poland; Implications for Increased Flooding Frequency. Biogeochemistry 2009, 92, 247–262. [Google Scholar] [CrossRef]
- De-Campos, A.B.; Huang, C.; Johnston, C.T. Biogeochemistry of Terrestrial Soils as Influenced by Short-Term Flooding. Biogeochemistry 2012, 111, 239–252. [Google Scholar] [CrossRef]
- Akpoveta, V.O.; Osakwe, S.A.; Ize-Iyamu, O.K.; Medjor, W.O.; Egharevba, F. Post Flooding Effect on Soil Quality in Nigeria: The Asaba, Onitsha Experience. Open J. Soil. Sci. 2014, 4, 72–80. [Google Scholar] [CrossRef]
- Kumaragamage, D.; Amarawansha, G.S.; Indraratne, S.P.; Jayarathne, K.; Flaten, D.N.; Zvomuya, F.; Akinremi, O.O. Degree of Phosphorus Saturation as a Predictor of Redox-Induced Phosphorus Release from Flooded Soils to Floodwater. J. Environ. Qual. 2019, 48, 1817–1825. [Google Scholar] [CrossRef]
- Khan, S.U.; Hooda, P.S.; Blackwell, M.S.A.; Busquets, R. Effects of Drying and Simulated Flooding on Soil Phosphorus Dynamics from Two Contrasting UK Grassland Soils. Eur. J. Soil. Sci. 2022, 73, e13196. [Google Scholar] [CrossRef]
- Tang, J.; Liu, E.; Li, Y.; Tang, Y.; Tian, Y.; Du, S.; Li, H.; Wan, L.; Zhang, Q. Afforestation Promotes Soil Organic Carbon and Soil Microbial Residual Carbon Accrual in a Seasonally Flooded Marshland. Forests 2024, 15, 1542. [Google Scholar] [CrossRef]
- Gervais-Beaulac, V. Organic Carbon Distribution in Alluvial Soils According to Different Flood Risk Zones. J. Soil. Sci. Environ. Manag. 2013, 4, 169–177. [Google Scholar] [CrossRef]
- Jelinski, N.A.; Kucharik, C.J. Land-use Effects on Soil Carbon and Nitrogen on a U.S. Midwestern Floodplain. Soil. Sci. Soc. Am. J. 2009, 73, 217–225. [Google Scholar] [CrossRef]
- Schoenholtz, S.H.; Miegroet, H.V.; Burger, J.A. A Review of Chemical and Physical Properties as Indicators of Forest Soil Quality: Challenges and Opportunities. For. Ecol. Manag. 2000, 138, 335–356. [Google Scholar] [CrossRef]
- Kiedrzyńska, E.; Kiedrzyński, M.; Zalewski, M. Sustainable Floodplain Management for Flood Prevention and Water Quality Improvement. Nat. Hazards 2015, 76, 955–977. [Google Scholar] [CrossRef]
- Paradis, R.; Saint-Laurent, D. Spatial Distribution of Organic Carbon and Nitrogen in Soils Related to Flood Recurrence Intervals and Land Use Changes in Southern Qubec, Canada. J. Soil. Sci. Environ. Manag. 2017, 8, 25–36. [Google Scholar] [CrossRef][Green Version]
- Williams, C. Assessing the Effect of Undirected Forest Restoration and Flooding on the Soil Quality in an Agricultural Floodplain. Figshare. 2025. Dataset. Available online: https://figshare.com/articles/dataset/Assessing_the_Effect_of_Undirected_Forest_Restoration_and_Flooding_on_the_Soil_Quality_in_an_Agricultural_Floodplain/29803547/1 (accessed on 2 August 2025).
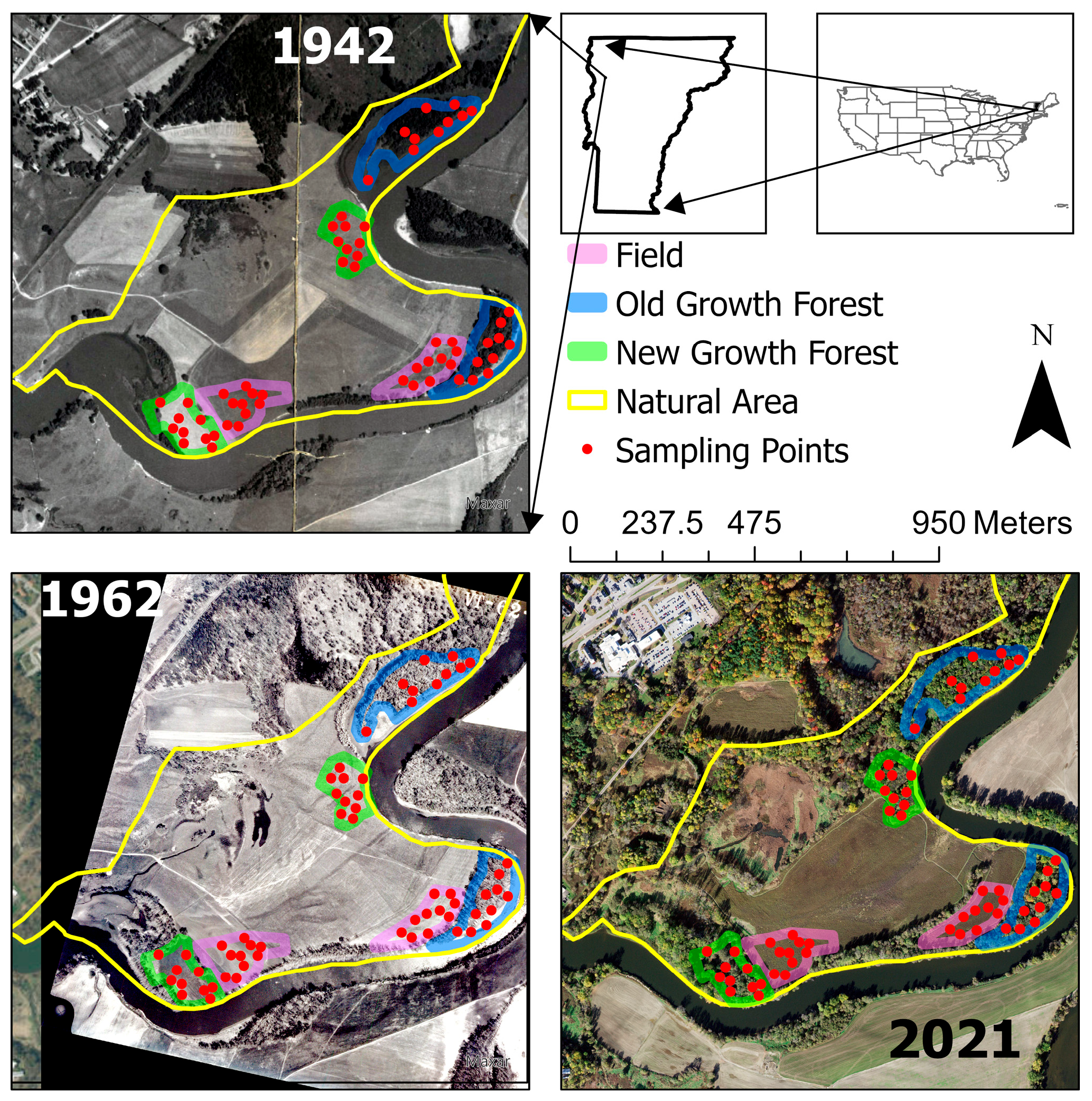
| Zone | Pre 1900 | 1900 to 1942 | 1942 to 1962 | 1962 to 2018 | 2018 to Present |
|---|---|---|---|---|---|
| Field Plot A and B | Unknown | Used for sheep, cattle, and potato farming | Used for sheep, cattle, and potato farming | Conventional row crop, corn agriculture | Naturally recolonizing with grasses and trees |
| New-growth Plot A and B | Unknown | Used for sheep, cattle, and potato farming | Used for sheep, cattle, and potato farming | Reforested | Forested |
| Old-growth Plot A | Unknown | Forested riparian | Forested riparian | Forested riparian | Forested riparian |
| Old-growth Plot B | River island presumed forested | Forested river island | Forested part of Natural Area | Forested riparian | Forested riparian |
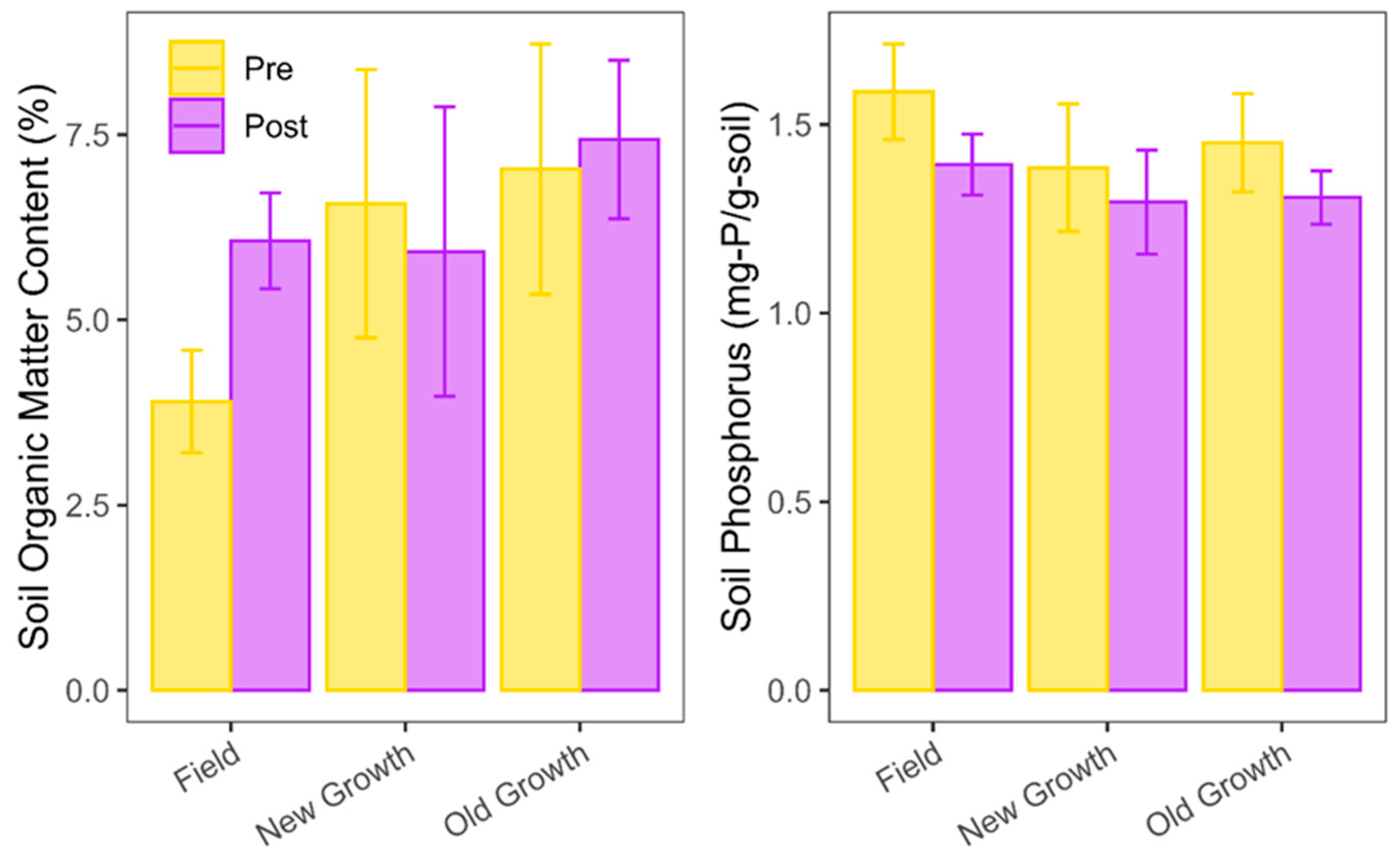
| Zone | Temp (°C) | Moisture (%) | pH | LOI (%) | Extractable Phosphorus (mg-P/g soil) |
|---|---|---|---|---|---|
| Field Plot A | 17.2 ± 3.3 a | 39.6 ± 12.7 a | 6.4 ± 0.2 a | 4.1 ± 0.7 a | 1.59 ± 0.14 a |
| Field Plot B | 14.7 ± 2.7 a | 46.7 ± 5.2 a | 6.3 ± 0.1 a | 3.7 ± 0.7 a | 1.58 ± 0.12 a |
| New-growth Plot A | 15.4 ± 0.7 b | 86.6 ± 8.6 b | 5.9 ± 0.2 b | 7.7 ± 1.8 b | 1.55 ± 0.07 b |
| New-growth Plot B | 18.1 ± 1.0 b | 60.3 ± 7.7 b | 6.1 ± 0.1 b | 5.4 ± 0.9 b | 1.27 ± 0.12 b |
| Old-growth Plot A | 17.9 ± 1.3 b | 89.0 ± 16.8 c | 5.7 ± 0.2 c | 6.1 ± 0.4 b | 1.41 ± 0.11 b |
| Old-growth Plot B | 15.9 ± 1.0 b | 83.5 ± 6.6 c | 5.9 ± 0.2 c | 8.0 ± 2.0 b | 1.49 ± 0.14 b |
| Zone | Flood Period | SR | BA | HIX | Peak A | Peak C | Peak D | Peak E | Peak M | Peak N | Peak T |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h water extraction | |||||||||||
| Field | Pre | 0.72 ± 0.02 | 0.65 ± 0.05 | 0.67 ± 0.05 | 26.7 ± 1.5 | 17.2 ± 0.8 | 6.2 ± 0.4 | 2 ± 0.2 | 17.6 ± 1.4 | 13.3 ± 1.1 | 17.2 ± 2 |
| New-growth | Pre | 0.81 ± 0.05 | 0.56 ± 0.02 | 0.79 ± 0.03 | 28.9 ± 0.9 | 18.3 ± 0.8 | 7.1 ± 0.7 | 2.1 ± 0.2 | 19.1 ± 1.1 | 11.3 ± 0.6 | 13.4 ± 1.8 |
| Old-growth | Pre | 0.81 ± 0.04 | 0.54 ± 0.05 | 0.79 ± 0.04 | 28.7 ± 1.1 | 18.8 ± 0.8 | 7 ± 0.6 | 2 ± 0.1 | 19.3 ± 0.9 | 11.1 ± 0.7 | 13.1 ± 1.7 |
| 30 min water extraction | |||||||||||
| Field | |||||||||||
| Pre | 0.97 ± 0.08 | 0.53 ± 0.07 | 0.68 ± 0.04 | 25.7 ± 1.5 | 19 ± 0.9 | 5.9 ± 0.4 | 2.1 ± 0.2 | 19.8 ± 0.8 | 12.9 ± 1.5 | 14.7 ± 2.3 | |
| Post | 1.03 ± 0.09 | 0.5 ± 0.02 | 0.77 ± 0.04 | 29.1 ± 1.8 | 18.9 ± 0.8 | 6.8 ± 0.4 | 2 ± 0.1 | 20.5 ± 1 | 10.8 ± 1.6 | 11.8 ± 2.3 | |
| New-growth | |||||||||||
| Pre | 1.11 ± 0.13 | 0.49 ± 0.05 | 0.82 ± 0.04 | 29.6 ± 2.4 | 19.2 ± 1 | 7.3 ± 0.8 | 2.7 ± 0.4 | 20.4 ± 0.8 | 10.0 ± 1.4 | 10.8 ± 3.4 | |
| Post | 0.98 ± 0.07 | 0.51 ± 0.02 | 0.79 ± 0.03 | 29.3 ± 1.3 | 18.4 ± 1.1 | 7.4 ± 0.4 | 2.2 ± 0.2 | 19.8 ± 0.8 | 10.5 ± 1.3 | 12.3 ± 2 | |
| Old-growth | |||||||||||
| Pre | 1.03 ± 0.08 | 0.47 ± 0.03 | 0.82 ± 0.03 | 29 ± 2.1 | 19.1 ± 1.4 | 7 ± 0.7 | 2.5 ± 0.2 | 22 ± 2.3 | 9.9 ± 1.4 | 10.5 ± 2.5 | |
| Post | 0.96 ± 0.07 | 0.5 ± 0.02 | 0.79 ± 0.02 | 28.7 ± 1.5 | 18.4 ± 1.1 | 7.1 ± 0.4 | 2.2 ± 0.1 | 21.2 ± 1.1 | 10.4 ± 1 | 11.9 ± 1.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wessinger, A.; Juarez, A.; Williams, C.J. Assessing the Effect of Undirected Forest Restoration and Flooding on the Soil Quality in an Agricultural Floodplain. Soil Syst. 2025, 9, 88. https://doi.org/10.3390/soilsystems9030088
Wessinger A, Juarez A, Williams CJ. Assessing the Effect of Undirected Forest Restoration and Flooding on the Soil Quality in an Agricultural Floodplain. Soil Systems. 2025; 9(3):88. https://doi.org/10.3390/soilsystems9030088
Chicago/Turabian StyleWessinger, Addison, Anna Juarez, and Clayton J. Williams. 2025. "Assessing the Effect of Undirected Forest Restoration and Flooding on the Soil Quality in an Agricultural Floodplain" Soil Systems 9, no. 3: 88. https://doi.org/10.3390/soilsystems9030088
APA StyleWessinger, A., Juarez, A., & Williams, C. J. (2025). Assessing the Effect of Undirected Forest Restoration and Flooding on the Soil Quality in an Agricultural Floodplain. Soil Systems, 9(3), 88. https://doi.org/10.3390/soilsystems9030088







