Conservation Reserve Program Soils Show Potential as a Soil Health Benchmark—A Southern Minnesota Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling and Processing
2.3. Soil Mangement Assessment Framework
2.4. Statistical Analysis
3. Results and Discussion
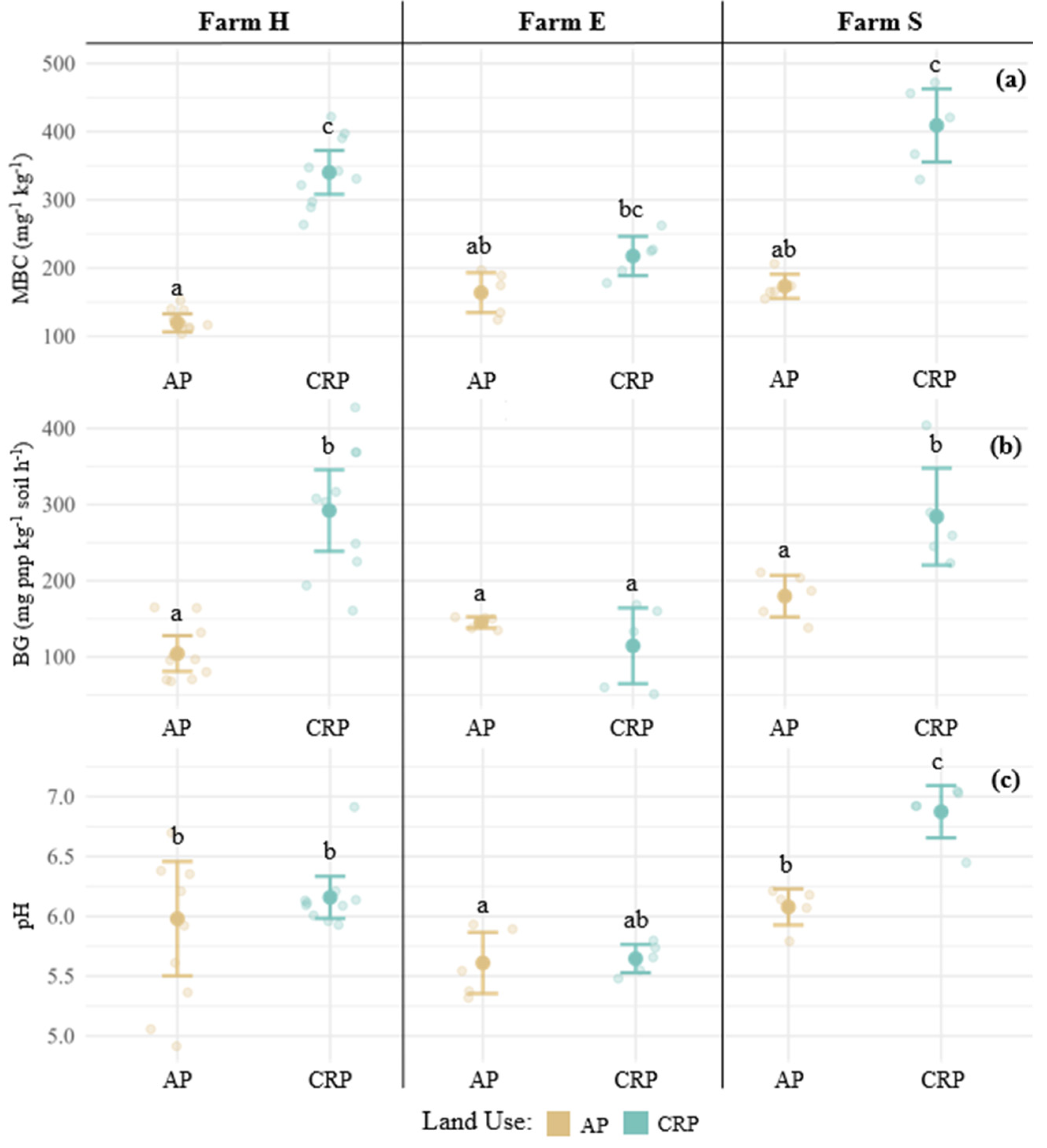
3.1. Soil Management Assessment Framework Indicators
3.2. Soil Management Assessment Framework Scores
3.3. The Soil Health Gap: AP vs. CRP
3.4. Individual Farm Pairs—Soil Health Comparison
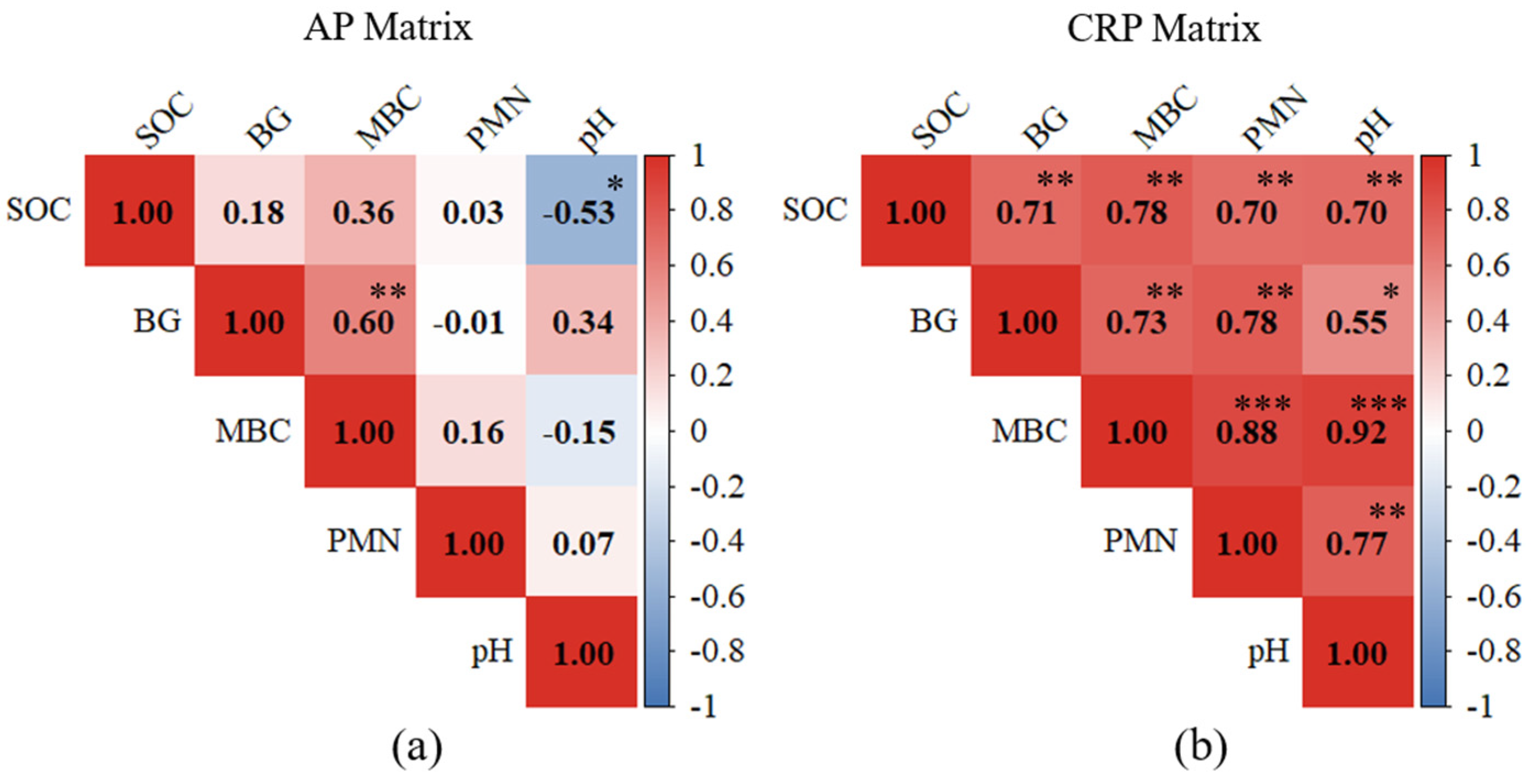
3.5. Effect of pH and Tillage on Biological Soil Health Indicators
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMAF | Soil Management Assessment Framework |
| SHG | Soil health gap |
| CRP | Conservation Reserve Program |
| SHI | Soil health index |
| AP | Agricultural production |
References
- Jain, H.K. Transition to Twenty-First Century Agriculture: Change of Direction. Agric. Res. 2012, 1, 12–17. [Google Scholar] [CrossRef]
- Karlen, D.L.; Veum, K.S.; Sudduth, K.A.; Obrycki, J.F.; Nunes, M.R. Soil Health Assessment: Past Accomplishments, Current Activities, and Future Opportunities. Soil Tillage Res. 2019, 195, 104365. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The Concept and Future Prospects of Soil Health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Wood, S.A.; Bueno De Mesquita, C.P. How Microbes Can, and Cannot, Be Used to Assess Soil Health. Soil Biol. Biochem. 2021, 153, 108111. [Google Scholar] [CrossRef]
- Wade, J.; Culman, S.W.; Gasch, C.K.; Lazcano, C.; Maltais-Landry, G.; Margenot, A.J.; Martin, T.K.; Potter, T.S.; Roper, W.R.; Ruark, M.D.; et al. Rigorous, Empirical, and Quantitative: A Proposed Pipeline for Soil Health Assessments. Soil Biol. Biochem. 2022, 170, 108710. [Google Scholar] [CrossRef]
- Norris, C.E.; Bean, G.M.; Cappellazzi, S.B.; Cope, M.; Greub, K.L.H.; Liptzin, D.; Rieke, E.L.; Tracy, P.W.; Morgan, C.L.S.; Honeycutt, C.W. Introducing the North American Project to Evaluate Soil Health Measurements. Agron. J. 2020, 112, 3195–3215. [Google Scholar] [CrossRef]
- USDA-NRCS Soil Health Page. Available online: https://www.nrcs.usda.gov/conservation-basics/natural-resource-concerns/soils/soil-health (accessed on 7 May 2025).
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The Soil Management Assessment Framework: A Quantitative Soil Quality Evaluation Method. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Apesteguía, M.; Virto, I.; Orcaray, L.; Bescansa, P.; Enrique, A.; Imaz, M.J.; Karlen, D.L. Tillage Effects on Soil Quality after Three Years of Irrigation in Northern Spain. Sustainability 2017, 9, 1476. [Google Scholar] [CrossRef]
- Nunes, M.R.; Karlen, D.L.; Veum, K.S.; Moorman, T.B. A SMAF Assessment of U.S. Tillage and Crop Management Strategies. Environ. Sustain. Indic. 2020, 8, 100072. [Google Scholar] [CrossRef]
- Jiménez, L.; Jiménez, W.; Felicito, D.; Fierro, N.; Quichimbo, P.; Sánchez, D.; Capa-Mora, D. Rediscovering the edaphic knowledgee of smallholder farmers in southern Ecuador. Geoderma 2022, 406, 115468. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Karlen, D.L.; Franco, A.L.C.; Cerri, C.E.P.; Tormena, C.A.; Cerri, C.C. A soil management assessment framework (SMAF) evaluation of sugarcane expansion on soil quality. Soil Sci. Soc. Am. J. 2016, 80, 215–226. [Google Scholar] [CrossRef]
- Shawver, C.; Ippolito, J.A.; Brummer, J.; Ahola, J.; Rhoades, R. Soil health changes following transition from an annual cropping to perennial management-intensive grazing agroecosystem. Agro. Geosci. Environ. 2021, 4, e20181. [Google Scholar] [CrossRef]
- Trimarco, T.; Brummer, J.E.; Buchanan, C.; Ippolito, J.A. Tracking Soil Health Changes in a Management-Intensive Grazing Agroecosystem. Soil Syst. 2023, 7, 94. [Google Scholar] [CrossRef]
- Maharjan, B.; Das, S.; Acharya, B.S. Soil Health Gap: A Concept to Establish a Benchmark for Soil Health Management. Glob. Ecol. Conserv. 2020, 23, e01116. [Google Scholar] [CrossRef]
- Stubbs, M. Conservation Reserve Program (CRP): Status and Issues; Library of Congress, Congressional Research Service: Washington, DC, USA, 2014. [Google Scholar]
- Karlen, D.L.; Rosek, M.J.; Gardner, J.C.; Allan, D.L.; Alms, M.J.; Bezdicek, D.F.; Flock, M.; Huggins, D.R.; Miller, B.S.; Staben, M.L. Conservation Reserve Program effects on soil quality indicators. J. Soil Water Conserv. 1999, 54, 439–444. [Google Scholar] [CrossRef]
- Johnson, K.A.; Dalzell, B.J.; Donahue, M.; Gourevitch, J.; Johnson, D.L.; Karlovits, G.S.; Keeler, B.; Smith, J.T. Conservation Reserve Program (CRP) lands provide ecosystem service benefits that exceed land rental payment costs. Ecosys. Serv. 2016, 18, 175–185. [Google Scholar] [CrossRef]
- Dunn, C.P.; Stearns, F.; Guntenspergen, G.R.; Sharpe, D.M. Ecological benefits of the Conservation Reserve Program. Conserv. Biol. 1993, 7, 132–139. [Google Scholar] [CrossRef]
- GLISA Albert Lea Page. Available online: https://glisa.umich.edu/station/albert-lea-3-se/ (accessed on 7 May 2025).
- Faribault County Minnesota; County Profile; 2022 Census of Agriculture; USDA-NASS: Washington, DC, USA, 2022. Available online: https://www.nass.usda.gov/Publications/AgCensus/2022/Online_Resources/County_Profiles/Minnesota/cp27043.pdf (accessed on 5 May 2025).
- Ashworth, J.; Keyes, D.; Kirk, R.; Lessard, R. Standard procedure in the hydrometer method for particle size analysis. Commun. Soil Sci. Plant Anal. 2001, 32, 633–642. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate Stability and Size Distribution. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; SSSA Book Series; Klute, A., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and Soil Acidity. In Methods of Soil Analysis: Part 3 Chemical Methods; SSSA Book Series; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar] [CrossRef]
- Rhoades, J.D. Salinity: Electrical Conductivity and Total Dissolved Solids. In Methods of Soil Analysis: Part 3 Chemical Methods; SSSA Book Series; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 Soil Test Extractant: A Modification of Mehlich 2 Extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Green, V.; Stott, D.; Cruz, J.; Curi, N. Tillage Impacts on Soil Biological Activity and Aggregation in a Brazilian Cerrado Oxisol. Soil Tillage Res. 2007, 92, 114–121. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen-Inorganic Forms. In Methods of Soil Analysis: Part 3 Chemical Methods; SSSA Book Series; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis: Part 3 Chemical Methods; SSSA Book Series; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Sherrod, L.A.; Dunn, G.; Peterson, G.A.; Kolberg, R.L. Inorganic Carbon Analysis by Modified Pressure-Calcimeter Method. Soil Sci. Soc. Am. J. 2002, 66, 299–305. [Google Scholar] [CrossRef]
- Beck, T.; Joergensen, R.G.; Kandeler, E.; Makeschin, F.; Nuss, E.; Oberholzer, H.R.; Scheu, S. An Inter-Laboratory Comparison of Ten Different Ways of Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1997, 29, 1023–1032. [Google Scholar] [CrossRef]
- Stott, D.E.; Andrews, S.S.; Liebig, M.A.; Wienhold, B.J.; Karlen, D.L. Evaluation of β-Glucosidase Activity as a Soil Quality Indicator for the Soil Management Assessment Framework. Soil Sci. Soc. Am. J. 2010, 74, 107–119. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Ducey, T.F.; Diaz, K.; Barbarick, K.A. Long-Term Biosolids Land Application Influences Soil Health. Sci. Total Environ. 2021, 791, 148344. [Google Scholar] [CrossRef]
- Li, C.; Fultz, L.M.; Moore-Kucera, J.; Acosta-Martínez, V.; Kakarla, M.; Weindorf, D.C. Soil Microbial Community Restoration in Conservation Reserve Program Semi-Arid Grasslands. Soil Biol. Biochem. 2018, 118, 166–177. [Google Scholar] [CrossRef]
- Tyler, H.L.; Locke, M.A.; Moore, M.T.; Steinriede, R.W. Impact of Conservation Land Management Practices on Soil Microbial Function in an Agricultural Watershed. J. Soil Water Conserv. 2016, 71, 396–403. [Google Scholar] [CrossRef]
- De, M.; Riopel, J.A.; Cihacek, L.J.; Lawrinenko, M.; Baldwin-Kordick, R.; Hall, S.J.; McDaniel, M.D. Soil Health Recovery after Grassland Reestablishment on Cropland: The Effects of Time and Topographic Position. Soil Sci. Soc. Am. J. 2020, 84, 568–586. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Some Thoughts on the Biogeochemical Cycling of Potassium in Terrestrial Ecosystems. Biogeochemistry 2021, 154, 427–432. [Google Scholar] [CrossRef]
- Schoenau, J.J.; Stewart, J.W.B.; Bettany, J.R. Forms and Cycling of Phosphorus in Prairie and Boreal Forest Soils. Biogeochemistry 1989, 8, 223–237. [Google Scholar] [CrossRef]
- Kasper, M.; Buchan, G.D.; Mentler, A.; Blum, W.E.H. Influence of Soil Tillage Systems on Aggregate Stability and the Distribution of C and N in Different Aggregate Fractions. Soil Tillage Res. 2009, 105, 192–199. [Google Scholar] [CrossRef]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Kim, N.; Zabaloy, M.C.; Guan, K.; Villamil, M.B. Do Cover Crops Benefit Soil Microbiome? A Meta-Analysis of Current Research. Soil Biol. Biochem. 2020, 142, 107701. [Google Scholar] [CrossRef]
- Zuber, S.M.; Villamil, M.B. Meta-Analysis Approach to Assess Effect of Tillage on Microbial Biomass and Enzyme Activities. Soil Biol. Biochem. 2016, 97, 176–187. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil pH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Stegen, J.C.; Kim, M.; Dong, K.; Adams, J.M.; Lee, Y.K. Soil pH Mediates the Balance between Stochastic and Deterministic Assembly of Bacteria. ISME J. 2018, 12, 1072–1083. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Tabatabai, M.A. Enzyme Activities in a Limed Agricultural Soil. Biol. Fertil. Soils 2000, 31, 85–91. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; Van Agtmaal, M.; et al. Land Use Driven Change in Soil pH Affects Microbial Carbon Cycling Processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A Global Atlas of the Dominant Bacteria Found in Soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Stott, D.E. Recommended Soil Health Indicators and Associated Laboratory Procedures; Soil Health Technical Note 450-03; USDA-NRCS: Washington, DC, USA, 2019. Available online: https://www.naptprogram.org/files/napt/publications/method-papers/2019-nrcs-technote-450-03.pdf (accessed on 7 May 2025).
- Nunes, M.R.; Veum, K.S.; Parker, P.A.; Holan, S.H.; Amsili, J.P.; Van Es, H.M.; Wills, S.A.; Seybold, C.A.; Karlen, D.L. SHAPEv1.0 Scoring Curves and Peer Group Benchmarks for Dynamic Soil Health Indicators. Soil Sci. Soc. Am. J. 2024, 88, 858–875. [Google Scholar] [CrossRef]
- Nunes, M.R.; Veum, K.S.; Parker, P.A.; Holan, S.H.; Karlen, D.L.; Amsili, J.P.; Van Es, H.M.; Wills, S.A.; Seybold, C.A.; Moorman, T.B. The Soil Health Assessment Protocol and Evaluation Applied to Soil Organic Carbon. Soil Sci. Soc. Am. J. 2021, 85, 1196–1213. [Google Scholar] [CrossRef]
| Descriptor | Farm H | Farm E | Farm S |
|---|---|---|---|
| AP- | |||
| Longitude | −93.860312 | −93.942425 | −94.071673 |
| Latitude | 43.581294 | 43.639020 | 43.635242 |
| Dominant Soil Series | Spillville Loam | Truman Silt Loam | Shorewood Silty Clay Loam |
| World Reference Base Soil Group | Cumulic Phaeozem | Calcaric Phaeozem | Stagnc Luvic Stagnosol |
| Measured Texture | Clay Loam | Clay Loam | Clay |
| Size (ha) | 6.7 | 6.0 | 5.0 |
| Crop at Sampling | Soybean | Corn | Cover Crop |
| Tillage Level | Aggressive | Minimal | None |
| a Soil Health Principles Utilized | None | 1, 2, 3, and 4 | 1, 2, 3, and 4 |
| Soil Health Practices Implemented | None | Reduced tillage and cover crops | No-till, cover crops, third crop, and manure |
| Crop Rotation | b C-C | c C-S | d C-S-P |
| CRP- | |||
| Latitude | −93.865420 | −93.941628 | −94.074464 |
| Longitude | 43.581724 | 43.636721 | 43.635062 |
| Dominant Soil Series | Spillville Loam | Truman Silt Loam | Coland Clay Loam |
| Measured Soil Texture | Clay Loam | Sandy Clay Loam | Clay |
| World Reference Base Soil Group | Cumulic Phaeozem | Calcaric Phaeozem | Gleyic Cumulic Phaeozem |
| Size (ha) | 3.6 | 5.3 | 3.6 |
| CRP Type | CRP | CREP | CREP |
| Establishment Year | 2001 | 2001 | 2001 |
| Seeding Mix | e MN CP25 Standard (643) | e MN CP25 Standard (643) | e MN CP25 Standard (643) |
| Land Use | Farm | Physical Indicators | Chemical Indicators | Nutrient Indicators | Biological Indicators | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clay % | Bd g cm−1 | WSA % | pH | EC dS m−1 | M-3P mg kg −1 | M-3K mg kg −1 | SOC % | MBC mg kg −1 | PMN mg kg −1 | BG mg pnp a kg−1 soil h−1 | ||
| AP | H | 30 (2) a | 1.36 (0.04) b | 67 (3) a | 6.0 (0.2) | 0.07 (0.02) a | 34 (5) a | 101 (24) a | 2.48 (0.17) a | 120 (7) a | 37 (2) | 104 (12) a |
| E | 29 (2) a | 1.23 (0.02) b | 83 (2) b | 5.6 (0.1) | 0.36 (0.05) b | 55 (4) ab | 232 (22) ab | 3.16 (0.21) b | 164 (15) b | 38 (3) | 145 (4) ab | |
| S | 46 (2) b | 1.03 (0.04) a | 83 (1) b | 6.1 (0.1) | 0.32 (0.04) b | 92 (23) b | 459 (168) b | 2.98 (0.10) ab | 173 (9) b | 40 (2) | 180 (14) b | |
| p-value | 0.004 | 0.002 | 0.005 | 0.250 | <0.001 | 0.030 | 0.004 | 0.040 | 0.005 | 0.530 | 0.010 | |
| CRP | H | 31 (1) a | 1.18 (0.02) | 90 (1) | 6.2 (0.1) b | 0.09 (0.02) b | 29 (4) | 126 (10) | 3.41 (0.16) a | 340 (16) b | 67 (5) b | 292 (27) b |
| E | 28 (2) a | 1.21 (0.01) | 92 (1) | 5.7 (0.1) a | 0.03 (0.00) a | 19 (2) | 201 (31) | 2.96 (0.26) a | 218 (14) a | 35 (3) a | 115 (25) a | |
| S | 41 (3) b | 1.16 (0.06) | 90 (1) | 6.9 (0.1) c | 0.20 (0.05) c | 43 (9) | 229 (38) | 4.24 (0.36) b | 409 (27) b | 72 (7) b | 284 (32) b | |
| p-value | 0.040 | 0.500 | 0.230 | <0.001 | <0.001 | 0.060 | 0.060 | 0.020 | 0.002 | 0.004 | 0.006 | |
| AP | 34 (2) | 1.25 (0.04) | 75 (2) | 5.9 (0.1) | 0.21 (0.04) | 54 (8) | 223 (53) | 2.77 (0.12) | 144 (8) | 38 (1) | 133 (10) | |
| CRP | 33 (1) | 1.18 (0.02) | 90 (0.5) | 6.2 (0.1) | 0.10 (0.02) | 30 (3) | 170 (16) | 3.51 (0.17) | 327 (19) | 60 (4) | 246 (24) | |
| p-value | 0.650 | 0.080 | <0.001 | 0.150 | 0.030 | 0.010 | 0.780 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Land Use | Farm | Physical Indicators | Chemical Indicators | Nutrient Indicators | Biological Indicators | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bd | WSA | pH | EC | Plant-Available P | Plant-Available K | SOC | MBC | PMN | BG | ||
| AP | H | 0.55 (0.07) a | 1.00 (0.00) | 0.93 (0.02) | 1.00 (0.00) | 0.92 (0.05) | 0.69 (0.05) a | 0.50 (0.05) a | 0.12 (0.01) a | 1.00 (0.00) | 0.09 (0.01) a |
| E | 0.80 (0.07) ab | 1.00 (0.00) | 0.94 (0.02) | 1.00 (0.00) | 1.00 (0.00) | 0.97 (0.03) b | 0.74 (0.04) b | 0.22 (0.05) ab | 1.00 (0.00) | 0.13 (0.01) ab | |
| S | 0.97 (0.03) b | 1.00 (0.00) | 0.99 (0.01) | 1.00 (0.00) | 1.00 (0.00) | 0.98 (0.02) b | 0.66 (0.03) ab | 0.20 (0.02) b | 1.00 (0.00) | 0.19 (0.03) b | |
| p-value | 0.005 | N/A | 0.120 | N/A | 0.190 | 0.004 | 0.020 | 0.010 | N/A | 0.010 | |
| CRP | H | 0.82 (0.04) | 1.00 (0.00) | 0.99 (0.00) b | 1.00 (0.00) | 0.93 (0.05) | 0.86 (0.03) | 0.77 (0.04) a | 0.67 (0.04) b | 1.00 (0.00) | 0.51 (0.08) b |
| E | 0.91 (0.05) | 1.00 (0.00) | 0.97 (0.01) a | 1.00 (0.00) | 0.87 (0.08) | 0.89 (0.05) | 0.74 (0.06) a | 0.43 (0.04) a | 1.00 (0.00) | 0.11 (0.03) a | |
| S | 0.82 (0.07) | 1.00 (0.00) | 0.97 (0.01) a | 1.00 (0.00) | 0.93 (0.07) | 0.95 (0.05) | 0.91 (0.03) b | 0.82 (0.05) b | 1.00 (0.00) | 0.47 (0.09) b | |
| p-value | 0.440 | N/A | 0.004 | N/A | 0.760 | 0.170 | 0.040 | 0.004 | N/A | 0.006 | |
| AP | 0.71 (0.06) | 1.00 (0.00) | 0.95 (0.01) | 1.00 (0.00) | 0.96 (0.02) | 0.83 (0.04) | 0.60 (0.04) | 0.16 (0.02) | 1.00 (0.00) | 0.12 (0.01) | |
| CRP | 0.84 (0.03) | 1.00 (0.00) | 0.98 (0.00) | 1.00 (0.00) | 0.92 (0.04) | 0.89 (0.02) | 0.80 (0.03) | 0.65 (0.04) | 1.00 (0.00) | 0.40 (0.06) | |
| p-value | 0.120 | N/A | 0.170 | N/A | 0.420 | 0.610 | <0.001 | <0.001 | N/A | <0.001 | |
| Land Use | Farm | Physical SHI | Chemical SHI | Nutrient SHI | Biological SHI | Overall SHI |
|---|---|---|---|---|---|---|
| AP | H | 0.77 (0.03) a | 0.96 (0.01) | 0.80 (0.04) a | 0.43 (0.01) a | 0.68 (0.01) a |
| E | 0.90 (0.04) ab | 0.97 (0.01) | 0.98 (0.02) b | 0.52 (0.01) b | 0.78 (0.01) b | |
| S | 0.98 (0.01) b | 1.00 (0.00) | 0.99 (0.01) b | 0.51 (0.01) b | 0.80 (0.01) b | |
| p-value | 0.004 | 0.120 | 0.004 | 0.001 | <0.001 | |
| CRP | H | 0.91 (0.02) | 1.00 (0.00) b | 0.89 (0.04) | 0.74 (0.04) b | 0.86 (0.02) b |
| E | 0.96 (0.03) | 0.99 (0.00) a | 0.88 (0.06) | 0.57 (0.03) a | 0.79 (0.01) a | |
| S | 0.91 (0.04) | 0.98 (0.00) a | 0.94 (0.06) | 0.80 (0.04) b | 0.89 (0.03) b | |
| p-value | 0.440 | 0.004 | 0.140 | 0.020 | 0.040 | |
| AP | All | 0.86 (0.03) | 0.97 (0.01) | 0.90 (0.03) | 0.47 (0.01) | 0.73 (0.01) |
| CRP | All | 0.92 (0.02) | 0.99 (0.00) | 0.90 (0.03) | 0.71 (0.03) | 0.85 (0.01) |
| p-value | 0.120 | 0.170 | 0.860 | <0.001 | <0.001 | |
| Soil Health Gap | H | 0.14 | 0.04 | 0.09 | 0.31 | 0.18 |
| E | 0.06 | 0.02 | −0.10 | 0.05 | 0.01 | |
| S | −0.07 | −0.02 | −0.05 | 0.29 | 0.09 | |
| All | 0.06 | 0.02 | 0.00 | 0.24 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffman, O.; Chorpenning, C.E.; Trimarco, T.; Carr, N.; Buchanan, C.; Ippolito, J.A. Conservation Reserve Program Soils Show Potential as a Soil Health Benchmark—A Southern Minnesota Case Study. Soil Syst. 2025, 9, 46. https://doi.org/10.3390/soilsystems9020046
Hoffman O, Chorpenning CE, Trimarco T, Carr N, Buchanan C, Ippolito JA. Conservation Reserve Program Soils Show Potential as a Soil Health Benchmark—A Southern Minnesota Case Study. Soil Systems. 2025; 9(2):46. https://doi.org/10.3390/soilsystems9020046
Chicago/Turabian StyleHoffman, Oliver, Christopher E. Chorpenning, Tad Trimarco, Nathan Carr, Cassidy Buchanan, and James A. Ippolito. 2025. "Conservation Reserve Program Soils Show Potential as a Soil Health Benchmark—A Southern Minnesota Case Study" Soil Systems 9, no. 2: 46. https://doi.org/10.3390/soilsystems9020046
APA StyleHoffman, O., Chorpenning, C. E., Trimarco, T., Carr, N., Buchanan, C., & Ippolito, J. A. (2025). Conservation Reserve Program Soils Show Potential as a Soil Health Benchmark—A Southern Minnesota Case Study. Soil Systems, 9(2), 46. https://doi.org/10.3390/soilsystems9020046







