Seasonality of Arbuscular Mycorrhizal Fungal Diversity and Glomalin in Sodic Soils of Grasslands Under Contrasting Grazing Intensities
Abstract
1. Introduction
2. Materials and Methods
2.1. Selected Sites and Soil Sampling Procedure
2.2. Taxonomic Identification of AMF Spores in the Soil
2.3. Diversity of the AMF Community
2.4. Hyphal Density in the Soil
2.5. Glomalin
2.6. Soil Analyses
2.7. Statistical Analyses
3. Results
3.1. Soil Chemical Properties
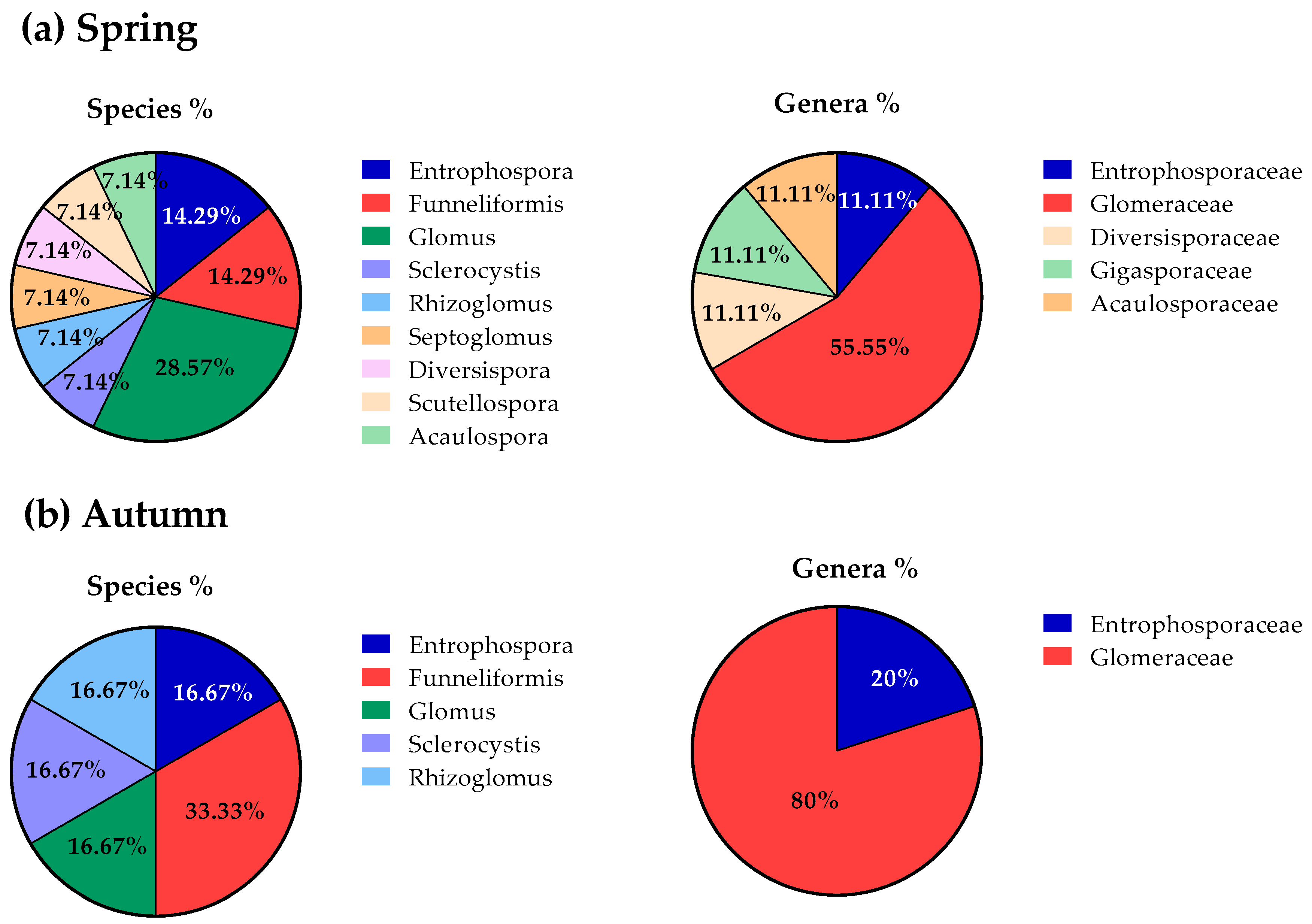
3.2. AMF Community
3.3. Relationship Between AMF Community and Soil Properties
4. Discussion
4.1. Seasonality of Soil Properties Under Different Grazing Intensities
4.2. Seasonal Effect on the Diversity of the AMF Community Under Different Grazing Intensities
4.3. Seasonal Relationships Between Soil Properties and AMF Variables Under Grazing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMF | Arbuscular mycorrhizal fungi |
| T-GRSPs | Total glomalin-related soil proteins |
| EE-GRSPs | Easily extractable glomalin-related soil proteins |
| ESP | Exchangeable sodium percentage |
References
- Conant, R.T.; Cerri, C.E.P.; Osborne, B.B.; Paustian, K. Grassland management impacts on soil carbon stocks: A new synthesis. Ecol. Appl. 2017, 27, 662–668. [Google Scholar] [CrossRef]
- Scholtz, R.; Twidwell, D. The last continuous grasslands on Earth: Identification and conservation importance. Conserv. Sci. Pract. 2022, 4, e626. [Google Scholar] [CrossRef]
- Escudero, V.; Mendoza, R. Seasonal variation of arbuscular mycorrhizal fungi in temperate grasslands along a wide hydrologic gradient. Mycorrhiza 2005, 15, 291–299. [Google Scholar] [CrossRef]
- García, I.V.; Covacevich, F.; Fernández-López, C.; Cabello, M. Lotus tenuis maintains high arbuscular mycorrhizal diversity in grasslands regardless of soil properties or management. Rhizosphere 2023, 27, 100754. [Google Scholar] [CrossRef]
- Lugo, M.A.; Ontivero, R.E.; Iriarte, H.J.; Yelikbayev, B.; Pagano, M.C. The Diversity of Arbuscular Mycorrhizal Fungi and Their Associations in South America: A Case Study of Argentinean and Brazilian Cattle Raising Productive Ecosystems: A Review. Diversity 2023, 15, 1006. [Google Scholar] [CrossRef]
- Vecchio, M.C.; Bolanos, V.A.; Golluscio, R.A.; Rodríguez, A.M. Rotational grazing and exclosure improves grassland condition of the halophytic steppe in Flooding Pampa (Argentina) compared with continuous grazing. Rangel. J. 2019, 41, 1–12. [Google Scholar] [CrossRef]
- Bai, Z.; Jia, A.; Liu, D.; Zhang, C.; Wang, M. How Seasonal Grazing Exclusion Affects Grassland Productivity and Plant Community Diversity. Grasses 2022, 1, 12–29. [Google Scholar] [CrossRef]
- Teague, W.R.; Dowhower, S.L.; Baker, S.A.; Haile, N.; DeLaune, P.B.; Conover, D.M. Grazing management impacts on vegetation, soil biota and soil chemical, physical and hydrological properties in tall grass prairie. Agricul. Ecosys. Environ. 2011, 141, 310–322. [Google Scholar] [CrossRef]
- van der Heyde, M.; Bennett, J.A.; Piter, J.; Hart, M.M. Long-term effects of grazing on the arbuscular mycorrhizal symbiosis. Agric. Ecosys. Environ. 2017, 243, 27–33. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Asmelash, F.; Bekele, T.; Birhane, E. The potential role of arbuscular mycorrhizal fungi in the restoration of degraded lands. Front. Microbiol. 2016, 7, 1095. [Google Scholar] [CrossRef]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoono, S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Roy, T.; Mandal, U.; Mandal, D.; Yadav, D. Role of arbuscular mycorrhizal fungi in soil and water conservation: A potentially unexplored domain. Curr. Sci. 2021, 120, 1573–1577. [Google Scholar] [CrossRef]
- Nie, W.; He, Q.; Guo, H.; Zhang, W.; Ma, L.; Li, J.; Wen, D. Arbuscular Mycorrhizal fungi: Boosting Crop Resilience to Environmental Stresses. Microorganisms 2024, 12, 2448. [Google Scholar] [CrossRef]
- García, I. Lotus tenuis in association with arbuscular mycorrhizal fungi is more tolerant to partial submergence than to high-intensity defoliation. Int. J. Plant Biol. 2025, 16, 47. [Google Scholar] [CrossRef]
- Ren, H.; Gui, W.; Bai, Y.; Stein, C.; Rodrigues, J.L.M.; Wilson, G.W.T.; Cobb, A.B.; Zhang, Y.; Yang, G. Long-term effects of grazing and topography on extra-radical hyphae of arbuscular mycorrhizal fungi in semi-arid grasslands. Mycorrhiza 2018, 28, 117–127. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Shen, Y.; Dong, F.; Chen, J. Global negative effects of livestock grazing on arbuscular mycorrhizas: A meta-analysis. Sci Total Environ. 2020, 708, 134553. [Google Scholar] [CrossRef]
- Faghihinia, M.; Zou, Y.; Chen, Z.; Bai, Y.; Li, W.; Marrs, R.; Staddon, P.L. Environmental drivers of grazing effects on arbuscular mycorrhizal fungi in grasslands. Appl. Soil Ecol. 2020, 153, 103591. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Liang, C.; Ai, Z.; Wu, Y.; Xu, H.; Xue, S.; Liu, G. Glomalin-related soil protein affects soil aggregation and recovery of soil nutrient following natural revegetation on the Loess Plateau. Geoderma 2020, 357, 113921. [Google Scholar] [CrossRef]
- Commatteo, J.G.; Barbieri, P.A.; Corral, R.A.; Covacevich, F. The potential of glomalin-related soil proteins as a sensitive indicator of changes in different cropping systems in the Argentine Pampas. Environ. Sustain. 2023, 6, 183–194. [Google Scholar] [CrossRef]
- Šarapatka, B.; Alvarado-Solano, D.P.; Čižmár, D. Can glomalin content be used as an indicator for erosion damage to soil and related changes in organic matter characteristics and nutrients? CATENA 2019, 18, 104078. [Google Scholar] [CrossRef]
- Davison, J.; Moora, M.; Semchenko, M.; Adenan, S.B.; Ahmed, T.; Akhmetzhanova, A.A.; S Alatalo, J.M.; Al-Quraishy, S.; Andriyanova, E.; Anslan, S.; et al. Temperature and pH define the realised niche space of arbuscular mycorrhizal fungi. New Phytol. 2021, 231, 763–776. [Google Scholar] [CrossRef]
- Matinizadeh, M.; Nouri, E.; Bayranvand, M.; Kolarikova, Z.; Janoušková, M. Arbuscular mycorrhiza and rhizosphere soil enzymatic activities as modulated by grazing intensity and plant species identity in a semi-arid grassland. Rhizosphere 2024, 30, 100893. [Google Scholar] [CrossRef]
- Barceló, M.; van Bodegom, P.M.; Tedersoo, L.; den Haan, N.; Veen, G.F.; Ostonen, I.; Trimbos, K.; Soudzilovskaia, N.A. The abundance of arbuscular mycorrhiza in soils is linked to the total length of roots colonized at ecosystem level. PLoS ONE 2020, 15, e0237256. [Google Scholar] [CrossRef] [PubMed]
- Baral, N.K.; Giri, A.; Shah, P.K.; Kemmelmeier, K.; Stürmer, S.L.; Gyawali, S.; Raut, J.K. Diversity of arbuscular mycorrhizal fungi (Glomeromycota) in adjacent areas of different land use in Nepal. GSC Biol. Pharm. Sci. 2021, 15, 141–150. [Google Scholar] [CrossRef]
- Lutgen, E.R.; Muir-Clairmont, D.; Graham, J.; Rillig, M.C. Seasonality of arbuscular mycorrhizal hyphae and glomalin in a western Montana grassland. Plant Soil 2003, 257, 71–83. [Google Scholar] [CrossRef]
- García, I.V.; Mendoza, R.E. Arbuscular mycorrhizal fungi and plant symbiosis in a saline-sodic soil. Mycorrhiza 2007, 17, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Daget, P.; Poissonet, J. Uné méthode d’analyse phytologique des prairies. Critéres d’application. Ann. Agron. 1971, 22, 5–41. [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Walker, C.; Mize, W.; McNabb, H.S. Populations of endogonaceous fungi at two populations in central Iowa. Canad. J Bot. 1982, 60, 2518–2529. [Google Scholar] [CrossRef]
- Omar, M.B.; Bolland, L.; Heather, W.A.P.V.A. (polivinil alcohol). A permanent mounting medium for fungi. Bull. Br. Mycol. Soc. 1979, 13, 31–32. [Google Scholar] [CrossRef]
- Schenck, N.C.; Peréz, Y. Manual for the Identification of VA Mycorrhizal Fungi, 2nd ed.; International Culture Collection of VA Mycorrhizal Fungi: Gainsville, FL, USA, 1990. [Google Scholar]
- Morris, E.; Tancredi Caruso, K.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D.; et al. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, L.-D.; Liu, R.-J. Survey of arbuscular mycorrhizal fungi in deforested and natural forest land in the subtropical region of Dujiangyan, southwest China. Plant Soil 2004, 261, 257–263. [Google Scholar] [CrossRef]
- Malik, J.A.; Dar, B.A.; Alqarawi, A.A.; Assaeed, A.M.; Alotaibi, F.; Alkhasha, A.; Adam, A.M.; Abd-ElGawad, A.M. Species Richness of Arbuscular Mycorrhizal Fungi in Heterogenous Saline Environments. Diversity 2025, 17, 183. [Google Scholar] [CrossRef]
- Abbott, L.K.; Robson, A.D.; De Boer, G. The effect of phosphorus on the formation of hyphae in soil by the vesicular–arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytol. 1984, 97, 437–446. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil. 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chapman, H.D. Cation exchange capacity. In Methods of Soil Analysis, Part 2—Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 891–901. [Google Scholar]
- Walkley, A.; Armstrong Black, I. An examination of the Degljareff method for determining soil organic matter, and proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Marbán, L.; Ratto, S. (Eds.) Nitrógeno del Suelo. Tecnologías en Análisis de Suelos; Asociación Argentina de la Ciencia del Suelo: Buenos Aires, Argentina, 2005; pp. 117–122. ISBN 987-21419-1-6. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total organic and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Version. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Cordoba. Argentina. 2019. Available online: http://www.infostat.com.ar (accessed on 1 February 2020).
- Richards, L. Diagnóstico y Rehabilitación de Suelos Salinos y Sódicos; Editorial Limusa: Mexico City, Mexico, 1974. [Google Scholar]
- Medina-Roldán, E.; Arredondo, J.T.; Huber-Sannwald, E.; Chapa-Vargas, L.; Olalde- Portugal, V. Grazing effects nitrogen on fungal root symbionts and carbon and storage in a shortgrass steppe in Central Mexico. J. Arid Environ. 2008, 72, 546–556. [Google Scholar] [CrossRef]
- Egan, G.; Crawley, M.J.; Fornara, D.A. Effects of long-term grassland management on the carbon and nitrogen pools of different soil aggregate fractions. Sci. Total Environ. 2018, 613, 810–819. [Google Scholar] [CrossRef]
- García, I.; Mendoza, R. Relationships among soil properties, plant nutrition and arbuscular mycorrhizal fungi–plant symbioses in a temperate grassland along hydrologic, saline and sodic gradients. FEMS Microbiol. Ecol. 2008, 63, 359–371. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, Y.; Liu, X.; Du, G. Spatio-temporal dynamics of arbuscular mycorrhizal fungi associated with glomalin-related soil protein and soil enzymes in different managed semiarid steppes. Mycorrhiza 2014, 24, 525–538. [Google Scholar] [CrossRef]
- Černý, J.; Balík, J.; Suran, P.; Sedlář, O.; Procházková, S.; Kulhánek, M. The content of soil glomalin concerning selected indicators of soil fertility. Agronomy 2024, 14, 1731. [Google Scholar] [CrossRef]
- Smilauer, P.; Smilauerov, M. Contrasting effects of host identity, plant community, and local species pool on the composition and colonization levels of arbuscular mycorrhizal fungal community in a temperate grassland. New Phytol. 2020, 225, 461–473. [Google Scholar] [CrossRef]
- Stevens, B.M.; Propster, J.R.; Öpik, M.; Wilson, G.W.T.; Alloway, S.L.; Mayemba, E.; Johnson, N.C. Arbuscular mycorrhizal fungi in roots and soil respond differently to biotic and abiotic factors in the Serengeti. Mycorrhiza 2020, 30, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Y.; Guo, L.D. Arbuscular mycorrhizal fungi in non-grazed, restored and overgrazed grassland in the Inner Mongolia steppe. Mycorrhiza 2007, 17, 689–693. [Google Scholar] [CrossRef]
- Chagnon, P.-L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef]
- Parihar, M.; Rakshita, A.; Singhb, H.B.; Rana, K. Diversity of arbuscular mycorrhizal fungi in alkaline soils of hot sub humid ecoregion of Middle Gangetic Plains of India. Acta Agric. Scand. Sect. B. 2021, 69, 386–397. [Google Scholar] [CrossRef]
- Ontivero, R.E.; Risio Allione, L.V.; Castellarini, F.; Lugo, M.A. Composición de las comunidades de hongos micorrícicos arbusculares en diferentes usos de suelo en el Caldenal, Argentina. Asoc. Argent. Ecol. Ecol. Austral. 2023, 33, 95–107. [Google Scholar] [CrossRef]
- Li, Z.-F.; Lü, P.-P.; Wang, Y.-L.; Yao, H.; Maitra, P.; Sun, X.; Zheng, Y.; Guo, L.-D. Response of arbuscular mycorrhizal fungal community in soil and roots to grazing differs in a wetland on the Qinghai-Tibet plateau. PeerJ 2020, 8, e9375. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Zhu, X.; Chen, C.; Wu, J.; Yang, B.; Zakari, S.; Jiang, X.J.; Singh, N.; Liu, W. The role of glomalin in mitigation of multiple soil degradation problems. Crit. Rev. Environ. Sci. Technol. 2020, 52, 1604–1638. [Google Scholar] [CrossRef]
- Onyeaka, H.N.; Akinsemolu, A.A.; Siyanbola, K.F.; Adetunji, V.A. Green Microbe Profile: Rhizophagus intraradices—A Review of Benevolent Fungi Promoting Plant Health and Sustainability. Microbiol. Res. 2024, 15, 1028–1049. [Google Scholar] [CrossRef]
- Uhlmann, E.; Görke, C.; Petersen, A.; Oberwinkler, F. Arbuscular mycorrhizae from semiarid region of Namibia. J. Arid. Environ. 2006, 64, 221–237. [Google Scholar] [CrossRef]




| Grassland Site | Grazing Intensity | Location | Soil Classification | Na+ (cmol/kg) | Dominant Plant Species | Stocking Rate (LU/ha) | Period of Management (Years) |
|---|---|---|---|---|---|---|---|
| 1L | low | Chascomús | Typic Natraquoll | 7.07 | Cynodon dactylon (L.) Pers. | 1 | 13 |
| Festuca arundinacea Schreb. | |||||||
| 2H | high | Chascomús | Typic Natraquoll | 5.99 | Cynodon dactylon | 0.85 | 35 |
| Festuca arundinacea | |||||||
| 3L | low | San Cristobal | Typic Natraquoll | 7.03 | Cynodon dactylon | 1.23 | 26 |
| Festuca arundinacea | |||||||
| 4H | high | San Cristobal | Typic Natraquoll | 4.56 | Cynodon dactylon | 0.57 | 30 |
| 5L | low | San Cristobal | Typic Natraqualf | 6.69 | Sporobolus spartinus (Trin.) P.M. Peterson & Saarela | 1.23 | 26 |
| Cynodon dactylon | |||||||
| 6H | high | San Cristobal | Typic Natraqualf | 5.71 | Sporobolus spartinus | 0.57 | 30 |
| Cynodon dactylon |
| AMF Species | 1L | 2H | 3L | 4H | 5L | 6H | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | |
| Family Entrophosporaceae | ||||||||||||
| Entrophospora etunicata | 2.33 | 0 | 4.67 | 0 | 1.33 | 0 | 0 | 0 | 0 | 0 | 0.67 | 0 |
| Entrophospora claroidea | 0 | 5 | 1.67 | 0.33 | 12 | 7.33 | 11.33 | 6 | 5 | 22.67 | 23.33 | 7 |
| Family Glomeraceae | ||||||||||||
| Funneliformis geosporus | 29.67 | 38.67 | 22.33 | 56.33 | 23.33 | 37.67 | 33.67 | 51.67 | 41.67 | 52 | 34 | 39 |
| Funneliformis mosseae | 0 | 13.33 | 0.67 | 7.33 | 3.67 | 0 | 0 | 0 | 7.33 | 0 | 6.67 | 10.67 |
| Glomus brohultii | 31.66 | 33 | 16.67 | 28 | 34.67 | 30 | 32.33 | 32.33 | 29.33 | 10 | 22 | 36.67 |
| Glomus sp. 1 | 0 | 0 | 0 | 0 | 2.33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Glomus sp. 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.67 | 0 |
| Glomus fuegianum | 0 | 0 | 0 | 0 | 0.67 | 0 | 1.33 | 0 | 0 | 0 | 0 | 0 |
| Sclerocystis sinuosa | 12.33 | 0 | 16.33 | 0 | 0 | 0 | 2.67 | 6.33 | 0 | 0 | 0 | 0 |
| Rhizoglomus intraradices | 13.00 | 9.67 | 0 | 1.67 | 7.67 | 0.67 | 25.33 | 0 | 3 | 14.67 | 0 | 6.67 |
| Septoglomus constrictum | 10.66 | 0 | 26 | 0 | 6.67 | 0 | 14.33 | 0 | 1.33 | 0 | 3.67 | 0 |
| Family Diversisporaceae | ||||||||||||
| Diversispora spurca | 0 | 0 | 0 | 0 | 13 | 0 | 4 | 0 | 15.67 | 0 | 5.67 | 0 |
| Family Gigasporaceae | ||||||||||||
| Scutellospora sp. | 0 | 0 | 0 | 0 | 1.33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Family Acaulosporaceae | ||||||||||||
| Acaulospora sp. | 0 | 0 | 2.33 | 0 | 7.50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H | 1.83 | 1.65 | 2.18 | 1.50 | 2.35 | 1.62 | 1.98 | 1.38 | 1.91 | 1.47 | 2.29 | 1.91 |
| R | 4.33 | 4.67 | 5.67 | 4.67 | 7.33 | 3.33 | 4.67 | 3.33 | 4.67 | 3.33 | 6.67 | 5.33 |
| E | 0.93 | 0.73 | 0.89 | 0.72 | 0.82 | 0.94 | 0.90 | 0.83 | 0.86 | 0.90 | 0.85 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, I.; Cáceres-Mago, K.; Becerra, A.G. Seasonality of Arbuscular Mycorrhizal Fungal Diversity and Glomalin in Sodic Soils of Grasslands Under Contrasting Grazing Intensities. Soil Syst. 2025, 9, 87. https://doi.org/10.3390/soilsystems9030087
García I, Cáceres-Mago K, Becerra AG. Seasonality of Arbuscular Mycorrhizal Fungal Diversity and Glomalin in Sodic Soils of Grasslands Under Contrasting Grazing Intensities. Soil Systems. 2025; 9(3):87. https://doi.org/10.3390/soilsystems9030087
Chicago/Turabian StyleGarcía, Ileana, Karla Cáceres-Mago, and Alejandra Gabriela Becerra. 2025. "Seasonality of Arbuscular Mycorrhizal Fungal Diversity and Glomalin in Sodic Soils of Grasslands Under Contrasting Grazing Intensities" Soil Systems 9, no. 3: 87. https://doi.org/10.3390/soilsystems9030087
APA StyleGarcía, I., Cáceres-Mago, K., & Becerra, A. G. (2025). Seasonality of Arbuscular Mycorrhizal Fungal Diversity and Glomalin in Sodic Soils of Grasslands Under Contrasting Grazing Intensities. Soil Systems, 9(3), 87. https://doi.org/10.3390/soilsystems9030087






