A Novel Biostimulant–Biochar Strategy for Improving Soil Quality and Salinity Tolerance in Medicinal Mint (Mentha longifolia L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar, Spirulina, Soil, and Plant Materials
2.2. Pot Experiment
2.3. Soil, Plant, Biochar, and Spirulina Analysis
2.4. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of Biochar
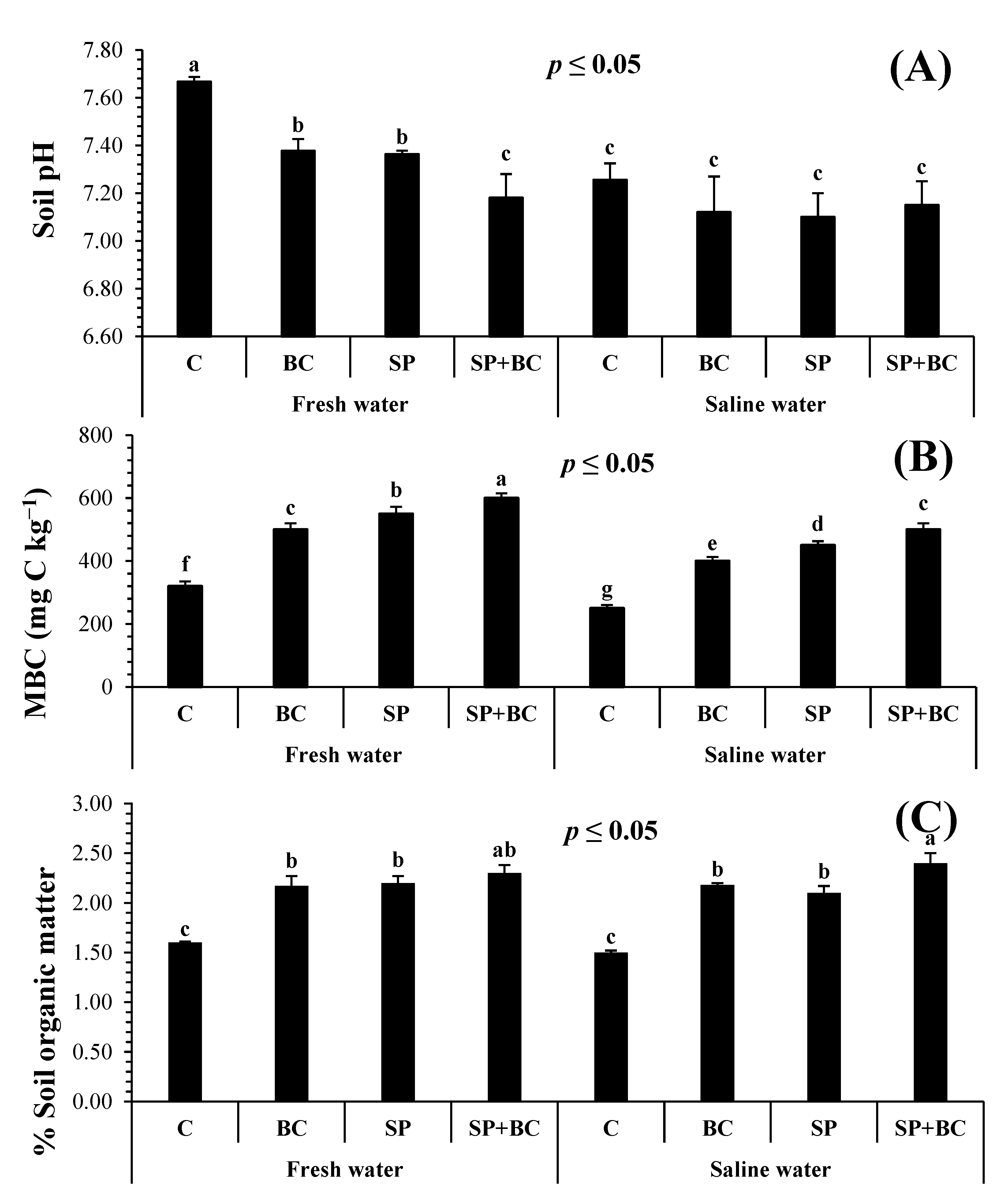
3.2. Biochar and Spirulina Effects on Soil Quality
3.2.1. Biochar and Spirulina Effects on Soil Biochemical and Physicochemical Characterization
3.2.2. Biochar and Spirulina Effects on Soil Soluble Cations
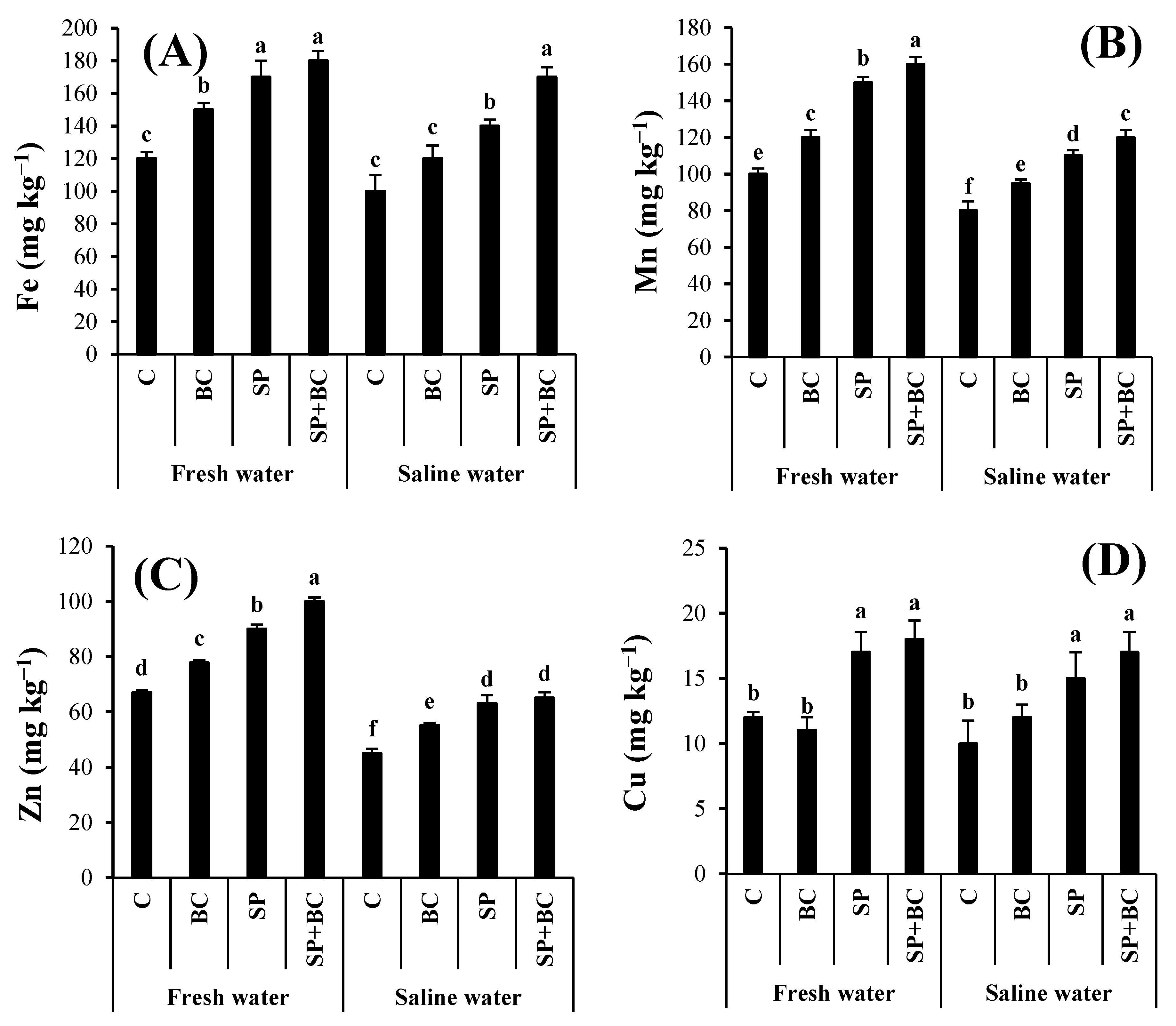
3.3. Effect of Biochar and Spirulina on Nutrient Availability
3.4. Effect of Biochar and Spirulina on Nutrients and Uptake by Mint Plants
3.5. Effect of Biochar and Spirulina on Ionic Balance
3.6. Effect of Biochar and Spirulina on Mint Growth Under Salinity Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mbuni, Y.M.; Wang, S.; Mwangi, B.N.; Mbari, N.J.; Musili, P.M.; Walter, N.O.; Wang, Q. Medicinal plants and their traditional uses in local communities around Cherangani Hills, Western Kenya. Plants 2020, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- El-Alakmy, A.A.; Hassan, H.; Badawy, M.Y.; Ali, M.A. Improving productivity of wild mint (Mentha longifolia L.) plants by using humic acid under saline water irrigation conditions. Sinai J. Appl. Sci. 2017, 6, 89–100. [Google Scholar] [CrossRef][Green Version]
- Hosseini, S.J.; Tahmasebi-Sarvestani, Z.; Mokhtassi-Bidgoli, A.; Keshavarz, H.; Kazemi, S.; Khalvandi, M.; Pirdashti, H.; Bovand, F.; Abassian, A. Do various levels of salinity change chlorophyll fluorescence, nutrient uptake, and physiological characteristics of Mentha ecotypes? Ind. Crops Prod. 2023, 203, 117199. [Google Scholar] [CrossRef]
- Hosseini, S.J.; Tahmasebi-Sarvestani, Z.; Mokhtassi-Bidgoli, A.; Keshavarz, H.; Kazemi, S.; Khalvandi, M.; Pirdashti, H.; Bovand, F.; Abassian, A. Growth, oil and physiological parameters of three mint species grown under saline stress levels. Physiol. Mol. Biol. Plants 2023, 29, 1061–1072. [Google Scholar]
- Lawrence, B.M. Mint: The Genus Mentha; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Singh, R.; Shushni, M.A.; Belkheir, A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef]
- Murtaza, G.; Usman, M.; Iqbal, J.; Tahir, M.N.; Elshikh, M.S.; Alkahtani, J.; Toleikienė, M.; Iqbal, R.; Akram, M.I.; Gruda, N.S. The impact of biochar addition on morpho-physiological characteristics, yield and water use efficiency of tomato plants under drought and salinity stress. BMC Plant Biol. 2024, 24, 1–15. [Google Scholar] [CrossRef]
- Fekri, M.O.; Gomah, H.H.; Eissa, M.A. Growth improvement of sweet basil (Ocimum basilicum L.) irrigated with saline water using biochar and Spirulina algae extract. Assiut J. Agric. Sci. 2024, 55, 260–275. [Google Scholar] [CrossRef]
- Alghamdi, S.A.; Alharby, H.F.; Abdelfattah, M.A.; Mohamed, I.A.A.; Hakeem, K.R.; Rady, M.M.; Shaaban, A. Spirulina platensis-inoculated humified compost boosts rhizosphere soil hydro-physico-chemical properties and Atriplex nummularia forage yield and quality in an arid saline calcareous soil. J. Soil Sci. Plant Nutr. 2023, 23, 2215–2236. [Google Scholar] [CrossRef]
- Hailu, B.; Mehari, H. Impacts of soil salinity/sodicity on soil-water relations and plant growth in dry land areas: A review. J. Nat. Sci. Res. 2021, 12, 1–10. [Google Scholar]
- Wang, B.; Kuang, S.; Shao, H.; Cheng, F.; Wang, H. Improving soil fertility by driving microbial community changes in saline soils of Yellow River Delta under petroleum pollution. J. Environ. Manag. 2022, 304, 114265. [Google Scholar] [CrossRef]
- Rath, K.M.; Murphy, D.N.; Rousk, J. The microbial community size, structure, and process rates along natural gradients of soil salinity. Soil. Biol. Biochem. 2019, 138, 107607. [Google Scholar] [CrossRef]
- Wang, L.; Luo, P.; Guo, X.; Zhang, M.; Li, H.; Liu, F.; Wu, J. Leaching of soil legacy nitrogen in intact soil columns and significance of soil macropore structure. Sci. Total Environ. 2024, 906, 167546. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.; Mousa, M.A.; Ibrahim, O.H.; Alshareef, N.O.; Eissa, M.A. Calcium-rich biochar stimulates salt resistance in pearl millet (Pennisetum glaucum L.) plants by improving soil quality and enhancing the antioxidant defense. Plants 2022, 11, 1301. [Google Scholar] [CrossRef]
- Abd El-Wahed, M.H.; Eissa, M.A.; Almasoudi, N.M.; Abo-Elyousr, K.A. Macronutrient-rich biochar induces boron nanoparticles in improving the salt tolerance of pomegranate (Punica granatum L.) in arid degraded soils. Sci. Hortic. 2023, 313, 111908. [Google Scholar] [CrossRef]
- Arahou, F.; Lijassi, I.; Wahby, A.; Rhazi, L.; Arahou, M.; Wahby, I. Spirulina-based biostimulants for sustainable agriculture: Yield improvement and market trends. BioEnergy Res. 2023, 16, 1401–1416. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Shedeed, Z.A.; Gheda, S.; Elsanadily, S.; Alharbi, K.; Osman, M.E. Spirulina platensis biofertilization for enhancing growth, photosynthetic capacity and yield of Lupinus luteus. Agriculture 2022, 12, 781. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R. Evaluating biomas-derived biochar on seed germination and early seedling growth of maize and black gram. Biomass Conv. Bioref. 2022, 12, 5663–5676. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, L.X.; Zhang, H.P.; Li, X.Y.; Shen, Y.F.; Li, S.Q. Soil amendment with biochar increases maize yields in a semi-arid region by improving soil quality and root growth. Crop Pasture Sci. 2016, 67, 495–507. [Google Scholar] [CrossRef]
- Pandit, N.R.; Mulder, J.; Hale, S.E.; Martinsen, V.; Schmidt, H.P.; Cornelissen, G. Biochar improves maize growth by alleviation of nutrient stress in a moderately acidic low-input Nepalese soil. Sci. Total Environ. 2018, 625, 1380–1389. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, J.; Liu, X.; Ma, Y.; Wei, Z.; Wan, H.; Liu, F. Effect of biochar addition and reduced irrigation regimes on growth, physiology and water use efficiency of cotton plants under salt stress. Ind. Crops Prod. 2023, 198, 116702. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Zhang, L.; Zheng, Y.; Liu, X.; Zhang, Y. The critical role of biochar to mitigate the adverse impacts of drought and salinity stress in plants. Front. Plant Sci. 2023, 14, 1163451. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Sa’adah, N.; Vijayanathan, J.; Abdullah, R.; Yaacob, J.S.; Mazlan, M.A.; Ahmad RElham, P.; Kassim, A.S. Influence of different pyrolysis temperature on the characteristics of forestry waste biochar for sodium adsorption. J. Mater. Cycles Waste Manag. 2024, 26, 894–907. [Google Scholar] [CrossRef]
- Soil Survey Staff (2016) Keys to Soil Taxonomy, 11th ed.; USDA-Natural Resources Conservation Services: Washington, DC, USA, 1997.
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. Station. Circ. 1950, 347, 1–32. [Google Scholar]
- Burt, R. Soil Survey Laboratory Methods Manual; Soil Survey Investigations Report No. 42, Version 4.0; Natural Resources Conservation Service, United States Department of Agriculture: Washington, DC, USA, 2004. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Methods of Soil Analysis; Part 3. Chemical Methods. Soil Science Society of America Book Series no.5; John Wiley & Sons: New York, NY, USA, 1996; pp. 961–1010. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1973; p. 100. [Google Scholar]
- Parkinson, J.A.; Allen, S.E. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil. Sci. Plant Anal. 1975, 6, 1–11. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzym. 1987, 148, 350–382. [Google Scholar]
- Mostafa, M.M.; Hammad, D.M.; Reda, M.M.; El-Sayed, A.E.K.B. Water extracts of Spirulina platensis and Chlorella vulgaris enhance tomato (Solanum lycopersicum L.) tolerance against saline water irrigation. Biomass Convers. Biorefinery 2023, 14, 21181–21191. [Google Scholar] [CrossRef]
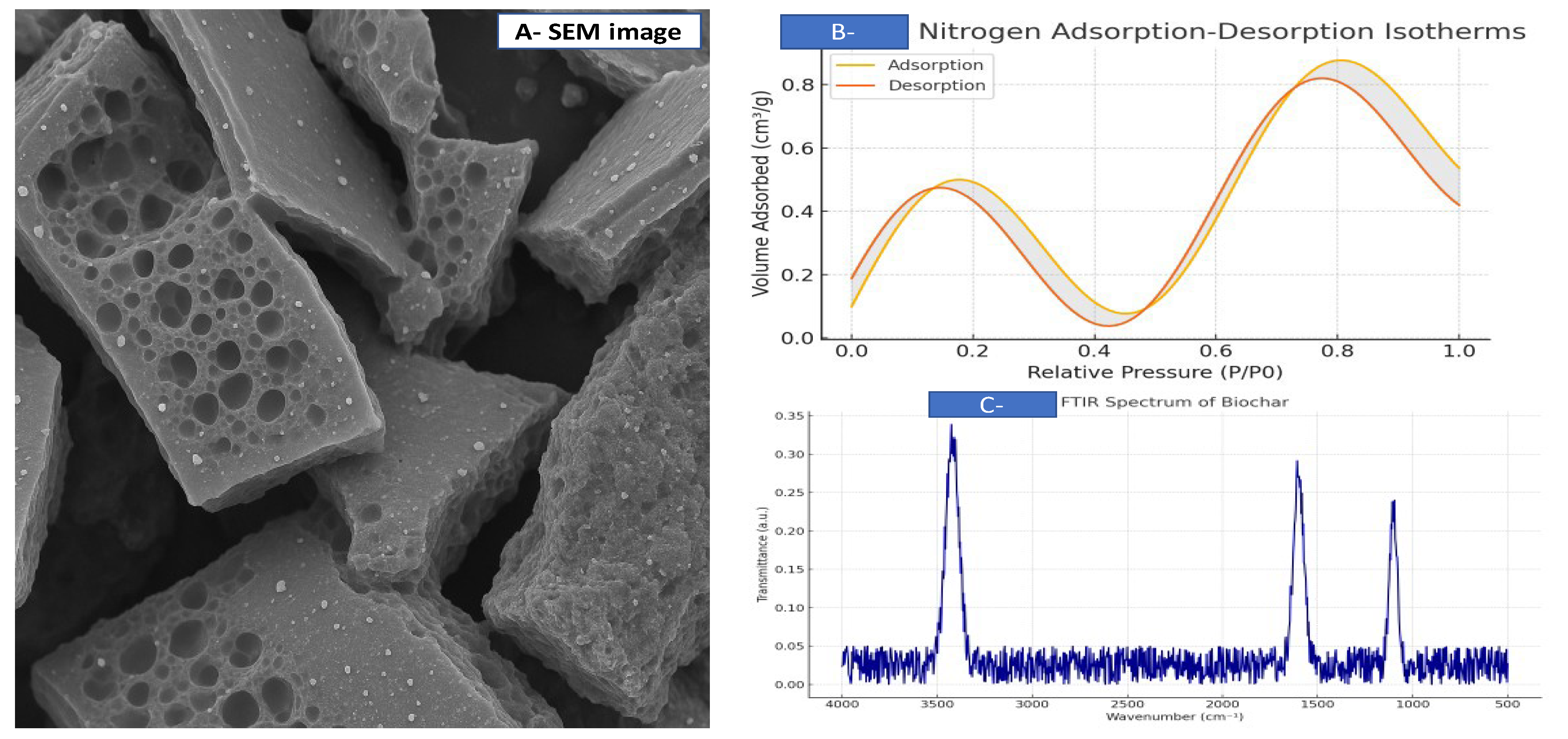
| Soil | Spirulina | Biochar | |
|---|---|---|---|
| pH | 7.82 ± 0.05 | 6.52 ± 0.08 | 9.55 ± 0.15 |
| Salinity (d Sm−1) | 1.8 ± 0.2 | 4.5 ± 0.1 | 3.7 ± 0.2 |
| OM (%) | 1.5 ± 0.1 | 86 ± 3 | 72 ± 4 |
| N (%) | 250 ± 12 | 10 ± 1 | 0.2 ± 0.0 |
| P (%) | 10 ± 1 | 0.35 ± 0.04 | 0.22 ± 0.05 |
| K (%) | 550 ± 16 | 1.2 ± 0.2 | 0.11 ± 1 |
| Ca (%) | 400 ± 12 | 3.5 ± 0.4 | 1.4 ± 0.2 |
| Fe (mg kg−1) | 8.2 ± 1 | 750 ± 10 | 60 ± 3 |
| Mn (mg kg−1) | 20 ± 2 | 70 ± 3 | 40 ± 3 |
| Zn (mg kg−1) | 1.5 ± 0.1 | 100 ± 4 | 50 ± 1 |
| Cu (mg kg−1) | 0.58 ± 0.00 | 75 ± 3 | 12 ± 1 |
| Water Type | Treatment | CEC (cmol kg−1) | Soluble Cations in Soil Solution (mg L−1) | ||||
|---|---|---|---|---|---|---|---|
| Na | Ca | K | Ca/Na | K/Na | |||
| FW | C | 17.1 ± 0.8 b | 250 ± 8 e | 250 ± 12 d | 420 ± 5 d | 1.0 ± 0.1 d | 1.7 ± 0.1 c |
| BC | 20.2 ± 0.9 a | 220 ± 6 a | 350 ± 18 c | 480 ± 8 c | 1.6 ± 0.0 b | 2.2 ± 0.1 b | |
| SP | 20.3 ± 0.7 a | 210 ± 5 d | 370 ± 10 c | 500 ± 12 b | 1.8 ± 0.2 ab | 2.4 ± 0.2 b | |
| BC + SP | 21.5 ± 1.0 a | 200 ± 9 b | 400 ± 6 b | 570 ± 16 a | 2.0 ± 0.1 a | 2.9 ± 0.1 a | |
| SW | C | 16.8 ± 0.4 b | 510 ± 15 a | 320 ± 5 c | 400 ± 10 d | 0.6 ± 0.1 d | 0.8 ± 0.1 d |
| BC | 19.8 ± 1.0 a | 430 ± 20 b | 380 ± 15 c | 470 ± 8 c | 0.9 ± 0.0 d | 1.1 ± 0.0 d | |
| SP | 21.0 ± 0.8 a | 420 ± 17 b | 400 ± 8 b | 510 ± 13 b | 1.0 ± 0.1 d | 1.2 ± 0.1 d | |
| BC + SP | 22.0 ± 1.0 a | 300 ± 12 c | 420 ± 12 a | 580 ± 20 a | 1.4 ± 0.0 c | 1.9 ± 0.2 c | |
| Water Type | Treatment | N | P | K | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| FW | C | 350 ± 15 a | 8.5 ± 0.3 e | 530 ± 25 c | 380 ± 22 d | 6.7 ± 0.4 c | 15 ± 2 b | 1.7 ± 0.4 c | 0.50 ± 0.04 c |
| BC | 300 ± 27 a | 13.2 ± 0.2 c | 590 ± 18 b | 490 ± 18 c | 7.2 ± 0.2 b | 17 ± 3 b | 2.2 ± 0.2 b | 0.41 ± 0.02 d | |
| SP | 320 ± 18 a | 15.1 ± 0.1 b | 610 ± 22 b | 490 ± 13 c | 9.4 ± 0.3 a | 23 ± 4 a | 2.9 ± 0.3 a | 0.92 ± 0.04 a | |
| BC + SP | 328 ± 20 a | 16.2 ± 0.3 a | 680 ± 26 a | 530 ± 22 b | 9.5 ± 0.4 a | 20 ± 3 a | 2.6 ± 0.4 a | 0.62 ± 0.03 b | |
| SW | C | 330 ± 15 a | 7.2 ± 0.4 e | 460 ± 15 d | 450 ± 25 c | 6.2 ± 0.2 c | 17 ± 1 b | 1.3 ± 0.2 c | 0.56 ± 0.02 c |
| BC | 335 ± 18 a | 11.4 ± 0.7 d | 580 ± 28 b | 480 ± 19 c | 7.5 ± 0.1 b | 14 ± 2 b | 2.0 ± 0.1 b | 0.38 ± 0.06 d | |
| SP | 318 ± 20 a | 13.8 ± 0.8 c | 620 ± 18 b | 570 ± 15 a | 9.4 ± 0.3 a | 25 ± 4 a | 2.8 ± 0.3 a | 0.95 ± 0.02 a | |
| BC + SP | 315 ± 25 a | 15.6 ± 1.3 a | 700 ± 30 a | 590 ± 22 a | 9.7 ± 0.2 a | 21 ± 2 a | 2.7 ± 0.2 a | 0.68 ± 0.05 b |
| Water Type | Treatment | N | P | K | Ca | Na | K/Na | Ca/Na |
|---|---|---|---|---|---|---|---|---|
| FW | C | 1.6 ± 0.0 c | 0.32 ± 0.0 d | 1.0 ± 0.1 d | 1.8 ± 0.1 d | 0.19 ± 0.0 d | 5.6 ± 0.1 e | 10.0 ± 0.2 c |
| BC | 2.0 ± 0.1 b | 0.38 ± 0.05 c | 1.5 ± 0.0 bc | 2.4 ± 0.1 b | 0.22 ± 0.02 d | 6.8 ± 0.1 c | 10.9 ± 0.3 c | |
| SP | 2.6 ± 0.2 a | 0.43 ± 0.01 b | 1.6 ± 0.1 b | 2.6 ± 0.1 ab | 0.21 ± 0.01 d | 7.6 ± 0.3 b | 12.4 ± 0.2 b | |
| BC + SP | 2.8 ± 0.2 a | 0.47 ± 0.03 a | 2.0 ± 0.1 a | 2.8 ± 0.1 a | 0.20 ± 0.03 d | 10.0 ± 0.0 a | 14.0 ± 0.3 a | |
| SW | C | 1.0 ± 0.0 d | 0.18 ± 0.02 f | 0.8 ± 0.3 d | 1.2 ± 0.1 e | 0.50 ± 0.00 a | 1.6 ± 0.3 h | 2.4 ± 0.0 g |
| BC | 1.6 ± 0.2 c | 0.25 ± 0.03 e | 1.2 ± 0.2 c | 1.5 ± 0.2 d | 0.34 ± 0.03 b | 3.5 ± 0.2 g | 4.4 ± 0.2 f | |
| SP | 1.7 ± 0.1 c | 0.32 ± 0.02 d | 1.3 ± 0.2 c | 1.7 ± 0.1 d | 0.32 ± 0.03 b | 4.1 ± 0.0 f | 5.3 ± 0.1 e | |
| BC + SP | 2.2 ± 0.1 b | 0.37 ± 0.01 c | 1.5 ± 0.2 bc | 2.0 ± 0.2 c | 0.25 ± 0.01 c | 6.0 ± 0.0 d | 8.0 ± 0.0 d |
| Water Type | Treatment | PH | FW | DW | TC |
|---|---|---|---|---|---|
| FW | C | 23 ± 3 c | 60 ± 2 c | 20 ± 2 c | 2.9 ± 0.1 c |
| BC | 28 ± 1 b | 70 ± 5 b | 21 ± 1 c | 3.1 ± 0.2 c | |
| SP | 34 ± 1 a | 82 ± 3 a | 25 ± 1 b | 3.6 ± 0.1 ab | |
| BC + SP | 35 ± 3 a | 88 ± 5 a | 30 ± 1 a | 3.8 ± 0.2 a | |
| SW | C | 18 ± 2 c | 40 ± 2 d | 15 ± 1 d | 2.2 ± 0.2 d |
| BC | 26 ± 2 b | 50 ± 2 e | 22 ± 1 c | 2.5 ± 0.2 d | |
| SP | 27 ± 2 b | 62 ± 2 c | 25 ± 1 b | 3.0 ± 0.1 c | |
| BC + SP | 30 ± 2 b | 70 ± 3 b | 27 ± 1 a | 3.4 ± 0.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eissa, M.A.; Alotaibi, M.O.; Alotibi, M.M.; Aljuaid, A.; Aldayel, T.H.; Ghoneim, A.M. A Novel Biostimulant–Biochar Strategy for Improving Soil Quality and Salinity Tolerance in Medicinal Mint (Mentha longifolia L.). Soil Syst. 2025, 9, 58. https://doi.org/10.3390/soilsystems9020058
Eissa MA, Alotaibi MO, Alotibi MM, Aljuaid A, Aldayel TH, Ghoneim AM. A Novel Biostimulant–Biochar Strategy for Improving Soil Quality and Salinity Tolerance in Medicinal Mint (Mentha longifolia L.). Soil Systems. 2025; 9(2):58. https://doi.org/10.3390/soilsystems9020058
Chicago/Turabian StyleEissa, Mamdouh A., Modhi O. Alotaibi, Mashael M. Alotibi, Alya Aljuaid, Taghreed Hamad Aldayel, and Adel M. Ghoneim. 2025. "A Novel Biostimulant–Biochar Strategy for Improving Soil Quality and Salinity Tolerance in Medicinal Mint (Mentha longifolia L.)" Soil Systems 9, no. 2: 58. https://doi.org/10.3390/soilsystems9020058
APA StyleEissa, M. A., Alotaibi, M. O., Alotibi, M. M., Aljuaid, A., Aldayel, T. H., & Ghoneim, A. M. (2025). A Novel Biostimulant–Biochar Strategy for Improving Soil Quality and Salinity Tolerance in Medicinal Mint (Mentha longifolia L.). Soil Systems, 9(2), 58. https://doi.org/10.3390/soilsystems9020058








