Urban Market Gardening Improves Soil Health: A Case Study in Burkina Faso
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
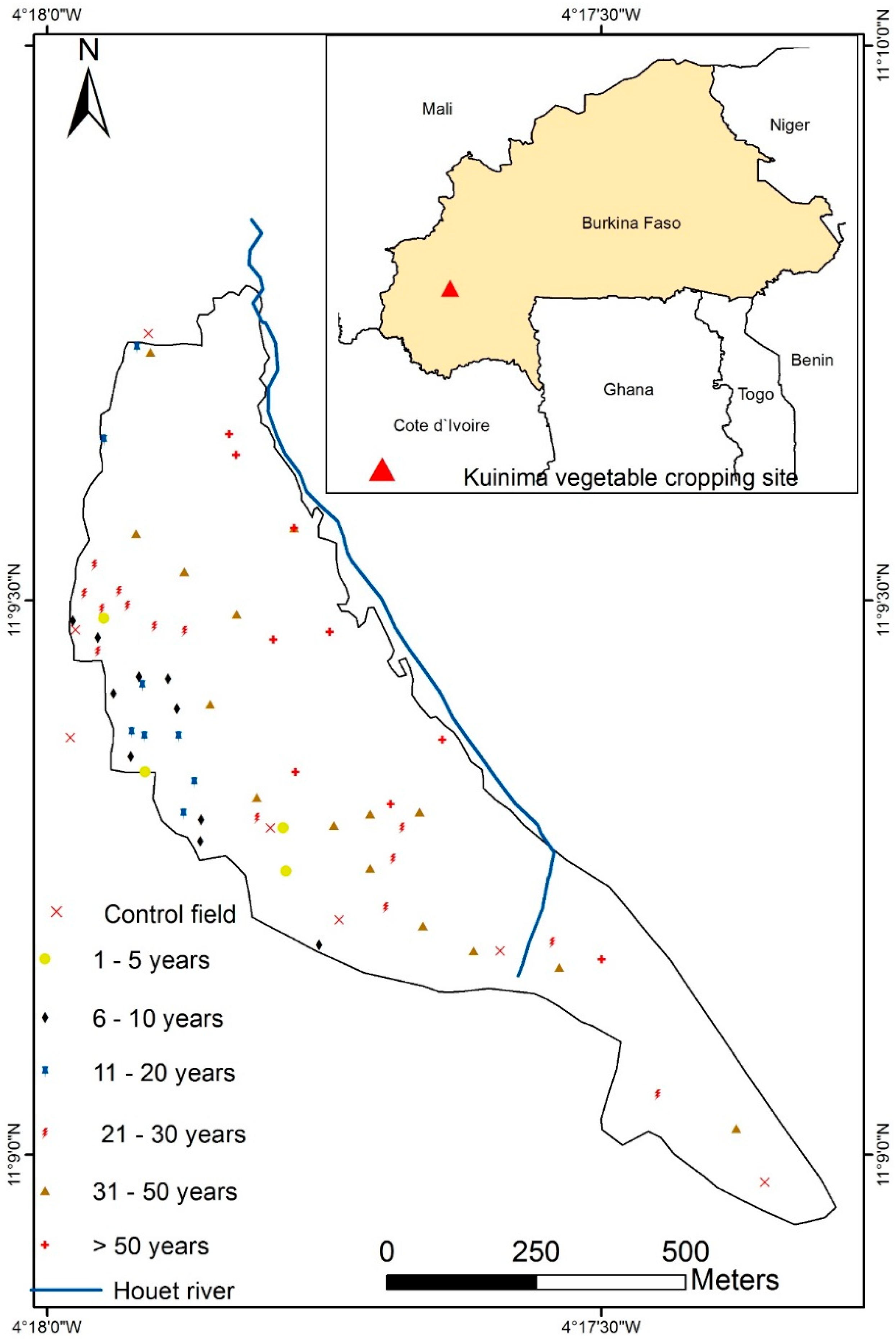
2.2. Field and Soil Sampling
2.3. Analysis of Soil Samples
2.3.1. Soil Texture and Chemical Parameters
2.3.2. Enzymatic Activity
2.3.3. Biochemical Activity
2.3.4. Soil Physical Properties
2.4. Evaluation of the Soil Health Index
2.5. Statistical Analysis
3. Results
3.1. Soil Chemical, Biological, and Physical Properties
3.2. Selection of Indicators
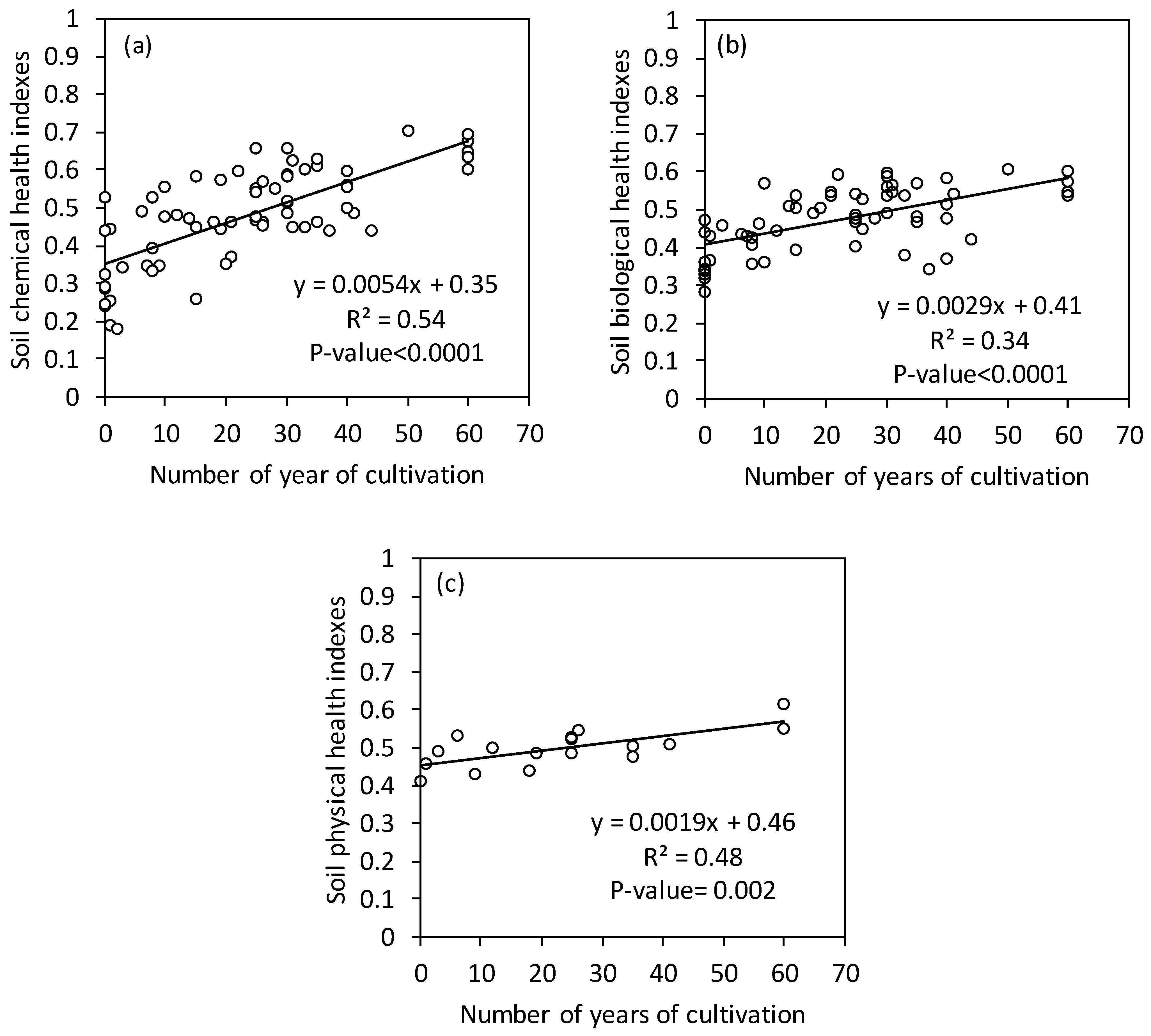
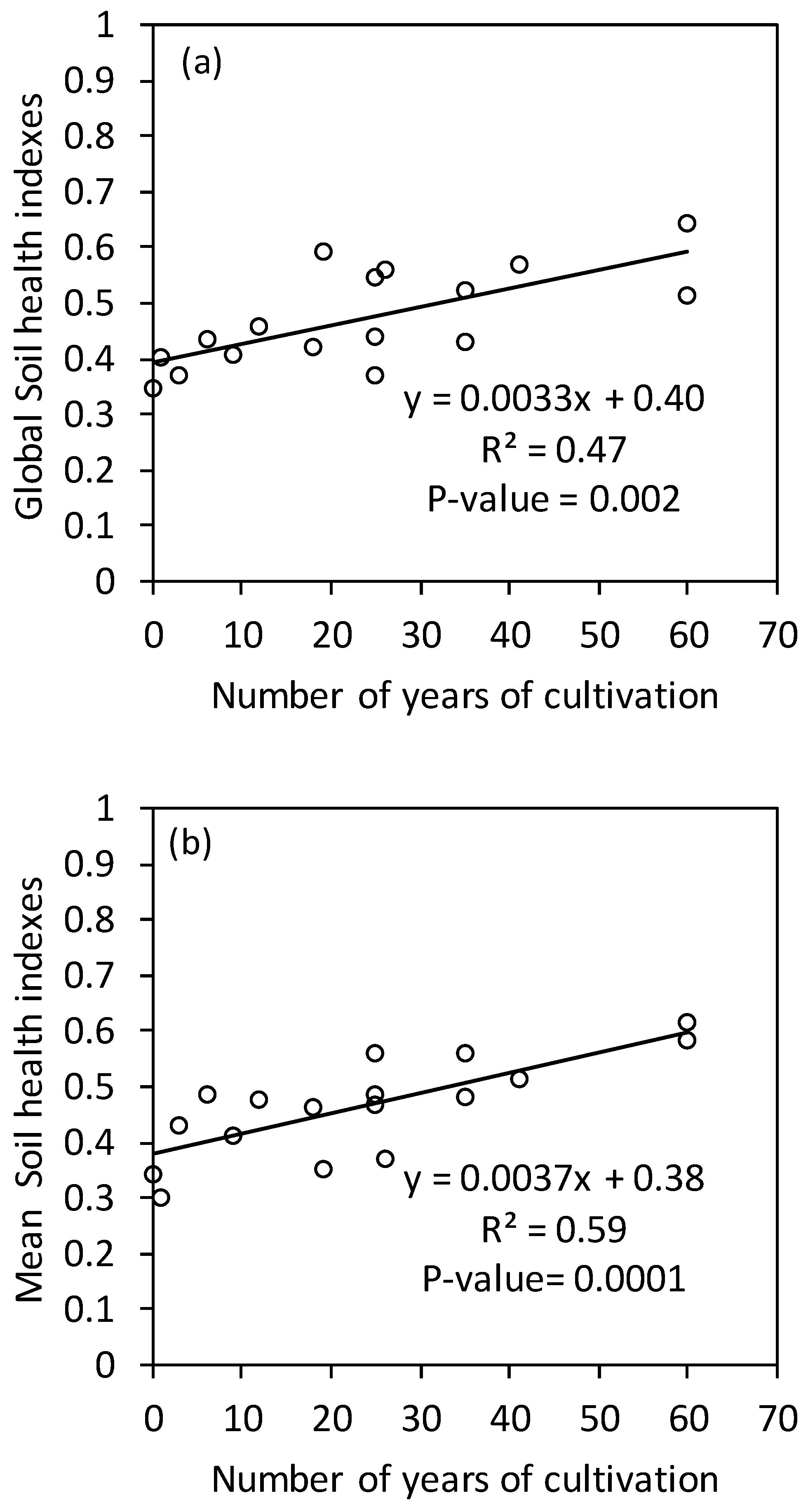
3.3. Effects of the Duration of Cultivation on the Soil Health Index
4. Discussion
4.1. Soil Chemical, Biological, and Physical Characteristics
4.2. Principal Component Analysis
4.3. Evolution of the Soil Health Index
4.4. SHI Methodology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Wienhold, B.J.; Andrews, S.S.; Karlen, D.L. Soil Quality: A Review of the Science and Experiences in the USA. Environ. Geochem. Health 2004, 26, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Rojas, M. Soil quality indicators: Critical tools in ecosystem restoration. Curr. Opin. Environ. Sci. Heal. 2018, 5, 47–52. [Google Scholar] [CrossRef]
- Granatstein, D.; Bezdicek, D.F. The Need for a Soil Quality Index: Local and Regional Perspectives. Am. J. Altern. Agric. 1992, 7, 12–16. [Google Scholar] [CrossRef]
- Chahal, I.; Hooker, D.C.; Deen, B.; Janovicek, K.; Eerd, L.L. Van. Soil & Tillage Research Long-term effects of crop rotation, tillage, and fertilizer nitrogen on soil health indicators and crop productivity in a temperate climate. Soil Tillage Res. 2021, 213, 105121. [Google Scholar] [CrossRef]
- Bai, Z.; Caspari, T.; Gonzalez, M.R.; Batjes, N.H.; Mäder, P.; Bünemann, E.K.; Tóth, Z. Effects of agricultural management practices on soil quality: A review of long-term experiments for Europe and China. Agric. Ecosyst. Environ. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Koishi, A.; Bragazza, L.; Maltas, A.; Guillaume, T.; Sinaj, S. Long-term effects of organic amendments on soil organic matter quantity and quality in conventional cropping systems in Switzerland. Agronomy 2020, 10, 1977. [Google Scholar] [CrossRef]
- Koulibaly, B.; Dakuo, D.; Traoré, O.; Ouattara, K. Long-term Effects of Crops Residues Management on Soil Chemical Properties and Yields in Cotton—Maize—Sorghum Rotation System in Burkina Faso. J. Agric. Ecol. Res. 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Norris, C.E.; Congreves, K.A. Alternative management practices improve soil health indices in intensive vegetable cropping systems: A review. Front. Environ. Sci. 2018, 6, 1–18. [Google Scholar] [CrossRef]
- Son, D.; Somda, I.; Legreve, A.; Schiffers, B. Pratiques phytosanitaires des producteurs de tomates du Burkina Faso et risques pour la santé et l’environnement. Cahiers Agric. 2017, 26, 25005. [Google Scholar] [CrossRef]
- Lompo, D.J.-P.; Compaoré, E.; Sedogo, M.P.; Melapie, M.; Bielders, C.L.; Schlecht, E.; Buerkert, A. Horizontal Flows of Nitrogen, Potassium, and Carbon in Urban Vegetables Gardens of Bobo Dioulasso, Burkina Faso. Nutr. Cycl. Agroecosyst. 2019, 115, 189–199. [Google Scholar] [CrossRef]
- Ouédraogo, R.A.; Kambiré, F.C.; Kestemont, M.; Bielders, C.L. Caractériser La Diversité Des Exploitations Maraîchères de La Région de Bobo-Dioulasso Au Burkina Faso Pour Faciliter Leur Transition Agroécologique. Cah. Agric. 2019, 28, 20. [Google Scholar] [CrossRef]
- Zerbo, I.; Dabiré, K.; Kaboré, S.A.; Sawadogo, C.M.; Thiombiano, A. Plant Agrobiodiversity, Agricultural and Phytosanitary Practices of Market Garden Crops in the Centre-East Region of Burkina Faso. J. Sustain. Agric. Environ. 2024, 3, 1–15. [Google Scholar] [CrossRef]
- Ridder, N.; Keulen, H. Some Aspects of the Role of Organic Matter in Sustainable Intensified Arable Farming Systems in the West-African Semi-Arid-Tropics (SAT). Fertil. Res. 1990, 26, 299–310. [Google Scholar] [CrossRef]
- Abdulkadir, A.; Leffelaar, P.A.; Agbenin, J.O.; Giller, K.E. Nutrient Flows and Balances in Urban and Peri-Urban Agroecosystems of Kano, Nigeria. Nutr. Cycl. Agroecosyst. 2013, 95, 231–254. [Google Scholar] [CrossRef]
- Ahouangninou, C.; Martin, T.; Edorh, P.; Bio-Bangana, S.; Samuel, O.; St-Laurent, L.; Dion, S.; Fayomi, B. Characterization of Health and Environmental Risks of Pesticide Use in Market-Gardening in the Rural City of Tori-Bossito in Benin, West Africa. J. Environ. Prot. 2012, 3, 241–248. [Google Scholar] [CrossRef]
- Kiba, D.I.; Zongo, N.A.; Lompo, F.; Jansa, J.; Compaore, E.; Sedogo, P.M.; Frossard, E. The Diversity of Fertilization Practices Affects Soil and Crop Quality in Urban Vegetable Sites of Burkina Faso. Eur. J. Agron. 2012, 38, 12–21. [Google Scholar] [CrossRef]
- Doran, J.W.; Sarrantonio, M.; Liebig, M.A. Soil health and sustainability. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: San Diego, CA, USA, 1996; Volume 56, pp. 1–54. [Google Scholar] [CrossRef]
- Andrews, S.S.; Mitchell, J.P.; Mancinelli, R.; Karlen, D.L.; Hartz, T.K.; Horwath, W.R.; Pettygrove, G.S.; Scow, K.M.; Munk, D.S. On-Farm Assessment of Soil Quality in California’s Central Valley. Agron. J. 2002, 94, 12–23. [Google Scholar] [CrossRef]
- Qu, M.; Chen, Y.; Khan, A.; Liu, P. Enhancing maize yield stability, soil health, and microbial diversity via long-term manure practices: Insights from a 14-year trial. Environ. Technol. Innov. 2025, 38, 104177. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Yi, X.; Zheng, H.; Ni, K.; Ma, Q.; Cai, Y.; Ma, L.; Shi, Y.; Yang, X.; et al. Partially replacing chemical fertilizer with manure improves soil quality and ecosystem multifunctionality in a tea plantation. Agric. Ecosyst. Environ. 2025, 378, 109284. [Google Scholar] [CrossRef]
- Rezapour, S.; Nouri, A.; Jalil, H.M.; Hawkins, S.A.; Lukas, S.B. Influence of treated wastewater irrigation on soil nutritional-chemical attributes using soil quality index. Sustainability 2021, 13, 1952. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil Quality—A Critical Review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Jasrotia, P.; Hamilton, S.K.; Robertson, G.P. Ecological management of intensively cropped agro-ecosystems improves soil quality with sustained productivity. Agric. Ecosyst. Environ. 2011, 140, 419–429. [Google Scholar] [CrossRef]
- Jones, A.; Breuning-Madsen, H.; Brossard, M.; Chapelle, J.; Dampha, A.; Deckers, J.; Dewitte, O.; Dondeyne, S.; Gallali, T.; Hallett, S.; et al. (Eds.) Atlas Des Sols D’Afrique; Publications Office: Hong Kong, China, 2015. [Google Scholar]
- Ouédraogo, R.A.; Chartin, C.; Kambiré, F.C.; van Wesemael, B.; Delvaux, B.; Milogo, H.; Bielders, C.L. Short and Long-Term Impact of Urban Gardening on Soil Organic Carbon Fractions in Lixisols (Burkina Faso). Geoderma 2020, 362, 114110. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic and available forms of phosphorus insoils. Soil. Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Okalebo, J.R.; Gathua, K.W.; Paul, L.W. Laboratory Methods of Soil and Plant Analysis: A Working Manual, 2nd ed.; SACRED Africa: Nairobi, Kenya, 2002; pp. 1–131. [Google Scholar]
- Hayano, K.A. Method for the Determination of β-Glucosidase Activity in Soil. Soil Sci. Plant Nutr. 1973, 19, 103–108. [Google Scholar] [CrossRef]
- Makoi, J.H.J.R.; Ndakidemi, P.A. Selected soil enzymes: Examples of their potential roles in the ecosystem. African J. Biotechnol. 2008, 7, 181–191. Available online: https://www.ajol.info/index.php/ajb/article/view/58355 (accessed on 11 March 2025).
- Tabatabai, M.A.; Bremner, J.M. Use of P-Nitrophenyl Phosphate for Assay of Soil Phosphatase Activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a Sensitive and Rapid Method for the Measurement of Total Microbial Activity Using Fluorescein Diacetate (FDA) in a Range of Soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gascó, G.; Gutiérrez, B.; Méndez, A. Soil Biochemical Activities and the Geometric Mean of Enzyme Activities after Application of Sewage Sludge and Sewage Sludge Biochar to Soil. Biol. Fertil. Soils 2012, 48, 511–517. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass, C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Rössner, H.; Kuhnert-Finkernagel, R.; Öhlinger, R.; Beck, T.; Baumgarten, A.; Heilmann, B. Indirect estimation of microbial biomass. In Methods in Soil Biology; Schinner, F., Ohlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 47–75. [Google Scholar]
- Rutgers, M.; Schouten, A.J.; Bloem, J.; van Eekeren, N.; de Goede, R.G.M.; Jagersop Akkerhuis, G.A.J.M.; vander Wal, A.; Mulder, C.; Brussaard, L.; Breure, A.M. Biological Measurements in a Nationwide Soil Monitoring Network. Eur. J. Soil Sci. 2009, 60, 820–832. [Google Scholar] [CrossRef]
- Schjønning, P.; McBride, R.A.; Keller, T.; Obour, P.B. Corrigendum to ‘Predicting Soil Particle Density from Clay and Soil Organic Matter Contents’ [Geoderma 286 (2017) 83–87]. Geoderma 2017, 292, 150. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, J.W., Bezdicek, D.F., Eds.; Special Publications, ASA-SSSA; Soil Science Society of Amer: Madison, WI, USA, 1994; Volume 35, pp. 3–21. [Google Scholar]
- Andrews, S.S.; Flora, C.B.; Mitchell, J.P.; Karlen, D.L. Growers’ perceptions and acceptance of soil quality indices. Geoderma 2003, 114, 187–213. [Google Scholar] [CrossRef]
- Bastida, F.; Luis Moreno, J.; Hernández, T.; García, C. Microbiological Degradation Index of Soils in a Semiarid Climate. Soil Biol. Biochem. 2006, 38, 3463–3473. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Li, Z.; Liu, M.; Xu, C.; Zhang, R.; Luo, W. Effects of vegetation restoration on soil quality in degraded karst landscapes of southwest China. Sci. Total Environ. 2019, 650, 2657–2665. [Google Scholar] [CrossRef]
- Sharma, K.L.; Mandal, U.K.; Srinivas, K.; Vittal, K.P.R.; Mandal, B.; Grace, J.K.; Ramesh, V. Long-Term Soil Management Effects on Crop Yields and Soil Quality in a Dryland Alfisol. Soil Tillage Res. 2005, 83, 246–259. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Alternative Soil Quality Indices for Evaluating the Effect of Intensive Cropping, Fertilisation and Manuring for 31 Years in the Semi-Arid Soils of India. Environ. Monit. Assess. 2008, 136, 419–435. [Google Scholar] [CrossRef]
- da Costa, A.C.S.; Junior, I.G.d.S.; Canton, L.C.; Gil, L.G.; Figueiredo, R. Contribution of the chemical and mineralogical properties of sandy-loam tropical soils to the cation exchange capacity. Rev. Bras. Cienc. Solo 2020, 44, 1–18. [Google Scholar] [CrossRef]
- Liptzin, D.; Silver, W.L. Effects of Carbon Additions on Iron Reduction and Phosphorus Availability in a Humid Tropical Forest Soil. Soil Biol. Biochem. 2009, 41, 1696–1702. [Google Scholar] [CrossRef]
- Ebhin Masto, R.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Changes in Soil Biological and Biochemical Characteristics in a Long-Term Field Trial on a Sub-Tropical Inceptisol. Soil Biol. Biochem. 2006, 38, 1577–1582. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Zobeck, T.M.; Gill, T.E.; Kennedy, A.C. Enzyme Activities and Microbial Community Structure in Semiarid Agricultural Soils. Biol. Fertil. Soils 2003, 38, 216–227. [Google Scholar] [CrossRef]
- Smukler, S.M.; Jackson, L.E.; Murphree, L.; Yokota, R.; Koike, S.T.; Smith, R.F. Transition to Large-Scale Organic Vegetable Production in the Salinas Valley, California. Agric. Ecosyst. Environ. 2008, 126, 168–188. [Google Scholar] [CrossRef]
- Kallenbach, C.; Grandy, A.S. Controls over Soil Microbial Biomass Responses to Carbon Amendments in Agricultural Systems: A Meta-Analysis. Agric. Ecosyst. Environ. 2011, 144, 241–252. [Google Scholar] [CrossRef]
- Li, J.; Cooper, J.M.; Lin, Z.; Li, Y.; Yang, X.; Zhao, B. Soil Microbial Community Structure and Function Are Significantly Affected by Long-Term Organic and Mineral Fertilization Regimes in the North China Plain. Appl. Soil Ecol. 2015, 96, 75–87. [Google Scholar] [CrossRef]
- Abdu, N.; Abdulkadir, A.; Agbenin, J.O.; Buerkert, A. Vertical Distribution of Heavy Metals in Wastewater-Irrigated Vegetable Garden Soils of Three West African Cities. Nutr. Cycl. Agroecosyst. 2011, 89, 387–397. [Google Scholar] [CrossRef]
- Sanchez, P.A. Properties and Management of Soils in the Tropics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar] [CrossRef]
- Gondal, A.H.; Hussain, I.; Ijaz, A.B.; Zafar, A.; Imran, B.; Zafar, H.; Sohai, M.D.; Niazi, H.; Touseef, M.; Khan, A.A.; et al. Influence of Soil Ph and Microbes on Mineral Solubility and Plant Nutrition: A Review. Int. J. Agric. Biol. Sci. 2021, 5, 71–81. [Google Scholar]
- Andrews, S.S.; Carroll, C.R. Designing a Soil Quality Assessment Tool for Sustainable Agroecosystem Management. Ecological. Appl. 2001, 11, 1573–1585. [Google Scholar] [CrossRef]
- Kirchmann, H.; Haberhauer, G.; Kandeler, E.; Sessitsch, A.; Gerzabek, M.H. Effects of level and quality of organic matter input on carbon storage and biological activity in soil: Synthesis of a long-term experiment. Global. Biogeochem. Cycles 2004, 18, 1–9. [Google Scholar] [CrossRef]
- Arshad, M.A.; Martin, S. Identifying critical limits for soil quality indicators in agro-ecosystems. Agric. Ecosyst. Environ. 2002, 88, 153–160. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Mitchell, J.P. A Comparison of Soil Quality Indexing Methods for Vegetable Production Systems in Northern California. Agric. Ecosyst. Environ. 2002, 90, 25–45. [Google Scholar] [CrossRef]
- Svoray, T.; Michailov, E.; Cohen, A.; Rokah, L.; Sturm, A. Predicting Gully Initiation: Comparing Data Mining Techniques, Analytical Hierarchy Processes and the Topographic Threshold. Earth Surf. Process. Landf. 2012, 37, 607–619. [Google Scholar] [CrossRef]
- Truog, E. Soil Reaction Influence on Availability of Plant Nutrients. Soil Sci. Soc. Am. J. 1947, 11, 305–308. [Google Scholar] [CrossRef]
| Characteristic | Variable | Unit | Min | Max | Median | Mean | St.Dev. | CV | n |
|---|---|---|---|---|---|---|---|---|---|
| Chemical | Ctot | g kg−1 | 5.5 | 34.7 | 19.5 | 18.7 | 7.1 | 0.38 | 69 |
| Ntot | g kg−1 | 0.56 | 2.55 | 1.44 | 1.46 | 0.5 | 0.34 | 69 | |
| pH | - | 5.0 | 7.6 | 6.6 | 6.6 | 0.58 | 0.09 | 69 | |
| SBE | cmol kg−1 | 2.4 | 17.1 | 6.8 | 6.8 | 2.6 | 0.38 | 69 | |
| CEC | cmol kg−1 | 3.4 | 17.6 | 10.5 | 10.5 | 3.5 | 0.33 | 69 | |
| P Bray | mg kg−1 | 21.3 | 169.3 | 53.3 | 62.3 | 31.4 | 0.50 | 69 | |
| EC | µS cm−1 | 17 | 255 | 95 | 101 | 44 | 0.44 | 69 | |
| Biological | Betaglu-cosidase | µg g−1 h−1 | 11.0 | 66.5 | 33.1 | 34.3 | 11 | 0.32 | 69 |
| Phosphatase | µg g−1 h−1 | 57.0 | 280.0 | 179.0 | 177.9 | 57.8 | 0.32 | 69 | |
| FDA | µg g−1 h−1 | 50.9 | 217.9 | 142.0 | 142.3 | 36.5 | 0.26 | 69 | |
| Uréase | µg g−1 h−1 | 175.0 | 830.1 | 445.5 | 436.2 | 145.8 | 0.33 | 69 | |
| GME | µg g−1 h−1 | 64.6 | 212.4 | 139.6 | 137.3 | 33.1 | 0.24 | 69 | |
| Resp | µg g−1 h−1 | 0.39 | 1.98 | 1.00 | 0.99 | 0.31 | 0.31 | 69 | |
| MBC | µg g−1 | 12.0 | 114.0 | 43.0 | 48 | 19.9 | 0.41 | 69 | |
| QCO2 | µg C-CO2 µg−1 MBC h−1 | 0.005 | 0.08 | 0.02 | 0.03 | 0.02 | 0.67 | 69 | |
| NH4+ | µg g−1 | 2.0 | 41.2 | 2.8 | 6.3 | 7 | 1.11 | 69 | |
| NO3− | µg g−1 | 9.5 | 114.8 | 39.2 | 41.8 | 21.4 | 0.51 | 69 | |
| Nmin/Ntot | mg g−1 | 1.55 | 8.53 | 3.06 | 3.02 | 1.2 | 0.40 | 69 | |
| C/N | - | 9.6 | 15.9 | 12.7 | 12.8 | 1.7 | 0.13 | 69 | |
| Qmic | mg g−1 | 0.59 | 9.90 | 2.74 | 2.91 | 1.64 | 0.56 | 69 | |
| Physical | Clay | % | 8 | 23 | 11 | 12 | 3.1 | 0.26 | 69 |
| Silt | % | 8 | 16 | 12 | 13 | 1.8 | 0.14 | 69 | |
| Sand | % | 64 | 82 | 76 | 75 | 3.6 | 0.05 | 69 | |
| BD | g cm−3 | 1.05 | 1.34 | 1.13 | 1.16 | 0.08 | 0.07 | 18 | |
| PAWC | % | 14 | 26 | 21 | 21 | 3 | 0.14 | 18 | |
| Macpo | % | 22 | 37 | 29 | 29 | 4 | 0.14 | 18 | |
| KS | m s−1 | 2.4 × 10−6 | 4.3 × 10−5 | 1.2 × 10−5 | 1.5 × 10−5 | 1.1 × 10−5 | 0.73 | 18 |
| Variable | Years | SHIchem | SHIbiol | SHIphys | SHImean | SHIglobal |
|---|---|---|---|---|---|---|
| Years | 1 | 0.65 | 0.65 | 0.67 | 0.75 | 0.66 |
| SHIchem | 0.65 | 1 | 0.78 | 0.67 | 0.66 | 0.83 |
| SHIbiol | 0.65 | 0.78 | 1 | 0.48 | 0.68 | 0.75 |
| SHIphys | 0.67 | 0.67 | 0.48 | 1 | 0.63 | 0.58 |
| SHImean | 0.75 | 0.66 | 0.68 | 0.63 | 1 | 0.51 |
| SHIglobal | 0.66 | 0.83 | 0.75 | 0.58 | 0.51 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouédraogo, R.A.; Kambiré, F.C.; Cournac, L.; Bielders, C.L. Urban Market Gardening Improves Soil Health: A Case Study in Burkina Faso. Soil Syst. 2025, 9, 59. https://doi.org/10.3390/soilsystems9020059
Ouédraogo RA, Kambiré FC, Cournac L, Bielders CL. Urban Market Gardening Improves Soil Health: A Case Study in Burkina Faso. Soil Systems. 2025; 9(2):59. https://doi.org/10.3390/soilsystems9020059
Chicago/Turabian StyleOuédraogo, Rayangnéwendé Adèle, Fabèkourè Cédric Kambiré, Laurent Cournac, and Charles L. Bielders. 2025. "Urban Market Gardening Improves Soil Health: A Case Study in Burkina Faso" Soil Systems 9, no. 2: 59. https://doi.org/10.3390/soilsystems9020059
APA StyleOuédraogo, R. A., Kambiré, F. C., Cournac, L., & Bielders, C. L. (2025). Urban Market Gardening Improves Soil Health: A Case Study in Burkina Faso. Soil Systems, 9(2), 59. https://doi.org/10.3390/soilsystems9020059







