Cutting-Edge Technology Using Blended Controlled-Release Fertilizers and Conventional Monoammonium Phosphate as a Strategy to Improve Phosphorus Coffee Nutrition During the Coffee Development Phase
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Design and Description of Treatments
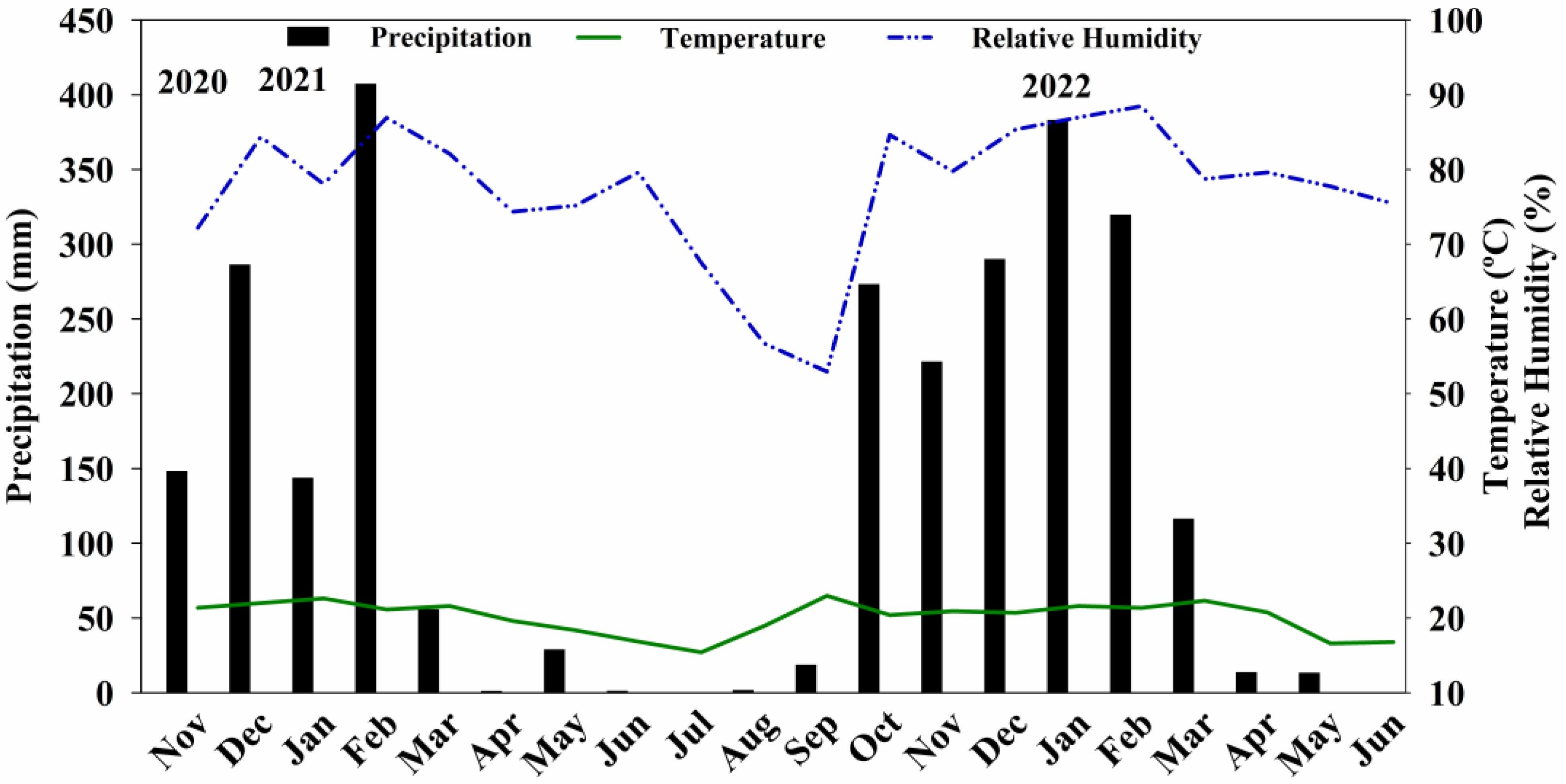
2.2. Coffee Crop Management
2.3. Characterization of the Controlled-Release Fertilizers and Nutrient Release in Field Conditions
Nutrient Release from the Fertilizer Blends
2.4. Leaf Area, Biomass, and Coffee Yield
2.4.1. Leaf Area
2.4.2. Dry Biomass
2.4.3. Coffee Bean Yield
2.5. Available Phosphorus in Soil
2.6. Statistical Analysis
3. Results
3.1. Thickness of the Coatings and Nutrient Release from Fertilizers
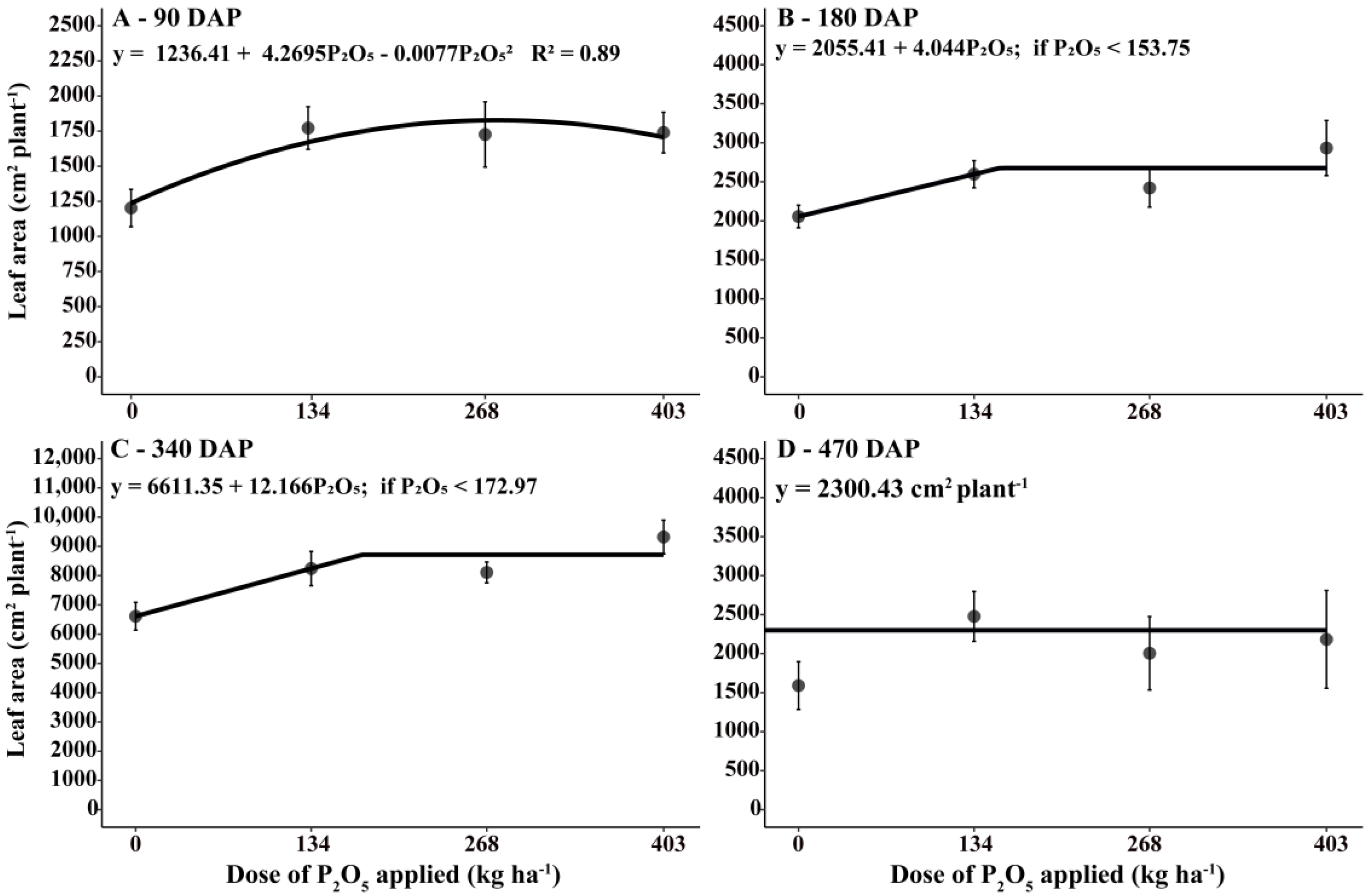
3.2. Leaf Area Increment in Coffee Plants
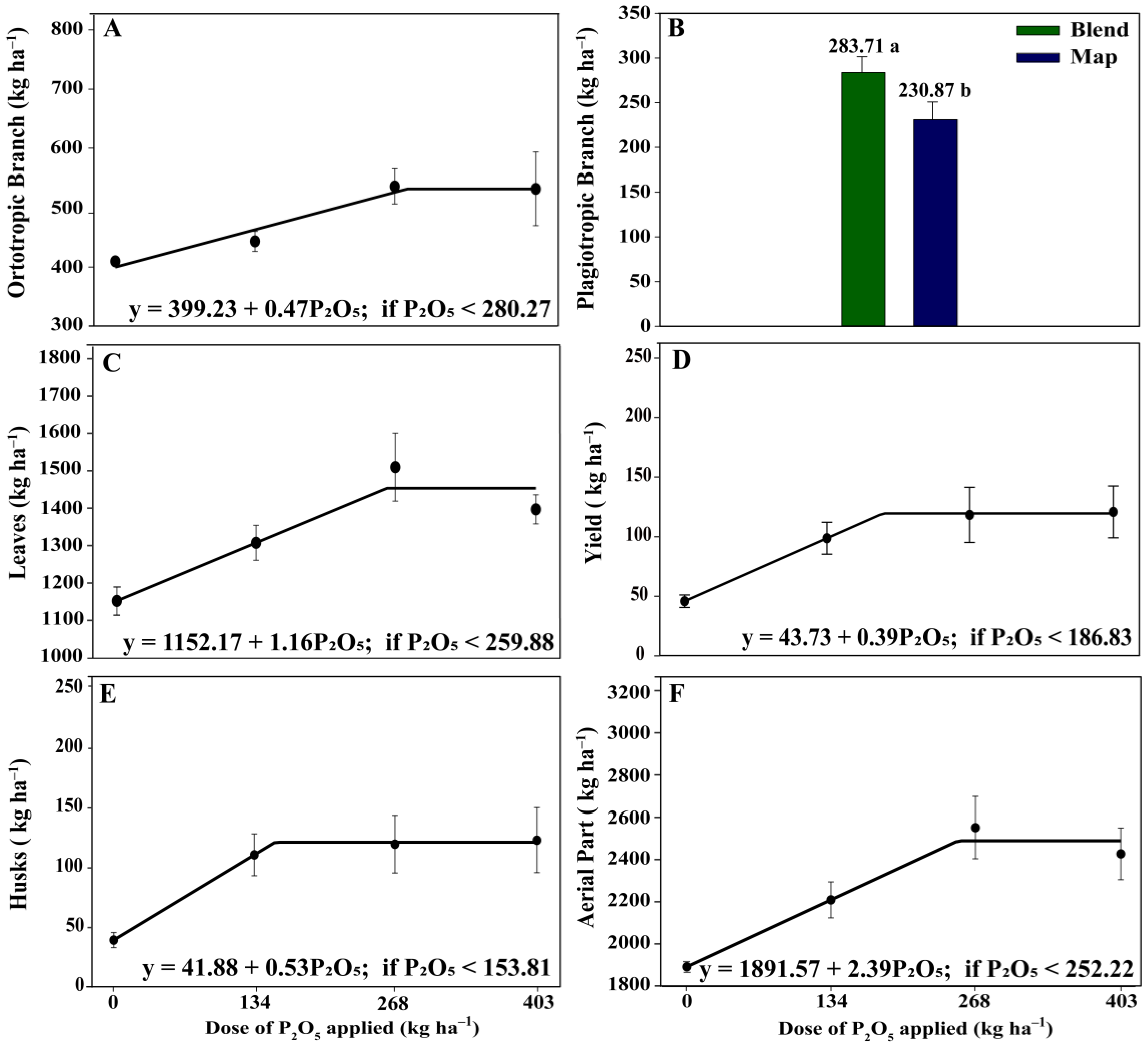
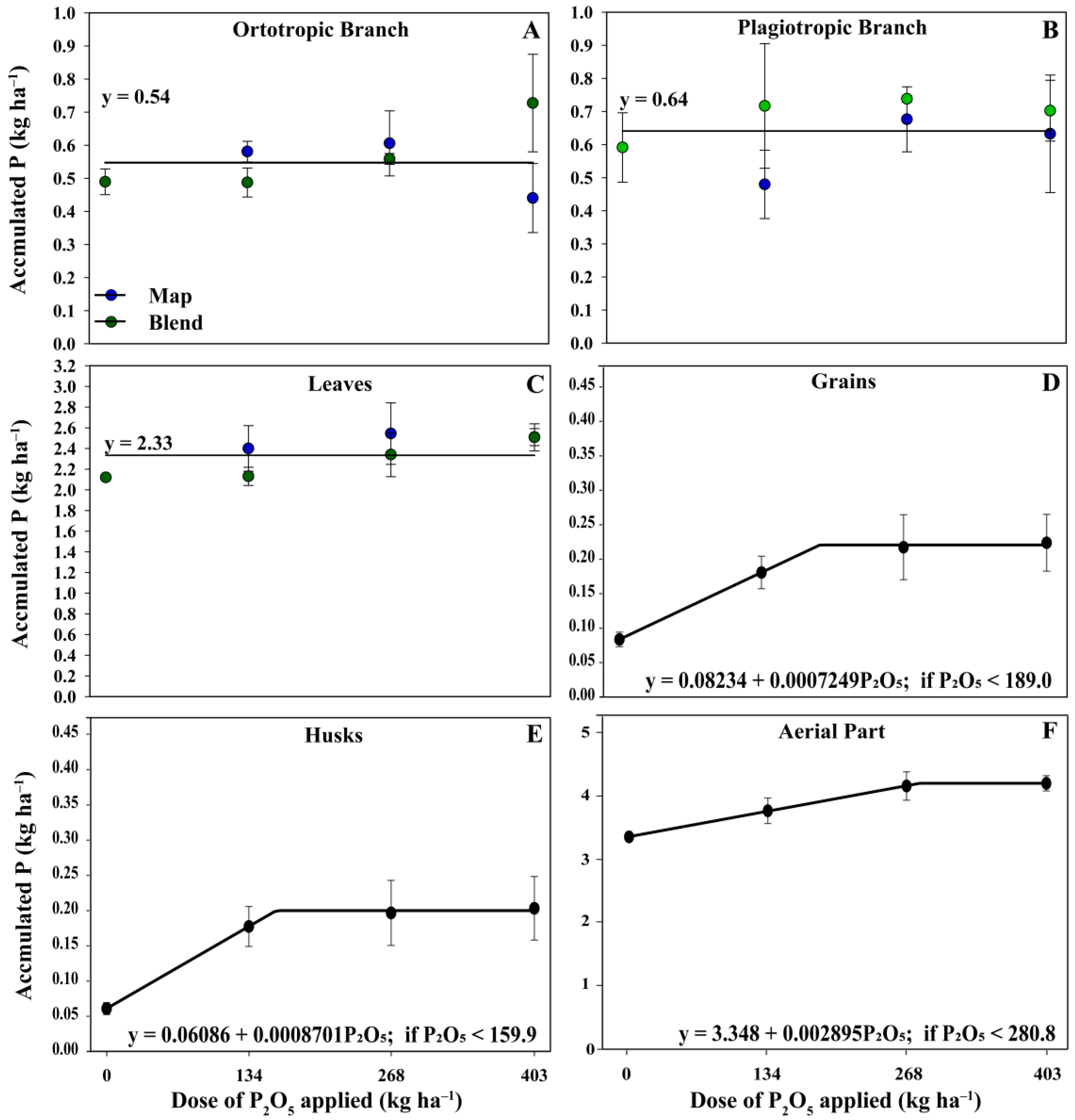
3.3. Yield, Biomass, and Nutrient Accumulation
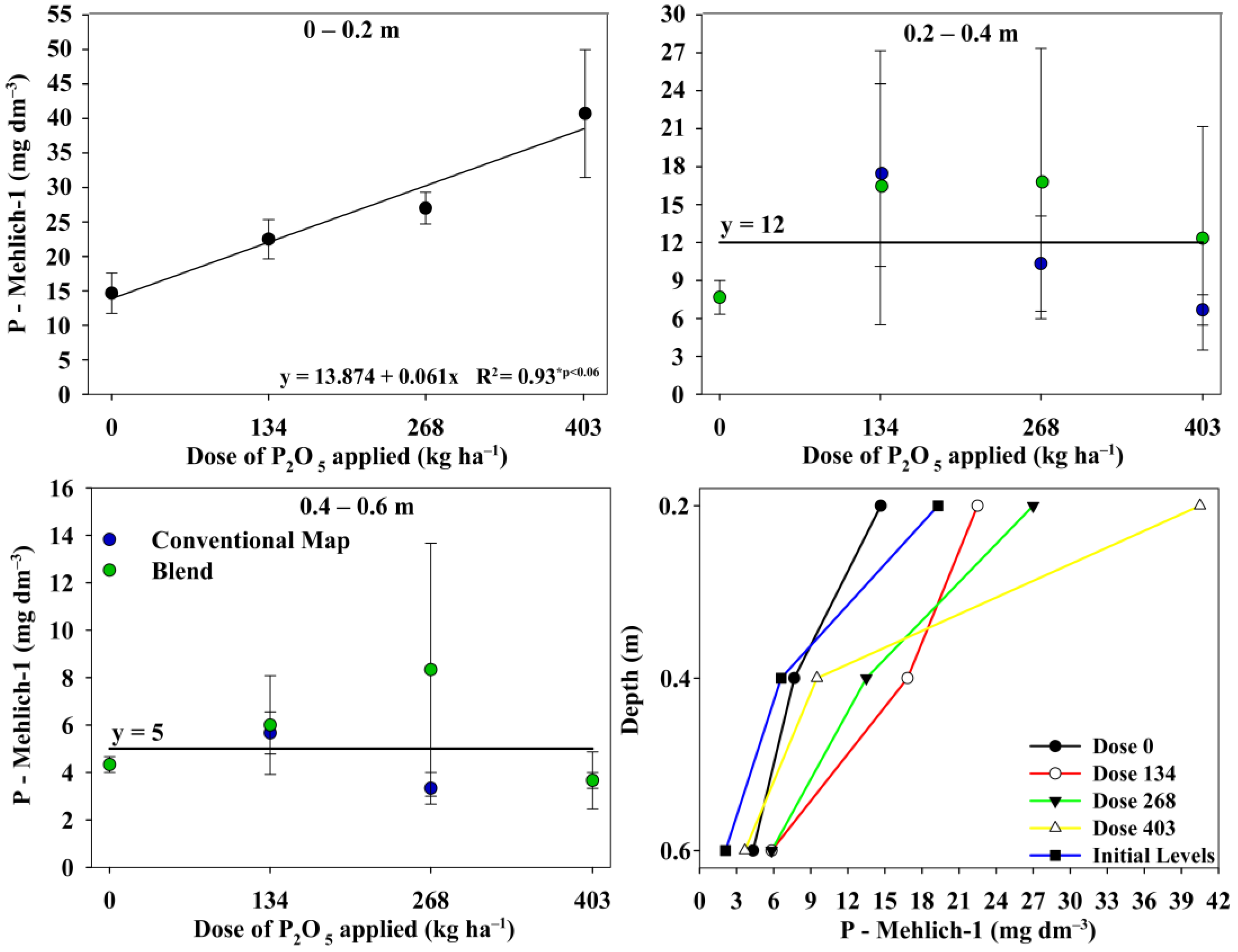
3.4. Available Phosphorus in Soil
4. Discussion
4.1. Nutrient Release Dynamics
4.2. Agronomic Performance of Coffee Plants Associated with the Application of Fertilizer Blends and Phosphorus Doses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DaMatta, F.M.; Martins, S.C.V.; Ramalho, J.D.C. Ecophysiology of Coffee Growth and Production in a Context of Climate Changes. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Guimarães, P.T.G.; Reis, T.H.P.; Guelfi, D.; Mattiello, E.M.; Montanari, M. Correção e adubação de solo em cafeeiros em produção–cultivo de sequeiro. In Cafeicultura do Cerrado; Carvalho, G.R., Ferreira, A.D., Andrade, V.T., Botelho, C.E., Carvalho, J.P.F., Eds.; Epamig: Belo Horizonte, Brazil, 2021; pp. 141–172. (In Portuguese) [Google Scholar]
- Vilela, D.J.M.; Sousa Coelho, L.; Ramos Guelfi Silva, D.; Rodrigues Carvalho, G.; Elias Botelho, C.; Dominghetti Ferreira, A. Nutritional Efficiency in Phosphorus of Arabica Coffee Genotypes. Coffee Sci. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Chagas, W.F.T.; Guelfi, D.R.; Emrich, E.B.; Silva, A.L.; Faquin, V. Agronomic Efficiency of Polymer-Coated Triple Superphosphate in Onion Cultivated in Contrasting Texture Soils. Rev. Cienc. Agron. 2016, 47, 439–446. [Google Scholar] [CrossRef][Green Version]
- Laviola, B.G.; Martinez, H.E.P.; de Souza, R.B.; Salomão, L.C.C.; Cruz, C.D. Macronutrient Accumulation in Coffee Fruits at Brazilian Zona Da Mata Conditions. J. Plant Nutr. 2009, 32, 980–995. [Google Scholar] [CrossRef]
- Araújo, F.H.V.; Cruz, R.d.S.; Porto, D.W.B.; Machado, C.M.M.; França, A.C. Effects of Mycorrhizal Association and Phosphate Fertilization on the Initial Growth of Coffee Plants. Pesqui. Agropecu. Trop. 2020, 50, e58646. [Google Scholar] [CrossRef]
- Pavinato, P.S.; Cherubin, M.R.; Soltangheisi, A.; Rocha, G.C.; Chadwick, D.R.; Jones, D.L. Revealing Soil Legacy Phosphorus to Promote Sustainable Agriculture in Brazil. Sci. Rep. 2020, 10, 15615. [Google Scholar] [CrossRef]
- Roy, E.D.; Richards, P.D.; Martinelli, L.A.; Coletta, L.D.; Lins, S.R.M.; Vazquez, F.F.; Willig, E.; Spera, S.A.; VanWey, L.K.; Porder, S. The Phosphorus Cost of Agricultural Intensification in the Tropics. Nat. Plants 2016, 2, 16043. [Google Scholar] [CrossRef]
- de Lima, T.M.; Weindorf, D.C.; Curi, N.; Guilherme, L.R.G.; Lana, R.M.Q.; Ribeiro, B.T. Elemental Analysis of Cerrado Agricultural Soils via Portable X-Ray Fluorescence Spectrometry: Inferences for Soil Fertility Assessment. Geoderma 2019, 353, 264–272. [Google Scholar] [CrossRef]
- Favarin, J.L.; Souza, L.T.; Mazzafera, P.; Dimenstein, L. Correção de solo e adubação do cafeeiro irrigado em produção. In Cafeicultura do Cerrado; Carvalho, G.R., Ferreira, A.D., Andrade, V.T., Botelho, C.E., Carvalho, J.P.F., Eds.; Epamig: Belo Horizonte, Brazil, 2021; pp. 173–208. (In Portuguese) [Google Scholar]
- dos Reis, R.A., Jr.; Pazzetti, G.; Guelfi, D.R. Enhanced Efficiency Phosphorus Fertilizer Impact on Physiological Characteristics of Coffee Seedlings. Adv. Biosci. Biotechnol. 2023, 14, 492–504. [Google Scholar] [CrossRef]
- Neto, A.P.; Favarin, J.L.; Hammond, J.P.; Tezotto, T.; Couto, H.T.Z. Analysis of Phosphorus Use Efficiency Traits in Coffea Genotypes Reveals Coffea Arabica and Coffea canephora Have Contrasting Phosphorus Uptake and Utilization Efficiencies. Front. Plant Sci. 2016, 7, 408. [Google Scholar] [CrossRef]
- Voltolini, G.B.; Vilela, M.S.; de Alecrim, A.O.; da Silva, L.C.; José Guimarães, R.; Netto, P.M.; Rezende, T.T. Techniques for Sustainable Coffee Crop Management: Impacts on Growth and Yield. Arch. Agron. Soil Sci. 2022, 69, 2413–2429. [Google Scholar] [CrossRef]
- Volf, M.R.; Rosolem, C.A. Soil P Diffusion and Availability Modified by Controlled-Release P Fertilizers. J. Soil Sci. Plant Nutr. 2021, 21, 162–172. [Google Scholar] [CrossRef]
- Sanchez, P.A. Properties and Management of Soils in the Tropics, 2nd ed.; Cambridge University Press (Virtual Publishing): Cambridge, UK, 2019; ISBN 9781316814802. [Google Scholar]
- Andrade, F.V.; Stauffer, E.; Siman, F.C.; de Sá Mendonça, E. Productivity and Nutrition of Conilon Coffee (Coffea canephora) in Response to Slow-Realease Fertilizers. Commun. Soil Sci. Plant Anal. 2024, 56, 800–810. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Casey, P.; Muster, T.; Gill, H.; Adhikari, B. Enhanced Efficiency Fertilisers: A Review of Formulation and Nutrient Release Patterns: Enhanced Efficiency Fertilizers. J. Sci. Food Agric. 2015, 95, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.M.C.; Nguyen, T.H.; Wang, S.-L.; Nguyen, A.D. Preparation of NPK Nanofertilizer Based on Chitosan Nanoparticles and Its Effect on Biophysical Characteristics and Growth of Coffee in Green House. Res. Chem. Intermed. 2019, 45, 51–63. [Google Scholar] [CrossRef]
- Embrapa. Sistema Brasileiro de Classificação de Solos, 15th ed.; Embrapa: Brasília, Brazil, 2018. (In Portuguese) [Google Scholar]
- MAPA. Manual de Métodos Analíticos Oficiais Para Fertilizantes e Corretivos; Secretaria de Defesa Agropecuária: Brasília, Brazil, 2017. (In Portuguese) [Google Scholar]
- Antunes, W.C.; Pompelli, M.F.; Carretero, D.M.; DaMatta, F.M. Allometric Models for Non-Destructive Leaf Area Estimation in Coffee (Coffea arabica and Coffea canephora). Ann. Appl. Biol. 2008, 153, 33–40. [Google Scholar] [CrossRef]
- Bremner, J.M.; Breitenbeck, G.A. A Simple Method for Determination of Ammonium in semimicro-Kjeldahl Analysis of Soils and Plant Materials Using a Block Digester. Commun. Soil Sci. Plant Anal. 1983, 14, 905–913. [Google Scholar] [CrossRef]
- Mehlich, A. Rapid Determination of Cation and Anion Exchange Properties and pHe of Soils. J. AOAC Int. 1953, 36, 445–457. [Google Scholar] [CrossRef][Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 15 June 2024).
- Pena, E.A.; Slate, E.H. Global Validation of Linear Model Assumptions. J. Am. Stat. Assoc. 2006, 101, 341–354. [Google Scholar] [CrossRef]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: An R Package for ANOVA and Experimental Designs. Appl. Math. 2014, 05, 2952–2958. [Google Scholar] [CrossRef]
- SigmaPlot, Version 12.5; Systat Software, Inc.: San Jose, CA, USA, 2013.
- ISO 21263:2017; Slow-Release Fertilizers-Determination of the Release of the Nutrients-Method for Coated Fertilizers. ISO: Geneva, Switzerland, 2017.
- Mattiello, E.M.; Pereira, M.G.; Zonta, E.; Mauri, J.; Matiello, J.D.; Meireles, P.G.; Silva, I.R. da Produção de Matéria Seca, Crescimento Radicular e Absorção de Cálcio, Fósforo e Alumínio Por Coffea canephora e Coffea Arabica Sob Influência Da Atividade Do Alumínio Em Solução. Rev. Bras. Cienc. Solo 2008, 32, 425–434. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Yi, D.; Low, S.; Goh, H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, H.; Tang, S.Y.; et al. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled Release Fertilizer: A Review on Developments, Applications and Potential in Agriculture. J. Control Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- da Silva, L.; Marchiori, P.E.R.; Maciel, C.P.; Machado, E.C.; Ribeiro, R.V. Fotossíntese, Relações Hídricas e Crescimento de Cafeeiros Jovens Em Relação à Disponibilidade de Fósforo. Pesqui. Agropecu. Bras. 2010, 45, 965–972. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Cakmak, I.; Coskun, D.; Kok, D.; Lambers, L.J.; Schjoerring, H.; White, J.K. Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Academic Press: Cambridge, MA, USA, 2023. [Google Scholar]
- DaMatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. Ecophysiology of Coffee Growth and Production. Braz. J. Plant Physiol. 2007, 19, 485–510. [Google Scholar] [CrossRef]
- do Amaral, J.A.T.; Rena, A.B.; Amaral, J.F.T. do Crescimento Vegetativo Sazonal Do Cafeeiro e Sua Relação Com Fotoperíodo, Frutificação, Resistência Estomática e Fotossíntese. Pesqui. Agropecu. Bras. 2006, 41, 377–384. [Google Scholar] [CrossRef]
- do Amaral, J.F.T.; Martinez, H.E.P.; Laviola, B.G.; Fernandes Filho, E.I.; Cruz, C.D. Eficiência de Utilização de Nutrientes Por Cultivares de Cafeeiro. Cienc. Rural 2011, 41, 621–629. [Google Scholar] [CrossRef]
- Fenilli, T.A.B.; Reichart, K.; Bacchi, O.O.S.; Trivelin, P.C.O.; Dourado-Neto, D. The 15N Isotope to Evaluate Fertilizer Nitrogen Absorption Efficiency by the Coffee Plant. An. Acad. Bras. Cienc. 2007, 79, 767–776. [Google Scholar] [CrossRef]
- Bruno, I.P.; Unkovich, M.J.; Bortolotto, R.P.; Bacchi, O.O.S.; Dourado-Neto, D.; Reichardt, K. Fertilizer Nitrogen in Fertigated Coffee Crop: Absorption Changes in Plant Compartments over Time. Field Crops Res. 2011, 124, 369–377. [Google Scholar] [CrossRef]
- Fenilli, T.A.B.; Reichardt, K.; Favarin, J.L.; Bacchi, O.O.S.; Silva, A.L.; Timm, L.C. Fertilizer 15N Balance in a Coffee Cropping System: A Case Study in Brazil. Rev. Bras. Cienc. Solo 2008, 32, 1459–1469. [Google Scholar] [CrossRef]



| Nutrients | Equations | R2 |
|---|---|---|
| Development | ||
| N | 0.97 | |
| P2O5 | 0.97 | |
| K2O | 0.97 | |
| Post-development | ||
| N | 0.99 | |
| P2O5 | 0.99 | |
| K2O | 0.99 | |
| Rates of P2O5 (kg ha−1) | Orthotropic Branch | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| kg ha−1 | g ha−1 | |||||||||
| N | K | Ca | Mg | S | B | Cu | Fe | Mn | Zn | |
| 0 | 4.66 | 4.54 | 1.60 | 0.41 | 0.23 | 10.96 | 8.60 | 60.11 | 5.71 | 4.95 |
| 143 | 4.65 | 3.18 | 1.53 | 0.46 | 0.22 | 8.64 | 9.75 | 74.39 | 5.81 | 5.34 |
| 268 | 5.04 | 3.92 | 1.75 | 0.49 | 0.24 | 9.75 | 11.68 | 68.87 | 8.90 | 5.84 |
| 403 | 5.83 | 3.83 | 1.58 | 0.42 | 0.23 | 10.52 | 11.20 | 76.82 | 7.39 | 5.70 |
| Plagiotropic Branch | ||||||||||
| 0 | 4.49 | 5.48 | 1.73 | 0.82 | 0.31 | 8.17 | 8.80 | 35.94 | 9.04 | 7.18 |
| 143 | 4.76 | 5.81 | 1.74 | 0.75 | 0.28 | 8.28 | 8.43 | 41.14 | 8.96 | 9.49 |
| 268 | 4.82 | 5.92 | 1.79 | 0.75 | 0.29 | 9.57 | 9.27 | 36.56 | 9.92 | 7.89 |
| 403 | 4.83 | 5.41 | 1.83 | 0.82 | 0.31 | 8.52 | 9.10 | 43.70 | 9.42 | 8.96 |
| Leaves | ||||||||||
| 0 | 36.02 | 24.40 | 14.58 | 4.04 | 2.64 | 94.80 | 38.62 | 338.31 | 132.29 | 20.39 |
| 143 | 38.78 | 29.86 | 16.28 | 4.43 | 3.00 | 128.11 | 43.30 | 452.41 | 115.33 | 24.48 |
| 268 | 43.47 | 29.79 | 17.93 | 4.95 | 2.83 | 132.03 | 45.33 | 353.22 | 124.66 | 24.40 |
| 403 | 43.53 | 31.52 | 17.05 | 4.80 | 2.74 | 130.44 | 43.29 | 471.06 | 95.98 | 24.43 |
| Husks | ||||||||||
| 0 | 0.78 | 0.98 | 0.14 | 0.04 | 0.07 | 1.78 | 0.94 | 15.98 | 0.71 | 0.58 |
| 143 | 2.12 | 2.68 | 0.33 | 0.12 | 0.18 | 5.36 | 2.65 | 52.08 | 1.81 | 1.78 |
| 268 | 2.33 | 3.15 | 0.36 | 0.13 | 0.20 | 6.09 | 2.63 | 59.48 | 1.98 | 1.91 |
| 403 | 2.67 | 3.25 | 0.36 | 0.14 | 0.21 | 5.28 | 2.84 | 56.57 | 2.01 | 2.47 |
| Beans | ||||||||||
| 0 | 1.13 | 0.77 | 0.04 | 0.11 | 0.07 | 0.73 | 0.87 | 3.86 | 0.89 | 0.47 |
| 143 | 2.78 | 1.70 | 0.09 | 0.23 | 0.16 | 2.12 | 1.84 | 7.53 | 1.71 | 0.87 |
| 268 | 3.25 | 2.08 | 0.11 | 0.28 | 0.20 | 2.24 | 2.10 | 9.21 | 2.14 | 1.09 |
| 403 | 3.40 | 2.13 | 0.10 | 0.29 | 0.21 | 1.75 | 2.20 | 8.85 | 2.12 | 1.03 |
| Aerial Parts | ||||||||||
| 0 | 47.07 | 36.16 | 18.09 | 5.43 | 3.32 | 116.45 | 57.83 | 454.20 | 148.64 | 33.58 |
| 143 | 53.09 | 43.23 | 19.98 | 5.99 | 3.84 | 152.50 | 65.96 | 627.56 | 133.61 | 41.96 |
| 268 | 58.91 | 44.86 | 21.94 | 6.60 | 3.77 | 159.68 | 71.01 | 527.34 | 147.60 | 41.12 |
| 403 | 60.26 | 46.14 | 20.91 | 6.47 | 3.70 | 156.51 | 68.63 | 657.00 | 116.91 | 42.59 |
| Mean | 54.83 | 42.60 | 20.23 | 6.12 | 3.66 | 146.28 | 65.86 | 566.53 | 136.69 | 39.81 |
| Structure | Proportion of the Nutrients in the Aerial Parts (%) | |||||||||
| Orthotropic | 9.20 | 9.08 | 7.99 | 7.31 | 6.29 | 6.81 | 15.65 | 12.36 | 5.08 | 13.70 |
| Plagiotropic | 8.62 | 13.28 | 8.76 | 12.81 | 8.17 | 5.90 | 13.51 | 6.94 | 6.83 | 21.05 |
| Leaves | 73.77 | 67.83 | 81.36 | 74.39 | 76.67 | 82.95 | 64.74 | 71.27 | 85.64 | 58.84 |
| Husk | 3.60 | 5.90 | 1.47 | 1.78 | 4.48 | 3.16 | 3.44 | 8.13 | 1.19 | 4.23 |
| Beans | 4.81 | 3.92 | 0.42 | 3.71 | 4.39 | 1.17 | 2.66 | 1.30 | 1.25 | 2.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutra, M.P.; Sarkis, L.F.; Oliveira, D.P.; Santiago, H.d.A.; Resende, G.T.d.S.; de Melo, M.E.A.; da Fonseca, A.B.; López, C.J.H.; Silva, E.d.S.; Zaqueu, A.d.S.; et al. Cutting-Edge Technology Using Blended Controlled-Release Fertilizers and Conventional Monoammonium Phosphate as a Strategy to Improve Phosphorus Coffee Nutrition During the Coffee Development Phase. Soil Syst. 2025, 9, 47. https://doi.org/10.3390/soilsystems9020047
Dutra MP, Sarkis LF, Oliveira DP, Santiago HdA, Resende GTdS, de Melo MEA, da Fonseca AB, López CJH, Silva EdS, Zaqueu AdS, et al. Cutting-Edge Technology Using Blended Controlled-Release Fertilizers and Conventional Monoammonium Phosphate as a Strategy to Improve Phosphorus Coffee Nutrition During the Coffee Development Phase. Soil Systems. 2025; 9(2):47. https://doi.org/10.3390/soilsystems9020047
Chicago/Turabian StyleDutra, Mateus Portes, Leonardo Fernandes Sarkis, Damiany Pádua Oliveira, Hugo de Almeida Santiago, Gustavo Tadeu de Sousa Resende, Maria Elisa Araújo de Melo, Adrianne Braga da Fonseca, Cristhian José Hernández López, Euler dos Santos Silva, Aline dos Santos Zaqueu, and et al. 2025. "Cutting-Edge Technology Using Blended Controlled-Release Fertilizers and Conventional Monoammonium Phosphate as a Strategy to Improve Phosphorus Coffee Nutrition During the Coffee Development Phase" Soil Systems 9, no. 2: 47. https://doi.org/10.3390/soilsystems9020047
APA StyleDutra, M. P., Sarkis, L. F., Oliveira, D. P., Santiago, H. d. A., Resende, G. T. d. S., de Melo, M. E. A., da Fonseca, A. B., López, C. J. H., Silva, E. d. S., Zaqueu, A. d. S., de Lima, G. H. F., Silva, J. M., Pozza, A. A. A., & Guelfi, D. (2025). Cutting-Edge Technology Using Blended Controlled-Release Fertilizers and Conventional Monoammonium Phosphate as a Strategy to Improve Phosphorus Coffee Nutrition During the Coffee Development Phase. Soil Systems, 9(2), 47. https://doi.org/10.3390/soilsystems9020047










