Spatiotemporal Variations Affect DTPA-Extractable Heavy Metals in Coastal Salt-Affected Soils of Arid Regions
Abstract
1. Introduction
2. Materials and Methods
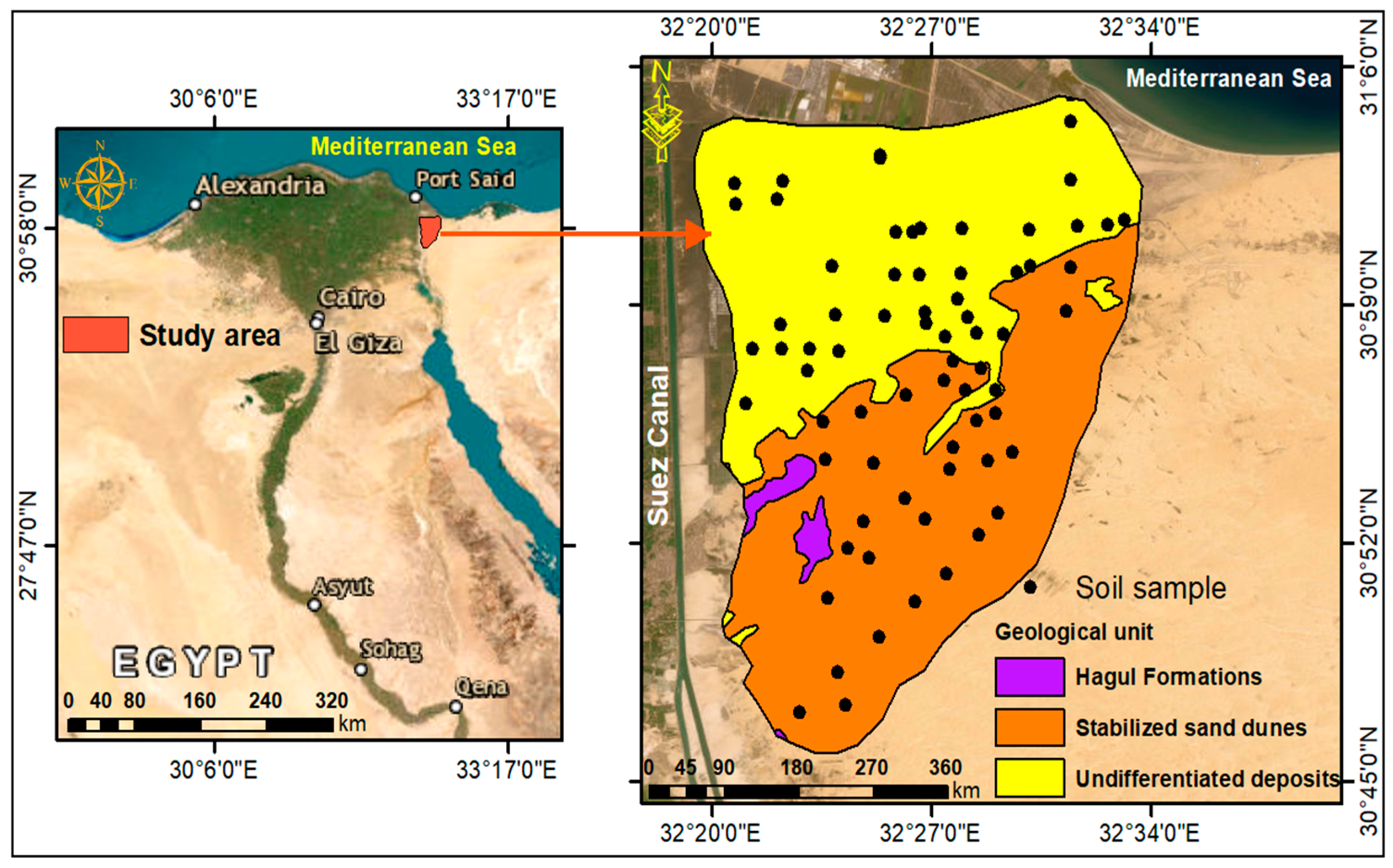
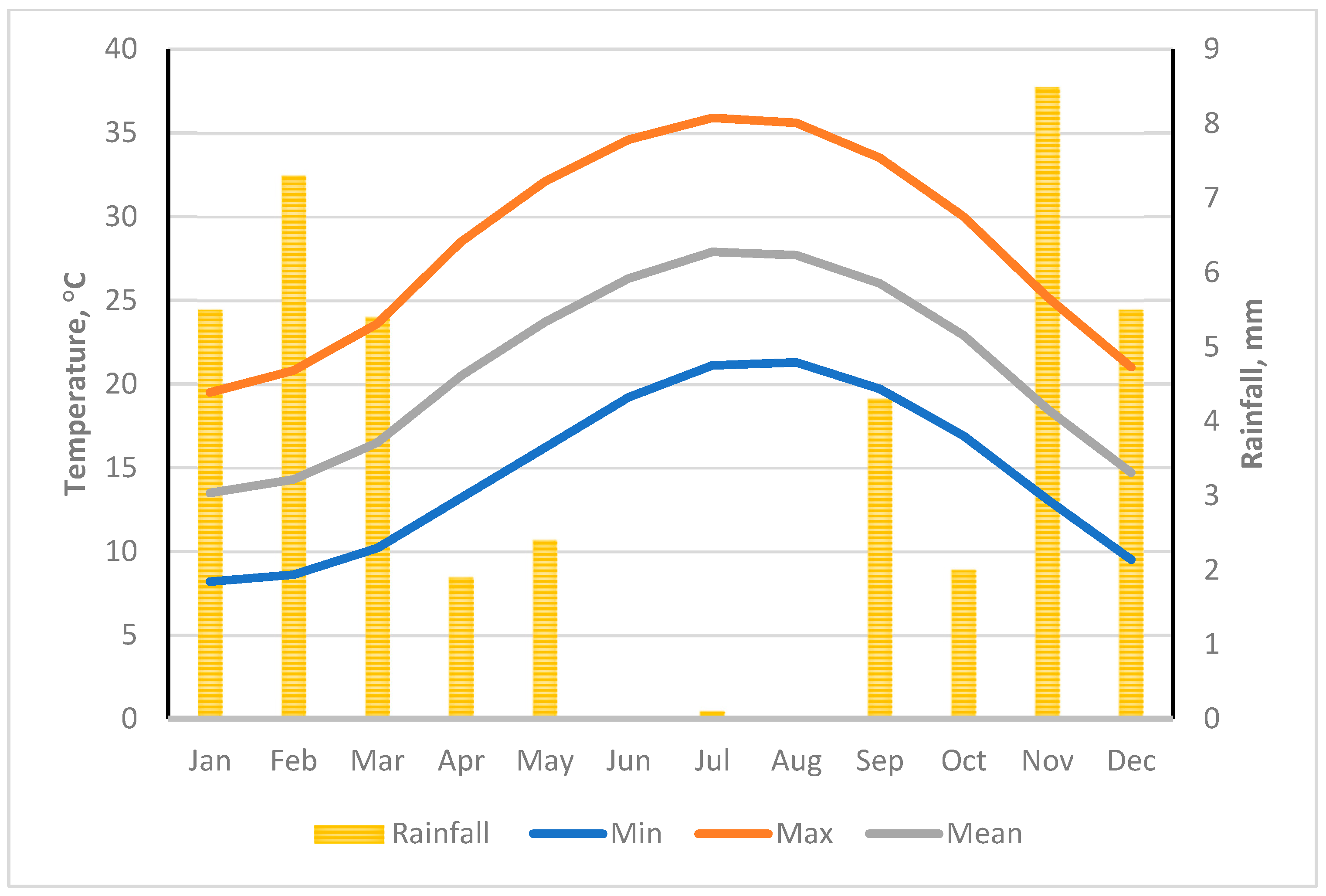
2.1. Description of the Study Area
2.2. Field Work and Laboratory Analysis
2.3. Statistical Analysis
2.4. Geostatistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Soil Metal Bioavailability
3.3. Metal Relationships in Soils
3.4. Potential Sources of Metals in Soils
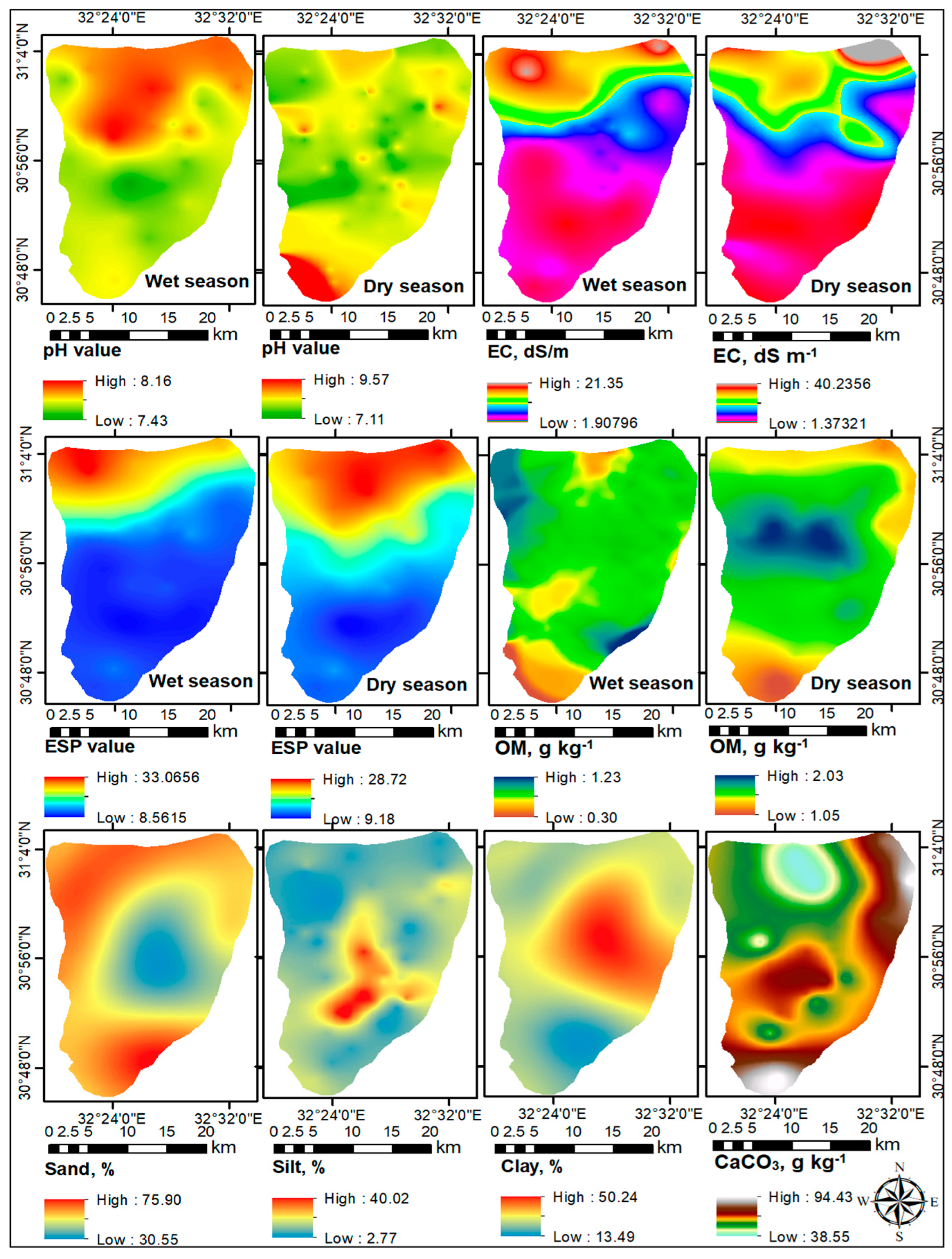
3.5. Spatial Variability of Soil Properties and Heavy Metals
4. Discussion
4.1. Seasonal Variability of Soil Properties and Metal Bioavailability
4.2. Metal Associations in Soils
4.3. Potential Metal Contamination Sources
4.4. Spatial Variability of Soil Properties and HMs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adnan, M.; Xiao, B.; Ali, M.U.; Xiao, P.; Zhao, P.; Wang, H.; Bibi, S. Heavy metals pollution from smelting activities: A threat to soil and groundwater. Ecotoxicol. Environ. Saf. 2024, 274, 116189. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhang, X.; Zhu, Y.; Zhuo, R. Recent advances in phyto-combined remediation of heavy metal pollution in soil. Biotechnol. Adv. 2024, 72, 108337. [Google Scholar] [CrossRef]
- Akansha, J.; Thakur, S.; Chaithanya, M.S.; Gupta, B.S.; Das, S.; Das, B.; Rajasekar, N.; Priya, K. Technological and economic analysis of electrokinetic remediation of contaminated soil: A global perspective and its application in Indian scenario. Heliyon 2024, 10, e24293. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Wang, K.; He, X.; Yang, Z.; Wang, L. Characterization of physicochemical parameters and bioavailable heavy metals and their interactions with microbial community in arsenic-contaminated soils and sediments. Environ. Sci. Pollut. Res. 2022, 29, 49672–49683. [Google Scholar] [CrossRef] [PubMed]
- Rate, A.W. Inorganic contaminants in urban soils. In Urban Soils: Principles and Practice; Rate, A.W., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 153–199. [Google Scholar]
- Yang, Q.F.; Wang, S.L.; Nan, Z.R. Migration, accumulation, and risk assessment of potentially toxic elements in soil-plant (shrub and herbage) systems at typical polymetallic mines in Northwest China. Environ. Sci. Pollut. Res. 2023, 30, 46092–46106. [Google Scholar] [CrossRef]
- Abuzaid, A.S.; Bassouny, M.A.; Jahin, H.S.; Abdelhafez, A.A. Stabilization of lead and copper in a contaminated Typic Torripsament soil using humic substances. CLEAN Soil, Air, Water 2019, 47, 1800309. [Google Scholar] [CrossRef]
- Li, Z.; Jiao, W.; Li, R.; Yu, Z.; Song, N.; Liu, J.; Zong, H.; Wang, F. Source apportionment and source-specific risk assessment of bioavailable metals in river sediments of an anthropogenically influenced watershed in China. Sci. Total Environ. 2024, 912, 169367. [Google Scholar] [CrossRef]
- Alamgir, M. The effects of soil properties to the extent of soil contamination with metals. In Environmental Remediation Technologies for Metal-Contaminated Soils; Hasegawa, H., Rahman, I.M.M., Rahman, M.A., Eds.; Springer: Tokyo, Japan, 2016; pp. 1–19. [Google Scholar]
- Abuzaid, A.S.; El-Komy, M.S.; Shokr, M.S.; El Baroudy, A.A.; Mohamed, E.S.; Rebouh, N.Y.; Abdel-Hai, M.S. Predicting dynamics of soil salinity and sodicity using remote sensing techniques: A landscape-scale assessment in the northeastern Egypt. Sustainability 2023, 15, 9440. [Google Scholar] [CrossRef]
- Wang, Z.; Han, R.X.; Muhammad, A.; Guan, D.X.; Zama, E.; Li, G. Correlative distribution of DOM and heavy metals in the soils of the Zhangxi watershed in Ningbo city, East of China. Environ. Pollut. 2022, 299, 118811. [Google Scholar] [CrossRef]
- Ren, J.; Chen, J.; Han, L.; Wang, M.; Yang, B.; Du, P.; Li, F. Spatial distribution of heavy metals, salinity and alkalinity in soils around bauxite residue disposal area. Sci. Total Environ. 2018, 628–629, 1200–1208. [Google Scholar] [CrossRef]
- Zhen, J.; Pei, T.; Xie, S. Kriging methods with auxiliary nighttime lights data to detect potentially toxic metals concentrations in soil. Sci. Total Environ. 2019, 659, 363–371. [Google Scholar] [CrossRef]
- Shi, C.; Wang, Y. Non-parametric machine learning methods for interpolation of spatially varying non-stationary and non-Gaussian geotechnical properties. Geosci. Front. 2021, 12, 339–350. [Google Scholar] [CrossRef]
- Abuzaid, A.S.; Mazrou, Y.S.A.; El Baroudy, A.A.; Ding, Z.; Shokr, M.S. Multi-Indicator and geospatial based approaches for assessing variation of land quality in arid agroecosystems. Sustainability 2022, 14, 5840. [Google Scholar] [CrossRef]
- Golden, N.; Zhang, C.; Potito, A.; Gibson, P.J.; Bargary, N.; Morrison, L. Use of ordinary cokriging with magnetic susceptibility for mapping lead concentrations in soils of an urban contaminated site. J. Soils Sed. 2020, 20, 1357–1370. [Google Scholar] [CrossRef]
- Dad, J.M.; Ul Shafiq, M. Spatial variability and delineation of management zones based on soil micronutrient status in apple orchard soils of Kashmir valley, India. Environ. Monit. Assess. 2021, 193, 797. [Google Scholar] [CrossRef]
- Dagar, J.C.; Gupta, S.R.; Gaur, A. Tree-based farming systems for improving productivity and ecosystem services in saline environments of dry regions: An overview. Farming Syst. 2023, 1, 100003. [Google Scholar] [CrossRef]
- Devkota, K.P.; Devkota, M.; Rezaei, M.; Oosterbaan, R. Managing salinity for sustainable agricultural production in salt-affected soils of irrigated drylands. Agric. Syst. 2022, 198, 103390. [Google Scholar] [CrossRef]
- Stavi, I.; Thevs, N.; Priori, S. Soil salinity and sodicity in drylands: A review of causes, effects, monitoring, and restoration measures. Front. Environ. Sci. 2021, 9, 712831. [Google Scholar] [CrossRef]
- Kebede, F. Status, drivers, and suggested management scenarios of salt-affected soils in Africa. In Biosaline Agriculture as a Climate Change Adaptation for Food Security; Choukr-Allah, R., Ragab, R., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 259–284. [Google Scholar]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef]
- Chu, B.; Chen, X.J.; Li, Q.S.; Yang, Y.F.; Mei, X.Q.; He, B.Y.; Li, H.; Tan, L. Effects of salinity on the transformation of heavy metals in tropical estuary wetland soil. Chem. Ecol. 2015, 31, 186–198. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Chen, Z.; Li, Y.; Ding, K.; Liu, W.; Liu, Y.; Yuan, Y.; Zhang, M.; Baker, A.J.M.; Yang, W.; et al. Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 2020, 400, 123289. [Google Scholar] [CrossRef]
- Ouda, S.; Khalifa, H.; Mohamadin, A.; Zohry, A.E.-H. Sustainable use of soil and water resources to combat degradation. In Global Segradation of Soil and Water Resources: Regional Assessment and Strategies; Li, R., Napier, T.L., El-Swaify, S.A., Sabir, M., Rienzi, E., Eds.; Springer Nature: Singapore, 2022; pp. 61–74. [Google Scholar]
- Soil Survey Staff. Soil Survey Field and Laboratory Methods Manual; Soil Survey Investigations Report No. 51, Version 2.0; Burt, R., Soil Survey Staff, Eds.; U.S. Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Abuzaid, A.S.; Jahin, H.S. Combinations of multivariate statistical analysis and analytical hierarchical process for indexing surface water quality under arid conditions. J. Contam. Hydrol. 2022, 248, 104005. [Google Scholar] [CrossRef]
- Bai, J.H.; Zhao, Q.Q.; Wang, W.; Wang, X.; Jia, J.; Cui, B.S.; Liu, X.H. Arsenic and heavy metals pollution along a salinity gradient in drained coastal wetland soils: Depth distributions, sources and toxic risks. Ecol. Indic. 2019, 96, 91–98. [Google Scholar] [CrossRef]
- Santra, P.; Kumar, M.; Panwar, N.R.; Yadav, R.S. Digital soil mapping: The future need of sustainable soil management. In Geospatial Technologies for Crops and Soils; Mitran, T., Meena, R.S., Chakraborty, A., Eds.; Springer: Singapore, 2021; pp. 319–355. [Google Scholar]
- Sebei, A.; Chaabani, A.; Abdelmalek-Babbou, C.; Helali, M.A.; Dhahri, F.; Chaabani, F. Evaluation of pollution by heavy metals of an abandoned Pb-Zn mine in northern Tunisia using sequential fractionation and geostatistical mapping. Environ. Sci. Pollut. Res. 2020, 27, 43942–43957. [Google Scholar] [CrossRef]
- Mammadov, E.; Denk, M.; Riedel, F.; Lewinska, K.; Kazmierowski, C.; Glaesser, C. Visible and near-infrared reflectance spectroscopy for assessment of soil properties in the Caucasus Mountains, Azerbaijan. Commun. Soil Sci. Plant Anal. 2020, 51, 2111–2136. [Google Scholar] [CrossRef]
- FAO. Guidelines for Soil Description, 4th ed.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2006. [Google Scholar]
- Yang, Z.; Jing, F.; Chen, X.; Liu, W.; Guo, B.; Lin, G.; Huang, R.; Liu, W. Spatial distribution and sources of seven available heavy metals in the paddy soil of red region in Hunan Province of China. Environ. Monit. Assess. 2018, 190, 611. [Google Scholar] [CrossRef]
- Rezapour, S.; Kouhinezhad, P.; Samadi, A. Trace metals toxicity in relation to long-term intensive agricultural production in a calcareous environment with different soil types. Nat. Hazards 2020, 100, 551–570. [Google Scholar] [CrossRef]
- Jafari, M.; Tavili, A.; Panahi, F.; Zandi Esfahan, E.; Ghorbani, M. Characteristics of arid and desert ecosystems. In Reclamation of arid Lands; Jafari, M., Tavili, A., Panahi, F., Zandi Esfahan, E., Ghorbani, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 21–91. [Google Scholar]
- Wang, J.; Ding, J.; Yu, D.; Ma, X.; Zhang, Z.; Ge, X.; Teng, D.; Li, X.; Liang, J.; Lizaga, I.; et al. Capability of Sentinel-2 MSI data for monitoring and mapping of soil salinity in dry and wet seasons in the Ebinur Lake region, Xinjiang, China. Geoderma 2019, 353, 172–187. [Google Scholar] [CrossRef]
- Jia, P.; Shang, T.; Zhang, J.; Sun, Y. Inversion of soil pH during the dry and wet seasons in the Yinbei region of Ningxia, China, based on multi-source remote sensing data. Geoderma Reg. 2021, 25, e00399. [Google Scholar] [CrossRef]
- Hopkins, B.G.; Stark, J.C.; Kelling, K.A. Nutrient management. In Potato Production Systems; Stark, J.C., Thornton, M., Nolte, P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 155–202. [Google Scholar]
- Liang, G.; Reed, S.C.; Stark, J.M.; Waring, B.G. Unraveling mechanisms underlying effects of wetting–drying cycles on soil respiration in a dryland. Biogeochemistry 2023, 166, 23–37. [Google Scholar] [CrossRef]
- Weng, N.Y.; Wang, W.X. Seasonal fluctuations of metal bioaccumulation and reproductive health of local oyster populations in a large contaminated estuary. Environ. Pollut. 2019, 250, 175–185. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I. Heavy metals: Definition, toxicity, and uptake in plants. In Cellular and Molecular Phytotoxicity of Heavy Metals; Faisal, M., Saquib, Q., Alatar, A.A., Al-Khedhairy, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–17. [Google Scholar]
- Yang, Y.; Peng, Y.; Ma, Y.; Chen, G.; Li, F.; Liu, T. Effects of aging and reduction processes on Cr toxicity to wheat root elongation in Cr(VI) spiked soils. Environ. Pollut. 2022, 296, 118784. [Google Scholar] [CrossRef]
- Mostafa, M.T.; El-Nady, H.; Gomaa, R.M.; Salman, S.A.; Khalifa, I.H. Contamination and sediment quality evaluation of toxic metals enrichment in heavy mineral-rich beach sands of Arish City, Northeastern Egypt. Euro-Mediter. J. Environ. Integr. 2024, 9, 7–22. [Google Scholar] [CrossRef]
- Kong, X.; Wang, Y.; Ma, L.; Li, H.; Han, Z. Impact of δ-MnO2 on the chemical speciation and fractionation of Cr(III) in contaminated soils. Environ. Sci. Pollut. Res. 2022, 29, 45328–45337. [Google Scholar] [CrossRef]
- Gonnelli, C.; Renella, G. Chromium and nickel. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2013; pp. 313–333. [Google Scholar]
- He, G.D.; Zhang, Z.M.; Wu, X.L.; Cui, M.Y.; Zhang, J.C.; Huang, X.F. Adsorption of heavy metals on soil collected from Lixisol of typical karst areas in the presence of CaCO3 and soil clay and their competition behavior. Sustainability 2020, 12, 7315. [Google Scholar] [CrossRef]
- Abuzaid, A.S.; Bassouny, M.A. Total and DTPA-extractable forms of potentially toxic metals in soils of rice fields, north Nile Delta of Egypt. Environ. Technol. Innov. 2020, 18, 100717. [Google Scholar] [CrossRef]
- Abuzaid, A.S.; Jahin, H.S. Profile distribution and source identification of potentially toxic elements in north Nile Delta, Egypt. Soil Sedim. Contam. 2019, 28, 582–600. [Google Scholar] [CrossRef]
- Jahin, H.S.; Abuzaid, A.S.; Abdellatif, D.A. Using multivariate analysis to develop irrigation water quality index for surface water in Kafr El-Sheikh Governorate, Egypt. Environ. Technol. Innov. 2020, 17, 100532. [Google Scholar] [CrossRef]
- Hamdan, M.A.; Hassan, F.A. Quaternary of Egypt. In The Geology of Egypt; Hamimi, Z., El-Barkooky, A., Frías, J.M., Fritz, H., Abd El-Rahman, Y., Eds.; Springer Nature AG: Cham, Switzerland, 2020; pp. 445–494. [Google Scholar]
- Garzanti, E.; Andò, S.; Limonta, M.; Fielding, L.; Najman, Y. Diagenetic control on mineralogical suites in sand, silt, and mud (Cenozoic Nile Delta): Implications for provenance reconstructions. Earth-Sci. Rev. 2018, 185, 122–139. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Haider, F.U.; Ahmad, M.; Hussain, S.; Maqsood, M.F.; Ishfaq, M.; Shahzad, B.; Waqas, M.M.; Ali, B.; Tayyab, M.N.; et al. Chromium toxicity, speciation, and remediation strategies in soil-plant interface: A critical review. Front. Plant Sci. 2023, 13, 1081624. [Google Scholar] [CrossRef] [PubMed]
- Redwan, M.; Bamousa, A.O. Characterization and environmental impact assessment of gold mine tailings in arid regions: A case study of Barramiya gold mine area, Eastern Desert, Egypt. J. Afr. Earth Sci. 2019, 160, 103644. [Google Scholar] [CrossRef]
- Shokr, M.S.; Abdellatif, M.A.; El Behairy, R.A.; Abdelhameed, H.H.; El Baroudy, A.A.; Mohamed, E.S.; Rebouh, N.Y.; Ding, Z.; Abuzaid, A.S. Assessment of potential heavy metal contamination hazards based on GIS and multivariate analysis in some Mediterranean zones. Agronomy 2022, 12, 3220. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Elbehiry, F. Geology. In The Soils of Egypt; El-Ramady, H., Alshaal, T., Bakr, N., Elbana, T., Mohamed, E., Belal, A., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 93–110. [Google Scholar]
- Osman, K.T. Soil as a part of the lithosphere. In Soils: Principles, Properties and Management; Osman, K.T., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 9–16. [Google Scholar]
- Chhabra, R. Nature and origin of salts, classification, area and distribution of salt-affected soils. In Salt-Affected Soils and Marginal Waters: Global Perspectives and Sustainable Management; Chhabra, R., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–47. [Google Scholar]
- Abuzaid, A.S.; Jahin, H.S.; Shokr, M.S.; El Baroudy, A.A.; Mohamed, E.S.; Rebouh, N.Y.; Bassouny, M.A. A novel regional-scale assessment of soil metal pollution in arid agroecosystems. Agronomy 2023, 13, 161. [Google Scholar] [CrossRef]
- Arumugam, T.; Kinattinkara, S.; Nambron, D.; Velusamy, S.; Shanmugamoorthy, M.; Pradeep, T.; Mageshkumar, P. An integration of soil characteristics by using GIS based Geostatistics and multivariate statistics analysis sultan Batheri block, Wayanad District, India. Urban Clim. 2022, 46, 101339. [Google Scholar] [CrossRef]
- Grangeon, S.; Bataillard, P.; Coussy, S. The nature of manganese oxides in soils and their role as scavengers of trace elements: Implication for soil remediation. In Environmental Soil Remediation and Rehabilitation: Existing and Innovative Solutions; van Hullebusch, E.D., Huguenot, D., Pechaud, Y., Simonnot, M.-O., Colombano, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 399–429. [Google Scholar]

| Property | Unit | Season | Minimum | Maximum | Mean | CV, % |
|---|---|---|---|---|---|---|
| pH | --- | Wet | 7.10 | 8.52 | 7.83 a | 3.53 |
| Dry | 7.10 | 9.60 | 7.64 b | 4.74 | ||
| EC | dS m−1 | Wet | 0.17 | 31.30 | 7.32 b | 106.31 |
| Dry | 0.29 | 61.00 | 11.43 a | 127.29 | ||
| Na+ | mmolC L−1 | Wet | 1.10 | 270.58 | 52.24 b | 121.46 |
| Dry | 1.96 | 478.00 | 83.69 a | 130.87 | ||
| K+ | Wet | 0.02 | 3.74 | 0.68 b | 103.05 | |
| Dry | 0.02 | 7.80 | 1.41 a | 101.33 | ||
| Ca2+ | Wet | 0.10 | 49.90 | 9.55 b | 97.95 | |
| Dry | 0.50 | 77.20 | 17.61 a | 122.96 | ||
| Mg2+ | Wet | 0.14 | 49.58 | 11.07 a | 100.14 | |
| Dry | 0.25 | 90.80 | 12.49 a | 131.11 | ||
| Cl− | Wet | 0.04 | 296.20 | 61.08 a | 125.92 | |
| Dry | 1.80 | 470.00 | 69.44 a | 134.74 | ||
| SO42− | Wet | 0.00 | 76.99 | 10.87 b | 141.71 | |
| Dry | 0.69 | 137.00 | 26.44 b | 119.60 | ||
| HCO3− | Wet | 0.20 | 6.00 | 1.63 b | 66.26 | |
| Dry | 0.66 | 112.00 | 20.14 a | 138.94 | ||
| ESP | --- | Wet | 0.83 | 51.15 | 14.14 a | 83.42 |
| Dry | 3.35 | 46.13 | 17.98 a | 63.10 | ||
| OM | g kg−1 | Wet | 1.00 | 16.00 | 7.51 b | 56.12 |
| Dry | 1.00 | 21.00 | 12.24 a | 42.85 | ||
| Sand | % | All | 16.00 | 88.00 | 49.90 | 42.83 |
| Silt | 2.00 | 46.00 | 15.47 | 70.05 | ||
| Clay | 6.00 | 64.00 | 34.63 | 50.57 | ||
| CaCO3 | g kg−1 | 48.00 | 146.70 | 67.02 | 29.63 |
| Metal | Wet Season | Dry Season | MAC | ||||
|---|---|---|---|---|---|---|---|
| Range | Mean | CV, % | Range | Mean | CV, % | ||
| Cr | 0.04–0.282 | 0.16 a | 96.04 | 0.05–1.1 | 0.19 a | 96.16 | 0.50 1 |
| Co | 0.01–0.03 | 0.014 a | 112.50 | 0.01–0.04 | 0.016 a | 99.09 | NA |
| Cu | 0.14–3.12 | 0.82 a | 67.27 | 0.17–3.81 | 1.01 a | 67.53 | 0.20 1 |
| Fe | 0.01–39.45 | 14.21 a | 73.65 | 0.01–48.21 | 17.37 a | 73.65 | NA |
| Pb | 0.01–0.09 | 0.031 a | 73.12 | 0.01–0.11 | 0.034 a | 74.03 | 15.0 1 |
| Mn | 0.01–2.11 | 0.70 b | 60.12 | 0.02–2.58 | 0.86 a | 59.88 | 30.0 2 |
| Ni | 0.02–0.12 | 0.04 b | 42.12 | 0.02–0.15 | 0.06 a | 42.50 | 1.0 1 |
| Zn | 0.05–0.56 | 0.18 b | 53.57 | 0.07–0.68 | 0.23 a | 53.78 | 0.50 1 |
| Wet Season | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | EC | Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | ESP | Sand | Silt | Clay | OM | CaCO3 | Cr | Co | Cu | Fe | Pb | Mn | Ni | |
| Cr | −0.28 * | −0.16 | −0.14 | −0.08 | −0.14 | −0.18 | −0.14 | −0.15 | 0.12 | −0.09 | −0.14 | 0.30 * | −0.01 | −0.25 * | 0.20 | 1.00 | ||||||
| Co | 0.36 ** | 0.50 ** | 0.49 ** | 0.10 | 0.30 * | 0.42 ** | 0.51 ** | −0.03 | −0.38 ** | 0.51 ** | 0.31 * | −0.40 ** | −0.13 | 0.11 | −0.040 ** | −0.18 | 1.00 | |||||
| Cu | 0.05 | −0.04 | −0.02 | −0.10 | −0.13 | −0.05 | −0.06 | 0.11 | 0.24 | 0.04 | 0.05 | −0.11 | 0.01 | −0.14 | 0.17 | −0.12 | −0.12 | 1.00 | ||||
| Fe | 0.28 * | 0.51 ** | 0.51 ** | 0.12 | 0.26 * | 0.45 ** | 0.53 ** | −0.04 | −0.37 ** | 0.52 ** | 0.17 | −0.32 * | −0.01 | 0.02 | −0.41 ** | −0.14 | 0.90 ** | −0.02 | 1.00 | |||
| Pb | 0.00 | 0.03 | 0.07 | 0.07 | −0.13 | −0.10 | 0.05 | −0.08 | 0.13 | 0.09 | 0.03 | 0.05 | −0.06 | −0.21 | 0.31 * | 0.17 | −0.25 | 0.43 ** | −0.19 | 1.00 | ||
| Mn | 0.17 | 0.18 | 0.17 | −0.03 | 0.14 | 0.16 | 0.17 | 0.02 | 0.14 | 0.24 | 0.33 ** | −0.29 * | −0.22 | −0.07 | −0.05 | −0.15 | 0.43 ** | 0.31 * | 0.40 ** | 0.22 | 1.00 | |
| Ni | 0.21 | 0.33 ** | 0.34 ** | −0.03 | 0.14 | 0.23 | 0.36 ** | −0.11 | −0.15 | 0.38 ** | 0.14 | −0.23 | −0.03 | 0.15 | −0.02 | −0.03 | 0.60 ** | −0.05 | 0.58 ** | −0.08 | 0.310 * | 1.00 |
| Zn | 0.11 | −0.04 | −0.03 | −0.07 | −0.09 | −0.08 | −0.03 | −0.09 | 0.09 | 0.00 | 0.08 | −0.04 | −0.08 | 0.11 | 0.29 * | −0.20 | 0.05 | 0.26 * | 0.09 | 0.23 | 0.33 ** | 0.22 |
| Dry season | ||||||||||||||||||||||
| pH | EC | Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | ESP | Sand | Silt | Clay | OM | CaCO3 | Cr | Co | Cu | Fe | Pb | Mn | Ni | |
| Cr | 0.08 | −0.14 | −0.14 | −0.21 | −0.20 | −0.09 | −0.11 | −0.16 | −0.22 | −0.17 | −0.15 | 0.29 * | −0.01 | −0.12 | 0.20 | 1.00 | ||||||
| Co | 0.07 | 0.52 ** | 0.51 ** | 0.19 | 0.058 ** | 0.462 ** | 0.48 ** | 0.53 ** | 0.53 ** | 0.54 ** | 0.27 * | −0.42 ** | −0.07 | −0.12 | −0.34 * | −0.22 | 1.00 | |||||
| Cu | 0.13 | −0.11 | −0.10 | −0.20 | −0.09 | −0.13 | −0.11 | −0.14 | −0.03 | −0.04 | 0.05 | −0.11 | 0.01 | −0.19 | 0.17 | −0.12 | −0.01 | 1.00 | ||||
| Fe | 0.12 | 0.41 ** | 0.40 ** | 0.21 | 0.50 ** | 0.322 * | 0.035 ** | 0.43 ** | 0.53 ** | 0.50 ** | 0.17 | −0.32 * | −0.01 | −0.16 | −0.41 ** | −0.15 | 0.90 ** | −0.02 | 1.00 | |||
| Pb | 0.20 | 0.06 | 0.09 | −0.25 | −0.05 | 0.12 | 0.13 | 0.04 | −0.08 | 0.04 | 0.00 | 0.05 | −0.02 | −0.07 | 0.27 | 0.16 | −0.16 | 0.46 ** | −0.16 | 1.00 | ||
| Mn | 0.18 | 0.19 | 0.19 | 0.04 | 0.15 | 0.20 | 0.19 | 0.18 | 0.15 | 0.28 * | 0.33 ** | −0.29 * | −0.22 | −0.10 | −0.05 | −0.15 | 0.44 ** | 0.31 * | .40 ** | 0.22 | 1.00 | |
| Ni | 0.16 | 0.36 ** | 0.35 ** | 0.05 | 0.35 ** | 0.34 ** | 0.345 ** | 0.35 ** | 0.33 * | 0.35 ** | 0.18 | −0.21 | −0.08 | 0.11 | −0.02 | −0.04 | 0.62 ** | −0.05 | 0.58 ** | −0.06 | 0.33 ** | 1.00 |
| Zn | 0.18 | −0.07 | −0.07 | 0.22 | −0.10 | −0.06 | −0.07 | −0.07 | −0.06 | −0.01 | 0.09 | −0.03 | −0.09 | 0.33 * | 0.30 * | −0.20 | 0.08 | 0.26 * | 0.09 | 0.22 | 0.33 ** | 0.24 |
| Parameter | Wet Season | Parameter | Dry Season | ||||||||||||
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | ||
| Eigenvalue | 5.16 | 2.75 | 2.12 | 2.06 | 2.02 | 1.92 | 1.70 | Eigenvalue | 7.50 | 2.88 | 2.11 | 1.90 | 1.76 | 1.65 | 1.59 |
| Variance, % | 22.43 | 11.94 | 9.23 | 8.97 | 8.79 | 8.33 | 7.39 | Variance, % | 32.61 | 12.50 | 9.18 | 8.27 | 7.64 | 7.19 | 6.90 |
| Cumulative, % | 22.43 | 34.37 | 43.60 | 52.57 | 61.36 | 69.69 | 77.09 | Cumulative, % | 32.61 | 45.11 | 54.29 | 62.56 | 70.20 | 77.39 | 84.29 |
| Indicator | Eigenvectors | Indicator | Eigenvectors | ||||||||||||
| pH | 0.27 | 0.22 | −0.10 | 0.16 | −0.33 | 0.57 | −0.22 | pH | −0.17 | 0.41 | 0.36 | 0.25 | −0.12 | 0.03 | 0.05 |
| EC | 0.96 | 0.19 | 0.08 | −0.06 | 0.12 | 0.12 | −0.02 | EC | 0.99 | 0.12 | 0.01 | −0.01 | 0.02 | 0.05 | 0.00 |
| Na+ | 0.96 | 0.19 | 0.06 | −0.02 | −0.02 | 0.10 | −0.02 | Na+ | 0.99 | 0.10 | 0.01 | 0.02 | 0.02 | 0.02 | 0.00 |
| K+ | 0.00 | −0.08 | 0.07 | −0.03 | 0.42 | 0.69 | 0.11 | K+ | 0.11 | 0.01 | 0.06 | 0.05 | −0.06 | 0.91 | 0.17 |
| Ca2+ | 0.56 | 0.04 | 0.00 | −0.07 | 0.67 | 0.10 | −0.01 | Ca2+ | 0.85 | 0.23 | −0.03 | −0.27 | 0.10 | 0.20 | 0.01 |
| Mg2+ | 0.70 | 0.22 | 0.18 | −0.21 | 0.33 | 0.07 | −0.08 | Mg2+ | 0.97 | 0.03 | 0.05 | 0.12 | −0.04 | −0.07 | 0.01 |
| Cl− | 0.96 | 0.20 | 0.09 | −0.05 | −0.04 | 0.13 | −0.03 | Cl− | 0.98 | 0.06 | 0.06 | 0.07 | 0.01 | −0.01 | −0.02 |
| SO42− | 0.00 | −0.03 | −0.05 | −0.05 | 0.91 | −0.04 | 0.00 | SO42− | 0.99 | 0.08 | 0.02 | −0.01 | −0.03 | 0.05 | 0.01 |
| HCO3− | −0.13 | −0.21 | −0.03 | 0.32 | 0.24 | −0.72 | 0.13 | HCO3− | 0.61 | 0.36 | −0.19 | −0.35 | 0.13 | 0.28 | 0.06 |
| ESP | 0.93 | 0.23 | 0.09 | 0.08 | −0.01 | 0.05 | 0.04 | ESP | 0.89 | 0.18 | −0.06 | −0.04 | 0.22 | 0.17 | −0.11 |
| Sand | 0.21 | 0.15 | 0.94 | 0.05 | 0.05 | 0.01 | −0.16 | Sand | 0.18 | 0.12 | 0.91 | 0.00 | 0.32 | −0.07 | 0.04 |
| Silt | −0.30 | −0.19 | −0.36 | −0.14 | −0.27 | −0.17 | 0.43 | Silt | −0.40 | −0.13 | −0.28 | 0.10 | −0.67 | 0.18 | 0.04 |
| Clay | −0.07 | −0.06 | −0.93 | 0.02 | 0.10 | 0.08 | −0.06 | Clay | 0.07 | −0.05 | −0.93 | −0.07 | 0.08 | −0.05 | −0.08 |
| SOM | −0.11 | 0.07 | 0.06 | −0.18 | −0.22 | −0.03 | −0.68 | SOM | −0.02 | −0.17 | 0.08 | 0.00 | −0.11 | 0.01 | 0.85 |
| CaCO3 | −0.32 | −0.17 | 0.29 | 0.24 | −0.19 | −0.52 | 0.18 | CaCO3 | −0.42 | −0.03 | 0.27 | 0.29 | −0.08 | −0.55 | 0.36 |
| Cr | −0.11 | 0.01 | 0.00 | −0.19 | −0.14 | −0.17 | 0.82 | Cr | −0.17 | −0.11 | −0.01 | 0.08 | −0.56 | −0.30 | −0.58 |
| Co | 0.35 | 0.80 | 0.17 | −0.09 | 0.03 | 0.27 | −0.13 | Co | 0.38 | 0.80 | 0.09 | −0.21 | 0.20 | 0.07 | −0.12 |
| Cu | −0.08 | −0.01 | −0.08 | 0.76 | 0.02 | −0.12 | −0.09 | Cu | −0.25 | 0.03 | −0.06 | 0.37 | 0.73 | −0.02 | −0.11 |
| Fe | 0.36 | 0.82 | 0.03 | −0.05 | 0.00 | 0.24 | −0.07 | Fe | 0.16 | 0.85 | −0.01 | −0.21 | 0.12 | 0.18 | −0.27 |
| Pb | −0.01 | −0.22 | 0.07 | 0.73 | −0.19 | 0.06 | 0.35 | Pb | 0.11 | −0.23 | 0.03 | 0.86 | 0.14 | −0.12 | −0.06 |
| Mn | 0.11 | 0.54 | 0.22 | 0.57 | 0.14 | −0.03 | −0.08 | Mn | 0.12 | 0.47 | 0.17 | 0.43 | 0.41 | 0.15 | −0.01 |
| Ni | 0.24 | 0.78 | 0.05 | 0.06 | −0.08 | −0.07 | 0.01 | Ni | 0.25 | 0.80 | 0.09 | 0.08 | −0.04 | −0.21 | 0.13 |
| Zn | −0.16 | 0.27 | 0.07 | 0.51 | −0.13 | −0.24 | −0.24 | Zn | −0.16 | 0.31 | 0.15 | 0.61 | 0.04 | 0.34 | 0.46 |
| Variable | Season | Model | Nugget | Partial Sill | Sill | Nugget/ Sill | SPD | Range, km | Prediction Error | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ME | RMSE | MSE | RMSSE | ASE | |||||||||
| pH | Wet | Exponential | 0.04 | 0.05 | 0.09 | 0.42 | Moderate | 14.20 | 0.00 | 0.24 | 0.00 | 0.98 | 0.25 |
| Dry | Hole effect | 0.01 | 0.07 | 0.08 | 0.13 | Strong | 2.98 | 0.00 | 0.37 | 0.01 | 1.16 | 0.32 | |
| EC | Wet | Exponential | 0.24 | 0.58 | 0.82 | 0.29 | Moderate | 37.13 | 0.01 | 0.66 | 0.01 | 1.04 | 0.62 |
| Dry | Hole effect | 1.69 | 0.34 | 2.02 | 0.83 | Weak | 4.94 | 0.02 | 1.49 | 0.01 | 0.96 | 1.54 | |
| ESP | Wet | K-Bessel | 0.92 | 1.29 | 2.22 | 0.42 | Moderate | 35.84 | 0.00 | 1.05 | 0.01 | 1.03 | 1.02 |
| Dry | Exponential | 0.85 | 0.78 | 1.63 | 0.52 | Moderate | 37.13 | 0.01 | 1.03 | 0.00 | 0.99 | 1.05 | |
| OM | Wet | Tetraspherical | 0.17 | 0.02 | 0.18 | 0.91 | Weak | 6.40 | 0.00 | 0.45 | 0.01 | 1.02 | 0.44 |
| Dry | Exponential | 0.31 | 0.31 | 0.62 | 0.51 | Moderate | 37.13 | 0.01 | 0.73 | 0.01 | 1.13 | 0.64 | |
| Sand | All | J-Bessel | 2.49 | 2.98 | 5.47 | 0.46 | Moderate | 20.56 | −0.01 | 1.97 | 0.00 | 1.11 | 1.74 |
| Silt | Exponential | 0.07 | 0.49 | 0.56 | 0.13 | Strong | 11.23 | 0.05 | 9.19 | 0.05 | 1.09 | 9.97 | |
| Clay | Gaussian | 1.84 | 1.80 | 3.65 | 0.51 | Moderate | 12.42 | 0.02 | 1.61 | 0.01 | 1.06 | 1.52 | |
| CaCO3 | J-Bessel | 0.03 | 0.03 | 0.06 | 0.52 | Moderate | 37.13 | 0.06 | 1.81 | 0.04 | 1.28 | 1.34 | |
| Cr | Wet | Tetraspherical | 0.01 | 0.02 | 0.03 | 0.37 | Moderate | 10.17 | 0.00 | 0.15 | 0.00 | 1.01 | 0.15 |
| Dry | K-Bessel | 0.02 | 0.03 | 0.05 | 0.43 | Moderate | 7.81 | 0.00 | 0.17 | 0.00 | 1.01 | 0.18 | |
| Co | Wet | Gaussian | 0.00 | 0.00 | 0.00 | 0.69 | Moderate | 9.06 | 0.00 | 0.01 | 0.01 | 0.97 | 0.01 |
| Dry | K-Bessel | 0.00 | 0.00 | 0.00 | 0.92 | Weak | 4.21 | 0.00 | 0.01 | 0.00 | 1.01 | 0.01 | |
| Cu | Wet | Rational Quadratic | 0.04 | 0.22 | 0.27 | 0.17 | Strong | 5.59 | 0.00 | 0.53 | 0.00 | 1.11 | 0.46 |
| Dry | Hole Effect | 0.16 | 0.22 | 0.38 | 0.42 | Moderate | 6.27 | 0.00 | 0.66 | 0.00 | 1.16 | 0.55 | |
| Fe | Wet | Gaussian | 0.88 | 0.43 | 1.32 | 0.67 | Moderate | 26.79 | 0.00 | 0.95 | 0.00 | 1.00 | 0.99 |
| Dry | Gaussian | 1.32 | 0.65 | 1.97 | 0.67 | Moderate | 26.79 | 0.00 | 1.17 | 0.00 | 1.00 | 1.21 | |
| Pb | Wet | J-Bessel | 0.00 | 0.00 | 0.00 | 0.79 | Weak | 2.31 | 0.00 | 0.02 | 0.03 | 0.85 | 0.03 |
| Dry | Exponential | 0.00 | 0.00 | 0.00 | 0.59 | Moderate | 3.21 | 0.00 | 0.02 | 0.00 | 0.83 | 0.03 | |
| Mn | Wet | Exponential | 0.13 | 0.14 | 0.27 | 0.49 | Moderate | 37.57 | 0.00 | 0.45 | 0.00 | 1.10 | 0.41 |
| Dry | Circular | 0.21 | 0.26 | 0.47 | 0.44 | Moderate | 37.57 | 0.00 | 0.55 | 0.00 | 1.10 | 0.50 | |
| Ni | Wet | Gaussian | 0.00 | 0.00 | 0.00 | 0.75 | Weak | 4.07 | 0.00 | 0.02 | 0.01 | 1.07 | 0.02 |
| Dry | Gaussian | 0.00 | 0.00 | 0.00 | 0.79 | Weak | 4.21 | 0.00 | 0.03 | 0.01 | 1.06 | 0.03 | |
| Zn | Wet | Exponential | 0.00 | 0.01 | 0.01 | 0.30 | Moderate | 5.62 | 0.00 | 0.11 | 0.02 | 1.08 | 0.10 |
| Dry | Exponential | 0.01 | 0.01 | 0.02 | 0.32 | Moderate | 5.53 | 0.00 | 0.13 | 0.02 | 1.08 | 0.12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Komy, M.S.; Abuzaid, A.S.; Fadl, M.E.; Drosos, M.; Scopa, A.; Abdel-Hai, M.S. Spatiotemporal Variations Affect DTPA-Extractable Heavy Metals in Coastal Salt-Affected Soils of Arid Regions. Soil Syst. 2025, 9, 26. https://doi.org/10.3390/soilsystems9010026
El-Komy MS, Abuzaid AS, Fadl ME, Drosos M, Scopa A, Abdel-Hai MS. Spatiotemporal Variations Affect DTPA-Extractable Heavy Metals in Coastal Salt-Affected Soils of Arid Regions. Soil Systems. 2025; 9(1):26. https://doi.org/10.3390/soilsystems9010026
Chicago/Turabian StyleEl-Komy, Mostafa S., Ahmed S. Abuzaid, Mohamed E. Fadl, Marios Drosos, Antonio Scopa, and Mohamed S. Abdel-Hai. 2025. "Spatiotemporal Variations Affect DTPA-Extractable Heavy Metals in Coastal Salt-Affected Soils of Arid Regions" Soil Systems 9, no. 1: 26. https://doi.org/10.3390/soilsystems9010026
APA StyleEl-Komy, M. S., Abuzaid, A. S., Fadl, M. E., Drosos, M., Scopa, A., & Abdel-Hai, M. S. (2025). Spatiotemporal Variations Affect DTPA-Extractable Heavy Metals in Coastal Salt-Affected Soils of Arid Regions. Soil Systems, 9(1), 26. https://doi.org/10.3390/soilsystems9010026









