Abstract
Glauconite, a diagenetic sedimentary phyllosilicate mineral, holds significant importance in soil science, as it is commonly used in soil characterization (as in greensands) and can be identified in the field by its color and morphology. It is a potential fertilizer, rich in essential macronutrients like potassium, phosphorus, calcium, and numerous micronutrients such as manganese, zinc, copper, cobalt, and nickel. In this meticulously conducted study, we extracted five individual elements (potassium, calcium, magnesium, sodium, and zinc) from washed glauconite samples separated into five different size fractions using a suite of acids. The acids employed were nitric acid, hydrochloric acid, acetic acid, and phosphoric acid, each prepared at the same molarity of 0.1 M. Water was used as the control solubilizing medium. The extractant behavior of the acids was assessed by measuring concentrations of each element by the ICP-OES device. The results demonstrate that nitric acid consistently exhibits the highest efficacy in releasing elements, followed by hydrochloric acid and phosphoric acid, while acetic acid results in the lowest release of these nutrients. These findings support the use of acidification treatment of glauconite, enhancing elemental release and potentially reducing the amount of glauconite needed as an alternative fertilizer, thus adding economic value.
1. Introduction
The agricultural sector in Egypt faces various challenges, such as poor soil quality, erosion, and severe water scarcity, as well as limited use of machines and rising costs for essential tools like pesticides and fertilizers [1]. To address these issues, there is a need to improve soil fertility in agricultural practices. While Egypt produces nitrogen and phosphate fertilizers, it imports potash fertilizers and certain nitrogenous fertilizers to meet local demands [2]. The traditional use of fertilizers can lead to environmental concerns as plants cannot absorb all the released nutrients, resulting in soil pollution [3]. Plants absorb only about 2% of potassium from the soil in a season, but the use of concentrated potassium salts can cause an excessive buildup of non-exchangeable potassium in the soil, releasing surplus potassium into groundwater during off-seasons [4]. To tackle this, slow-release potassium fertilizers like glauconite are considered, as they serve the dual purpose of providing plant nutrients and maintaining low chemical levels in the soil [5,6]. Glauconite is a green, layered phyllosilicate mineral, primarily found in marine deposits as sediments [7,8,9,10,11] or less frequently in volcanic rocks [12], and has proven useful in agricultural practice [13,14]. Glauconite has significant value in soil science, as it is used in soil characterization (greensands) [10,15,16,17,18,19], and it can be identified in the field by its color and morphology (pellet-like) [12], while X-ray diffraction [20] and Fourier-transform infrared spectroscopy [21] are used to identify it in the laboratory. Exploring alternative potassium sources, such as potassium-bearing silicates like glauconite, can potentially reduce import dependence and save foreign exchange [22,23].
Furthermore, applying glauconite helps avoid soil salinization and prevents groundwater contamination with chlorine [24]. Additionally, glauconite encompasses a rich array of trace elements, including Cu, Zn, Fe, Mn, B, Se, Co, Mo, Cr, V, and Y, which serve as essential micronutrients crucial for plant growth [23]. Cu, Zn, and B enrichment has been further enhanced in modified glauconite nanocomposites [25]. Moreover, the incorporation of glauconite rocks in agricultural practices enhances soil texture, porosity, and permeability owing to their uniform pellet structure [23]. The heightened sorption capacity of glauconite further augments the moisture-retention capability of agricultural soils [26].
A few studies have focused on modifying the glauconite fertilization efficiency. For example, Rudmin et al. [27] prepared controlled-release nanocomposites by mechanically activating a mix of glauconite and nitrogen nutrients in a 1:1 ratio. Other modifications evaluated the efficiency of organic compounds in solubilizing nutrients from rocks where glauconite was incubated and composted with coffee husk and citric and humic acids [28].
Prior to this study, the authors published a related article [29] in which elemental availability from glauconite using neutral and mildly basic extractants, including potassium chloride (KCl), ammonium lactate solution, and the full three-step BCR sequential extraction procedure, was evaluated. The BCR scheme, in which metals are divided into acid-soluble/exchangeable, reducible, and oxidizable fractions, is currently the recommended method by the European Community Bureau of Reference, now the Standard Measurements and Testing Programme of the European Community. Alkaline extraction solvents, in particular NaOH, can be used to great advantage for the efficient extraction of arsenic from contaminated soils [30,31] and for the determination of the elemental fraction bound to organic matter [32].
However, the fact that the existing scientific literature lacks studies comparing the usage of acetic acid (CH3COOH), hydrochloric acid (HCl), phosphoric acid (H3PO4), and nitric acid (HNO3) on the release of calcium (Ca), potassium (K), magnesium (Mg), sodium (Na), and zinc (Zn) from glauconite prompted this research. Egyptian soils typically exhibit alkaline pH levels ranging from 7.8 to 8.5. In such environments, acid activation may enhance nutrient release and thus improve the agronomic value of glauconite as a slow-release fertilizer. Therefore, our study aims to simulate a pre-treatment strategy that could improve glauconite’s performance when applied to alkaline agricultural soils. Neutral solutions are ideal for the determination of easily soluble and exchangeable species fractions; moreover, these fractions are of paramount importance for environmental mobility. However, these solutions can also extract the easily soluble elemental fraction from the solid phase; thus, we focused on the more strongly bound, typically acid-soluble fraction.
Few studies have thoroughly examined and compared the impacts of different acids on nutrient release from glauconite. Furthermore, most existing studies have predominantly concentrated on potassium release, neglecting other essential nutrients. This investigation aims to provide valuable insights into optimizing the utilization of glauconite as a nutrient source in agricultural practices and to evaluate the total release potential of essential nutrients from glauconite under controlled chemical conditions. This approach aligns with our broader objective of assessing the suitability of glauconite as a slow-release potassium fertilizer, as explored in the subsequent plant experiment phase (data not yet published).
2. Materials and Methods
2.1. Glauconite Sample
Representative samples of glauconite sediments were procured from the El-Baharia Oasis, situated in the Western Desert of Egypt near the El-Gedida mining area with coordinates 28°28′26.11″ N, 29°11′4.18″ E, and an elevation of 187 m above sea level (Figure S1) [6]. Egypt boasts abundant mineral resources, with substantial ironstone reserves notably present in the El-Gedida mine [33]. The mining site encompasses iron ores and considerable deposits of glauconite [34]. These glauconite deposits, serving as overburden, are excavated to access the commercially valuable iron ore deposit. Widespread occurrences of glauconite are observed across various locations in the Western Desert. The initial step in sample preparation for analysis involved the reduction of the glauconite rock to particles smaller than 2 mm using a jaw crusher. Subsequently, the samples underwent fractionation through dry sieving, resulting in five distinct size fractions (<2–1, <1–0.5, <0.5–0.2, <0.2–0.1, and <0.1 mm). These size-fractionated samples were then utilized in the subsequent acid-extraction analysis.
2.2. Glauconite Washing
The initial analysis of the glauconite already used in this study unveiled a predominant water-soluble sodium fraction, constituting 96% of the total sodium content. Concurrently, the salinity quotient within the samples ranged between approximately 12 and 13 mS cm−1. Recognizing the potential deleterious implications of elevated salinity levels and excessive sodium content on soil fertility and plant health, a pre-treatment protocol involving the washing of glauconite samples with distilled water (d H2O) was implemented before adding acids to the samples, thereby enhancing the suitability of glauconite for agriculture usage as an alternative soil fertilizer without causing environmental harm.
Subsequently, 50 g of material from each size fraction were washed with 250 mL of d H2O. The washing process involved shaking the mixture for two hours, followed by filtration. The resulting filtrate was collected for the determination of elemental composition by inductively coupled plasma optical emission spectrometry (ICP-OES). The residue of the glauconite samples after filtration was dried in an oven at a temperature of 105 °C until it reached a constant mass.
2.3. Acidic Extraction
Acid treatment was performed by the Hungarian standard MSZ 1484-3:2006 protocol. The acid activation process was carried out by mixing the washed and dried glauconite samples with 0.1 M of extractive solvents (nitric acid, hydrochloric acid, acetic acid, or phosphoric acid) separately into polyethylene bottles with a ratio of 1:40. The polyethylene bottles were placed on a rotary agitator. The acidified glauconite mixtures were rotated at 24 rpm for 16 h. After mixing, the eluate was filtered using a 0.45 μm filter paper. The total released elemental content of the elute was analyzed by ICP-OES. For each analyzed parameter, three replicates were made to ensure the accuracy of the work.
2.4. Instrumental Analysis
Chemical analysis of the elements was carried out by ICP-OES using an Activa-M spectrometer (Horiba Jobin Yvonn, Palaiseau, France). The operating parameters were set based on the recommendations of the manufacturer for multi-elemental analysis, including 1300 W of incident power, 13.146 dm3 min−1 of plasma gas flow, 0.32 dm3 min−1 of sheath gas flow, 0.2 dm3 min−1 of auxiliary gas flow, 0.28 dm3 min−1 of nebulizer gas flow, 2.86 bar of nebulizer gas pressure, and 0.85 cm3 min−1 of nebulizer sample flow uptake. For each analyzed parameter, three replicates were made to ensure the accuracy of the work. Limit of detection (LOD) values were established for each element, determined as the concentration of the element causing a shift in the mean value of the background (blank) signal corresponding to three times its standard deviation. According to this determination, 0.02, 1.0, 0.02, 0.7, and 0.3 µg L−1 LOD values were calculated for Ca, K, Mg, Na, and Zn, respectively.
2.5. pH and Electrical Conductivity Measurements
Under practical field conditions, glauconite may be modified by acid before application as fertilizer. For simulation of environmental conditions, a separate set of glauconite samples was prepared by mixing glauconite with acids until saturation. For this treatment, glauconite extracts at a ratio of 1:2.5 (10 g of glauconite per 25 mL d H2O) were prepared. The pH and electrical conductivity assessments were conducted on various iterations of the glauconite sample: initially unwashed, washed glauconite without acid treatment, and washed glauconite with acid treatment of 0.1 M of nitric acid, hydrochloric acid, acetic acid, and phosphoric acid. Acid treatment involved adding each acid separately to each sample until saturation was achieved. Notably, all analyses were performed on the glauconite–water mixture.
The pH measurements were executed using a Jenway 3510 standard digital pH meter (Jenway–Cole Parmer Co., Stone, Staffordshire, UK). Meanwhile, the electrical conductivity measurements were carried out using the Jenway 4510 conductivity and total dissolved solids measurement instrument (Jenway–Cole Parmer Co., Staffordshire, UK).
2.6. Statistical Analysis
Effects of extractive solvents and glauconite fractions on the release of elements investigated were statistically analyzed using the Statistica 8.0 software (StatSoft, TIBCO Software Inc., Tulsa, OK, USA). Shapiro–Wilk and Levene’s or Bartlett’s tests at the significance level of 0.05 were applied to determine the normality of the data and the homogeneity of variance, respectively. Based on the results of normality and homogeneity tests, significant effects of extractive solvents and glauconite fractions were determined by one-way analysis of variance (ANOVA) or the Kruskal–Wallis test. Significant differences among treatments were determined by Tukey’s honest significant difference (HSD) or Student–Newman–Keuls (SNK) post hoc tests at the significance level of 0.050. The results of the release were grouped and statistically compared by extractant solvents and glauconite fractions.
3. Results and Discussion
Utilizing various acids for extraction represents the most straightforward element extraction method from ore, thus rendering it a widely employed technique across diverse industries [1]. The investigation revealed the effect of the acid extractants and glauconite pellet size on the release of five nutrient elements: calcium (Ca), potassium (K), magnesium (Mg), sodium (Na), and zinc (Zn).
3.1. Calcium
The investigation into the release of calcium from glauconite revealed distinctive patterns contingent upon the choice of acid for acidification (Figure 1). The highest calcium release, ranging from 1199 ± 17.6 to 1545 ± 71.0 mg·kg−1, was obtained when nitric acid (HNO3) was employed. On the contrary, acetic acid (CH3COOH) application resulted in the lowest calcium release, with values ranging from 147 ± 4.9 to 254 ± 6.4 mg·kg−1. This observed variability in calcium release under different acidifying conditions led to the establishment of a descending order: HNO3 > HCl > H3PO4 > CH3COOH.
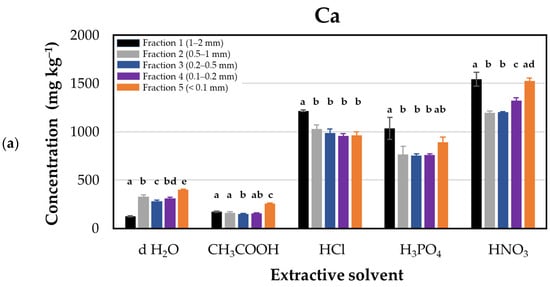
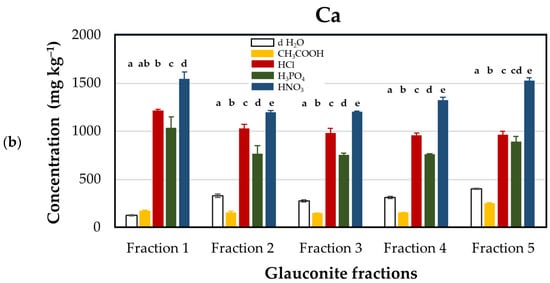
Figure 1.
Calcium concentrations (mg kg–1 dry matter) released from glauconite samples sieved for different particle sizes using distilled water and various acids as extractants. (a) Extraction efficacies plotted by extractants in particle size fractions: Fraction 1 (1–2 mm, black), Fraction 2 (0.5–1 mm, grey), Fraction 3 (0.2–0.5 mm, blue), Fraction 4 (0.1–0.2 mm, purple), and Fraction 5 (<0.1 mm, orange). (b) Extraction efficacies plotted by fractions using extractants: distilled water (d H2O, white), acetic acid (CH3COOH, yellow), hydrochloric acid (HCl, red), phosphoric acid (H3PO4, green), and nitric acid (HNO3, blue). Lower case Latin letters above the corresponding columns on the graph indicate significant differences: different lower case letters designate statistically significantly different categories within the groups (but not among different groups) by extractant solvents in Figure (a), and by glauconite fractions in Figure (b). Statistical comparisons were performed on a group-by-group (groups by solvents or groups by fractions) basis; thus, interpretations of significance for a given group do not represent any correlation with descriptors listed for other groups.
Calcium is available in glauconite as calcium oxide (CaO) [6]. The chemical reactions between calcium oxide and the respective acids elucidate the formation of calcium salts and water. Notably, the reaction with hydrochloric acid results in calcium chloride (CaCl2), treatment with nitric acid leads to calcium nitrate (Ca(NO3)2), and upon the reaction with phosphoric acid (H3PO4) calcium dihydrogen phosphate (Ca(H2PO4)2) is formed, while acetic acid (CH3COOH) forms calcium acetate (Ca(OCOC3)2). The result of salt formation in all cases indicates that acids are far more effective than simple d H2O extraction when it comes to extracting calcium from glauconite. Calcium chloride and calcium nitrate exhibit high solubility in water, reaching 745 g L−1 [35] and 1212 g L−1 [36] (20 °C), respectively, while the water solubility of calcium dihydrogen phosphate and calcium acetate are 180 and 347 g L−1 (20 °C), respectively, which is 3–6-fold lower than that of calcium nitrate. Our results demonstrated that nitric acid was substantially more effective than other acids in dissolving calcium from glauconite, predominantly due to the better water solubility of the calcium salts formed with nitric acid than those of salts formed with other inorganic or organic acids.
This information is valuable for understanding the chemical dynamics involved in releasing calcium from glauconitic sandstone. It has implications for potential applications in various fields, such as mineral processing and resource extraction.
3.2. Potassium
The liberation of potassium from glauconite was investigated, revealing notable variations in release efficiency under different acid treatments (Figure 2). Nitric acid (HNO3) demonstrated the highest efficacy, yielding a release ranging from 1011 ± 11.0 to 1118 ± 10.8 mg·kg−1. Hydrochloric acid (HCl) exhibited a comparable release, approximately equal to that of nitric acid; this result follows Tro [37], who stated that the solubility of both potassium chloride and potassium nitrate is the same at approximately 23 °C. Significantly, this release was three times higher than that observed with d H2O. Conversely, acetic acid (CH3COOH) resulted in the lowest potassium release, ranging from 455.7 ± 16.6 to 551 ± 4.5 mg·kg−1. The descending order of potassium release through acidification was determined as HNO3 ≈ HCl > H3PO4 > CH3COOH. In the case of potassium, relatively higher standard deviations were observed during the extraction process with phosphoric acid. This is because phosphoric acid can disturb the ionization balance of the plasma, which can affect the excitation state of potassium and thus its emission intensity. Since potassium has a low ionization energy, its ionization can be sensitive to the composition of the matrix. Phosphoric acid can affect the viscosity of the solution, which can alter the efficiency of the sample introduction system, and thus the amount and size distribution of the aerosol entering the plasma.
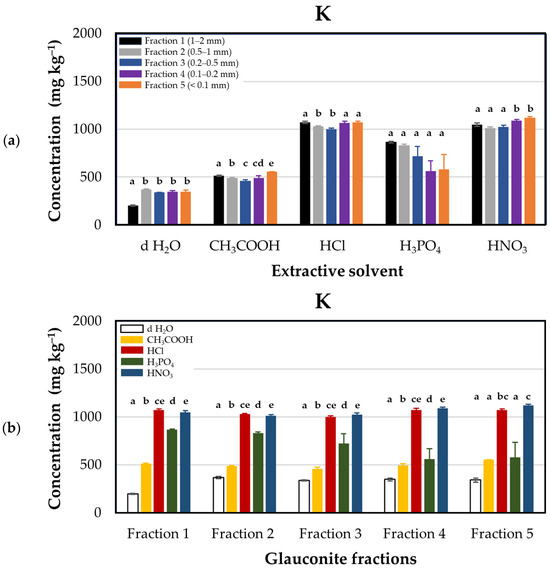
Figure 2.
Potassium concentrations (mg·kg−1 dry matter) released from glauconite samples sieved for different particle sizes using distilled water and various acids as extractants. (a) Extraction efficacies plotted by extractants in particle size fractions: Fraction 1 (1–2 mm, black), Fraction 2 (0.5–1 mm, grey), Fraction 3 (0.2–0.5 mm, blue), Fraction 4 (0.1–0.2 mm, purple), and Fraction 5 (<0.1 mm, orange). (b) Extraction efficacies plotted by fractions using extractants: distilled water (d H2O, white), acetic acid (CH3COOH, yellow), hydrochloric acid (HCl, red), phosphoric acid (H3PO4, green), and nitric acid (HNO3, blue). Lower case Latin letters above the corresponding columns on the graph indicate significant differences: different lower case letters designate statistically significantly different categories within the groups (but not among different groups) by extractant solvents in Figure (a), and by glauconite fractions in Figure (b). Statistical comparisons were performed on a group-by-group (groups by solvents or group by fractions) basis; thus, interpretations of significance for a given group do not represent any correlation with descriptors listed for other groups.
Table 1 summarizes previous studies on extracting potassium from glauconite by different acid treatments. For example, Shekhar et al. [38] used hydrochloric acid (HCl) to extract potassium from a concentrated glauconite sample, which resulted in a poor yield, with less than 12% of the potassium being recovered. Contrary to our results, HNO3 was more effective compared with a similar trend to HCl acid. Meanwhile, considering the use of acetic acid, Praveen and Tomar [39] reported poor release of potassium from glauconite, which is similar to our findings. This may generally reveal the poor extraction capacity of this acid compared to the other inorganic acids studied (HCl, HNO3, and H3PO4), but this may vary and depend on other factors like temperature and coke addition, as proven by Shekhar et al. [38].

Table 1.
Acid treatments of glauconite are reported in the scientific literature.
3.3. Magnesium
From the obtained results, d H2O extraction was the least effective method of extracting Mg from glauconite. Figure 3 illustrates that nitric acid treatment yielded the highest release of magnesium from glauconite, ranging from 979 ± 29.3 to 1142 ± 7.7 mg·kg−1; this release was three times higher than d H2O. Conversely, the lowest release of magnesium occurred when acidified with acetic acid, ranging from 119 ± 4.7 to 173 ± 3.2 mg·kg−1. The descending order of magnesium release through acidification was found to be HNO3 > HCl > H3PO4 > CH3COOH. This shows that the strong acids (e.g., HCl and HNO3) are better suited in terms of extraction compared to weak acids like acetic acid.
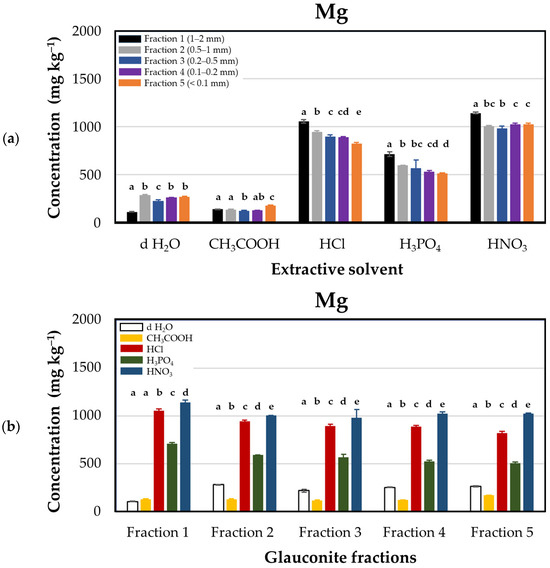
Figure 3.
Magnesium concentrations (mg·kg−1 dry matter) released from glauconite samples sieved for different particle sizes using distilled water and various acids as extractants. (a) Extraction efficacies plotted by extractants in particle size fractions: Fraction 1 (1–2 mm, black), Fraction 2 (0.5–1 mm, grey), Fraction 3 (0.2–0.5 mm, blue), Fraction 4 (0.1–0.2 mm, purple), and Fraction 5 (<0.1 mm, orange). (b) Extraction efficacies plotted by fractions using extractants: distilled water (d H2O, white), acetic acid (CH3COOH, yellow), hydrochloric acid (HCl, red), phosphoric acid (H3PO4, green), and nitric acid (HNO3, blue). Lower case Latin letters above the corresponding columns on the graph indicate significant differences: different lower case letters designate statistically significantly different categories within the groups (but not among different groups) by extractant solvents in Figure (a), and by glauconite fractions in Figure (b). Statistical comparisons were performed on a group-by-group (groups by solvents or group by fractions) basis; thus, interpretations of significance for a given group do not represent any correlation with descriptors listed for other groups.
3.4. Sodium
The effects of treatments on the release of sodium from glauconite (Figure 4) showed the highest result with d H2O, ranging from 3768 ± 61.2 to 8161 ± 135.0 mg·kg−1, resulting in the removal of over 50% of sodium content from approx. 8000 to 4000 mg·kg−1 before acidification. Acid treatment, however, does not have a noticeable impact on the release of sodium from glauconite, where all acids exhibited almost a uniform impact on sodium release.
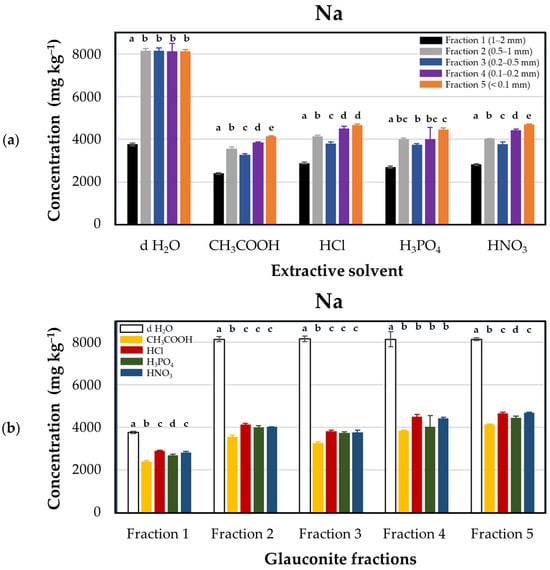
Figure 4.
Sodium concentrations (mg·kg−1 dry matter) released from glauconite samples sieved for different particle sizes using distilled water and various acids as extractants. (a) Extraction efficacies plotted by extractants in particle size fractions: Fraction 1 (1–2 mm, black), Fraction 2 (0.5–1 mm, grey), Fraction 3 (0.2–0.5 mm, blue), Fraction 4 (0.1–0.2 mm, purple), and Fraction 5 (<0.1 mm, orange). (b) Extraction efficacies plotted by fractions using extractants: distilled water (d H2O, white), acetic acid (CH3COOH, yellow), hydrochloric acid (HCl, red), phosphoric acid (H3PO4, green), and nitric acid (HNO3, blue). Lower case Latin letters above the corresponding columns on the graph indicate significant differences: different lower case letters designate statistically significantly different categories within the groups (but not among different groups) by extractant solvents in Figure (a), and by glauconite fractions in Figure (b). Statistical comparisons were performed on a group-by-group (groups by solvents or groups by fractions) basis; thus, interpretations of significance for a given group do not represent any correlation with descriptors listed for other groups.
This suggests that the washing process with d H2O is particularly effective in removing sodium from glauconite compared to acid treatment. Consequently, it is imperative to consider the washing process before utilizing glauconite as a fertilizer in agricultural applications due to the potential salinity-induced ramifications on plant growth [44]. Elevated salt concentrations can hinder plant growth by inducing nutritional imbalances, osmotic stress, and specific ion toxicity [45].
3.5. Zinc
Nitric acid (HNO3) treatment resulted in the highest release of zinc from glauconite (4.70 ± 0.12–7.38 ± 0.16 mg·kg−1), followed by hydrochloric acid (HCl), as illustrated in Figure 5. The descending order of zinc release through acidification was HNO3 > HCl > H3PO4 > CH3COOH. This trend reflects the acids’ varying chemical behavior and dissociation properties. Nitric acid, a strong monoprotic acid (pKa ≈ −1.4), fully dissociates in aqueous solution, providing a high concentration of H+ ions that facilitate proton exchange and structural breakdown of the glauconite matrix. This enables effective solubilization of zinc through reactions such as the following:
Zn2+ (bound) + 2H+ → Zn2+ (aq) + 2H+ (in structure)
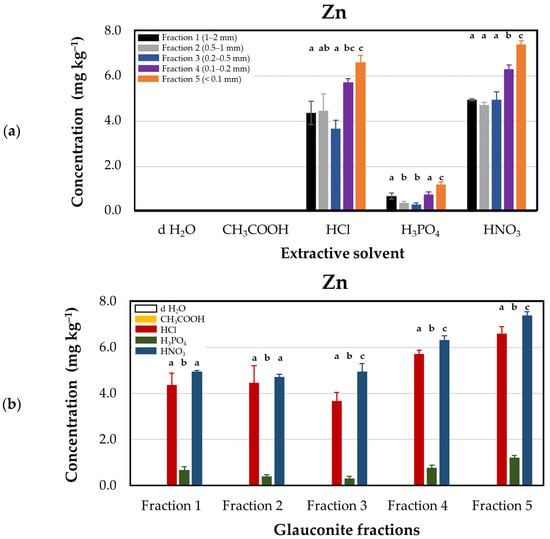
Figure 5.
Zinc concentrations (mg·kg–1 dry matter) released from glauconite samples sieved for different particle sizes using distilled water and various acids as extractants. (a) Extraction efficacies plotted by extractants in particle size fractions: Fraction 1 (1–2 mm, black), Fraction 2 (0.5–1 mm, grey), Fraction 3 (0.2–0.5 mm, blue), Fraction 4 (0.1–0.2 mm, purple), and Fraction 5 (<0.1 mm, orange). (b) Extraction efficacies plotted by fractions using extractants: distilled water (d H2O, white), acetic acid (CH3COOH, yellow), hydrochloric acid (HCl, red), phosphoric acid (H3PO4, green), and nitric acid (HNO3, blue). Lower case Latin letters above the corresponding columns on the graph indicate significant differences: different lower case letters designate statistically significantly different categories within the groups (but not among different groups) by extractant solvents in Figure (a), and by glauconite fractions in Figure (b). Statistical comparisons were performed on a group-by-group (groups by solvents or groups by fractions) basis; thus, interpretations of significance for a given group do not represent any correlation with descriptors listed for other groups.
In contrast, phosphoric acid (H3PO4), a triprotic acid with stepwise dissociation (pKa1 ≈ 2.15, pKa2 ≈ 7.20, pKa3 ≈ 12.35), releases fewer protons at pH values relevant to this study. Furthermore, its tendency to form poorly soluble metal phosphates like Zn3(PO4)2 may reduce measurable zinc concentrations in solution despite possible release from the solid phase. Zinc concentrations in the phosphoric acid treatment ranged only from 0.31 ± 0.08 to 1.20 ± 0.11 mg·kg−1. Among all acids tested, acetic acid (CH3COOH) resulted in the lowest zinc release, even less than that observed with deionized water. As a weak monoprotic acid (pKa ≈ 4.76), acetic acid dissociates only partially, producing limited H+ ions in solution. Consequently, it lacks the chemical strength necessary to break metal–oxygen bonds in the glauconite structure, which is reflected in its negligible effect on zinc mobilization. These results confirm that nitric acid is the most chemically effective acid for zinc release due to its strong acidity, full dissociation, and lack of interfering complexation or precipitation reactions.
3.6. Effect of Fraction Sizes of Glauconite on Element Release
The impact of glauconite particle size on the release of nutrient elements was investigated by dividing the glauconite sample into five size fractions and subjecting each to acid extraction.
Although a general trend of increasing elemental release with decreasing particle size was observed, most notably for the smallest fraction (<0.1 mm), this trend was not consistent or strong enough across all elements and extractants to justify treating size fractions separately. As illustrated by Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5, the <0.1 mm fraction occasionally exhibited slightly elevated extraction rates, and the variations among the five fractions were often marginal and not statistically or practically significant. For example, potassium, zinc, and sodium showed only slight increases in extraction from finer particles, while magnesium and calcium release remained virtually unaffected by particle size across all extractants. These results imply that the benefit of size separation for enhancing nutrient extraction is minimal. These results align with a previous study by Eldawwy et al. [29], where they found that glauconite pellets within the size fraction (<2.0–0.1mm) can be used without sifting, while the fractionation has a negligible effect on the elemental release. But this result is not compatible with previous research by Praveen and Tomar [39], who established a link between particle size and the effectiveness of glauconite nanoparticles. Their work suggests that as particle size decreases, efficacy generally improves. Similarly, Shekhar et al. [38] reported a correlation between finer particle size and higher extraction efficiency, specifically attributing this to the enhanced liberation of potassium at smaller fractions.
3.7. Changes in pH and Electrical Conductivity
Soil pH is a pivotal factor within soil dynamics, wielding influence over many physical, chemical, and biological processes that directly impact nutrient availability. These processes include but are not limited to the dissolution of metal solid phases and metal species’ precipitation, complexation, and acid–base interactions [46]. As shown in Table 2, the results of estimating the pH in the samples showed a slight change between the two glauconite samples before and after washing in a range between 5.8 and 6.1, while the pH decreased in the glauconite samples treated with acids to a lower value using hydrochloric acid accounting for a pH of 3.14. The order of the acids from the strongest to the least effective in reducing the pH number was as follows: HCl > HNO3 > H3PO4 > CH3COOH.

Table 2.
Electrical conductivity (EC) and pH values detected in 0.4 kg L−1 glauconite solutions (individual values reported as a mean ± standard deviation).
The investigation involved the assessment of electrical conductivity at room temperature (∼20 °C) utilizing a non-polarizing electrode. Discrepancies in electrical properties among the samples were ascribed to variations in elemental release induced by different acids employed in this study. The electrical properties exhibited alterations influenced by various factors, such as grain size, chemical composition, grain shape, facies, and porosity, as reported in previous studies [47,48].
The highest observed electrical conductivity was noted in the unwashed glauconite, registering a value of 12.5 mS cm−1. However, this conductivity decreased to 4.3 mS cm−1 after washing with water. Subsequent acidification resulted in a renewed increase in conductivity, with the highest value of 7.4 mS cm−1 observed in glauconite treated with HCl, followed by HNO3. The lowest value was recorded as 4.9 mS cm−1 for glauconite treated with CH3COOH.
4. Conclusions
This study aims to enhance the release of potassium and other essential elements such as calcium, magnesium, and zinc, thereby reducing the required quantities needed to optimize the utilization of glauconite as an alternative potassium fertilizer. The research compares the effects of acidification using four different acids on releasing these elements from glauconite across five distinct particle-size fractions following an initial washing with d H2O.
The results demonstrate significant variations in calcium, potassium, magnesium, sodium, and zinc release patterns under different acidification conditions. The impact of acidification on elemental release generally adhered to the following order: HNO3 > HCl > H3PO4 > CH3COOH (Table S1). Nitric acid consistently exhibits the highest efficacy in releasing calcium, potassium, magnesium, and zinc; therefore, the effective extraction ability of nitric acid also applies to glauconite. An important advantage of using nitric acid is that the metal nitrates formed by dissolving metal oxides, hydroxides, and salts of weaker acids are all highly soluble in water, in contrast to the limited solubility of some metal phosphates and chlorides. The nitrate ion is a weak complexing agent, so that dissolved metal ions are more easily detected in hydrated form. The above chemical properties explain the efficient extraction capacity of nitric acid, despite being a weaker acid (pKa~−2) than hydrochloric acid (pKa~−7), but clearly stronger than phosphoric acid (pKa1~2, pKa2~7, pKa3~12). Hydrochloric and phosphoric acids also show notable liberation, particularly for potassium, calcium, and magnesium. Conversely, acetic acid generally results in the lowest releases of these nutrients compared with other acids, highlighting its limited effectiveness in nutrient extraction from glauconite. Acetic acid is a weak acid, thus, it is expected that it extracts the lowest amount of nutrients. However, this acid mimics the real scenario of plant rhizospheres as it resembles the weak acidity of root exudates. Thus, if the ore is applied to soil directly as a fertilizer, the behavior observed with acetic acid may offer the most realistic indication of its performance in real agricultural systems. The use of more acidic extractants, however, can be used to model environmental situations where a very low pH aqueous phase is applied to the glauconite amended soil, e.g., acid mine drainage, industrial pollution, acid rain, or landfill leachate.
Interestingly, sodium release remained unaffected by acidification, indicating the stability of sodium content in glauconite under the tested conditions. This study’s comprehensive analysis of different size fractions provides valuable insights into the nutrient release dynamics from glauconite. While the smallest particle size fraction (<0.1 mm) occasionally exhibited slightly higher nutrient release, the differences among the remaining fractions were minimal, indicating that overall, particle size had a limited and inconsistent effect on elemental release from glauconite. Washing the glauconite samples also positively reduced the high salinity level from 12.5 to 4.3 mS cm−1 and eliminated more than 50% of their sodium content. The results encourage the acidification treatment of glauconite, enhancing elemental release and potentially reducing the amount of glauconite needed as an alternative fertilizer, thus adding economic value.
Further studies are needed to investigate the influence of the extraction time on the potential of various acids in nutrient release from glauconite. These studies could optimize the effectiveness of stronger acids in nutrient extraction. Additionally, exploring the impact of acid concentration is crucial. Utilizing a lower concentration of a stronger acid, such as nitric acid, may yield more efficient nutrient release compared to higher concentrations of a weaker acid, such as acetic acid. This approach could also reduce environmental impact and cost.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/soilsystems9020050/s1. Figure S1: The location of El-Gedida Iron Ore Mine with a geological map of El-Baharia Oasis, Western Desert, Egypt, and Google Earth image (Reproduced with permission). Table S1: Combined element concentrations in the extracts in all glauconite fractions obtained with different extractive solvents (individual concentration values reported as a mean ± standard deviation).
Author Contributions
Conceptualization, N.E. and M.H.; validation, E.T.; formal analysis, H.N., A.M.Z., G.H. and A.S.; investigation, N.E., M.H. and M.G.; writing—original draft preparation, N.E., H.N. and A.M.Z.; writing—review and editing, É.L., E.T. and A.S.; visualization, E.T. and A.S.; supervision, M.H. and M.G.; funding acquisition, E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Flagship Research Groups Program 2024 and the Research Excellence Program 2025 of the Hungarian University of Agriculture and Life Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the manuscript.
Acknowledgments
The authors thank the Centre for Circular Economy Analysis and Knowledge, founded by the Hungarian University of Agricultural and Life Sciences (MATE), for enrolling this international collaboration in its education profile.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jena, S.K. A review on potash recovery from different rock and mineral sources. Mining Metall. Explor. 2021, 38, 47–68. [Google Scholar] [CrossRef]
- Ahmed, M.M.B. An analytical economic study of the production and consumption of nitrogen fertilizers in Egypt. Alex. Sci. Exch. J. 2023, 44, 525–539. [Google Scholar] [CrossRef]
- Wang, Z.B.; Chen, J.; Mao, S.C.; Han, Y.C.; Chen, F.; Zhang, L.F.; Li, Y.B.; Li, C.D. Comparison of greenhouse gas emissions of chemical fertilizer types in China’s crop production. J. Clean. Prod. 2017, 141, 1267–1274. [Google Scholar] [CrossRef]
- Basak, B.B.; Sarkar, B.; Biswas, D.R.; Sarkar, S.; Sanderson, P.; Naidu, R. Bio-intervention of naturally occurring silicate minerals for alternative source of potassium: Challenges and opportunities. Adv. Agron. 2017, 141, 115–145. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, Q.; Zhang, W.; Liu, L.; Liang, H.; Quirino, R.L.; Chen, J.; Liu, M.; Lu, Q.; Zhang, C. Tunable thermo-physical performance of castor oil-based polyurethanes with tailored release of coated fertilizers. J. Clean. Prod. 2019, 210, 1207–1215. [Google Scholar] [CrossRef]
- El-Habaak, G.; Askalany, M.; Faraghaly, M.; Abdel-Hakeem, M. The economic potential of El-Gedida glauconite deposits, El-Bahariya Oasis, Western Desert, Egypt. J. Afr. Earth Sci. 2016, 120, 186–197. [Google Scholar] [CrossRef]
- Odom, I.E. Glauconite and celadonite minerals. Rev. Mineral. Geochem. 1984, 13, 545–584. [Google Scholar] [CrossRef]
- Rubio, B.; López-Pérez, A.E. Exploring the genesis of glaucony and verdine facies for paleoenvironmental interpretation: A review. Sediment. Geol. 2024, 461, 106579. [Google Scholar] [CrossRef]
- Mohammed, I.Q.; Sarin, T.; Singh, P.; Lawa, F.A.; Farouk, S.; Al-Kahtany, K.; Banerjee, S. The influence of depositional conditions on chemical and mineralogical composition of glauconite: Case study from the Late Cretaceous Dokan Basin in Kurdistan region of Iraq. Appl. Clay Sci. 2025, 263, 107639. [Google Scholar] [CrossRef]
- Wilmsen, M.; Bansal, U.; Metzner, N.; Böoning, P. Geochemical and depositional environment of an Upper Cretaceous greensand giant (Münsterland Cretaceous Basin, Germany). Chem. Geol. 2024, 661, 122168. [Google Scholar] [CrossRef]
- Baldermann, A.; Banerjee, S.; Löhr, S.C.; Rudmin, M.; Warr, L.N.; Chakraborty, A. Exploring reverse silicate weathering across geological time: A review. Clay Miner. 2025, 2025, 1–27. [Google Scholar] [CrossRef]
- Rudmin, M.; López-Quirós, A.; Banerjee, S.; Ruban, A.; Shaldybin, M.; Bernatonis, P.; Singh, P.; Dauletova, A.; Maximov, P. Origin of Fe-rich clay minerals in Early Devonian volcanic rocks of the Northern Minusa basin, Eastern Siberia. Appl. Clay Sci. 2023, 241, 107014. [Google Scholar] [CrossRef]
- Shekhar, S.; Kumari, V.; Sinha, S.; Mishra, D.; Sahu, K.K. A clean process for the recovery of potash fertilizer from glauconitic rock via hydrogen gas pre-treatment and mild acid leaching. JOM 2024, 76, 3343–3353. [Google Scholar] [CrossRef]
- Dasi, E.; Rudmin, M.; Banerjee, S. Glauconite applications in agriculture: A review of recent advances. Appl. Clay Sci. 2024, 253, 107368. [Google Scholar] [CrossRef]
- Loveland, P.J.; Findlay, D.C. Composition and development of some soils on glauconitic Cretaceous (Upper Greensand) rocks in southern England. J. Soil Sci. 1982, 33, 219–294. [Google Scholar] [CrossRef]
- Lynn, W.C.; Yeck, R.D. Redefinition of the glauconitic family in soil taxonomy. In Mineral Classification of Soils; Kittrick, A., Ed.; Soil Science Society of America, Inc., American Society of Agronomy, Inc.: Madison, WI, USA, 1985; Volume 16, pp. 125–133. [Google Scholar]
- Obasi, C.; Terry, D.O.; Myer, G.H.; Grandstaff, D.E. Glauconite composition and morphology, shocked quartz, and the origin of the Cretaceous(?) main fossiliferous layer (MFL) in Southern New Jersey, USA. J. Sediment. Res. 2011, 81, 479–494. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Mazurov, A. Compositional variation of glauconites in Upper Cretaceous-Paleogene sedimentary iron-ore deposits in South-eastern Western Siberia. Sediment. Geol. 2017, 355, 20–30. [Google Scholar] [CrossRef]
- Westgate, Z.J.; DeGroot, D.J.; McMullin, C.; Zou, Y.; Guo, D.; Van Haren, S.; Beemer, R.D.; Zeppilli, D.; Miller, K.G.; Browning, J.W. Effect of degradation on geotechnical behavior of glauconite sands from the U.S. Mid-Atlantic Coastal Plain. Ocean Eng. 2023, 283, 115081. [Google Scholar] [CrossRef]
- Drits, V.A.; Dainyak, L.G.; Muller, F.; Besson, G.; Manceau, A. Isomorphous cation distribution in celadonites, glauconites and Fe-illites determined by infrared, Mössbauer and EXAFS spectroscopies. Clay Miner. 1997, 32, 153–179. [Google Scholar] [CrossRef]
- Singh, P.; Banerjee, S.; Choudhury, T.R.; Bhattacharya, S.; Pande, K. Distinguishing celadonite from glauconite for environmental interpretations: A review. J. Palaeogeogr. 2023, 12, 179–194. [Google Scholar] [CrossRef]
- Torqueti, S.T.D.S.; Boldrin, K.V.F.; Nascimento, Â.M.P.D.; Paiva, P.D.D.O.; Furtini, A.E.; Luz, I.C.A. Alternative potassium source for the cultivation of ornamental sunflower. Ciên. Agrotec. 2016, 40, 257–264. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Mazurov, A.; Makarov, B.; Martemyanov, D. Economic potential of glauconitic rocks in Bakchar deposit (SE Western Siberia) for alternate potash fertilizer. Appl. Clay Sci. 2017, 150, 225–233. [Google Scholar] [CrossRef]
- Khitrin, I.; Maximov, P.; Dasi, E.; Ibraeva, K.; Ponomarev, K.; Maximova, N.; Belousov, P.; Ruban, A.; Rudmin, M. Glauconite-based nanocomposites with Zn/Cu/B: Multifunctional micronutrient fertilizers. Minerals 2025, 15, 12. [Google Scholar] [CrossRef]
- Franzosi, C.; Castro, L.N.; Celeda, A.M. Technical evaluation of glauconies as alternative potassium fertilizer from the Salamanca Formation, Patagonia, Southwest Argentina. Nat. Resour. Res. 2014, 23, 311–320. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Makarov, B.; Mazurov, A.; Ruban, A.; Oskina, Y.; Tolkachev, O.; Buyakov, A.; Shaldybin, M. An investigation of plant growth by the addition of glauconitic fertilizer. Appl. Clay Sci. 2019, 180, 105178. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Makarov, B.; Belousov, P.; Kurovsky, A.; Ibraeva, K.; Buyakov, A. Glauconite-urea nanocomposites as polyfunctional controlled-release fertilizers. J. Soil Sci. Plant Nutr. 2022, 22, 4035–4046. [Google Scholar] [CrossRef]
- Pessoa, R.S.; Silva, C.A.; Moretti, B.S.; Furtini, A.E.; Inda, A.V.; Curi, N. Solubilization of potassium from alternative rocks by humic and citric acids and coffee husk. Ciên. Agrotec. 2015, 39, 553–564. [Google Scholar] [CrossRef]
- Eldawwy, N.; Gulyás, M.; Naser, H.; Takács, A.; Lehoczky, É.; Horváth, M. Investigating the elemental composition of Egyptian glauconite sediments by applying BCR sequential extraction procedure and some single extractants. Agrokém. Talajt. 2024, 73, 42–58. [Google Scholar] [CrossRef]
- Yang, J.-S.; Lee, J.Y.; Baek, K.; Kwon, T.-S.; Choi, J. Extraction behavior of As, Pb, and Zn from mine tailings with acid and base solutions. J. Hazard. Mater. 2009, 171, 443–451. [Google Scholar] [CrossRef]
- Golia, E.E.; Tsiropoulos, N.G.; Vleioras, S.; Antoniadis, V. Investigation of extraction methods for the assessment of the pseudo-total concentration of potentially toxic elements in moderately contaminated soils of Central Greece. Water Air Soil Pollut. 2020, 231, 484. [Google Scholar] [CrossRef]
- Clemente, R.; Bernal, M.P. Fractionation of heavy metals and distribution of organic carbon in two contaminated soils amended with humic acids. Chemosphere 2006, 64, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Salama, W.; El Aref, M.; Gaupp, R. Mineralogical and geochemical investigations of the Middle Eocene ironstones, El Bahariya Depression, Western Desert, Egypt. Gondwana Res. 2012, 22, 717–736. [Google Scholar] [CrossRef]
- Hassan, M.; Baioumy, H. Characterization and origin of alunite in the El-Gideda iron mine (Egypt). Period. Mineral. 2007, 76, 11–24. [Google Scholar]
- Ropp, R.C. Group 17 (H, F, Cl, Br, I) alkaline earth compounds. In Encyclopedia of the Alkaline Earth Compounds; Elsevier: Kidlington, UK, 2013; pp. 25–104. [Google Scholar] [CrossRef]
- Kant, S.; Kafkafi, U. Fertigation. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Kidlington, UK, 2013; pp. 1–10. [Google Scholar] [CrossRef]
- Tro, N.J. Introductory Chemistry, 6th ed.; Pearson: London, UK, 2011; ISBN 978-01-3430-238-6. [Google Scholar]
- Shekhar, S.; Mishra, D.; Agrawal, A.; Sahu, K.K. Physico-chemical treatment of glauconitic sandstone to recover potash and magnetite. J. Clean. Prod. 2017, 147, 681–693. [Google Scholar] [CrossRef]
- Praveen, S.; Tomar, D.S. Solubility of glauconite nano-particle in root exudates. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 3315–3320. [Google Scholar] [CrossRef]
- Yadav, V.P.; Sharma, T. Leaching of Glauconitic sand stone in acid lixiviants. Miner. Eng. 1992, 5, 715–720. [Google Scholar] [CrossRef]
- Schimicoscki, R.S.; Oliveira, K.D.; Ávila-Neto, C.N. Potassium recovery from a Brazilian glauconitic siltstone via reaction with sulfuric acid in hydrothermal conditions. Hydrometallurgy 2020, 191, 105251. [Google Scholar] [CrossRef]
- Shekhar, S.; Mishra, D.; Agrawal, A.; Sahu, K.K. Physical and chemical characterization and recovery of potash fertilizer from glauconitic clay for agricultural application. Appl. Clay Sci. 2017, 143, 50–56. [Google Scholar] [CrossRef]
- Rao, B.R.; Rao, L.S.; Mazumdar, A.K.; Rao, T.C. Fluoride aided potassium extraction from glauconitic sandstone for liquid fertilizer. Miner. Eng. 1993, 6, 405–413. [Google Scholar] [CrossRef]
- Oze, C.; Smaill, J.B.; Reid, C.M.; Palin, M. Potassium and metal release related to glaucony dissolution in soils. Soil Syst. 2019, 3, 70. [Google Scholar] [CrossRef]
- Liu, J.; Xie, W.; Yang, J.; Yao, R.; Wang, X.; Li, W. Effect of different fertilization measures on soil salinity and nutrients in salt-affected soils. Water 2023, 15, 3274. [Google Scholar] [CrossRef]
- Fairbrother, A.; Wenstel, R.; Sappington, K.; Wood, W. Framework for metals risk assessment. Ecotox. Environ. Saf. 2007, 68, 145–227. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Anwar, E.A.; Gomaa, M.M. Electrical properties and geochemistry of carbonate rocks from the Qasr El-Sagha Formation, El-Faiyum, Egypt. Geophys. Prospect. 2013, 61, 630–644. [Google Scholar] [CrossRef]
- Meng, H.; Shi, Q.; Liu, T.; Liu, F.; Chen, P. The percolation properties of electrical conductivity and permeability for fractal porous media. Energies 2019, 12, 1085. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).