Poultry Manure-Derived Biochar Synthesis, Characterization, and Valorization in Agriculture: Effect of Pyrolysis Temperature and Metal-Salt Modification
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection and Characterization
2.2. Biochar Preparation and Characterization
2.3. Agricultural Valorization
2.4. Statistical Analysis
3. Results and Discussion
3.1. Agricultural Soil Characterization
3.2. Biochar Characterization
3.3. Impact of Biochar Amendment on Barley Growth
3.4. Nutrient Content in Plant Biomass
3.5. Correlation Analysis and Principal Component Analysis Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duyum, S. Report Name: Poultry and Products Annual; United States Department Agriculture Foreign Agricultural Service: Washington, DC, USA, 2023.
- USDA Livestock and Poultry: World Markets and Trade; United States Department Agriculture Foreign Agricultural Service: Washington, DC, USA, 2024; Volume 31.
- Knijnenburg, J.T.N.; Suwanree, S.; Macquarrie, D.; Kasemsiri, P.; Jetsrisuparb, K. Phosphorus Recovery from Animal Manures through Pyrolysis: Phosphorus Transformations, Release Mechanisms, and Applications of Manure Biochars in Agriculture. RSC Sustain. 2025, 3, 1084–1101. [Google Scholar] [CrossRef]
- Hamidu, J.A.; Osie-Adjei, A.; Oduro-Owusu, A.D. Poultry Waste Management-Manure, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 3, ISBN 9780323851251. [Google Scholar]
- Hollas, C.E.; Rodrigues, H.C.; Oyadomari, V.M.A.; Bolsan, A.C.; Venturin, B.; Bonassa, G.; Tápparo, D.C.; Abilhôa, H.C.Z.; da Silva, J.F.F.; Michelon, W.; et al. The Potential of Animal Manure Management Pathways toward a Circular Economy: A Bibliometric Analysis. Environ. Sci. Pollut. Res. 2022, 29, 73599–73621. [Google Scholar] [CrossRef]
- Low, Y.W.; Yee, K.F. A Review on Lignocellulosic Biomass Waste into Biochar-Derived Catalyst: Current Conversion Techniques, Sustainable Applications and Challenges. Biomass Bioenergy 2021, 154, 106245. [Google Scholar] [CrossRef]
- Campion, L.; Bekchanova, M.; Malina, R.; Kuppens, T. The Costs and Benefits of Biochar Production and Use: A Systematic Review. J. Clean. Prod. 2023, 408, 137138. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Zhu, Y.; Fang, W.; Tan, Y.; He, Z.; Liao, H. Research Status, Trends, and Mechanisms of Biochar Adsorption for Wastewater Treatment: A Scientometric Review. Environ. Sci. Eur. 2024, 36, 25. [Google Scholar] [CrossRef]
- Ullah, M.S.; Malekian, R.; Randhawa, G.S.; Gill, Y.S.; Singh, S.; Esau, T.J.; Zaman, Q.U.; Afzaal, H.; Du, D.L.; Farooque, A.A. The Potential of Biochar Incorporation into Agricultural Soils to Promote Sustainable Agriculture: Insights from Soil Health, Crop Productivity, Greenhouse Gas Emission Mitigation and Feasibility Perspectives—A Critical Review. Rev. Environ. Sci. Biotechnol. 2024, 23, 1105–1130. [Google Scholar] [CrossRef]
- Singh, R.; Goyal, A.; Sinha, S. Global Insights into Biochar: Production, Sustainable Applications, and Market Dynamics. Biomass Bioenergy 2025, 194, 107663. [Google Scholar] [CrossRef]
- Hadroug, S.; Khiari, B.; Jellali, S.; El-Bassi, L.; Al-Wardy, M.; Hamdi, W.; Jeguirim, M. Animal Manure Derived Biochars Synthesis, Characterization and Use for Wastewater Treatment and in Agriculture: A Recent Review. Sci. Total Environ. 2025, 985, 179751. [Google Scholar] [CrossRef] [PubMed]
- Hadroug, S.; Jellali, S.; Leahy, J.J.; Kwapinska, M.; Jeguirim, M.; Hamdi, H.; Kwapinski, W. Pyrolysis Process as a Sustainable Management Option of Poultry Manure: Characterization of the Derived Biochars and Assessment of Their Nutrient Release Capacities. Water 2019, 11, 2271. [Google Scholar] [CrossRef]
- Are, K.S.; Adelana, A.O.; Fademi, I.O.O.; Aina, O.A. Improving Physical Properties of Degraded Soil: Potential of Poultry Manure and Biochar. Agric. Nat. Resour. 2017, 51, 454–462. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Shafi, M.I.; Beamont, E.; Anawar, H.M. Poultry Litter Biochar Increases Mycorrhizal Colonisation, Soil Fertility and Cucumber Yield in a Fertigation System on Sandy Soil. Agriculture 2020, 10, 480. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Aboyeji, C.M.; Dunsin, O.; Simeon, V.T. Biochar and Poultry Manure Effects on Soil Properties and Radish (Raphanus sativus L.) Yield. Biol. Agric. Hortic. 2019, 35, 33–45. [Google Scholar] [CrossRef]
- Muema, F.M.; Richardson, Y.; Keita, A.; Sawadogo, M. An Interdisciplinary Overview on Biochar Production Engineering and Its Agronomic Applications. Biomass Bioenergy 2024, 190, 107416. [Google Scholar] [CrossRef]
- Deinert, L.; Hossen, S.; Ikoyi, I.; Kwapinksi, W.; Noll, M.; Schmalenberger, A. Poultry Litter Biochar Soil Amendment Affects Microbial Community Structures, Promotes Phosphorus Cycling and Growth of Barley (Hordeum Vulgare). Eur. J. Soil Biol. 2024, 120, 103591. [Google Scholar] [CrossRef]
- Piash, M.I.; Iwabuchi, K.; Itoh, T.; Uemura, K. Release of Essential Plant Nutrients from Manure- and Wood-Based Biochars. Geoderma 2021, 397, 115100. [Google Scholar] [CrossRef]
- Geisen, S.; Wall, D.H.; van der Putten, W.H. Challenges and Opportunities for Soil Biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R1036–R1044. [Google Scholar] [CrossRef]
- El-Bassi, L.; Azzaz, A.A.; Jellali, S.; Akrout, H.; Marks, E.A.N.; Ghimbeu, C.M.; Jeguirim, M. Application of Olive Mill Waste-Based Biochars in Agriculture: Impact on Soil Properties, Enzymatic Activities and Tomato Growth. Sci. Total Environ. 2021, 755, 142531. [Google Scholar] [CrossRef] [PubMed]
- Akça, M.O.; Namlı, A. Effects of Poultry Litter Biochar on Soil Enzyme Activities and Tomato, Pepper and Lettuce Plants Growth. Eurasian J. Soil Sci. 2015, 4, 161. [Google Scholar] [CrossRef][Green Version]
- Dróżdż, D.; Malińska, K.; Wystalska, K.; Meers, E.; Robles-Aguilar, A. The Influence of Poultry Manure-Derived Biochar and Compost on Soil Properties and Plant Biomass Growth. Materials 2023, 16, 6314. [Google Scholar] [CrossRef]
- Sayed, Y.A.; Ali, A.M.; Ibrahim, M.F.; Fadl, M.E.; Casucci, C.; Drosos, M.; Scopa, A.; Al-Sayed, H.M. Impact of Poultry Manure-Derived Biochar and Bio-Fertilizer Application to Boost Production of Black Cumin Plants (Nigella sativa L.) Grown on Sandy Loam Soil. Agriculture 2024, 14, 1801. [Google Scholar] [CrossRef]
- Agbede, T.M.; Oyewumi, A. Benefits of Biochar, Poultry Manure and Biochar–Poultry Manure for Improvement of Soil Properties and Sweet Potato Productivity in Degraded Tropical Agricultural Soils. Resour. Environ. Sustain. 2022, 7, 100051. [Google Scholar] [CrossRef]
- Subedi, R.; Taupe, N.; Ikoyi, I.; Bertora, C.; Zavattaro, L.; Schmalenberger, A.; Leahy, J.J.; Grignani, C. Chemically and Biologically-Mediated Fertilizing Value of Manure-Derived Biochar. Sci. Total Environ. 2016, 550, 924–933. [Google Scholar] [CrossRef]
- Gao, G.; Yan, L.; Tong, K.; Yu, H.; Lu, M.; Wang, L.; Niu, Y. The Potential and Prospects of Modified Biochar for Comprehensive Management of Salt-Affected Soils and Plants: A Critical Review. Sci. Total Environ. 2024, 912, 169618. [Google Scholar] [CrossRef] [PubMed]
- Wen, E.; Yang, X.; Chen, H.; Shaheen, S.M.; Sarkar, B.; Xu, S.; Song, H.; Liang, Y.; Rinklebe, J.; Hou, D.; et al. Iron-Modified Biochar and Water Management Regime-Induced Changes in Plant Growth, Enzyme Activities, and Phytoavailability of Arsenic, Cadmium and Lead in a Paddy Soil. J. Hazard. Mater. 2021, 407, 124344. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Y.; Wei, L.; Huang, H.; Zhou, C.; Ni, G. Reduced Cadmium(Cd) Accumulation in Lettuce Plants by Applying KMnO4 Modified Water Hyacinth Biochar. Heliyon 2022, 8, e11304. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the Characterization of Acidic and Basic Surface Sites on Carbons by Various Techniques. Carbon N. Y. 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Khater, S. Development of Soil Particle Size Distribution Model and Determination of All Related Coefficients. Ann. Agric. Sci. Moshtohor 2023, 61, 269–278. [Google Scholar] [CrossRef]
- Neenu, S.; Karthika, K.S. Aluminium Toxicity in Soil and Plants. Har. Dhara 2019, 2, 15–19. [Google Scholar]
- Mokrzycki, J.; Michalak, I.; Rutkowski, P. Tomato Green Waste Biochars as Sustainable Trivalent Chromium Sorbents. Environ. Sci. Pollut. Res. 2021, 28, 24245–24255. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Chen, Y.; Lu, Y.; Wang, Y.; Chen, J.; Zhao, Y.; Yang, M.; Tian, X. KMnO4 Modified Biochar Derived from Swine Manure for Tetracycline Removal. Water Pract. Technol. 2022, 17, 2422–2434. [Google Scholar] [CrossRef]
- Park, S.-J.; Lee, Y.-J.; Kang, J.-K.; Lee, J.-C.; Lee, C.-G. Application of Fe-Impregnated Biochar from Cattle Manure for Removing Pentavalent Antimony from Aqueous Solution. Appl. Sci. 2021, 11, 9257. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Matei Ghimbeu, C.; Jellai, S.; El-Bassi, L.; Jeguirim, M. Olive Mill By-Products Thermochemical Conversion via Hydrothermal Carbonization and Slow Pyrolysis: Detailed Comparison between the Generated Hydrochars and Biochars Characteristics. Processes 2022, 10, 231. [Google Scholar] [CrossRef]
- Álvarez, M.L.; Gascó, G.; Palacios, T.; Paz-Ferreiro, J.; Méndez, A. Fe Oxides-Biochar Composites Produced by Hydrothermal Carbonization and Pyrolysis of Biomass Waste. J. Anal. Appl. Pyrolysis 2020, 151, 104893. [Google Scholar] [CrossRef]
- Ibn Ferjani, A.; Jeguirim, M.; Jellali, S.; Limousy, L.; Courson, C.; Akrout, H.; Thevenin, N.; Ruidavets, L.; Muller, A.; Bennici, S. The Use of Exhausted Grape Marc to Produce Biofuels and Biofertilizers: Effect of Pyrolysis Temperatures on Biochars Properties. Renew. Sustain. Energy Rev. 2019, 107, 425–433. [Google Scholar] [CrossRef]
- Zuo, J.; Li, W.; Xia, Z.; Zhao, T.; Tan, C.; Wang, Y.; Li, J. Preparation of Modified Biochar and Its Adsorption of Cr(VI) in Aqueous Solution. Coatings 2023, 13, 1884. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, M.; Wang, H.; Zhou, S.; Li, W.; Wang, T.; Li, J. Study on Adsorption of Cu2+, Pb2+, Cd2+, and Zn2+ by the KMnO4 Modified Biochar Derived from Walnut Shell. Int. J. Environ. Sci. Technol. 2023, 20, 1551–1568. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.; Yang, W.; Niazi, N.K.; Wang, B.; Wu, P. A Novel Phosphate Rock-Magnetic Biochar for Pb2+ and Cd2+ Removal in Wastewater: Characterization, Performance and Mechanisms. Environ. Technol. Innov. 2023, 32, 103268. [Google Scholar] [CrossRef]
- Hadroug, S.; Jellali, S.; Azzaz, A.A.; Kwapinska, M.; Hamdi, H.; Leahy, J.J.; Jeguirim, M.; Kwapinski, W. Valorization of Salt Post-Modified Poultry Manure Biochars for Phosphorus Recovery from Aqueous Solutions: Investigations on Adsorption Properties and Involved Mechanism. Biomass Convers. Biorefinery 2022, 12, 4333–4348. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.; Qian, L.; Yong, X.; Wang, Y.; An, W.; Jia, H.; Zhou, J. Biochar Production Using Biogas Residue and Their Adsorption of Ammonium Nitrogen and Chemical Oxygen Demand in Wastewater. Biomass Convers. Biorefinery 2023, 13, 3881–3892. [Google Scholar] [CrossRef]
- Wystalska, K.; Malińska, K.; Barczak, M. Poultry Manure Derived Biochars—the Impact of Pyrolysis Temperature on Selected Properties and Potentials for Further Modifications. J. Sustain. Dev. Energy, Water Environ. Syst. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Chaves, L.H.G.; Fernandes, J.D.; Mendes, J.S.; Dantas, E.R.B.; Guerra, H.C.; Tito, G.A.; Silva, A.A.R.; Laurentino, L.G.S.; Souza, F.G.; Lima, W.B.; et al. Characterization of Poultry Litter Biochar for Agricultural Use. Sylwan 2020, 6, 164. [Google Scholar]
- Gunes, A.; Inal, A.; Sahin, O.; Taskin, M.B.; Atakol, O.; Yilmaz, N. Variations in Mineral Element Concentrations of Poultry Manure Biochar Obtained at Different Pyrolysis Temperatures, and Their Effects on Crop Growth and Mineral Nutrition. Soil Use Manag. 2015, 31, 429–437. [Google Scholar] [CrossRef]
- Tian, R.; Li, C.; Xie, S.; You, F.; Cao, Z.; Xu, Z.; Yu, G.; Wang, Y. Preparation of Biochar via Pyrolysis at Laboratory and Pilot Scales to Remove Antibiotics and Immobilize Heavy Metals in Livestock Feces. J. Soils Sediments 2019, 19, 2891–2902. [Google Scholar] [CrossRef]
- Bai, T.; Qu, W.; Yan, Y.; Ma, K.; Xu, Y.; Zhou, X.; Chen, Y.; Xu, Y. Influence of Pyrolysis Temperature on the Properties and Environmental Safety of Heavy Metals in Chicken Manure-Derived Biochars. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2020, 55, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Zolfi Bavariani, M.; Ronaghi, A.; Ghasemi, R. Influence of Pyrolysis Temperatures on FTIR Analysis, Nutrient Bioavailability, and Agricultural Use of Poultry Manure Biochars. Commun. Soil Sci. Plant Anal. 2019, 50, 402–411. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Y.; Nan, H.; Pei, L.; Huang, Y.; Cao, X.; Xu, X.; Zhao, L. Metal Chloride-Loaded Biochar for Phosphorus Recovery: Noteworthy Roles of Inherent Minerals in Precursor. Chemosphere 2021, 266, 128991. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, P.; Bogusz, A.; Dobrzyńska, J.; Dobrowolski, R.; Oleszczuk, P. Engineered Biochar Modified with Iron as a New Adsorbent for Treatment of Water Contaminated by Selenium. J. Saudi Chem. Soc. 2020, 24, 824–834. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, S.; Liu, X.; Wang, L.; Zhu, L.; Wang, Y. Adsorption Characteristics and Mechanisms of Cd(II) from Wastewater by Modified Chicken Manure Biochar. Environ. Sci. Pollut. Res. 2023, 31, 3800–3814. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Park, J.-H.; Li, R.; Dodla, S.K.; Zhang, Z. Biochar Produced from Mineral Salt-Impregnated Chicken Manure: Fertility Properties and Potential for Carbon Sequestration. Waste Manag. 2018, 78, 802–810. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Z.; Yu, Q.; Cheng, Y.; Liu, Z.; Zhang, T.; Zhou, S. Removal of Tetracycline from an Aqueous Solution Using Manganese Dioxide Modified Biochar Derived from Chinese Herbal Medicine Residues. Environ. Res. 2020, 183, 109195. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Yu, Z.; Zeng, G.; Luo, Y.; Jiang, L.; Yang, Z.; Qian, Y.; Wu, H. Amorphous MnO2 Modified Biochar Derived from Aerobically Composted Swine Manure for Adsorption of Pb(II) and Cd(II). ACS Sustain. Chem. Eng. 2017, 5, 5049–5058. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Fu, J.; Yuan, L.; Li, Z.; Liu, C.; Zhao, D.; Wang, X. A Novel Magnetic Biochar/MgFe-Layered Double Hydroxides Composite Removing Pb 2+ from Aqueous Solution: Isotherms, Kinetics and Thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 278–287. [Google Scholar] [CrossRef]
- Brocza, F.M.; Foster, S.J.; Peacock, C.L.; Jones, J.M. Synthesis and Applications of Manganese Oxide—Biochar Composites: A Systematic Review across Catalysis, Capacitor and Sorption Applications. Biomass Bioenergy 2024, 184, 107201. [Google Scholar] [CrossRef]
- Hussein, O.N.; AL-Jawad, S.M.H.; Imran, N.J. A Study on the Influence of Metal Fe, Ag, and Mn Doping and (Fe/Mn and Ag/Mn) Dual-Doping on the Structural, Morphological, Optical, and Antibacterial Activity of CuS Nanostructures. Plasmonics 2024, 19, 1101–1120. [Google Scholar] [CrossRef]
- Kavdır, Y.; İlay, R.; Güven, O.B.; Sungur, A. Characterization of Olive Pomace Biochar Produced at Different Temperatures and Their Temporal Effects on Soil Aggregation and Carbon Content. Biomass Convers. Biorefinery 2024, 14, 19305–19314. [Google Scholar] [CrossRef]
- Hadroug, S.; Jellali, S.; Jeguirim, M.; Kwapinska, M.; Hamdi, H.; Leahy, J.J.; Kwapinski, W. Static and Dynamic Investigations on Leaching/Retention of Nutrients from Raw Poultry Manure Biochars and Amended Agricultural Soil. Sustainability 2021, 13, 1212. [Google Scholar] [CrossRef]
- Erdem, H. The Effects of Biochars Produced in Different Pyrolsis Temperatures from Agricultural Wastes on Cadmium Uptake of Tobacco Plant. Saudi J. Biol. Sci. 2021, 28, 3965–3971. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhou, H.; Zeng, P.; Wang, S.L.; Yang, W.J.; Huang, F.; Huo, Y.; Yu, S.N.; Gu, J.F.; Liao, B.H. Nano-Fe3O4-Modified Biochar Promotes the Formation of Iron Plaque and Cadmium Immobilization in Rice Root. Chemosphere 2021, 276, 130212. [Google Scholar] [CrossRef]
- Razzaq, S.; Zhou, B.; Zia-ur-Rehman, M.; Aamer Maqsood, M.; Hussain, S.; Bakhsh, G.; Zhang, Z.; Yang, Q.; Altaf, A.R. Cadmium Stabilization and Redox Transformation Mechanism in Maize Using Nanoscale Zerovalent-Iron-Enriched Biochar in Cadmium-Contaminated Soil. Plants 2022, 11, 1074. [Google Scholar] [CrossRef]
- Lu, H.P.; Li, Z.A.; Gascó, G.; Méndez, A.; Shen, Y.; Paz-Ferreiro, J. Use of Magnetic Biochars for the Immobilization of Heavy Metals in a Multi-Contaminated Soil. Sci. Total Environ. 2018, 622–623, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, L.; Ding, X.; Liu, L.; Li, Y.; Fei, C.; Zhang, S. Fe-Modified Biochar Improved the Stability of Soil Aggregates and Organic Carbon: Evidence from Enzymatic Activity and Microbial Composition. L. Degrad. Dev. 2024, 35, 732–743. [Google Scholar] [CrossRef]
- Algethami, J.S.; Irshad, M.K.; Javed, W.; Alhamami, M.A.M.; Ibrahim, M. Iron-Modified Biochar Improves Plant Physiology, Soil Nutritional Status and Mitigates Pb and Cd-Hazard in Wheat (Triticum aestivum L.). Front. Plant Sci. 2023, 14, 1–12. [Google Scholar] [CrossRef]
- Zeng, G.; Ping, Y.; Xu, H.; Yang, Z.; Tang, C.; Yang, W.; Si, M.; Arinzechi, C.; Liu, L.; He, F.; et al. Transformation of As and Cd Associated with Fe–Mn-Modified Biochar during Simultaneous Remediation on the Contaminated Soil. Environ. Sci. Pollut. Res. 2024, 31, 47408–47419. [Google Scholar] [CrossRef]
- Masocha, B.L.; Dikinya, O. The Role of Poultry Litter and Its Biochar on Soil Fertility and Jatropha Curcas L. Growth on Sandy-Loam Soil. Appl. Sci. 2022, 12, 12294. [Google Scholar] [CrossRef]
- Waheed, A.; Xu, H.; Qiao, X.; Aili, A.; Yiremaikebayi, Y.; Haitao, D.; Muhammad, M. Biochar in Sustainable Agriculture and Climate Mitigation: Mechanisms, Challenges, and Applications in the Circular Bioeconomy. Biomass Bioenergy 2025, 193, 107531. [Google Scholar] [CrossRef]
- Gavili, E.; Moosavi, A.A.; Zahedifar, M. Integrated Effects of Cattle Manure-Derived Biochar and Soil Moisture Conditions on Soil Chemical Characteristics and Soybean Yield. Arch. Agron. Soil Sci. 2019, 65, 1758–1774. [Google Scholar] [CrossRef]
- Hammerschmiedt, T.; Holatko, J.; Kucerik, J.; Mustafa, A.; Radziemska, M.; Kintl, A.; Malicek, O.; Baltazar, T.; Latal, O.; Brtnicky, M. Manure Maturation with Biochar: Effects on Plant Biomass, Manure Quality and Soil Microbiological Characteristics. Agriculture 2022, 12, 314. [Google Scholar] [CrossRef]
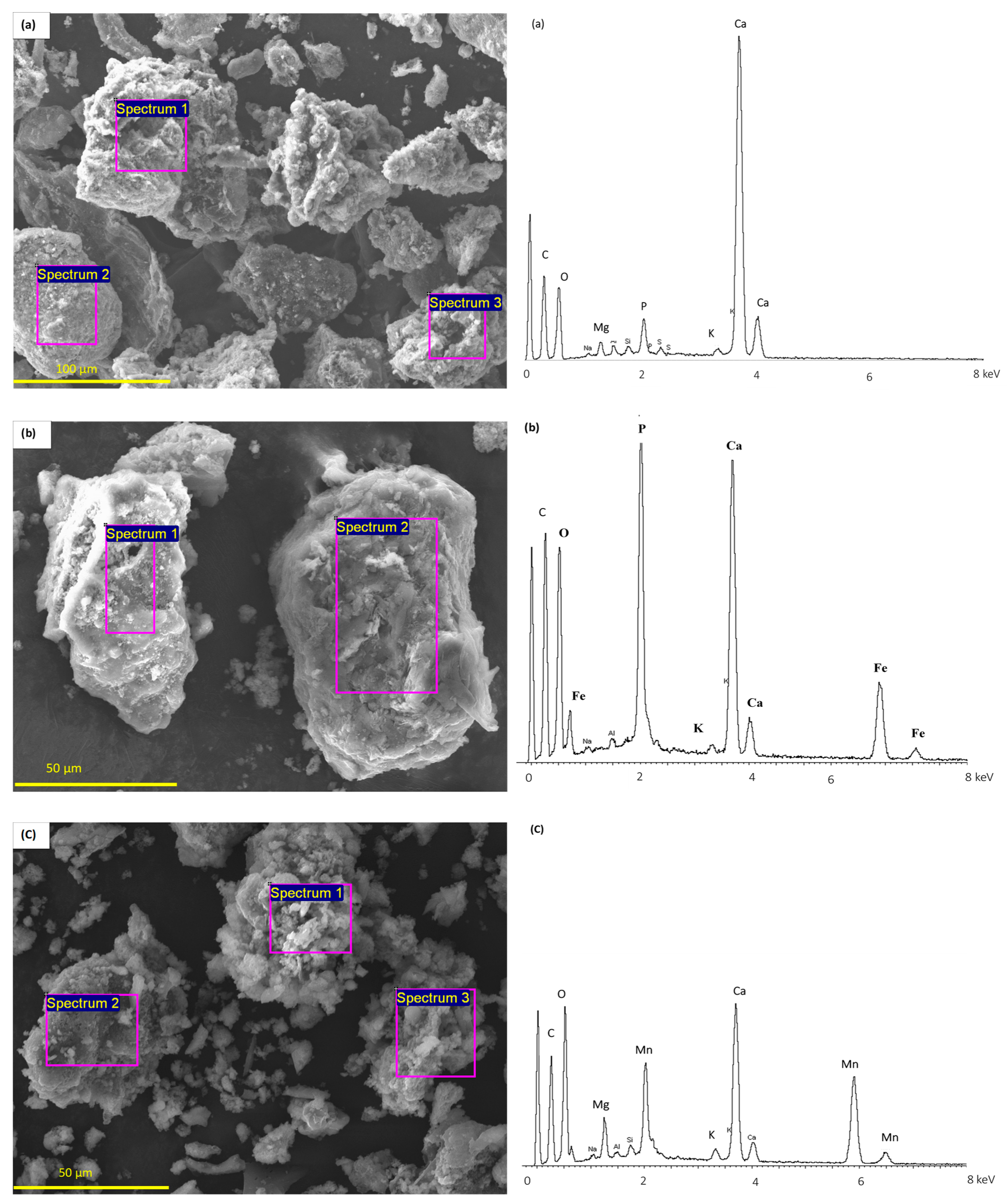

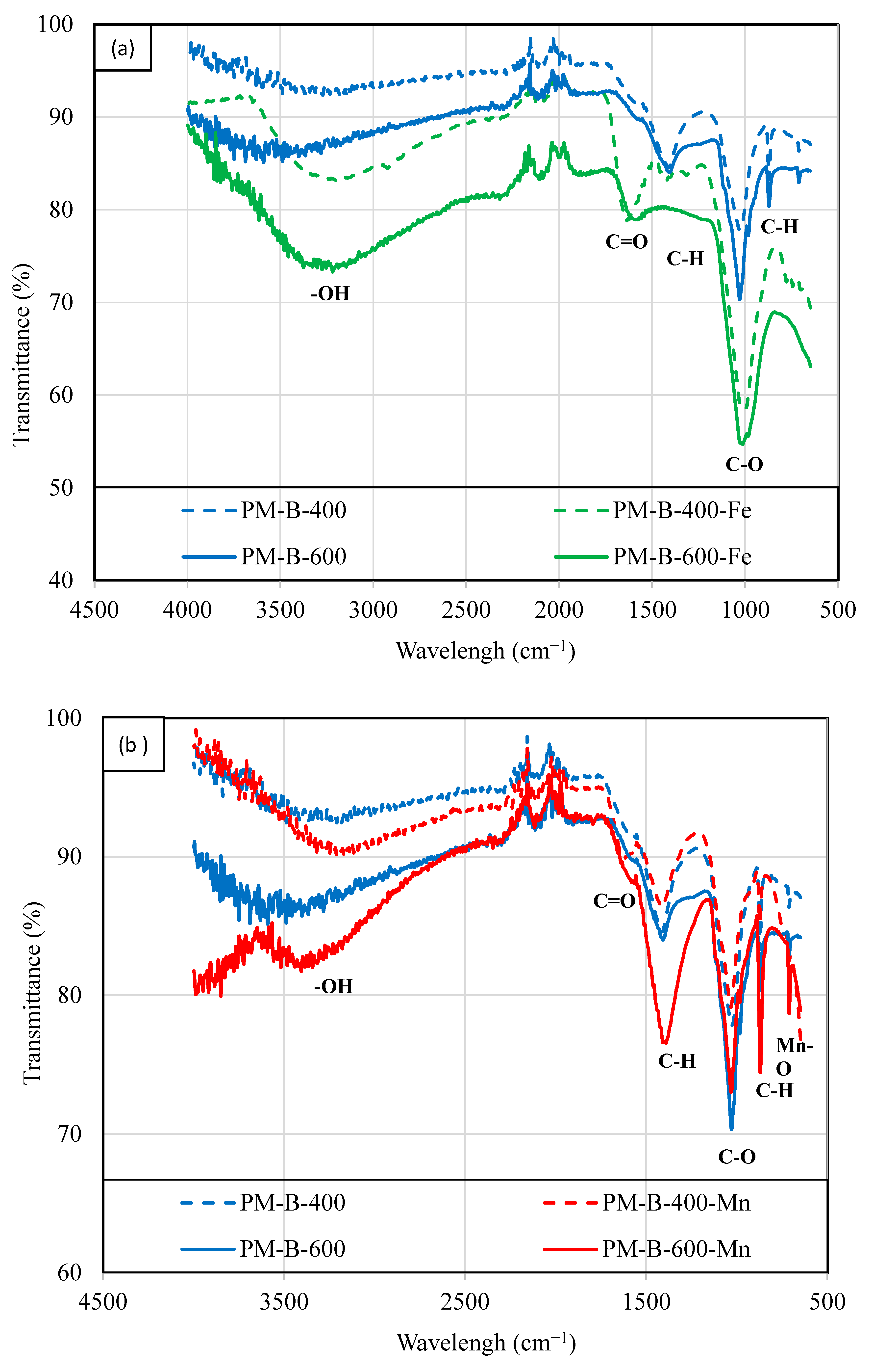
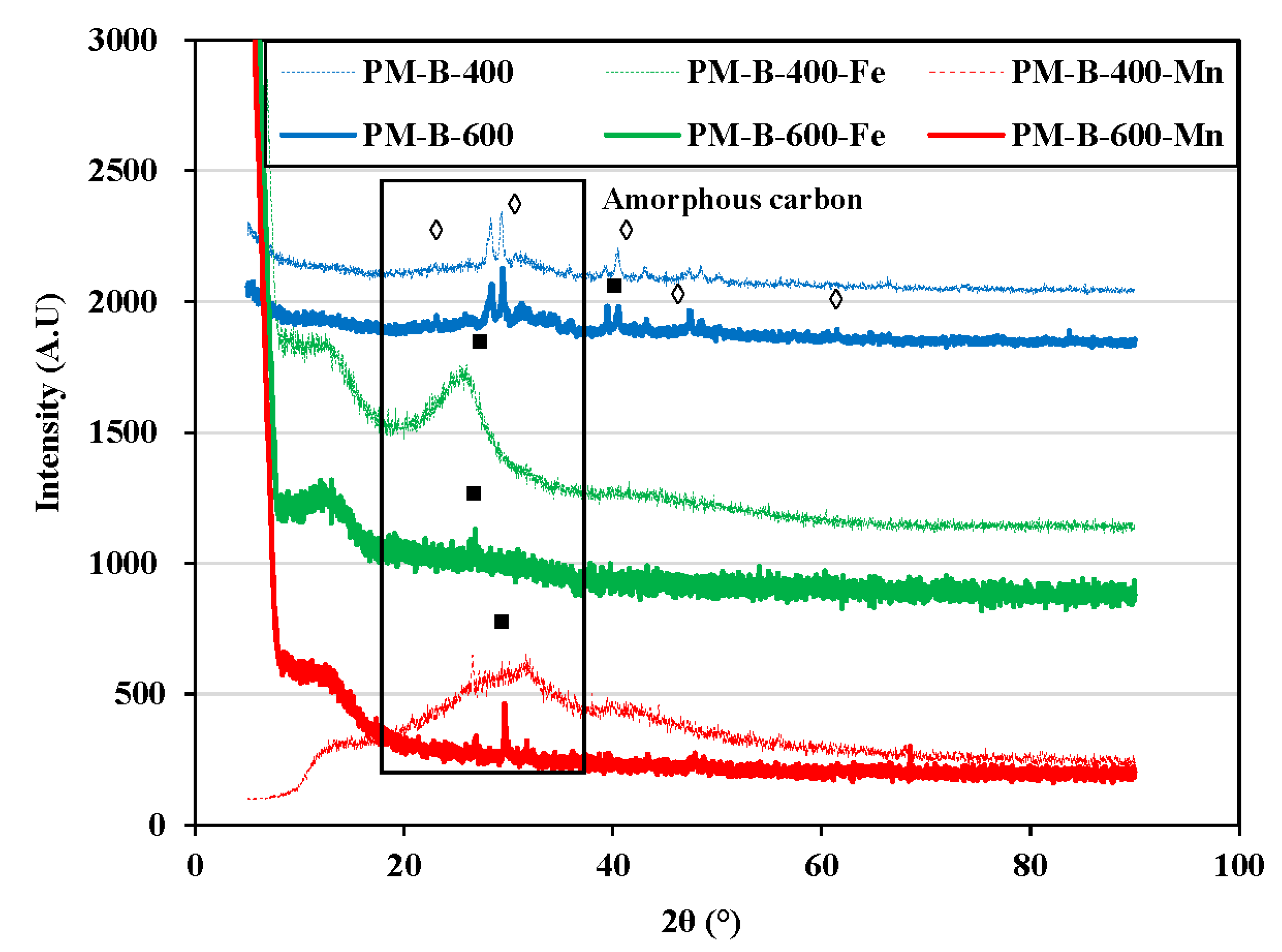

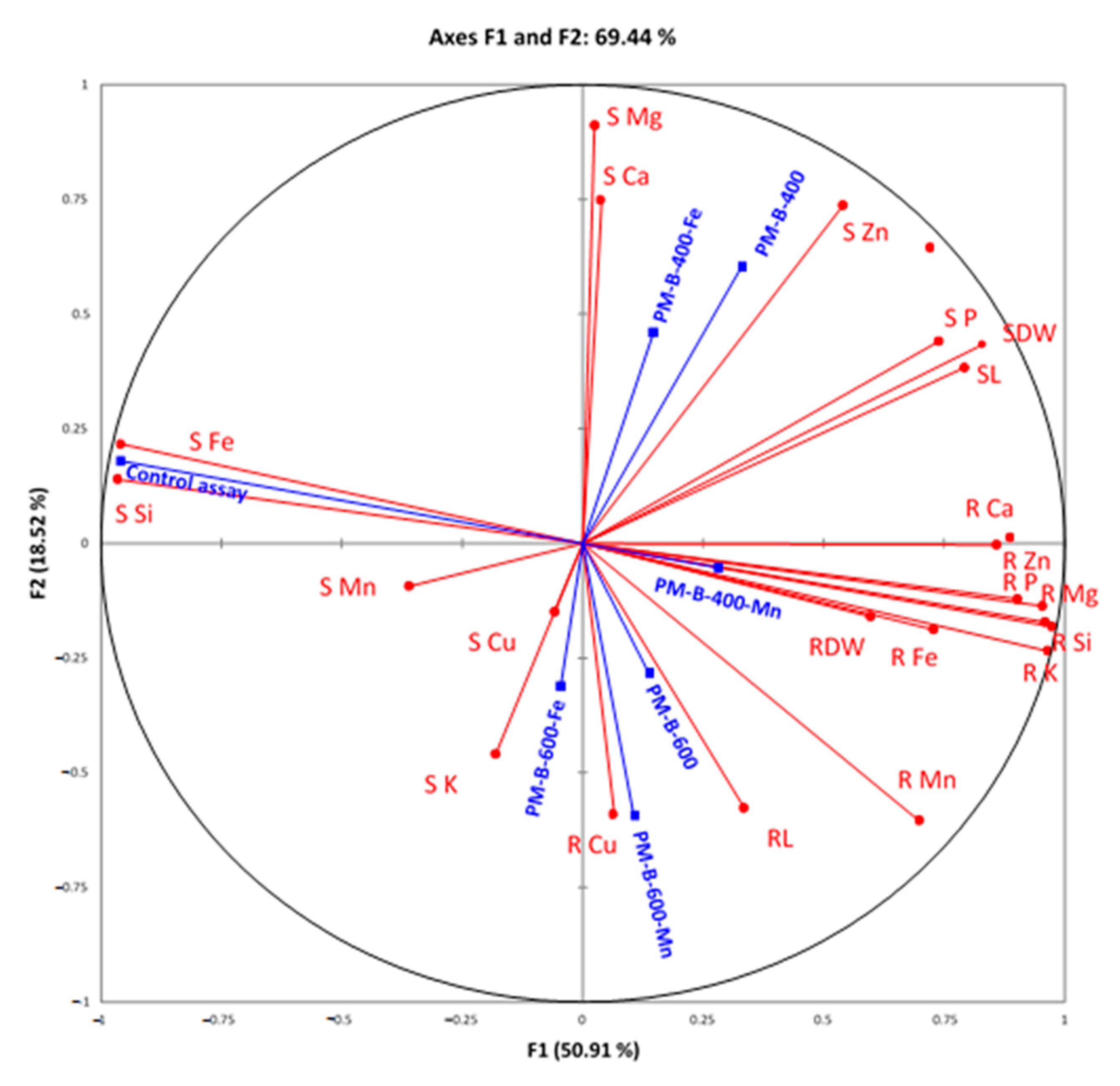
| Properties | PM-B-400 | PM-B-400-Fe | PM-B-400-Mn | PM-B-600 | PM-B-600-Fe | PM-B-600-Mn |
|---|---|---|---|---|---|---|
| d10 (mm) | 0.10 | 0.11 | 0.08 | 0.07 | 0.14 | 0.08 |
| d50 (mm) | 0.35 | 0.30 | 0.16 | 0.30 | 0.42 | 0.11 |
| d60 (mm) | 0.42 | 0.34 | 0.19 | 0.37 | 0.50 | 0.13 |
| Uniformity coefficient (UC = d60/d10) | 4.03 | 3.08 | 2.25 | 5.19 | 3.59 | 1.70 |
| Electrical conductivity (µs cm−1) | 333.0 | 750.3 | 376.7 | 1056 | 1335 | 1038 |
| pHpzc | 8.35 | 6.14 | 8.07 | 9.85 | 7.32 | 8.43 |
| Nutrient (%) | PM-B-400 | PM-B-400-Fe | PM-B-400-Mn | PM-B-600 | PM-B-600-Fe | PM-B-600-Mn |
|---|---|---|---|---|---|---|
| Mn | 0.08 ± 0.015 a | 0.01 ± 0.0008 b | 11.26 ± 0.06 b | 0.09 ± 0.0005 a | 0.02 ± 0.0008 b | 8.01 ± 0.01 b |
| Si | 1.82 ± 0.024 a | 1.1 ± 0.035 c | 1.03 ± 0.075 c | 1.61 ± 0.0590 a,b | 0.82 ± 0.092 c | 1.39 ± 0.027 b |
| Ca | 7.15 ± 0.538 a | 0.98 ± 0.071 b | 8.69 ± 0.034 a | 11.41 ± 0.078 c | 1.77 ± 0.078 b | 10.85 ± 0.069 c |
| K | 3.93 ± 0.710 a | 0.28 ± 0.07 b | 4.24 ± 0.080 a | 4.82 ± 0.0810 a | 0.45 ± 0.035 b | 4.76 ± 0.067 a |
| Mg | 1.45 ± 0.244 a | - | 1.31 ± 0.071 b | 1.81 ± 0.148 a | - | 1.63 ± 0.071 b |
| P | 4.63 ± 0.272 a | 4.56 ± 0.417 b | 3.62 ± 0.132 b | 5.85 ± 0.758 a | 4.39 ± 0.516 b | 4.45 ± 0.419 b |
| Al | 0.36 ± 0.312 a,b | 0.40 ± 0.080 a,b | 0.23 ± 0.081 a | 0.29 ± 0.085 b | 0.43 ± 0.068 a,b | 0.29 ± 0.027 a,b |
| Fe | 0.36 ± 0.123 a | 6.11 ± 0.085 b | 0.26 ± 0.068 a | 0.33 ± 0.101 a | 5.27 ± 0.106 c | 0.76 ± 0.063 d |
| Na | 1.10 ± 0.011 a | <DL | 0.36 ± 0.004 b | 1.50 ± 0.068 c | -<DL | 0.66 ± 0.009 d |
| S | 0.74 ± 0.116 a | 0.20 ± 0.039 b | 0.20 ± 0.003 b | 0.72 ± 0.035 a | 0.24 ± 0.07 b | 0.32 ± 0.07 b |
| Cr | 0.0061 ± 0.0007 a | 0.0034 ± 0.0016 b | 0.0027 ± 0.0007 a | 0.0041 ± 0.0002 a | 0.0036 ± 0.0005 a | 0.0027 ± 0.0004 a |
| Zn | 0.08 ± 0.0260 a,b | 0.01 ± 0.0030 a | 0.04 ± 0.0160 a,b | 0.08 ± 0.0300 a,b | 0.02 ± 0.008 a,b | 0.08 ± 0.0008 b |
| Ba | 0.0048 ± 0.0006 a,b | 0.0039 ± 0.0001 a | - | 0.0052 ± 0.0001 b | 0.0033 ± 0.00001 a | - |
| Ni | 0.0209 ± 0.007 a | 0.0210 ± 0.005 a | - | 0.0216 ± 0.001 a | 0.0238 ± 0.0003 a | - |
| Cu | 0.0122 ± 0.0002 a | 0.0137 ± 0.0004 a | - | 0.0142 ± 0.0052 a | 0.0139 ± 0.0065 a | - |
| Pb | - | 0.0007 ± 0.00005 a | - | 0.0003 ± 0.00006 b | 0.0009 ± 0.00001 c | - |
| Treatments | Shoot Length (cm) | Shoot Dry Weight (g) | Root Length (cm) | Root Dry Weight (g) |
|---|---|---|---|---|
| Control assay | 34.0 ± 0.957 a | 0.09 ± 0.013 a | 14.6 ± 0.520 a | 0.07 ± 0.010 a |
| PM-B-400 | 46.2 ± 2.872 c | 0.39 ± 0.054 d | 14.7 ± 0.656 a | 0.28 ± 0.032 b |
| PM-B-400-Fe | 47.5 ± 1.000 c | 0.36 ± 0.085 d | 23.8 ± 0.629 c | 0.62 ± 0.224 e |
| PM-B-400-Mn | 46.8 ± 1.315 c | 0.34 ± 0.028 d | 18.7 ± 0.816 b | 0.56 ± 0.169 e |
| PM-B-600 | 39.0 ± 1.500 b | 0.26 ± 0.047 c | 22.0 ± 1.291 c | 0.59 ± 0.170 e |
| PM-B-600-Fe | 41.5 ± 2.483 b | 0.22 ± 0.050 b | 24.7 ± 3.304 c | 0.49 ± 0.114 d |
| PM-B-600-Mn | 40.7 ± 2.217 b | 0.24 ± 0.056 bc | 24.0 ± 0.816 c | 0.35 ± 0.105 c |
| F | 27.190 | 16.462 | 37.777 | 7.029 |
| p | 7.25 × 10−9 | 5.832 × 10−7 | 3.423 × 10−10 | 3.3 × 10−4 |
| Treatment | Macronutrients Shoots (mg g−1) | Macronutrients Roots (mgg−1) | Micronutrients Shoots (mg g−1) | Micronutrients Roots (mg g−1) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Ca | P | Mg | K | Ca | P | Mg | Si | Fe | Mn | Cu | Zn | As | Si | Fe | Mn | Cu | Zn | As | |
| Control assay | 20.57 ± 1.9 a | 10.83 ± 1.7 b | 1.50 ± 1.1 a | 3.14 ± 0.9 d | 3.38 ± 1.5 a | 0.86 ± 0.6 a | 0.22 ± 0.1 a | 0.76 ± 0.06 a | 38.5 ± 2.1 b | 1.8 ± 0.05 c | 0.13 ± 0.02 a | 0.02 ± 0.001 a | 0.02 ± 0.001 a | 0.0034 ± 0.0002 b | 41.4 ± 0.001 a | 3.1 ± 0.001 a | 0.21 ± 0.001 a | 0.06 ± 0.078 a | 0.02 ± 0.001 a | 0.0215 ± 0.004 a |
| PM-B-400 | 25.76 ± 1.5 ab | 11.95 ± 1.5 b | 8.50 ± 2 d | 3.67 ± 0.8 d | 30.86 ± 2.5 c | 6.77 ± 1.5 e | 3.10 ± 1.0 e | 7.16 ± 1.0 c | 7.0 ± 1.2 a | 0.31 ± 0.2 b | 0.07 ± 0.004 a | 0.02 ± 0.001 a | 0.05 ± 0.005 b | 0.0001 ± 0.0001 a | 399.6 ± 0.001 c | 33.0 ± 0.001 b | 1.04 ± 0.001 b | 0.04 ± 0.002 a | 0.12 ± 0.001 b | 0.0178 ± 0.006 a |
| PM-B-400-Fe | 27.05 ± 1.5 b | 18.37 ± 1.5 c | 4.12 ± 1.9 b | 4.06 ± 1.01 e | 24.12 ± 1.9 b | 4.21 ± 1.0 c | 1.59 ± 0.6 b | 6.86 ± 1.0 bc | 8.4 ± 1.0 a | 0.33 ± 0.1 b | 0.06 ± 0.002 a | 0.02 ± 0.001 a | 0.03 ± 0.003 a | 0.0005 ± 0.0002 a | 356.0 ± 0.001 b | 36.9 ± 0.001 b | 1.11 ± 0.001 b | 0.05 ± 0.001 a | 0.08 ± 0.002 b | 0.0107 ± 0.004 a |
| PM-B-400-Mn | 37.86 ± 2.1 d | 9.62 ± 1.5 b | 6.70 ± 1.7 c | 2.67 ± 0.6 c | 31.03 ± 1.5 c | 5.56 ± 1.0 d | 2.47 ± 0.6 d | 7.19 ± 0.6 c | 8.7 ± 1.0 a | 0.24± 0.01 a | 0.13 ± 0.007 b | 0.012 ± 0.001 a | 0.03 ± 0.003 a | 0.0001 ± 0.0001 a | 378.4 ± 0.001 bc | 35.9 ± 0.001 b | 2.17 ± 0.002 d | 0.04 ± 0.003 a | 0.11 ± 0.001 b | 0.0063 ± 0.002 a |
| PM-B-600 | 31.18 ± 1.9 c | 6.82 ± 1.0 a | 4.46 ± 1.9 b | 2.44 ± 0.6 b | 29.91 ± 2.0 c | 5.68 ± 1.5 d | 2.62 ± 0.6 c | 6.98 ± 0.59 c | 8.6 ± 1.0 a | 0.21 ± 0.1 a | 0.04 ± 0.004 a | 0.011 ± 0.001 a | 0.02 ± 0.001 a | 0.0004 ± 0.0001 a | 373.4 ± 0.002 bc | 35.6 ± 0.001 b | 1.54 ± 0.001 c | 0.04 ± 0.02 a | 0.10 ± 0.001 b | 0.0143 ± 0.005 a |
| PM-B-600-Fe | 29.43 ± 2.0 bc | 8.88 ± 1.0 b | 2.47 ± 1.1 a | 2.16 ± 0.6 a | 25.23 ± 1.5 b | 2.61 ± 0.6 b | 1.73 ± 0.6 c | 6.04 ± 0.59 b | 11.3 ± 1.0 a | 0.31 ± 0.1 b | 0.05 ± 0.002 a | 0.02 ± 0.001 a | 0.02 ± 0.001 a | 0.0002 ± 0.0001 a | 385.3 ± 0.001 b | 38.0 ± 0.001 b | 1.30 ± 0.001 b | 0.05 ± 0.001 a | 0.09 ± 0.001 b | 0.0111 ± 0.008 a |
| PM-B-600-Mn | 34.09 ± 2.0 c | 8.19 ± 1.0 b | 3.48 ± 1 ab | 2.38 ± 0.5 ab | 31.18 ± 2.0 c | 5.68 ± 0.9 d | 2.58 ± 0.6 d | 7.60 ± 0.6 d | 9.5 ± 1.0 a | 0.28 ± 0.1 b | 0.12 ± 0.01 b | 0.04 ± 0.047 a | 0.02 ± 0.001 a | 0.0021 ± 0.0001 b | 377.5 ± 0.001 bc | 37.6 ± 0.001 d | 2.31 ± 0.001 d | 0.45 ± 0.001 b | 0.11 ± 0.001 b | 0.0119 ± 0.001 a |
| F | 22.485 | 12.49 | 5.914 | 3.932 | 84.64 | 12.34 | 10.12 | 37.96 | 974.1 | 2030 | 68.0 | 0.66 | 65.02 | 268.4 | 10,340 | 244.12 | 12,854 | 80.97 | 90.99 | 2.42 |
| p | 2 × 10−6 | 6 × 10−5 | 3 × 10−3 | 1.6 × 10−2 | 3 × 10−10 | 6 × 10−5 | 2 × 10−3 | 7 × 10−8 | 1 × 10−17 | 9 × 10−20 | 1 × 10−9 | 6.8 × 10−1 | 2 × 10−9 | 1.2 × 10−13 | 1 × 10−24 | 2.41 × 10−13 | 2 × 10−25 | 4 × 10−10 | 2 × 10−10 | 8.1 × 10−2 |
| Treatments | Control Assay | PM-B-400 | PM-B-400-Fe | PM-B-400-Mn | PM-B-600 | PM-B-600-Fe | PM-B-600-Mn | |
|---|---|---|---|---|---|---|---|---|
| Parameters | ||||||||
| SL | −0.704 | 0.345 | 0.417 | 0.402 | −0.273 | −0.057 | −0.129 | |
| RL | −0.559 | −0.552 | 0.339 | −0.165 | 0.160 | 0.421 | 0.356 | 1.000 |
| SDW | −0.700 | 0.461 | 0.346 | 0.283 | −0.100 | −0.190 | −0.100 | 0.8 |
| RDW | −0.647 | −0.255 | 0.358 | 0.254 | 0.309 | 0.119 | −0.138 | 0.600 |
| S P | −0.539 | 0.734 | −0.062 | 0.408 | 0.000 | −0.363 | −0.179 | 0.500 |
| S K | 0.197 | −0.426 | −0.327 | 0.501 | −0.011 | −0.145 | 0.211 | 0.400 |
| S Ca | 0.019 | 0.149 | 0.896 | −0.121 | −0.447 | −0.208 | −0.288 | 0.300 |
| S Mg | 0.129 | 0.455 | 0.695 | −0.160 | −0.304 | −0.475 | −0.341 | 0.200 |
| S Si | 0.998 | −0.203 | −0.176 | −0.170 | −0.173 | −0.122 | −0.155 | 0.100 |
| S Fe | 0.997 | −0.145 | −0.125 | −0.195 | −0.217 | −0.145 | −0.170 | 0.000 |
| S Mn | 0.482 | −0.165 | −0.264 | 0.492 | −0.476 | −0.439 | 0.370 | −0.100 |
| S Cu | 0.014 | −0.008 | 0.068 | −0.183 | −0.192 | −0.113 | 0.413 | −0.200 |
| S Zn | −0.324 | 0.820 | 0.161 | 0.209 | −0.155 | −0.392 | −0.320 | −0.300 |
| R P | −0.837 | 0.483 | −0.208 | 0.197 | 0.263 | −0.144 | 0.246 | −0.400 |
| R K | −0.957 | 0.254 | −0.043 | 0.261 | 0.212 | 0.006 | 0.268 | −0.500 |
| R Ca | −0.768 | 0.484 | −0.058 | 0.229 | 0.255 | −0.397 | 0.255 | −0.600 |
| R Mg | −0.980 | 0.198 | 0.143 | 0.204 | 0.165 | −0.008 | 0.279 | −0.700 |
| R Si | −0.995 | 0.239 | 0.089 | 0.166 | 0.149 | 0.190 | 0.163 | −0.8 |
| R Fe | −0.681 | −0.065 | 0.014 | 0.679 | −0.013 | 0.037 | 0.028 | −0.900 |
| R Mn | −0.723 | −0.207 | −0.164 | 0.482 | 0.100 | −0.060 | 0.573 | −1.000 |
| R Cu | −0.125 | −0.173 | −0.169 | −0.174 | −0.175 | −0.168 | 0.985 | |
| R Zn | −0.925 | 0.388 | −0.081 | 0.242 | 0.156 | 0.013 | 0.207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadroug, S.; El-Bassi, L.; Jellali, S.; Azzaz, A.A.; Jeguirim, M.; Hamdi, H.; Leahy, J.J.; Assadi, A.A.; Kwapinski, W. Poultry Manure-Derived Biochar Synthesis, Characterization, and Valorization in Agriculture: Effect of Pyrolysis Temperature and Metal-Salt Modification. Soil Syst. 2025, 9, 85. https://doi.org/10.3390/soilsystems9030085
Hadroug S, El-Bassi L, Jellali S, Azzaz AA, Jeguirim M, Hamdi H, Leahy JJ, Assadi AA, Kwapinski W. Poultry Manure-Derived Biochar Synthesis, Characterization, and Valorization in Agriculture: Effect of Pyrolysis Temperature and Metal-Salt Modification. Soil Systems. 2025; 9(3):85. https://doi.org/10.3390/soilsystems9030085
Chicago/Turabian StyleHadroug, Samar, Leila El-Bassi, Salah Jellali, Ahmed Amine Azzaz, Mejdi Jeguirim, Helmi Hamdi, James J. Leahy, Amine Aymen Assadi, and Witold Kwapinski. 2025. "Poultry Manure-Derived Biochar Synthesis, Characterization, and Valorization in Agriculture: Effect of Pyrolysis Temperature and Metal-Salt Modification" Soil Systems 9, no. 3: 85. https://doi.org/10.3390/soilsystems9030085
APA StyleHadroug, S., El-Bassi, L., Jellali, S., Azzaz, A. A., Jeguirim, M., Hamdi, H., Leahy, J. J., Assadi, A. A., & Kwapinski, W. (2025). Poultry Manure-Derived Biochar Synthesis, Characterization, and Valorization in Agriculture: Effect of Pyrolysis Temperature and Metal-Salt Modification. Soil Systems, 9(3), 85. https://doi.org/10.3390/soilsystems9030085










