Spatial and Temporal Variability of C Stocks and Fertility Levels After Repeated Compost Additions: A Case Study in a Converted Mediterranean Perennial Cropland
Abstract
1. Introduction
2. Materials and Methods
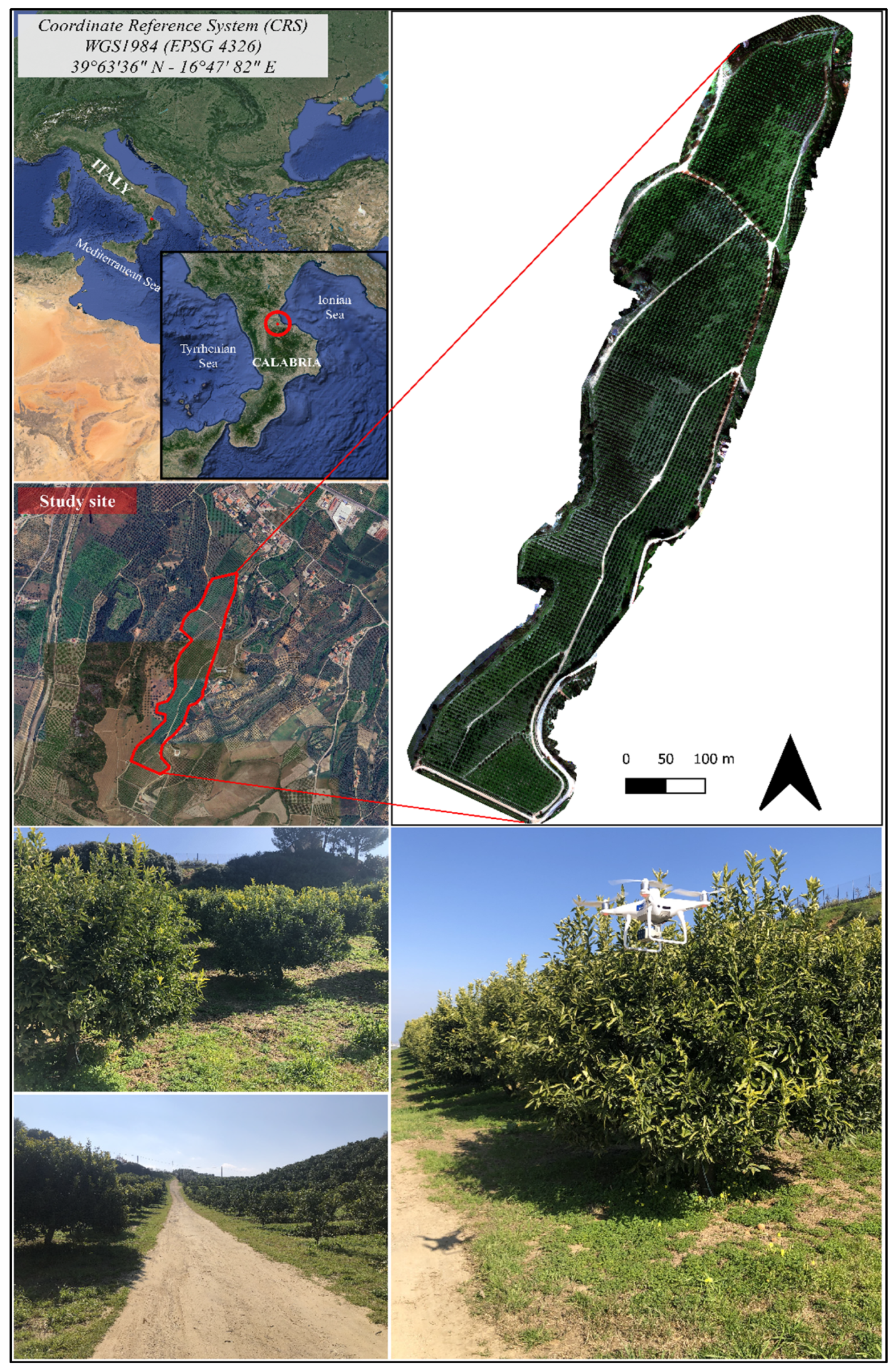
2.1. Site Description and Citrus Crop Management
2.2. Soil Sampling and Analysis
2.3. UAV Surveys and Image Processing
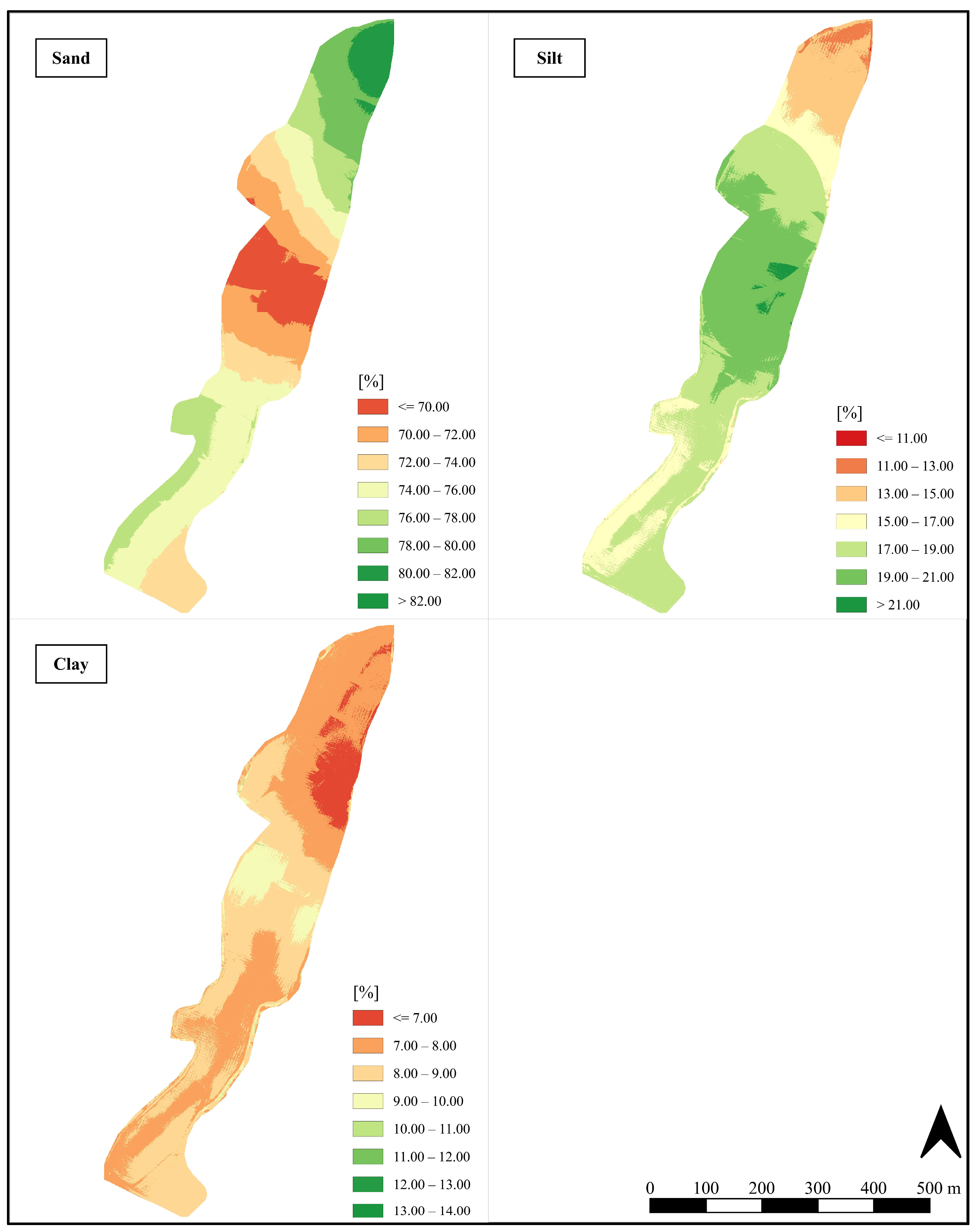
2.4. Spatialization of Soil Data (Kriging)
2.5. Statistics
3. Results and Discussion
3.1. Spatial Heterogeneity of Physico-Chemical Soil Properties and Si Pools After Land Use Change in the Soil of a Citrus Orchard
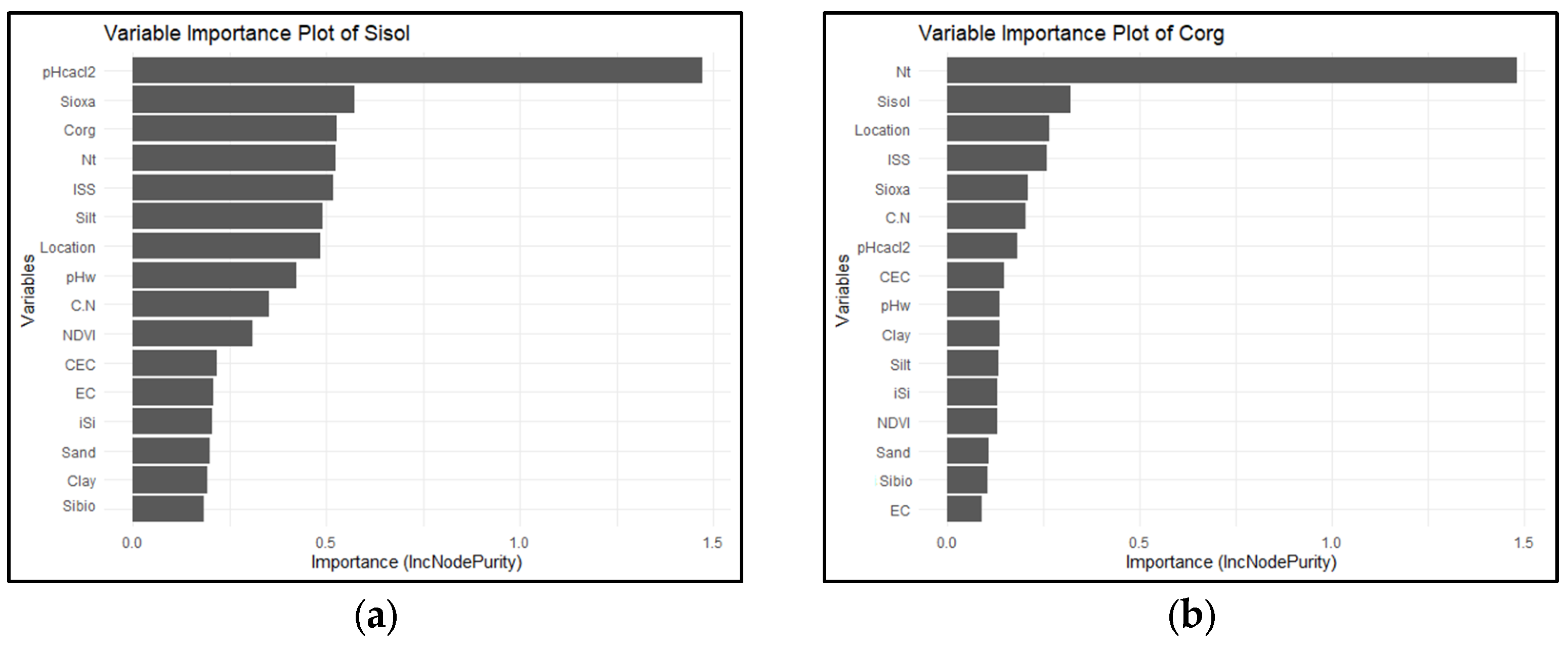
3.2. Relationships Between Si Pools and Physico-Chemical Soil Properties
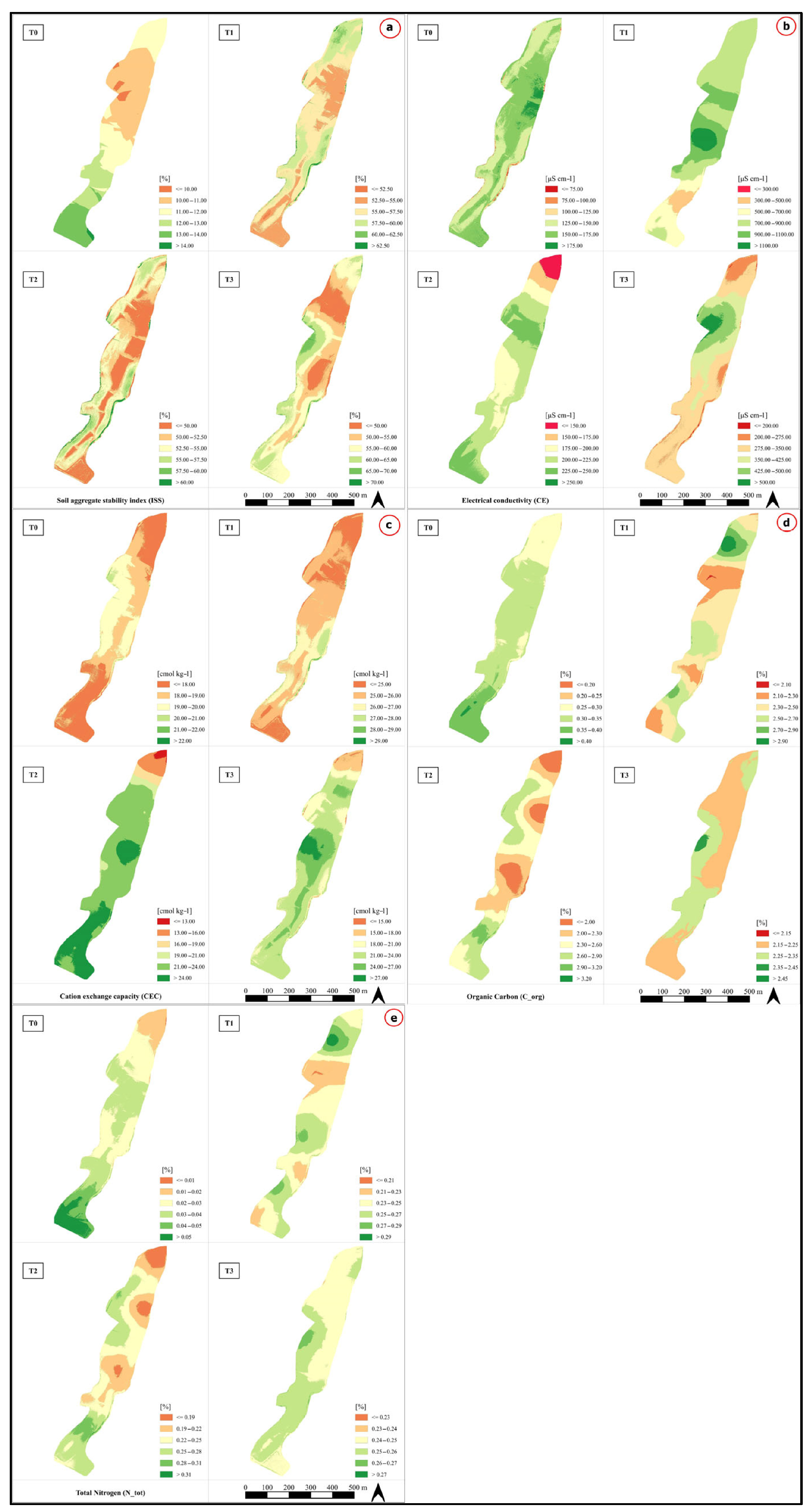
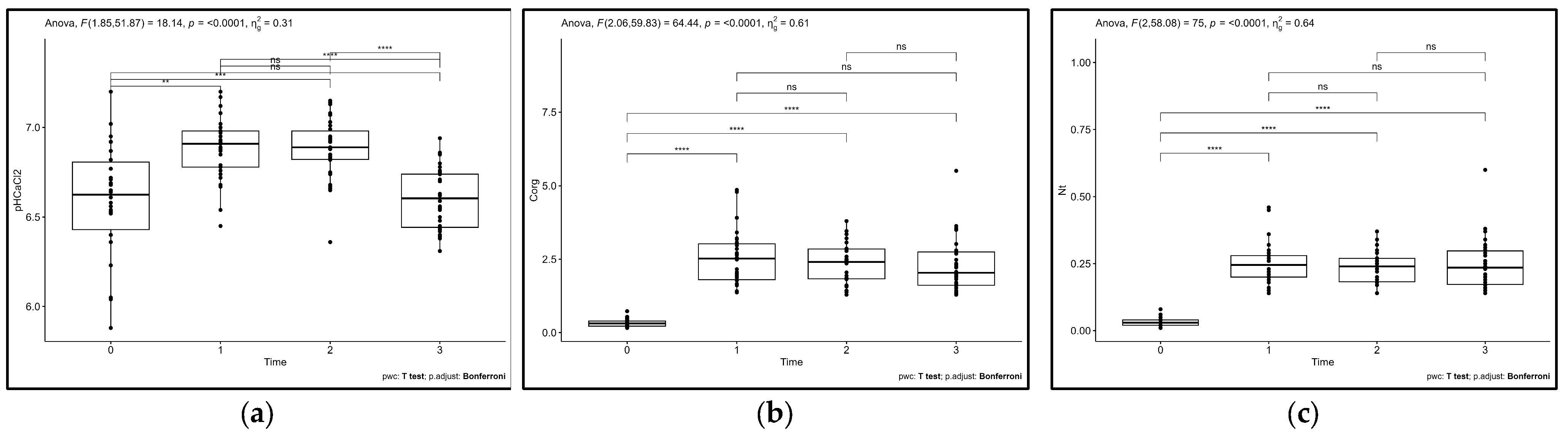
3.3. Temporal and Spatial Variations in Soil Properties and C Stocks to Repeated Compost Addition
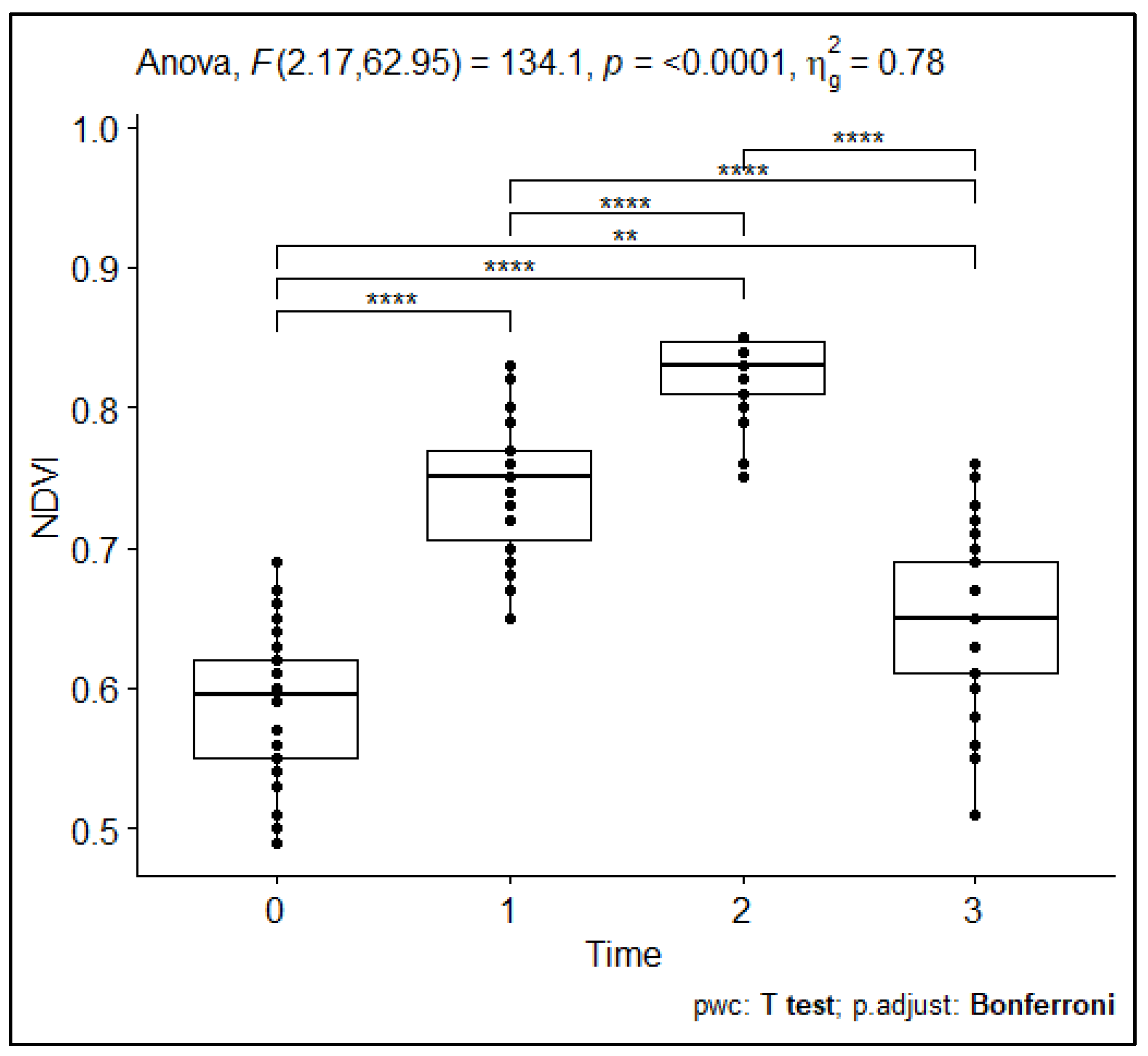
3.4. Temporal and Spatial Variations in Plant Responses to Repeated Compost Addition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOIs | Areas of interest |
| GNSS | Global navigation satellite system |
| NDVI | Normalized difference vegetation index |
| Sisol | CaCl2-extractable Si |
| Sioxa | Oxalate-extractable Si |
| iSi | Non-crystalline Na2CO3-extractable amorphous Si |
| Sibio | Biogenic Si |
| UAV | Uncrewed aerial vehicle |
References
- Molnar, T.J.; Kahn, P.C.; Ford, T.M.; Funk, C.J.; Funk, C.R. Tree crops, a permanent agriculture: Concepts from the past for a sustainable future. Resources 2013, 2, 457–488. [Google Scholar] [CrossRef]
- Lal, R.; Stewart, B.A. Soil Management for Sustaining Ecosystem Services. In Principles of Sustainable Soil Management in Agroecosystems, A Series in Advances in Soil Science; Lal, R., Stewart, B.A., Eds.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2013; pp. 521–536. ISBN 978-1-4665-1347-1. [Google Scholar]
- Lozano-García, B.; Muñoz-Rojas, M.; Parras-Alcántara, L. Climate and land use changes effects on soil organic carbon stocks in a Mediterranean seminatural area. Sci. Total Environ. 2016, 579, 1249–1259. [Google Scholar] [CrossRef]
- Francaviglia, R.; Renzi, G.; Ledda, L.; Benedetti, A. Organic carbon pools and soil biological fertility are affected by land use intensity in Mediterranean ecosystems of Sardinia, Italy. Sci. Total Environ. 2017, 599–600, 789–796. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Qiu, L.; Wei, X.; Liu, B.; Shao, M. Response of soil OC, N and P to land-use change and erosion in the black soil region of the Northeast China. Agric. Ecosys. Environ. 2020, 302, 107081. [Google Scholar] [CrossRef]
- Kim, D.-G.; Kirschbaum, M.U.F.; Eichler-Löbermann, B.; Giffort, R.M.; Liáng, L.L. The effect of land-use change on soil C, N, P, and their stoichiometries: A global synthesis. Agric. Ecosys. Environ. 2023, 348, 108402. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed. (global edition); Pearson Education: Harlow, UK, 2017; ISBN 978-1-292-16223-2. [Google Scholar]
- Baldock, J.A.; Nelson, P.N. Soil organic matter. In Handbook of Soil Science; Sumner, M.E., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 25–84. ISBN 0-8493-3136-6. [Google Scholar]
- Dick, W.A.; Gregorich, E.G. Developing and maintaining soil organic matter levels. In Managing Soil Quality: Challenges in Modern Agriculture; Schjønning, P., Elmholt, S., Christensen, B.T., Eds.; CABI Publishing: Oxon, UK, 2004; pp. 103–120. ISBN 0-85199-671-X. [Google Scholar]
- Naidu, R.; Megharaj, M.; Owens, G. Recyclable urban and industrial waste—Benefits and problems in agricultural use. In Managing Soil Quality: Challenges in Modern Agriculture; Schjønning, P., Elmholt, S., Christensen, B.T., Eds.; CABI Publishing: Oxon, UK, 2004; pp. 219–237. ISBN 0-85199-671-X. [Google Scholar]
- Panettieri, M.; Moreno, B.; de Sosa, L.L.; Benítez, E.; Madejón, E. Soil management and compost amendment are the main drivers of carbon sequestration in rainfed olive trees agroecosystems: An evaluation of chemical and biological markers. Catena 2022, 214, 106258. [Google Scholar] [CrossRef]
- Badagliacca, G.; Testa, G.; La Malfa, S.G.; Cafaro, V.; Lo Presti, E.; Monti, M. Organic fertilizers and bio-waste for sustainable soil management to support crops and control greenhouse gas emissions in Mediterranean agroecosystems: A review. Horticulturae 2024, 10, 427. [Google Scholar] [CrossRef]
- Fernández-Soler, C.; Garcia-Franco, N.; Almagro, M.; Díaz-Pereira, E.; Luján, R.; García, E.; Martínez-Mena, M. Cover crops improve the long-term stabilization of soil organic carbon and total nitrogen through physico-chemical protection in rainfed semiarid Mediterranean woody crop systems. Soil Use Manag. 2024, 40, e13066. [Google Scholar] [CrossRef]
- Gioacchini, P.; Baldi, E.; Montecchio, D.; Mazzon, M.; Quartieri, M.; Toselli, M.; Marzadori, C. Effect of long-term compost fertilization on the distribution of organic carbon and nitrogen in soil aggregates. Catena 2024, 240, 107968. [Google Scholar] [CrossRef]
- Khanal, S.; Fulton, J.; Shearer, S. An overview of current and potential applications of thermal remote sensing in precision agriculture. Comput. Electron. Agric. 2017, 139, 22–32. [Google Scholar] [CrossRef]
- Messina, G.; Fiozzo, V.; Praticò, S.; Siciliani, B.; Curcio, A.; Di Fazio, S.; Modica, G. Monitoring onion crops using multispectral imagery from unmanned aerial vehicle (UAV). In Smart Innovation, Systems and Technologies; Bevilacqua, C., Calabrò, F., Della Spina, L., Eds.; New Metropolitan Perspectives (NMP 2020); Springer: Cham, Switzerland, 2020; Volume 178. [Google Scholar] [CrossRef]
- Badagliacca, G.; Messina, G.; Praticò, S.; Lo Presti, E.; Preiti, G.; Monti, M.; Modica, G. Multispectral vegetation indices and machine learning approaches for durum wheat (Triticum durum Desf.) yield prediction across different varieties. AgriEngineering 2023, 5, 2032–2048. [Google Scholar] [CrossRef]
- Rouse, W.; Haas, R.H.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS, NASA SP-351. In Third Earth Resources Technology Satellite-l Symposium, Goddard Space Flight Center, Washington, DC, USA, 10–14 December 1973; National Aeronautics and Space Administration: Washington, DC, USA, 1974; Volume I: Technical Presentations Section A, Paper A-20; pp. 309–317. [Google Scholar]
- Yao, H.; Qin, R.; Chen, X. Unmanned aerial vehicle for remote sensing applications—A review. Remote Sens. 2019, 11, 1443. [Google Scholar] [CrossRef]
- Gago, J.; Douthe, C.; Coopman, R.E.; Gallego, P.P.; Ribas-Carbo, M.; Flexas, J.; Escalona, J.; Medrano, H. UAVs challenge to assess water stress for sustainable agriculture. Agric. Water Manag. 2015, 153, 9–19. [Google Scholar] [CrossRef]
- Messina, G.; Peña, J.M.; Vizzari, M.; Modica, G. A comparison of UAV and satellites multispectral imagery in monitoring onion crop. An application in the ‘cipolla rossa di Tropea’ (Italy). Remote Sens. 2020, 12, 3424. [Google Scholar] [CrossRef]
- Farias, G.D.; Bremm, C.; Bredemeier, C.; De Lima Menezes, J.; Alves, L.A.; Tiecher, T.; Martins, A.P.; Fioravanço, G.P.; Da Silva, G.P.; De Faccio Carvalho, P.C. Normalized Difference Vegetation Index (NDVI) for soybean biomass and nutrient uptake estimation in response to production systems and fertilization strategies. Front. Sustain. Food Syst. 2023, 6, 959681. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Lin, Y.; Guo, Z.; Guo, C.; Rao, X.; Liu, S.; Fu, S.; Ye, Q.; Hou, E. Increasing tree diversity reduces spatial heterogeneity of soil organic carbon and promotes carbon storage in subtropical forests. Agric. Ecosys. Environ. 2024, 371, 109077. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Nunan, N.; Schmidt, H.; Raynaud, X. The ecology of heterogeneity: Soil bacterial communities and C dynamics. Phil. Trans. R. Soc. B. 2020, 375, 20190249. [Google Scholar] [CrossRef] [PubMed]
- Bloor, J.M.G.; Tardif, A.; Pottier, J. Spatial heterogeneity of vegetation structure, plant N pools and soil N content in relation to grassland management. Agronomy 2020, 10, 716. [Google Scholar] [CrossRef]
- Patzold, S.; Mertens, F.M.; Bornemann, L.; Koleczek, B.; Franke, J.; Feilhauer, H.; Welp, G. Soil heterogeneity at the field scale: A challenge for precision crop protection. Precision Agric. 2008, 9, 367–390. [Google Scholar] [CrossRef]
- García-Palacios, P.; Maestre, F.T.; Bardgett, R.D.; de Kroon, H. Plant responses to soil heterogeneity and global environmental change. J. Ecol. 2012, 100, 1303–1314. [Google Scholar] [CrossRef]
- Lin, H.; Wheeler, D.; Bell, J.; Wilding, L. Assessment of soil spatial variability at multiple scales. Ecol. Model. 2005, 182, 271–290. [Google Scholar] [CrossRef]
- Nyengere, J.; Okamoto, Y.; Funakawa, S.; Shinjo, H. Analysis of spatial heterogeneity of soil physicochemical properties in Northern Malawi. Geoderma Reg. 2023, 35, e00733. [Google Scholar] [CrossRef]
- Tittonell, P. Spatial heterogeneity in agroecosystems. In A Systems Approach to Agroecology; Tittonell, P., Ed.; Springer Nature: Cham, Switzerland, 2023; pp. 241–280. [Google Scholar] [CrossRef]
- Shit, P.K.; Bhunia, G.S.; Maiti, R. Spatial analysis of soil properties using GIS based geostatistics models. Model Earth Syst. Environ. 2016, 2, 107. [Google Scholar] [CrossRef]
- Oliver, M.A. Geostatistical Applications for Precision Agriculture; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Liu, T.-L.; Juang, K.-W.; Lee, D.-Y. Interpolating soil properties using kriging combined with categorical information of soil maps. Soil Sci. Soc. Am. J. 2006, 70, 1200–1209. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon cycling in soils revisited. Plants 2021, 10, 295. [Google Scholar] [CrossRef]
- Cornelis, J.T.; Delvaux, B. Soil processes drive the biological silicon feedback loop. Funct. Ecol. 2016, 30, 1298–1310. [Google Scholar] [CrossRef]
- de Tombeur, F.; Cornelis, J.-T.; Laliberté, E.; Lambers, H.; Mahy, G.; Faucon, M.-P.; Turner, B.L. Impact of ecosystem water balance and soil parent material on silicon dynamics: Insights from three long-term chronosequences. Biogeochemistry 2021, 156, 335–350. [Google Scholar] [CrossRef]
- Ma, J.F.; Miyake, Y.; Takahashi, E. Silicon as a beneficial element for crop plants. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; 2001; pp. 17–39. [Google Scholar] [CrossRef]
- Mvondo-She, M.A.; Gatabazi, A.; Delmege Laing, M.; Rungano Ndhlala, A. A review on the role of silicon treatment in biotic stress mitigation and citrus production. Agronomy 2021, 11, 2198. [Google Scholar] [CrossRef]
- de Tombeur, F.; Roux, P.; Cornelis, J.-T. Silicon dynamics through the lens of soil-plant-animal interactions: Perspectives for agricultural practices. Plant Soil 2021, 467, 1–28. [Google Scholar] [CrossRef]
- Schaller, J.; Kleber, M.; Puppe, D.; Stein, M.; Sommer, M.; Rillig, M.C. The importance of reactive silica for maintaining soil health. Plant Soil 2025, 1–12. [Google Scholar] [CrossRef]
- Clymans, W.; Struyf, E.; Govers, G.; Vandevenne, F.; Conley, D.J. Anthropogenic impact on amorphous silica pools in temperate soils. Biogeosciences 2011, 8, 2281–2293. [Google Scholar] [CrossRef]
- Vandevenne, F.I.; Barão, L.; Ronchi, B.; Govers, G.; Meire, P.; Kelly, E.F.; Struyf, E. Silicon pools in human impacted soils of temperate zones. Glob. Biogeochem. Cycles 2015, 29, 1439–1450. [Google Scholar] [CrossRef]
- Schaller, J.; Turner, B.L.; Weissflog, A.; Pino, D.; Bielnicka, A.W.; Engelbrecht, B.M.J. Silicon in tropical forests: Large variation across soils and leaves suggests ecological significance. Biogeochemistry 2018, 140, 161–174. [Google Scholar] [CrossRef]
- Ishizawa, H.; Niiyama, K.; Iida, Y.; Shari, N.H.Z.; Ripin, A.; Kitajima, K. Spatial variations of soil silicon availability and biogenic silicon flux in a lowland tropical forest in Malaysia. Ecol. Res. 2019, 34, 548–559. [Google Scholar] [CrossRef]
- Cornu, S.; Meunier, J.-D.; Ratie, C.; Ouedraogo, F.; Lucas, Y.; Merdy, P.; Barboni, D.; Delvigne, C.; Borschneck, D.; Grauby, O.; et al. Allophanes, a significant soil pool of silicon for plants. Geoderma 2022, 412, 115722. [Google Scholar] [CrossRef]
- ARPACAL. Climatic Database of Calabrian Region. Available online: http://www.cfd.calabria.it (accessed on 18 February 2025).
- Soil Survey Staff. Keys to Soil Taxonomy; USDA-NRCS: Washington, DC, USA, 2010. [Google Scholar]
- IUSS Working Group. WRB World Reference Base for Soil Resources, 2nd ed.; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2006; 145p. [Google Scholar]
- Regulation (EU) No 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007. Off. J. Eur. Comm. 2018, L150/1, 1–92.
- UNI—Ente Nazionale di Unificazione. Compost: Classificazione, Requisiti e Modalità di Impiego; Norma UNI 10780, UNI: Milan, Italy, 1998. (In Italian) [Google Scholar]
- ANPA. Metodi di analisi del compost. In ANPA (Agenzia Nazionale per la Protezione dell’Ambiente)—Manuali e Linee Guida 3/2001; ANPA: Roma, Italy, 2001. (In Italian) [Google Scholar]
- Legislative decree n. 75/2010. Riordino e revisione della disciplina in materia di fertilizzanti, a norma dell’articolo 13 della legge 7 luglio 2009, n. 88. Gazz. Uff. Della Repubb. Ital. 2010, 121. Suppl. Ord. n. 106/L. (In Italian)
- Klute, A. Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods, 2nd ed.; SSSA and ASA: Madison, WI, USA, 1986. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis. Part 3. Chemical Methods; No. 5 in the SSSA Book Series; SSSA and ASA: Madison, WI, USA, 1996. [Google Scholar]
- Cornelis, J.-T.; Titeux, H.; Ranger, J.; Delvaux, B. Identification and distribution of the readily soluble silicon pool in a temperate forest soil below three distinct tree species. Plant Soil 2011, 342, 369–378. [Google Scholar] [CrossRef]
- Tamm, O. Eine Metode zur Bestimmung der anorganischen Komponenten des Gelkomplexes im Boden. Mddr. St. Skogsfor. 1922, 19, 387–404. (In German) [Google Scholar]
- Saccone, L.; Conley, D.J.; Koning, E.; Sauer, D.; Sommer, M.; Kaczorek, D.; Blecker, S.W.; Kelly, E.F. Assessing the extraction and quantification of amorphous silica in soils of forest and grassland ecosystems. Eur. J. Soil Sci. 2007, 58, 1446–1459. [Google Scholar] [CrossRef]
- DeMaster, D.J. The supply and accumulation of silica in the marine environment. Geochim. Cosmochim. Acta 1981, 45, 1715–1732. [Google Scholar] [CrossRef]
- Ghuffar, S. DEM generation from multi satellite Planetscope imagery. Remote Sens. 1922, 10, 1462. [Google Scholar] [CrossRef]
- Gribov, A.; Krivoruchko, K. Empirical Bayesian kriging implementation and usage. Sci. Total Environ. 2020, 722, 137290. [Google Scholar] [CrossRef]
- Pellicone, G.; Caloiero, T.; Modica, G.; Guagliardi, I. Application of several spatial interpolation techniques to monthly rainfall data in the Calabria region (southern Italy). Int. J. Climatol. 2018, 38, 3651–3666. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.3.1. RStudio Desktop. 2024. Available online: https://posit.co/download/rstudio-desktop/ (accessed on 18 July 2025).
- ARSSA. I Suoli della Calabria—Carta dei Suoli in Scala 1:250.000; Monografia Divulgativa; ARSSA: Soveria Mannelli, Italy, 2003. [Google Scholar]
- Zdruli, P. Land resources of the Mediterranean: Status, pressures, trends and impacts on future regional development. Land Degrad. Develop. 2012, 25, 373–384. [Google Scholar] [CrossRef]
- Sims, J.T. Soil fertility evaluation. In Handbook of Soil Science; Sumner, M.E., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. D113–D154. ISBN 0-8493-3136-6. [Google Scholar]
- Mvondo-She, M.A.; Marais, D. The investigation of silicon localization and accumulation in citrus. Plants 2019, 8, 200. [Google Scholar] [CrossRef]
- Nakamura, R.; Watanabe, T.; Onoda, Y. Contrasting silicon dynamics between aboveground vegetation and soil along a secondary successional gradient in a cool-temperate deciduous forest. Ecosystems 2023, 26, 1061–1076. [Google Scholar] [CrossRef]
- Tang, Y.; Lou, W.; Yan, X.; Li, S.; Wang, P.; Zhou, Y.; Zhan, T.; Zhang, S.; Hu, H.; Wang, X.; et al. The pivotal role of secondary nutrients and micronutrients in regulating fruit quality and root exudates metabolism profile of citrus. Plant Soil 2024, 500, 461–479. [Google Scholar] [CrossRef]
- Barão, L.; Teixeira, R.; Vandevenne, F.; Ronchi, B.; Unzué-Belmonte, D.; Struyf, E. Silicon mobilization in soils: The broader impact of land use. Silicon 2020, 12, 1529–1538. [Google Scholar] [CrossRef]
- Song, Z.; Müller, K.; Wang, H. Biogeochemical silicon cycle and carbon sequestration in agricultural ecosystems. Earth Sci. Rev. 2014, 139, 268–278. [Google Scholar] [CrossRef]
- Haynes, R.J. The nature of biogenic Si and its potential role in Si supply in agricultural soils. Agric. Ecosys. Environ. 2017, 245, 100–111. [Google Scholar] [CrossRef]
- Caubet, M.; Cornu, S.; Saby, N.P.A.; Meunier, J.-D. Agriculture increases the bioavailability of silicon, a beneficial element for crop, in temperate soils. Sci. Rep. 2020, 10, 19999. [Google Scholar] [CrossRef]
- de Tombeur, F.; Turner, B.L.; Laliberté, E.; Lambers, H.; Cornelis, J.-T. Silicon dynamics during 2 million years of soil development in a coastal dune chronosequence under a Mediterranean climate. Ecosystems 2020, 23, 1614–1630. [Google Scholar] [CrossRef]
- Yang, X.; Song, Z.; Van Zwieten, L.; Sun, X.; Yu, C.; Wang, W.; Liu, C.; Wang, H. Spatial distribution of plant-available silicon and its controlling factors in paddy fields of China. Geoderma 2021, 401, 115215. [Google Scholar] [CrossRef]
- Meunier, J.D.; Sandhya, K.; Prakash, N.B.; Borschnet, D.; Dussouillez, P. pH as a proxy for estimating plant-available Si? A case study in rice fields in Karnataka (South India). Plant Soil 2018, 432, 143–155. [Google Scholar] [CrossRef]
- Gelsomino, A.; Azzellino, A. Multivariate analysis of soils: Microbial biomass, metabolic activity and bacterial community structure and their relationships with soil depth and type. J. Plant Nutr. Soil Sci. 2011, 174, 381–394. [Google Scholar] [CrossRef]
- De Souza Junior, J.P.; Kadyampakeni, D.M.; Shahid, M.; Prado, R.; Fajardo, J.L.P. Mitigating abiotic stress in citrus: The role of silicon for enhanced productivity and quality. Plant Stress 2025, 16, 100837. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Singh, K.; Guha, S.; Choudhary, D.R.; Sonkar, A.; Mehta, S.; Husen, A. Exploring the role of silicon in enhancing sustainable plant growth, defense system, environmental stress mitigation and management. Disc. Appl. Sci. 2025, 7, 406. [Google Scholar] [CrossRef]
- Souri, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and plants: Current knowledge and future prospects. J. Plant Growth Regul. 2021, 40, 906–925. [Google Scholar] [CrossRef]
- Saad Ullah, M.; Mahmood, A.; Ameen, M.; Nayab, A.; Ayub, A. Multidimensional role of silicon to mitigate biotic and abiotic stresses in plants: A comprehensive review. Silicon 2024, 16, 5471–5500. [Google Scholar] [CrossRef]
- Parr, J.F.; Sullivan, L.A. Soil carbon sequestration in phytoliths. Soil Biol. Biochem. 2005, 37, 117–124. [Google Scholar] [CrossRef]
- Al-Omran, A.; Ibrahim, A.; Alharbi, A. Effects of biochar and compost on soil physical quality indices. Commun. Soil Sci. Plant Anal. 2021, 52, 2482–2499. [Google Scholar] [CrossRef]
- Steel, H.; Vandecasteele, B.; Willekens, K.; Sabbe, K.; Moens, T.; Bert, W. Nematode communities and macronutrients in composts and compost-amended soils as affected by feedstock composition. Appl. Soil Ecol. 2012, 61, 100–112. [Google Scholar] [CrossRef]
- Raviv, M. Composts in growing media: What’s new and what’s next? Acta Hort. 2013, 982, 39–52. [Google Scholar] [CrossRef]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R. A review of the use of composted municipal solid waste in agriculture. Agric. Ecosys. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Grattan, S.R.; Díaz, F.J.; Pedrero, F.; Vivaldi, G.A. Assessing the suitability of saline wastewaters for irrigation of Citrus spp.: Emphasis on boron and specific-ion interactions. Agric. Water Manag. 2015, 157, 48–58. [Google Scholar] [CrossRef]
- Quilty, J.R.; Cattle, S.R. Use and understanding of organic amendments in Australian agriculture: A review. Soil Res. 2011, 49, 1–16. [Google Scholar] [CrossRef]
- Gravuer, K.; Gennet, S.; Throop, H.L. Organic amendment additions to rangelands: A meta-analysis of multiple ecosystem outcomes. Glob. Change Biol. 2019, 25, 1152–1170. [Google Scholar] [CrossRef] [PubMed]
- Siedt, M.; Schäffer, A.; Smith, K.E.C.; Nabel, M.; Roß-Nickoll, M.; van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef]
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M.A. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Soil Sci. Plant Nutr. 2015, 15, 333–352. [Google Scholar] [CrossRef]
- Bonanomi, G.; De Filippis, F.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.; Abd-ElGawad, A. Repeated applications of organic amendments promote beneficial microbiota, improve soil fertility and increase crop yield. Appl. Soil Ecol. 2020, 156, 103714. [Google Scholar] [CrossRef]
- Piccolo, A.; Drosos, M. The essential role of humified organic matter in preserving soil health. Chem. Biol. Technol. Agric. 2025, 12, 21. [Google Scholar] [CrossRef]
- Chen, Y.; Camps-Arbestain, M.; Shen, Q.; Singh, B.; Cayuela, M.L. The long-term role of organic amendments in building soil nutrient fertility: A meta analysis and review. Nutr. Cycl. Agroecosyst. 2018, 111, 103–125. [Google Scholar] [CrossRef]
- Zhao, S.; Schmidt, S.; Qin, W.; Li, J.; Li, G.; Zhang, W. Towards the circular nitrogen economy—A global meta-analysis of composting technologies reveals much potential for mitigating nitrogen losses. Sci. Total Environ. 2020, 704, 135401. [Google Scholar] [CrossRef]
- Mondini, C.; Cayuela, M.L.; Sinicco, T.; Fornasier, F.; Galvez, A.; Sánchez-Monedero, M.A. Modification of the RothC model to simulate soil C mineralization of exogenous organic matter. Biogeosciences 2017, 14, 3253–3274. [Google Scholar] [CrossRef]
- Rumpel, C.; Amiraslani, F.; Chenu, C.; Garcia Cardenas, M.; Kaonga, M.; Koutika, L.-S.; Ladha, J.; Madari, B.; Shirato, Y.; Smith, P.; et al. The 4p1000 initiative: Opportunities, limitations and challenges for implementing soil organic carbon sequestration as a sustainable development strategy. Ambio 2020, 49, 350–360. [Google Scholar] [CrossRef]
- Srivastava, V.; de Araujo, A.S.F.; Vaish, B.; Bartelt-Hunt, S.; Singh, P.; Singh, R.P. Biological response of using municipal solid waste compost in agriculture as fertilizer supplement. Rev. Environ. Sci. Biotechnol. 2016, 15, 677–696. [Google Scholar] [CrossRef]
- Ros, M.; Klammer, S.; Knapp, B.; Aichberger, K.; Insam, H. Long-term effects of compost amendment of soil on functional and structural diversity and microbial activity. Soil Use Manag. 2006, 22, 209–218. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- D’Hose, T.; Cougnon, M.; De Vliegher, A.; Vandecasteele, B.; Viaene, N.; Cornelis, W.; Van Bockstaele, E.; Reheul, D. The positive relationship between soil quality and crop production: A case study on the effect of farm compost application. Appl. Soil Ecol. 2014, 75, 189–198. [Google Scholar] [CrossRef]
- Whetton, R.; Zhao, Y.; Shaddad, S.; Mouazen, A.M. Nonlinear parametric modelling to study how soil properties affect crop yields and NDVI. Comput. Electron. Agric. 2017, 138, 127–136. [Google Scholar] [CrossRef]
- Cao, M.-A.; Wang, P.; Hashem, A.; Wirth, S.; Abd_Allah, E.F.; Wu, Q.-S. Field inoculation of arbuscular mycorrhizal fungi improves fruit quality and root physiological activity of citrus. Agriculture 2021, 11, 1297. [Google Scholar] [CrossRef]
- Verhulst, N.; Govaerts, B.; Sayre, K.D.; Deckers, J.; François, I.M.; Dendooven, L. Using NDVI and soil quality analysis to assess influence of agronomic management on within-plot spatial variability and factors limiting production. Plant Soil 2009, 317, 41–59. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Capodilupo, M.; Scala, F. Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biol. Biochem. 2010, 42, 136–144. [Google Scholar] [CrossRef]
- Frouz, J.; Pižl, V.; Cienciala, E.; Kalčík, J. Carbon storage in post-mining forest soil, the role of tree biomass and soil bioturbation. Biogeochemistry 2009, 94, 111–121. [Google Scholar] [CrossRef]
- Melvin, A.M.; Goodale, C.L. Tree species and earthworm effects on soil nutrient distribution and turnover in a northeastern United States common garden. Can. J. For. Res. 2013, 43, 180–187. [Google Scholar] [CrossRef]
- Walmsley, A.; Vachová, P.; Hlava, J. Tree species identity governs the soil macrofauna community composition and soil development at reclaimed post-mining sites on calcium-rich clays. Eur. J. For. Res. 2019, 138, 753–761. [Google Scholar] [CrossRef]
- Woś, B.; Chodak, M.; Józefowska, A.; Pietrzykowski, M. Influence of tree species on carbon, nitrogen, and phosphorus stocks and stoichiometry under different soil regeneration scenarios on reclaimed and afforested mine and post-fire forest sites. Geoderma 2022, 415, 115782. [Google Scholar] [CrossRef]



| Parameter | Value |
|---|---|
| Sand (%) | 72.9 ± 6.8 |
| Silt (%) | 18.9 ± 4.5 |
| Clay (%) | 8.2 ± 3.6 |
| ISS (%) 1 | 11.9 ± 6.3 |
| pHH2O 2 | 6.52 ± 0.43 |
| pHCaCl2 3 | 6.60 ± 0.31 |
| EC (dS m−1 at 25 °C) 4 | 0.154 ± 0.054 |
| CEC (cmol kg−1) | 18.43 ± 3.08 |
| Ca (cmol kg−1) | 11.92 ± 0.71 |
| Mg (cmol kg−1) | 3.71 ± 0.19 |
| K (cmol kg−1) | 1.14 ± 0.06 |
| Na (cmol kg−1) | 1.66 ± 0.08 |
| Corg (%) | 0.32 ± 0.13 |
| Nt (%) | 0.03 ± 0.02 |
| C/N | 11.47 ± 3.47 |
| P-Olsen (mg kg−1) | 53 ± 3 |
| Sisol (mg g−1) | 15.3 ± 8.4 (×10−3) |
| Sibio (mg g−1) | 7.3 ± 3.0 |
| iSi (mg g−1) | 13.05 ± 5.57 |
| Sioxa (mg g−1) | 0.12 ± 0.06 |
| Parameter | Value | Mandatory Limit |
|---|---|---|
| Chemical analyses | ||
| Moisture (% fresh weight) | 18.6 ± 1.2 | <50 |
| pH 1 | 8.5 ± 0.3 | <6.0–8.8 |
| EC (dS m−1 at 25 °C) 2 | 2.4 ± 0.3 | − 5 |
| Corg (%) | 21.8 ± 2.0 | ≥20 |
| Nt (%) | 1.9 ± 0.2 | - |
| C/N | 11.6 ± 1.9 | <25 |
| Norg/Nt (%) | 94.0 ± 5.4 | ≥80 |
| CHA+FA (%) 3 | 7.2 ± 1.5 | ≥7.0 |
| NH4+-N (%) | <0.20 | - |
| P2O5 (%) | 1.46 ± 0.27 | - |
| K2O (%) | 2.52 ± 0.25 | - |
| Cd (mg kg−1) | 0.74 ± 0.15 | <1.5 |
| CrVI (mg kg−1) | <0.1 | <0.5 |
| Cu (mg kg−1) | 84 ± 16 | <230 |
| Hg (mg kg−1) | 0.15 ± 0.08 | <1.5 |
| Ni (mg kg−1) | 16 ± 4 | <100 |
| Pb (mg kg−1) | 20 ± 4 | <140 |
| Zn (mg kg−1) | 227 ± 45 | <500 |
| Na (mg kg−1) | 8220 ± 1070 | - |
| Total Cr (mg kg−1) | 30 ± 4 | - |
| Glass, metal, plastics (ø ≥ 2 mm; %) | 0.2 ± 0.0 | 0.5 |
| GI 4 (%) | 71 ± 7 | ≥60 |
| Microbiological analyses | ||
| Salmonella spp. (MPN 25 g−1) | Absent | Absent |
| Escherichia coli (CFU g−1) | <1 × 102 | 1 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Declet, A.; Rodinò, M.T.; Praticò, S.; Gelsomino, A.; Rombolà, A.D.; Modica, G.; Messina, G. Spatial and Temporal Variability of C Stocks and Fertility Levels After Repeated Compost Additions: A Case Study in a Converted Mediterranean Perennial Cropland. Soil Syst. 2025, 9, 86. https://doi.org/10.3390/soilsystems9030086
Rodríguez-Declet A, Rodinò MT, Praticò S, Gelsomino A, Rombolà AD, Modica G, Messina G. Spatial and Temporal Variability of C Stocks and Fertility Levels After Repeated Compost Additions: A Case Study in a Converted Mediterranean Perennial Cropland. Soil Systems. 2025; 9(3):86. https://doi.org/10.3390/soilsystems9030086
Chicago/Turabian StyleRodríguez-Declet, Arleen, Maria Teresa Rodinò, Salvatore Praticò, Antonio Gelsomino, Adamo Domenico Rombolà, Giuseppe Modica, and Gaetano Messina. 2025. "Spatial and Temporal Variability of C Stocks and Fertility Levels After Repeated Compost Additions: A Case Study in a Converted Mediterranean Perennial Cropland" Soil Systems 9, no. 3: 86. https://doi.org/10.3390/soilsystems9030086
APA StyleRodríguez-Declet, A., Rodinò, M. T., Praticò, S., Gelsomino, A., Rombolà, A. D., Modica, G., & Messina, G. (2025). Spatial and Temporal Variability of C Stocks and Fertility Levels After Repeated Compost Additions: A Case Study in a Converted Mediterranean Perennial Cropland. Soil Systems, 9(3), 86. https://doi.org/10.3390/soilsystems9030086










