Drought Stress Enhances Mycorrhizal Colonization in Rice Landraces Across Agroecological Zones of Far-West Nepal
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Rice Landraces
2.2. Experimental Design
2.3. Mycorrhizal Assessments
2.4. Soil Sampling and Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Parameters
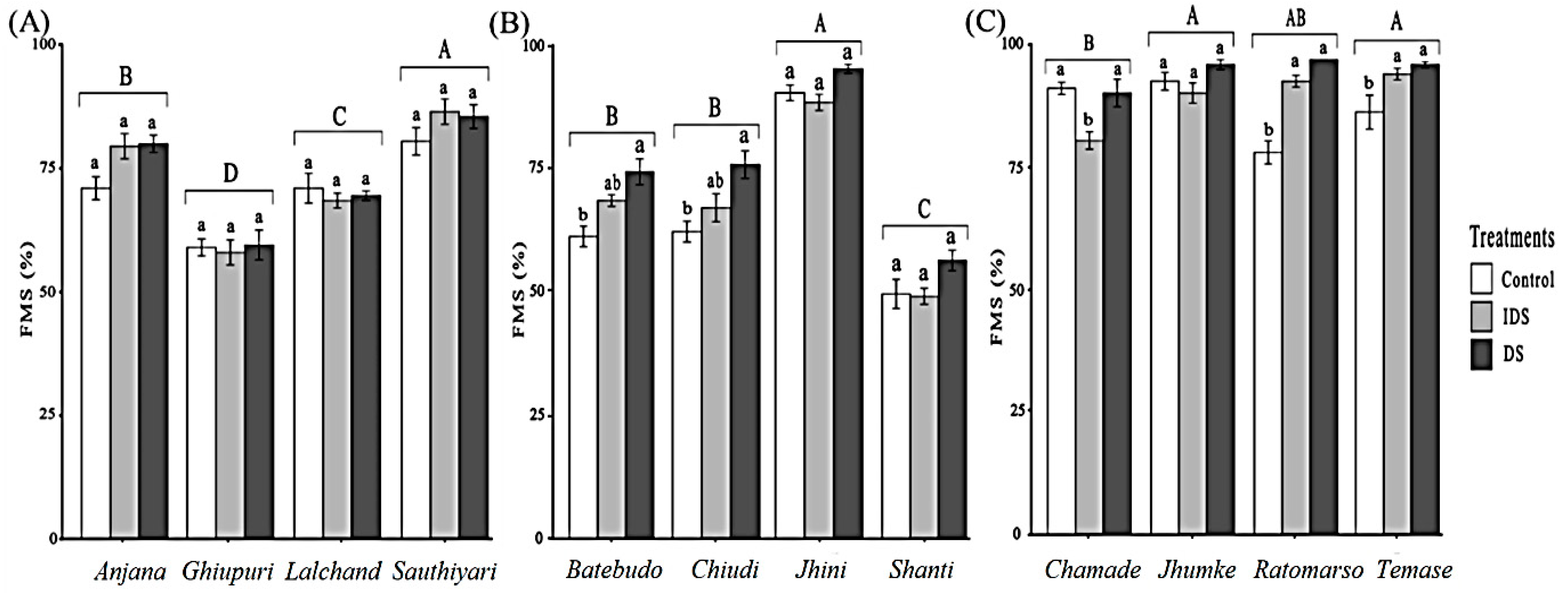
3.2. Frequency of Mycorrhizal Colonization in Root System (FMS)
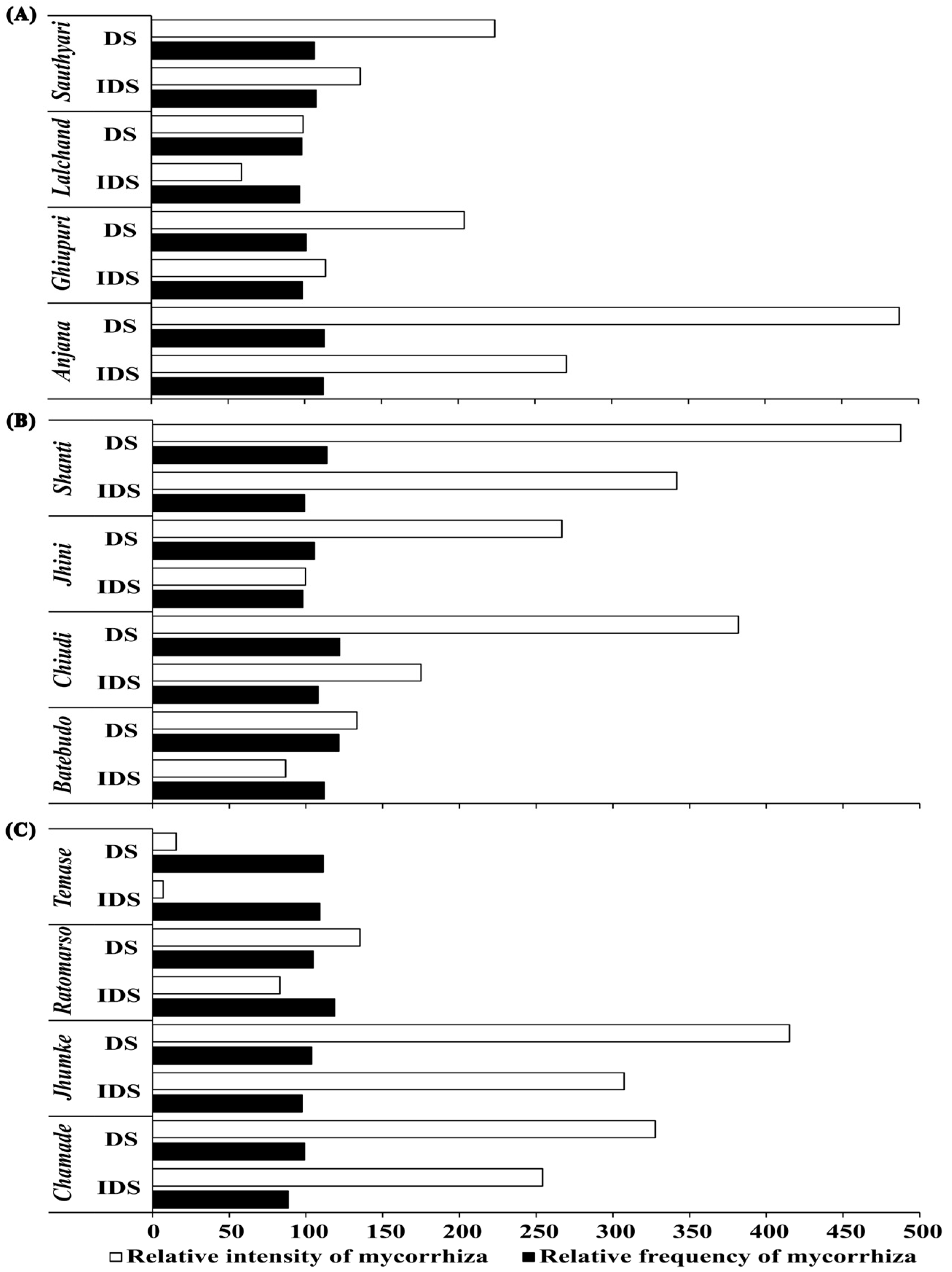
3.3. Intensity of Mycorrhizal Colonization in Root System (IRS)
3.4. Percentage Change in FMS and IRS
4. Discussion
4.1. The Effect of Ecological Zones and Rice Landraces on the FMS
4.2. FMS Response to Drought and Landrace-Specific Patterns
4.3. FMS and Drought: Percentage Change and Conservation Implications
4.4. IRS Varied with Soil Moisture and Landraces
4.5. Soil Properties, FMS, and IRS
4.6. Drought-Tolerant Landraces and Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohidem, N.A.; Hashim, N.; Shamsudin, R.; Che Man, H. Rice for Food Security: Revisiting Its Production, Diversity, Rice Milling Process and Nutrient Content. Agriculture 2022, 12, 741. [Google Scholar] [CrossRef]
- Dhungel, L.N.; Mahat, K.B.; Pant, S.K. Empowering Economic Development: A Case Study Analysis of Agricultural Commodity Production in Nepal’s Local Markets with a Focus on Farmers and Traders (2021/22). J. Adv. Acad. Res. 2024, 11, 24–35. [Google Scholar] [CrossRef]
- Lamichhane, N.; Dhami, U.; Dhakal, D.; Thapa, L.B. Rice Cultivation Area, Demographic Trends, and Trade Dynamics for Food Security in Nepal (2011–2021). Plant-Environ. Interact. 2024, 5, e70020. [Google Scholar] [CrossRef]
- Thagunna, R.S.; Chhetri, S.G.; Gautam, D.; Bhattarai, D.; Thapa, P.S. Climate Change, Climatic Disasters, and Adaptation Techniques: Learnings from the Lowlands of Nepal. Banko Janakari 2022, 32, 25–40. [Google Scholar] [CrossRef]
- Dahal, N.M.; Xiong, D.; Neupane, N.; Zhang, S.; Yuan, Y.; Zhang, B.; Fang, Y.; Zhao, W.; Wu, Y.; Deng, W. Estimating and Analyzing the Spatiotemporal Characteristics of Crop Yield Loss in Response to Drought in the Koshi River Basin, Nepal. Theor. Appl. Climatol. 2023, 152, 1053–1073. [Google Scholar] [CrossRef]
- Bernaola, L.; Cange, G.; Way, M.O.; Gore, J.; Hardke, J.; Stout, M. Natural Colonization of Rice by Arbuscular Mycorrhizal Fungi in Different Production Areas. Rice Sci. 2018, 25, 169–174. [Google Scholar] [CrossRef]
- Campo, S.; Martín-Cardoso, H.; Olivé, M.; Pla, E.; Catala-Forner, M.; Martínez-Eixarch, M.; San Segundo, B. Effect of Root Colonization by Arbuscular Mycorrhizal Fungi on Growth, Productivity and Blast Resistance in Rice. Rice 2020, 13, 42. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular Mycorrhizal Fungi Act as Biostimulants in Horticultural Crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Bowles, T.M.; Barrios-Masias, F.H.; Carlisle, E.A.; Cavagnaro, T.R.; Jackson, L.E. Effects of Arbuscular Mycorrhizae on Tomato Yield, Nutrient Uptake, Water Relations, and Soil Carbon Dynamics under Deficit Irrigation in Field Conditions. Sci. Total Environ. 2016, 566, 1223–1234. [Google Scholar] [CrossRef]
- Bücking, H.; Kafle, A. Role of Arbuscular Mycorrhizal Fungi in the Nitrogen Uptake of Plants: Current Knowledge and Research Gaps. Agronomy 2015, 5, 587–612. [Google Scholar] [CrossRef]
- Mitra, D.; Nayeri, F.D.; Sansinenea, E.; Ortiz, A.; Bhatta, B.B.; Adeyemi, N.O.; Janeeshma, E.; Tawfeeq Al-Ani, L.K.; Sharma, S.B.; Boutaj, H.; et al. Unraveling Arbuscular Mycorrhizal Fungi Interaction in Rice for Plant Growth Development and Enhancing Phosphorus Use Efficiency through Recent Development of Regulatory Genes. J. Plant Nutr. 2023, 46, 3184–3220. [Google Scholar] [CrossRef]
- Solaiman, M.Z.; Hirata, H. Responses of Directly Seeded Wetland Rice to Arbuscular Mycorrhizal Fungi Inoculation. J. Plant Nutr. 1997, 20, 1479–1487. [Google Scholar] [CrossRef]
- Cosme, M.; Stout, M.J.; Wurst, S. Effect of Arbuscular Mycorrhizal Fungi (Glomus intraradices) on the Oviposition of Rice Water Weevil (Lissorhoptrus oryzophilus). Mycorrhiza 2011, 21, 651–658. [Google Scholar] [CrossRef]
- John, S.A.; Ray, J.G. Optimization of Environmental and the Other Variables in the Application of Arbuscular Mycorrhizal Fungi as an Ecotechnological Tool for Sustainable Paddy Cultivation: A Critical Review. J. Appl. Microbiol. 2023, 134, lxad111. [Google Scholar] [CrossRef]
- MOALD. Statistical Information of Nepalese Agriculture; Ministry of Agriculture and Livestock Development, Government of Nepal: Kathmandu, Nepal, 2020.
- Kandel, B.P.; Shrestha, J. Characterization of Rice (Oryza sativa L.) Germplasm in Nepal: A Mini Review. Farming Manag. 2018, 3, 153–159. [Google Scholar] [CrossRef]
- CDD. Rice Varietal Mapping in Nepal: Implication for Development and Adoption; Crop Development Directorate (CDD), Department of Agriculture, Singhadurbar: Kathmandu, Nepal, 2015. [Google Scholar]
- Baniya, S.; Thapa, L.B.; Pant, D.R.; Pokhrel, C.P.; Pant, R.R.; Pal, K.B. Rice Accessions of Gorkha District of Nepal and Their Drought Tolerance Ability. Org. Agric. 2022, 12, 419–429. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased Arbuscular Mycorrhizal Fungal Colonization Reduces Yield Loss of Rice (Oryza sativa L.) under Drought. Mycorrhiza 2020, 30, 315–328. [Google Scholar] [CrossRef]
- Huang, R.; Li, Z.; Shen, X.; Choi, J.; Cao, Y. The Perspective of Arbuscular Mycorrhizal Symbiosis in Rice Domestication and Breeding. Int. J. Mol. Sci. 2022, 23, 12383. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method Which Gives an Objective Measure of Colonization of Roots by Vesicular-Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du Taux de Mycorhization VA d’un Système Radiculaire. Recherche de Méthodes d’Estimation Ayant une Signification Fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi Pearson, V., Gianinazzi, S., Eds.; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1967; p. 498. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; The American Society of Agronomy Inc.: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable Cations. In Agronomy Monographs; Page, A.L., Ed.; Wiley: Hoboken, NJ, USA, 1982; Volume 9, pp. 159–165. ISBN 978-0-89118-072-2. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 10 April 2024).
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-El-Mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ben Ahmed, H.; Mitsui, T.; Baslam, M.; Meddich, A. The Native Arbuscular Mycorrhizal Fungi and Vermicompost-Based Organic Amendments Enhance Soil Fertility, Growth Performance, and the Drought Stress Tolerance of Quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.M.; Reader, R.J. Taxonomic Basis for Variation in the Colonization Strategy of Arbuscular Mycorrhizal Fungi. New Phytol. 2002, 153, 335–344. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: London, UK, 2008. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Wilson, J.A.; Miller, R.M.; Bowker, M.A. Mycorrhizal Phenotypes and the L Aw of the M Inimum. New Phytol. 2015, 205, 1473–1484. [Google Scholar] [CrossRef]
- Maiti, D.; Variar, M.; Saha, J. Colonization of Upland Rice by Native VAM under Rainfed Mono-cropped Ecosystem. In Recent Advances in Phytopathological Research; Ray, A.K., Singha, K.K., Eds.; MD Publications: New Delhi, India, 1995; pp. 45–52. [Google Scholar]
- Augé, R.M. Water Relations, Drought and Vesicular-Arbuscular Mycorrhizal Symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Madouh, T.A.; Quoreshi, A.M. The Function of Arbuscular Mycorrhizal Fungi Associated with Drought Stress Resistance in Native Plants of Arid Desert Ecosystems: A Review. Diversity 2023, 15, 391. [Google Scholar] [CrossRef]
- Dey, S.; Bhattacharyya, R. The Mycorrhizoshpere Effect on Pedogenesis and Terrestrial Biomes. In Mycorrhizosphere and Pedogenesis; Varma, A., Choudhary, D.K., Eds.; Springer: Singapore, 2019; pp. 275–296. ISBN 9789811364792. [Google Scholar]
- Sendek, A.; Karakoç, C.; Wagg, C.; Domínguez-Begines, J.; do Couto, G.M.; van der Heijden, M.G.; Naz, A.A.; Lochner, A.; Chatzinotas, A.; Klotz, S. Drought Modulates Interactions between Arbuscular Mycorrhizal Fungal Diversity and Barley Genotype Diversity. Sci. Rep. 2019, 9, 9650. [Google Scholar] [CrossRef]
- Khan, M.H.; Meghvansi, M.K.; Prasad, K.; Siddiqui, S.; Varma, A. Arbuscular Mycorrhizal Symbiosis and Nutrient Resource Limitation: Predicting the Linkages and Effectiveness of Partnership. In Mycorrhiza—Nutrient Uptake, Biocontrol, Ecorestoration; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 115–130. ISBN 978-3-319-68866-4. [Google Scholar]
- Khalil, S.; Loynachan, T.E.; McNabb, H.S. Colonization of Soybean by Mycorrhizal Fungi and Spore Populations in Iowa Soils. Agron. J. 1992, 84, 832–836. [Google Scholar] [CrossRef]
- Kawahara, A.; An, G.-H.; Miyakawa, S.; Sonoda, J.; Ezawa, T. Nestedness in Arbuscular Mycorrhizal Fungal Communities along Soil pH Gradients in Early Primary Succession: Acid-Tolerant Fungi Are pH Generalists. PLoS ONE 2016, 11, e0165035. [Google Scholar] [CrossRef]
- Carrenho, R.; Trufem, S.F.B.; Bononi, V.L.R.; Silva, E.S. The Effect of Different Soil Properties on Arbuscular Mycorrhizal Colonization of Peanuts, Sorghum and Maize. Acta Bot. Bras. 2007, 21, 723–730. [Google Scholar] [CrossRef]
- Boddington, C.L.; Dodd, J.C. The Effect of Agricultural Practices on the Development of Indigenous Arbuscular Mycorrhizal Fungi. II. Studies in Experimental Microcosms. Plant Soil 2000, 218, 145–157. [Google Scholar] [CrossRef]
- Sivakumar, N. Effect of Edaphic Factors and Seasonal Variation on Spore Density and Root Colonization of Arbuscular Mycorrhizal Fungi in Sugarcane Fields. Ann. Microbiol. 2013, 63, 151–160. [Google Scholar] [CrossRef]
- Kuyper, T.W.; Wang, X.; Muchane, M.N. The Interplay Between Roots and Arbuscular Mycorrhizal Fungi Influencing Water and Nutrient Acquisition and Use Efficiency. In The Root Systems in Sustainable Agricultural Intensification; Rengel, Z., Djalovic, I., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2021; pp. 193–220. [Google Scholar] [CrossRef]
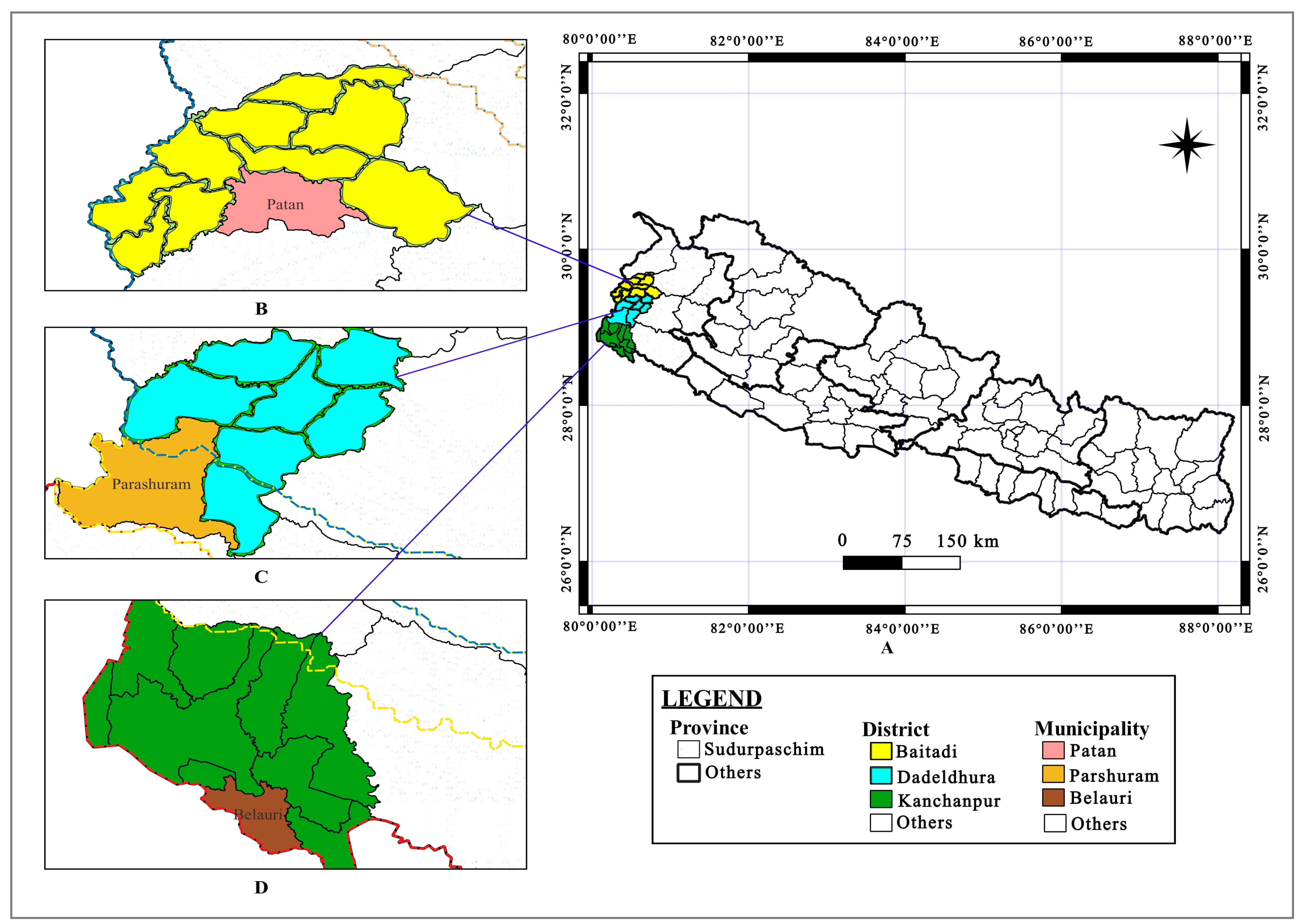


| S.N. | Agroecological Zone | District | Municipality/Site | Location | Elevation (masl.) |
|---|---|---|---|---|---|
| 1. | Tarai | Kanchanpur | Belauri/Shantipur | 28.696° N, 80.376° E | 180 |
| 2. | Inner-Tarai | Dadeldhura | Parashuram/Parigaun | 29.160° N, 80.286° E | 353 |
| 3. | Mid-hill | Baitadi | Patan/Kagsyali | 29.478° N, 80.617° E | 1666 |
| S.N. | Location | Varieties Selected |
|---|---|---|
| 1. | Tarai | Anjana, Ghiupuri, Lalchand, and Sauthyari |
| 2. | Inner-Tarai | Batebudho, Chiudi, Jhini, and Shanti |
| 3. | Mid-hill | Chamade, Jhumke, Ratomarso, and Temase |
| BD (g/cm3) | Clay (%) | Silt (%) | Sand (%) | pH | SOM (%) | N (%) | P (P2O5 mg/kg) | K (kg/ha) | |
|---|---|---|---|---|---|---|---|---|---|
| Mid-hill | 0.88 ± 0.13 b | 12.70 ± 1.4 ab | 26.74 ± 2.9 b | 60.56 ± 3.8 b | 5.15 ± 0.12 c | 5.81 ± 0.49 a | 0.27 ± 0.05 a | 0.62 ± 0.11 a | 112.8 ± 37.6 a |
| Inner-Tarai | 1.29 ± 0.13 a | 9.22 ± 2.2 b | 20.88 ± 3.3 c | 69.90 ± 4.6 a | 6.41 ± 0.14 b | 4.58 ± 0.51 b | 0.13 ± 0.05 b | 0.29 ± 0.11 b | 107.8 ± 24.3 a |
| Tarai | 1.25 ± 0.09 a | 12.76 ± 2.4 a | 40.40 ± 1.7 a | 46.84 ± 2.2 c | 7.27 ± 0.31 a | 1.87 ± 0.26 c | 0.08 ± 0.01 b | 0.18 ± 0.03 b | 72.0 ± 20.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhami, U.; Lamichhane, N.; Bhandari, S.; Pant, G.; Thapa, L.B.; Pokhrel, C.P.; Monokrousos, N.; Yadav, R.K.P. Drought Stress Enhances Mycorrhizal Colonization in Rice Landraces Across Agroecological Zones of Far-West Nepal. Soil Syst. 2025, 9, 72. https://doi.org/10.3390/soilsystems9030072
Dhami U, Lamichhane N, Bhandari S, Pant G, Thapa LB, Pokhrel CP, Monokrousos N, Yadav RKP. Drought Stress Enhances Mycorrhizal Colonization in Rice Landraces Across Agroecological Zones of Far-West Nepal. Soil Systems. 2025; 9(3):72. https://doi.org/10.3390/soilsystems9030072
Chicago/Turabian StyleDhami, Urmila, Nabin Lamichhane, Sudan Bhandari, Gunanand Pant, Lal Bahadur Thapa, Chandra Prasad Pokhrel, Nikolaos Monokrousos, and Ram Kailash Prasad Yadav. 2025. "Drought Stress Enhances Mycorrhizal Colonization in Rice Landraces Across Agroecological Zones of Far-West Nepal" Soil Systems 9, no. 3: 72. https://doi.org/10.3390/soilsystems9030072
APA StyleDhami, U., Lamichhane, N., Bhandari, S., Pant, G., Thapa, L. B., Pokhrel, C. P., Monokrousos, N., & Yadav, R. K. P. (2025). Drought Stress Enhances Mycorrhizal Colonization in Rice Landraces Across Agroecological Zones of Far-West Nepal. Soil Systems, 9(3), 72. https://doi.org/10.3390/soilsystems9030072







