Abstract
This research is part of a Hungarian Research OTKA project that examines the vegetation of sandy grasslands along the Danube. During this study, Festuca wagneri and Festuca tomanii were identified as potentially suitable grass species for urban planting and turf establishment based on preliminary research. Our aim was to determine the germination success of seeds from aesthetically selected individuals and to identify the growing media on which they germinate most effectively. From the collected Festuca individuals, we analyzed 30 specimens of each taxon under garden conditions and selected the individuals for germination. The Festuca tomanii individuals were uniform, so we selected only 5 individuals. The Festuca wagneri individuals were categorized into three groups: leaves and inflorescence densely upright, inflorescence shoots spread out, and low ’dwarf’ form (compact and dense but short in stature). It was assumed that Festuca species seeds would germinate better in sandy soils. To test our hypothesis, seeds from ten Festuca wagneri and five Festuca tomanii individuals, selected based on aesthetic criteria, were sown in six different substrates: a sand–peat mixture, sand, coconut fiber, peat, coconut fiber–sand mixture, and native sandy soil (Calcaric Arenosol). Contrary to our expectations, the growth and germination rates of seeds sown in peat and coconut fiber substrates were higher than those in native sandy soil. These results suggest that Festuca seeds germinate better on substrates resembling dead plant debris with a peat-like structure or on the surface of live mosses rather than on bare sand. Among the examined individuals, the seeds from the spreading Festuca wagneri group exhibited the highest germination rate, making this group particularly suitable for urban environments. Additionally, one of the upright Festuca wagneri individuals showed the highest leaf average length and should also be considered for urban planting. In contrast, despite their uniform appearance, the Festuca tomanii individuals did not demonstrate similar germination trends. In fact, the seeds from two clumps did not germinate at all, indicating that further research is necessary.
1. Introduction
The popularity of ornamental grasses in urban environments, parks, and slopes for protection against erosion has been increasing worldwide in the recent period [1,2,3]. In the United States, the urban use of native and non-native lawn grasses is common [4,5,6]. According to Steinegger [7], the Festuca genus, along with Miscantus, Pennisetum, and Panicum, ranks among the most popular and frequently planted ornamental grasses [8,9,10]. Love et al. [11] consider several Festuca species as ornamental grasses, including F. amethystina, F. glauca, F. idahoensis, F. punctoria, and F. valesiaca. They particularly highlight the red-tinted flower stalk of F. amethystina. This species, along with Festuca ovina ‘Glauca’, is recommended by Meyer and Mower [12] for use in perennial beds as background plants, focal points, ground cover, and in rock gardens. Staub and Robbins [13] investigated a hybrid of Festuca idahoensis and Festuca ovina. They suggested that the clone could be suitable as a low-maintenance ornamental plant for urban areas in semi-arid regions without irrigation. A special value of the hybrid is the presence of individuals with red, orange, and yellow discoloration, with both upright and drooping inflorescences. Several Festuca species are used globally as turf grass, such as Festuca ovina, Festuca arundinacea, and Festuca trachyphylla.
Festuca species can also be used in fiber circles on several continents [14,15]. In Europe, they are characterized by a widespread geographic distribution and a large number of their species [16,17,18,19,20,21,22,23], some of which can be used as ornamental plants as well [24]. Blue fescue (Festuca pallens) is the only one with fine leaves that is widely utilized for decorative purposes in the western United States [25,26]. According to Schmidt [27], Festuca gautieri thrives in rock gardens and is also suitable as an edging plant and ground cover. Festuca pallens is an excellent ground cover but is also suitable for container planting and border beds, according to Zsohár and Zsohárné [28]. Many Festuca species are native to New Zealand [29]. The fine-leaved Festuca coxii, native to the Chatham Islands, is widely promoted for landscaping purposes as a native blue-foliage grass [28].
The aesthetic appeal of these grasses is also noteworthy, as it is an essential consideration in the design of urban green spaces. Additionally, it is well-documented that grasses contribute to air purification and the reduction of urban surface temperatures [30] as well as soil health and environment protection [31].
However, the establishment of urban green spaces often involves the use of non-native grass species, which carries the risk of these species becoming invasive and threatening our natural grasslands. For instance, the spontaneous appearance of Eleusine indica, first recorded in Budapest in the early 20th century, is now observed in over a hundred locations throughout the city (e.g., Csontos, Schmotzer), and Sporobolus cryptandrus poses a threat to our sandy substrates [32,33]. Future invasive grass species are likely to be perennial, tall grasses [33].
Hypothesis and Questions
During the examination of the dry sandy grasslands along the Danube, it was hypothesized that drought-resistant individuals of the dominant grass species could be suitable for urban planting. This hypothesis is particularly relevant as only a few species can endure the extremely dry conditions frequently found in urban environments without intensive maintenance. These grasses could also be utilized on green roofs, where attributes like shallow root systems, low mass, drought tolerance, and wind resistance confer significant advantages. Therefore, it would be wise to utilize native Festuca species. The aim of this study was to determine whether the collected material could be classified, identify the morphological and phenotypic differences, and ascertain which taxa, if morphologically distinct. In the case of their application in urban environments, the determination of germination rate has particular importance. Urban soils are highly variable, often anthropogenic, disturbed, sealed, contaminated, and/or characterized by unfavorable physical, chemical, and biological properties. These conditions require plants that can adapt to extreme circumstances. We aimed to contribute to the selection of grass species that have a higher germination success under various conditions to ensure their sustainable application in urban environments. We also carried out morphological tests to determine the ornamental appearance of plant individuals. Could they be grouped by appearance? At the beginning of our germination studies, we hypothesized that the native sandy soil (Calcaric Arenosol) would yield the highest germination percentage, since this is the original substrate of the studied native Festuca species.
2. Materials and Methods
In 2018, 60 Festuca specimens were planted in the pilot area of the MATE Georgikon Campus, Department of Horticulture, in Keszthely, Hungary (Figure 1).

Figure 1.
Planted specimens in (A) 2019 and (B) 2023.
The planted individuals of Festuca originated from two different locations and could potentially be attributed to two species. Specimens collected near Kunpeszér belonged to the taxon Festuca wagneri (W1–W30), while those collected from the Újpesti Homoktövis Nature Reserve represented a newly discovered species of the Hungarian flora, Festuca tomanii (T1–T30). From these specimens, we selected three morphologically distinct types of Festuca wagneri, while the fourth group consisted of selected specimens of Festuca tomanii.
2.1. Studied Species
2.1.1. Festuca wagneri
Taxonomy of the species was clarified when it was described as a separate species [34]. Specimens of Festuca wagneri were collected by Wagner from the sandy grassland at Deliblato in 1904–1905. Botanical studies of the grasslands considered it as a new variety of Festuca sulcata. In the Hungarian title, the name was Festuca wagneri Degen Thaisz et Flatt. Festuca wagneri is different from all other Festuca species of the Carpathian Basin when considering the sclerenchyma and epidermis of its leaves, because it has long hairs on the lower surface of the leaf. Finally, based on the locus classicus and specimens from native areas, Festuca wagneri is a narrow-bladed species with five sclerenchyma bundles. Long hairs are missing on the epidermis of the lower surface of the leaf of the native and Carpathian Basin narrow-leaved Festuca species, so these individuals can be distinguished clearly from those of other Festuca species [34].
2.1.2. Festuca tomanii
Festuca tomanii Korneck & T. Gregor sp. nov. from the sand dunes of the northern Upper Rhine, the Middle Main, and the Elbe valleys in the Czech Republic has been described. It is a blue–green, tetraploid species which grows on base-rich sands [35].
2.2. Observed Data
The specimens were grown under the same conditions, and their inflorescences were analyzed for the following parameters. Inflorescence parameters of investigated Festuca taxa (legend of the measured parameters) included: (1) length of generative stem; (2) length of inflorescence; (3) length of the longest branch on the 1st node; (4) length of the branch on the 2nd node; (5) length of the 4th spikelet from the top of branch; (6) length of 4th spikelet from the top of inflorescence; (7) length of the 1st internode of the inflorescence; (8) floral number of spikelet from the top of branch; (9) length of upper glume from the top of branch; (10) length of lower glume from the top of branch; (11) length of the 2nd flower’s lemma from the top of branch; (12) length of the 2nd flower’s awn from the top of branch; (13) hair of spikelet from the top of branch; (14) length of the 1st flower’s lemma from the top of branch; (15) length of the 1st flower’s awn from the top of branch; (16) hair of upper glume; (17) floral number of spikelet from the top of inflorescence; (18) length of upper glume from the top of inflorescence; (19) length of lower glume from the top of inflorescence; (20) length of the 2nd flower’s lemma from the top of inflorescence; (21) length of the 2nd flower’s awn from the top of inflorescence; (22) hair of spikelet from the top of inflorescence; (23) length of the 1st flower’s lemma from the top of inflorescence; (24) length of the 1st flower’s awn from the top of inflorescence [34].
We measured the average length of plant leaves, the length of the flowering stem (inflorescence axis), and the total length of the panicle. Additionally, we observed changes in the color and shape of the flowering stem and panicle. These parameters are important considerations for the marketability of the respective species. During this study, we recorded the color of the plants (s: silver, g: green) and identified three types based on habit: 1 = erect, 2 = spreading, and 3 = low-growing.
We sowed the seeds of ten selected individuals of Festuca wagneri and individuals of Festuca tomanii into seven different substrates, based on preliminary investigations. The seven substrates were:
- Sand–peat mixture (EC (mS/cm): 0.56; pH: 5.71; O.M. (%) (organic matter): 16.5; composition: 57% peat, 43% river sand);
- Sand (EC (mS/cm): 0.2 4; pH: 7.56; O.M. (%) (organic matter): 0.5; composition: 100% sand);
- Coconut coir (EC (mS/cm): 0.61; pH: 5.02; O.M. (%) (organic matter): 62.3; composition: 100% coconut fiber);
- Peat (EC (mS/cm): 0.82; pH: 3.64; O.M. (%) (organic matter): 35; composition: 100% peat);
- Sand–coconut coir mixture (EC (mS/cm): 0.46; pH: 7.54; O.M. (%) (organic matter): 37; composition: 64% coconut fiber, 36% sand);
- Natural sandy soil (Calcaric Arenosol) from the substrate of Festuca wagneri (EC (mS/cm): 0.1; pH: 7.61; O.M. (%) (organic matter): 2.7; composition: [not specified]);
- Natural sandy soil (Calcaric Arenosol) from the substrate of Festuca tomanii (Homoktövis TVT) (EC (mS/cm): 0.35; pH: 7.23; O.M. (%) (organic matter): 3.5; composition: [not specified]).
Before germination, the seeds were stored in a dry, dark environment in a paper bag at 4 °C. Germination was conducted under greenhouse conditions in 16 × 24 cell seed trays, maintained at 20 °C in January (Figure 2). The greenhouse was set to a day/night temperature cycle of 18 °C/21 °C with a 14 h photoperiod [36].
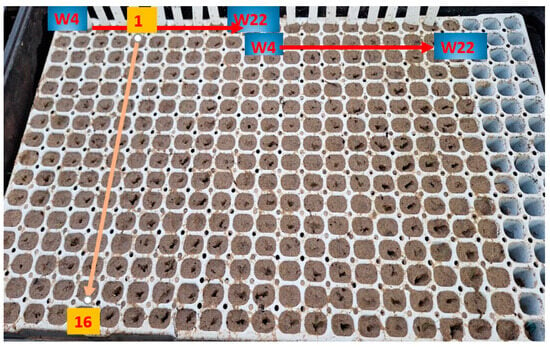
Figure 2.
The seed tray layout.
In total, we germinated 3840 seeds of Festuca wagneri and 640 seeds of Festuca tomanii. Each seed was individually placed into a 1 cm3 cell within the seed trays.
For Festuca wagneri, seeds were collected from ten individuals and sown into six different substrates with four replicates per substrate. Each seed from an individual was placed in 16 cells, one seed per cell (10 × 6 × 4 × 16). Similarly, for Festuca tomanii, seeds were collected from five individuals and sown into different substrates with four replicates. As with Festuca wagneri, each seed was placed in 16 individual cells (5 × 2 × 4 × 16).
To prevent drying, a water-filled tray was placed beneath the seed trays, ensuring that the seed trays remained in water at all times. Bottom heating was provided via heated tables. Irrigation was conducted using tap water, with the water in the soaking trays being changed every four days. Starting from the tenth day after sowing, we recorded the number of germinated seeds and measured the height of the seedlings over a 20-day period.
We sowed seeds from selected individuals identified during the habitus examination. We selected 10 individuals which were: W4, W5, W9, W10, W14, W15, W16, W17, W20, and W22. Additionally, 5 individuals were selected from Festuca tomanii: T1, T11, T21, T23, and T27.
2.3. Statistical Analysis
For the statistical analysis, we utilized Python and R in RStudio with the help of the ‘reticulate’ [37], leveraging various packages to perform the necessary tests and visualizations. The Shapiro–Wilk test, implemented using the ‘scipy.stats’ Python module [38], was conducted to assess the normality of the data for germinated (pcs), germination rate (%), and plant height (cm). As the data did not follow a normal distribution, the Kruskal–Wallis H test was used to compare genotypes within each substrate using the ‘scipy.stats’ module. The Scheirer–Ray–Hare test was performed using Python with the NumPy [39] and SciPy [37,40] libraries. Visualization of the germination rate (%) and plant height (cm) across different genotypes and growing media was achieved with the ‘seaborn’ Python library [39] and the ‘ggplot2’ [41,42] R package.
3. Results
During this study, we observed differences in the appearance of the plants based on their color and habitus. These differences were evident in the first year and were persistent. Based on this, we distinguished three groups among the Festuca wagneri individuals which are listed with the numbers of the selected clumps:
- Leaves and inflorescence are densely upright (W4, W17, and W22);
- Inflorescence shoots are spread out (W5, W15, and W20);
- Low ‘dwarf’ form plants that are compact and dense but short in stature (W9, W10, W14, and W16).
The fourth group consisted of the Festuca tomanii specimens, which were also tall with upright shoots (Figure 3).

Figure 3.
The distinct Festuca morphotypes. 1. W4, W17, and W22; 2. W5, W15, and W20; 3. W5, W15, and W20; 4. Festuca tomanii specimens.
Based on the measurements of the inflorescence parameters through the stereomicroscope, we could distinguish four groups. In the first group of specimens, a high intensity of hairs was observed on the lemmas of the spikelets. The individuals of the second group had larger spikelets in contrast to specimens of the third group, which had much smaller spikelets. In the fourth group, specimens were supposed to be transitional, characterized by a greater number of flowers and more spikelets per specimen than in the others, or in other cases, they were much smaller in size than the others.
It can be seen from the classification analysis that the most significant differences between the potential taxa were the length of the spikelet, the length of the upper glume, the length of the lemmas, and the length of the awn of the lemmas (Figure 4).
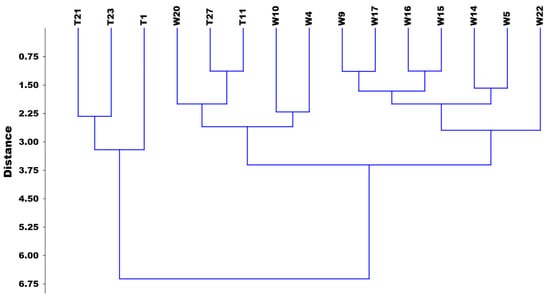
Figure 4.
Classification based on the morphological traits of the studied Festuca individuals. (W: Festuca wagneri; T: Festuca tomanii).
The spikelets of the individuals were the shortest in the first group, while the longest spikelets were found in the second group. The ones in the fourth group showed the widest range in the length of the spikelets. To explore the separation within the taxa and identify which morphological traits were responsible for this differentiation, a PCA analysis was performed. The results indicated that the following traits most distinctly separate the individuals: inflorescence length, length of the fourth spikelet from the top of the branch, length of the first internode of the inflorescence, and the number of florets per spikelet (Figure 5).
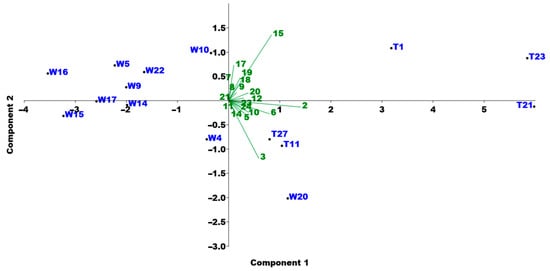
Figure 5.
PCA analysis based on the morphological traits of the examined Festuca individuals (W: Festuca wagneri; T: Festuca tomanii).
3.1. Result of Festuca wagneri Germination
3.1.1. Normality Analysis Results
The normality analysis was conducted on two variables: germination rate (%) and plant height (cm). The Shapiro–Wilk test was used to determine whether the data follows a normal distribution (Table 1).

Table 1.
The main variables and results.
As the p-values for all two variables were significantly less than 0.05, we rejected the null hypothesis that the data are normally distributed. This indicates that the data for germination rate (%) and plant height (cm) do not follow a normal distribution. These findings suggest that non-parametric statistical methods may be more appropriate for analyzing these datasets.
3.1.2. Kruskal–Wallis H Test Results by Growing Media
The Kruskal–Wallis H test was performed to compare the genotypes within each growing media based on the germination rate (%) and plant height (cm) variables (Table 2).

Table 2.
Results of Kruskal–Wallis H Test carried out on Festuca wagneri.
The Kruskal–Wallis H test results indicate statistically significant differences in the germination rate (%) variable across genotypes for all growing media except for coconut fiber, where the plant height did not show significant differences (p = 0.253, p = 0.253, p = 0.253). These findings suggest that the genotype has a significant impact on germination and growth metrics for most growing media types, underscoring the importance of genetic variation when assessing plant performance across different environments (Figure 6 and Figure 7).
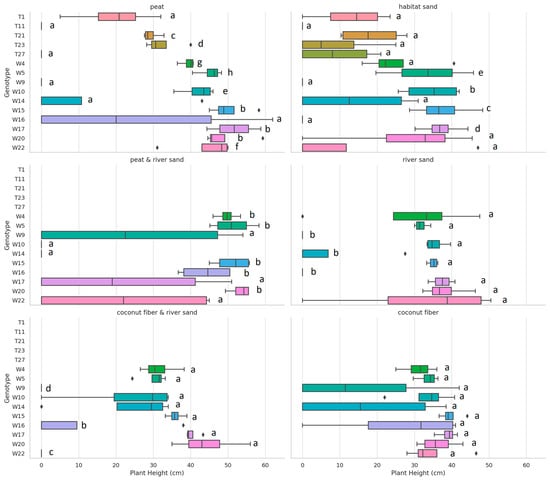
Figure 6.
Festuca tomanii (T) and Festuca wagneri (W) plant height by genotype and growing media. Genotypes sharing the same letter within the same growing medium did not have statistically significant differences at the p = 0.05 level. Different letters indicate significant differences in performance.
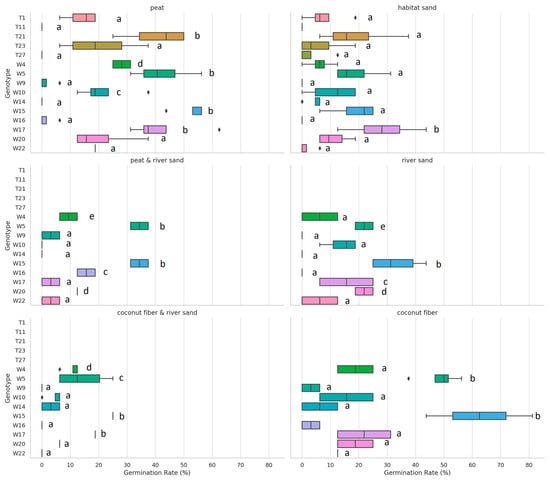
Figure 7.
Festuca tomanii (T) and Festuca wagneri (W) germination rate by genotype and growing media. Genotypes sharing the same letter within the same growing medium did not have statistically significant differences at the p = 0.05 level. Different letters indicate significant differences in performance.
3.2. Results for Festuca tomanii
Kruskal–Wallis Test Results by Growing Media of Festuca tomanii
The Kruskal–Wallis H test was performed to compare the genotypes within each medium based on germination rate (%) and plant height (cm) (Table 3).

Table 3.
Results of Kruskal–-Wallis H Test carried out on Festuca tomanii.
These results indicate that there are statistically significant differences among the genotypes for both germination rate and plant height in case of the peat substrate. However, no significant differences were found among the genotypes in the case of the sand substrate for both variables.
3.3. Genotype and Environmental Interactions
The germination rate and plant height of Festuca wagneri and Festuca tomanii were analyzed across different genotypes and growing media using the Scheirer–Ray–Hare test. This non-parametric test allowed us to assess the main effects of genotype and growing medium, as well as their interaction, on the germination success of these two species (Table 4).

Table 4.
Scheirer–Ray–Hare Test results for the effects of genotype and growing medium on germination rate and plant height in Festuca wagneri and Festuca tomanii.
The analysis of Festuca tomanii revealed significant effects for both the genotype and growing medium on the germination rate and plant height. Specifically, the test indicated that the genotype had a highly significant effect on both the germination rate (H = 82.02, p < 0.001) and plant height (H = 71.97, p < 0.001). This suggests that genetic variability among the Festuca tomanii individuals plays a crucial role in determining both the success of seed germination and the subsequent growth of seedlings.
The growing medium also significantly influenced both the germination rate (H = 5.58, p = 0.0182) and plant height (H = 7.69, p = 0.0055). The interaction between genotype and growing medium was significant for the plant height (H = 22.62, p = 0.00015) but showed a weaker effect on the germination rate (H = 9.20, p = 0.0562), suggesting that while the genotype and growing substrate interact to influence seedling growth, their combined effect on germination success is less pronounced.
In contrast, Festuca wagneri demonstrated even more pronounced effects. The genotype had an extremely significant impact on the germination rate (H = 786.15, p < 0.001) and plant height (H = 167.29, p < 0.001). Similarly, the growing medium showed a highly significant effect on the germination rate (H = 121.97, p < 0.001) and plant height (H = 67.53, p < 0.001). The interaction between the genotype and growing medium was also highly significant for both the germination rate (H = 242.51, p < 0.001) and plant height (H = 121.39, p < 0.001), indicating that the performance of Festuca wagneri genotypes is strongly dependent on the growing medium.
These findings emphasize the importance of both genetic diversity and environmental conditions in the germination and growth of Festuca species. The significant interaction effects observed in Festuca wagneri suggest that particular genotypes may be better suited to specific growing media, highlighting the need for targeted cultivation strategies in urban landscaping and ecological restoration projects.
4. Discussion
The results of the cluster analysis show that the three selected Festuca wagneri groups are distinctly separated not only from the Festuca tomanii clumps, which diverge at the highest level of dissimilarity, but also from each other. This confirms the selection based on appearance. Our results emphasize that while both Festuca tomanii and Festuca wagneri show variability in germination and growth across different the genotypes and growing conditions, Festuca wagneri exhibits a more complex interaction between genotype and environment, making it a more versatile candidate for urban planting under diverse conditions.
Germination Rate and Plant Height
The facet-wrapped boxplots for germination rate (%) and plant height (cm) across the different growing media reveal significant variability among the genotypes. In terms of germination rate, genotypes like W4 and W5 consistently show higher performance in several media, while others, such as W9, exhibit lower germination rates, particularly in the sand substrate. For plant height, genotypes such as W22 and W9 demonstrate taller plants across multiple growing media, indicating robust growth performance. Notably, peat and peat–river sand media tend to support higher variability in both germination rate and plant height, suggesting that these substrates may influence genotype performance more diversely. These findings underscore the importance of genotype selection and medium consideration in optimizing germination and growth outcomes for Festuca species.
The boxplots for Festuca tomanii illustrate the distribution of germination rate (%) and plant height (cm) for various genotypes across two media: peat and sand substrate. In the peat medium, the germination rates of the different genotypes show considerable variability, with Genotype T1 demonstrating a relatively higher and more consistent germination rate compared to others. Some genotypes, such as T11 and T21, display wider variability, indicating inconsistent performance in this medium. In contrast, the sand substrate also exhibits variability among the genotypes, with Genotype T23 having a relatively higher germination rate, while others such as T11 and T27 show lower rates. This suggests that the sand substrate may not be as conducive to germination for some genotypes as the peat medium.
Our findings indicate significant differences in seed germination across various planting media. The initially expected outcome, that the most optimal germination would occur in the native sandy substrate, was not confirmed. Instead, peat emerged as the most favorable substrate for germination. Peat is widely used in horticulture and agriculture as a vital substrate for soil improvement as well as an organic fertilizer [43,44,45].
Although the studied species grow in sandy soils, native sand (Calcaric Arenosol) did not favor seed germination, as was corroborated in this study [46]. Thus, the germination strategy of Festuca wagneri and Festuca tomanii diverged from the expected results. The river sand yielded the lowest germination percentages, and values were also low on native sand substrates. The individuals germinated most successfully in peat or peat-mixed media, where the average leaf length was also the most significant. Numerous horticultural studies have confirmed that peat is the most suitable germination medium for many plant seeds [44,45].
According to the literature, the outcomes of germination studies largely depend on the specific requirements of the Festuca species and the environmental conditions present [47]. Such studies help identify the optimal conditions necessary for successful seed germination and subsequent plant growth. During sample selection, taxa were chosen from substrates most similar to urban environments, specifically from the central, driest, and warmest regions of the Carpathian Basin. The phytosociological status of Festuca wagneri was assessed by Pócs [48], who documented it as a species of sandy steppe meadows. This species has extensive populations primarily in the Kiskunság region [49], which is characterized by an extremely dry climate. Considering seed viability is crucial when establishing natural grasslands and sustainable urban turf. Seed germination is a fundamental phase in a plant’s life cycle [46,50]. Even though a plant species may be drought-tolerant, it requires water during the initial germination phase, similar to other plants [39,48,49]. However, various factors can negatively impact germination success in urban environments. Several studies have investigated the germination of Festuca and other grass species, typically focusing on the effects of various environmental factors, such as temperature, light, moisture, soil type [49,51,52,53], soil salinity, and nutrients [54], and evaluated how different treatments affect germination percentage and speed [55,56,57]. Our study further emphasizes the significance of these factors, as substantial differences were observed even under identical conditions.
Festuca species’ seeds germinate within various temperature ranges, but the optimal temperature is typically 15–25 °C. Extremely low or high temperatures can reduce germination rates and slow the germination process [58,59,60]. Short heat treatments can sometimes improve germination rates, particularly in areas prone to natural fires. The highest germination rates for Festuca arundinacea seeds were observed at temperatures between 20 and 25 °C and soil moisture levels of 60–80% [61,62]. Therefore, in our experiments, we aimed to maintain a constant temperature of 20 °C (which in reality fluctuated between 18 °C and 21 °C), with consistent humidity.
Seeds are often exposed to cold and wet conditions before germination, which can benefit the germination of certain Festuca species [63]. Cold stratification significantly improved the germination rate of Festuca ovina seeds, particularly in their natural substrates, where cool, wet winters alternate [61]. We applied a similar approach in our study.
Some studies have also examined other factors. Most Festuca species do not require light for germination, meaning the seeds can germinate in darkness [62]. Festuca rubra seeds showed the best germination results in darkness at temperatures around 15 °C. However, in some species, the presence of light can accelerate germination [62]. In our experiment, a fourteen-hour light cycle was provided.
The soil structure and its water retention capacity also influence germination rates. Sandy soils usually have a weak structure (or are even structureless), high permeability, and poor water retention properties, and thus can dry out quickly [61]. An insufficient moisture content can impede seed germination, which we addressed by ensuring the germinating seeds received a controlled amount of water, although the limited capillary rise and low water holding capacity of sands still could result in water deficiency during germination.
In certain species, acidic treatments can enhance germination by softening the seed coat [58], and gibberellic acid treatments can also positively influence germination [63]. It is likely that in our study, the acidic nature of the peat substrate (pH 3.5–4) facilitated germination [64].
Although seed germination is fueled by the nutrients stored within the seed, the nutrient content of the soil can also affect post-germination growth [45,65].
There was also a correlation between morphological and ornamental groups and germination success. The selected four types, consisting of three groups of Festuca wagneri and one group of Festuca tomanii, exhibited varying germination success across all groups. However, three out of the four individuals in the Festuca wagneri third group, identified as the low ‘dwarf’ form, compact and dense but short in stature group (W10, W14, and W16), did not germinate or barely germinated. The most successful germination occurred in the second group, characterized by inflorescence shoots spread out, where all individuals germinated successfully. In the first group, with leaves and inflorescence densely upright, two individuals germinated successfully. Despite having the largest morphological parameters, the W22 plant in this group had low germination success. Among the selected Festuca tomanii individuals, two (T11 and T27) failed to germinate, but the remaining individuals germinated successfully. The unsuccessful germinators, T11 and T27 and W4 and W10, from the Festuca wagneri group, were grouped together, and they also stood out based on morphological parameters. The W20 individual was also associated with this group. The PCA analysis indicated that this individual had significantly large morphological parameters, and during germination, it produced the longest leaves, yet its germination success was low.
5. Conclusions
Our study demonstrates significant morphological differences between the two taxa, though some overlap was observed among individual specimens based on the classification method. This overlap is particularly important for horticultural applications, as it indicates that both taxa can be utilized with greater variability in urban environments. The morphological diversity observed among the three Festuca wagneri specimens further highlights their horticultural potential. Investigating the success of seed germination was crucial for assessing their applicability, revealing that the individuals of the present taxa are capable of germinating in various substrates, particularly in peat or peat-mixed media.
The germination tests conducted on seven different growing media indicated that native sandy soil is far from ideal for the germination and subsequent development of Festuca wagneri and Festuca tomanii, probably because of the limited water supply provided by the sandy soil texture. In contrast, peat and peat-mixed soils proved to be much more suitable. These findings suggest that Festuca species employ a specialized germination strategy. In their natural substrate, these species are typically found in mosaic environments of open or closing sandy grasslands, characterized by patches of moss and lichen. In such settings, living moss, dead plant debris, and peaty material may facilitate the germination of Festuca seeds. Similarly, in urban environments, the addition of organic matter to the soil could enhance their germination capacity through the effect of increased water holding capacity.
The question of whether these new Festuca species can be successfully utilized in urban settings can be affirmatively answered based on our germination studies. By understanding their germination strategies, we can more effectively provide the necessary conditions for the early stages of their life cycles. Their morphological diversity further supports this potential. Within the morphologically distinct groups, individuals with a significant germination potential can be identified, except for members of the third group, which includes the low ‘dwarf’ form, compact and dense but short in stature. As a result of our research, we recommend excluding this third group from urban lawn applications.
Author Contributions
Conceptualization, L.O., J.H. and K.P.; methodology, T.S.-S., Á.T. and É.H.B.; software, D.S. and Z.K.; formal analysis, T.S.-S., M.F., K.R. and J.H.; investigation, L.P., S.K., É.H.B. and J.H.; writing—original draft preparation, T.S.-S., L.P., Á.T., K.R., K.P., J.H., M.F., D.S. and P.C.; writing—review and editing, Á.T., K.P., P.C., J.R.K., K.R. and J.H.; supervision, K.P. and J.H.; funding acquisition, L.O. and K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grant OTKA K-147342 and the Research Excellence Programme of the Hungarian University of Agriculture and Life Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the first author.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the author 13's contact information. This change does not affect the scientific content of the article.
References
- Staub, J.E.; Robbins, M.D.; Larson, S.R.; Johnson, P.G. Multicolored Ornamental Festuca Grass Cultivars Freedom Fire Francy, Vida, Heidi, and Kim for Low-input Applications in Semiarid Environments. Hortscience 2017, 52, 925–931. [Google Scholar] [CrossRef]
- Loram, A.; Warren, P.H.; Gaston, K.J. Urban domestic gardens (XIV): The characteristics of gardens in five cities. Environ. Mgt. 2008, 42, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Clayton, W.D.; Harman, K.T.; Williamson, H. World Grass Species—Synonymy database; The Board of Trustees of the Royal Botanic Gardens, Kew: London, UK, 2006. [Google Scholar]
- Beard, J.B.; Green, R.L. The role of turfgrasses in environmental protection and their benefits to humans. J. Environ. Qual. 1994, 23, 452–460. [Google Scholar] [CrossRef]
- Fender, D. Urban perennial grasses in time of water crisis: Benefits and concerns. In Water Quality and Quantity Issues for Turfgrasses in Urban Landscapes; Council for Agricultural Science and Technology (CAST): Las Vegas, NV, USA, 2006; pp. 33–53. [Google Scholar]
- Hockenberry, M.M.; White, D.B.; Pellett, H. Ornamental Grasses For Minnesota. J. Environ. Hort. 1994, 12, 159–163. [Google Scholar]
- Steinegger, D.; Fech, J.C.; Lindgren, D.T.; Streich, A. Ornamental Grasses in Nebraska Landscapes; Historical Materials from University of Nebraska-Lincoln Extension: Lincoln, NE, USA, 1996. [Google Scholar]
- Botelho, F.C.; Rodrigues, S.; Bruzi, A. Ornamental Plant Breeding. Horticulture 2015, 21, 9–16. [Google Scholar] [CrossRef]
- Meyer, H.M.; White, D.B.; Pellet, H. Ornamental Grasses for Cold Climates; Department of Horicultural Science, University of Minnesota: Minneapolis, MN, USA, 2020. [Google Scholar]
- Stukonis, V.; Lemežienė, N.; Kanapeckas, J. Suitability of narrow-leaved Festuca species for turf. Agron. Res. 2010, 8, 729–734. [Google Scholar]
- Love, S.L.; Noble, K.; Parkinson, S.; Bell, S. Herbaceous Ornamentals Annuals, Perennials, and Ornamental Grasses; University of Idaho: Moscow, ID, USA, 2009. [Google Scholar]
- Meyer, H.M.; Mower, G.R. Ornamental Grasses for the Home and Garden; Cornell University: New York, NY, USA, 1986. [Google Scholar]
- Staub, J.E.; Robbins, M.D. Phenotypic and Genotypic Analysis of a U.S. Native Fine-leaved Festuca Population Reveals Its Potential Use for Low-input Urban Landscapes. J. Am. Soc. Hort. Sci. 2014, 139, 706–715. [Google Scholar] [CrossRef]
- Dobignard, D.; Chatelain, C. Index Synonymique de la Flore d’Afrique du Nord; Éditions des Conservatoire et Jardin Botaniques Genève: Geneva, Switzerland, 2010; pp. 401–441. [Google Scholar]
- Giraldo-Cañas, D. Catálogo de la familia Poaceae en Colombia. Darwiniana 2011, 49, 139–247. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea 5; Cambridge University Press: Cambridge, UK, 1980; pp. 1–494. [Google Scholar]
- Stace, C. New Flora of the British Isles 4; C & M: Cambridge, UK, 2019. [Google Scholar]
- Patzke, E. Zur Kenntnis der Sammelart Festuca ovina L. im südlichen Niedersachsen. Götting. Flor. Rundbr. 1968, 4, 14–17. [Google Scholar]
- Pils, G. Systematik, Karyologie und Verbreitung der Festuca valesiaca-gruppe (Poaceae) in Österreich und Südtirol. Phyton 1984, 24, 35–77. [Google Scholar]
- Dostal, J. Nová Kvetena ČSSR I-II; Academia: Praha, Czech Republic, 1989. [Google Scholar]
- Adler, W.; Oswald, K.; Fischer, R. Exkursionflora von Österreich; Ulmer: Stuttgart, Germany; Vienna, Austria, 1994. [Google Scholar]
- Rothmaler, W.; Echerhart, J.; Jäger, E.J.; Klaus, W. Exkursionflora von Deutschland. Band 4 Kritischer Band; Spektrum Akademischer Verlag Heidelberg: Berlin, Germany, 2002. [Google Scholar]
- Săvulescu, T. Flora Reipublicae Socialisticae Romania XII; Edit. Academiae Reipublicae Socialisticae Romănia: București, Romania, 1972. [Google Scholar]
- Brookes, J. Book of Gardens; Officina Nova Kiadó: Budapest, Hungary, 1991. [Google Scholar]
- Dąbrowska, A. Evaluation of the decorative value of wild-grown Festuca trachyphylla (Hack.) Krajina in the southeastern part of Poland. Folia Hort. 2013, 25, 13–19. [Google Scholar] [CrossRef]
- Tomaškin, J.; Tomaškinová, J.; Kizekova, M. Ornamental grasses as part of public green, their ecosystem services and use in vegetative arrangements in urban environment. Thaiszia J. Bot. 2015, 25, 1–13. [Google Scholar]
- Schmidt, G. Cultivation, Knowledge and Use of Perennial Ornamental Plants; Corvinus Kiadó: Budapest, Hungary, 2005. [Google Scholar]
- Zsohár, C.S.; Zsohárné Ambrus, M. Perennial Ornamental Plants; Botanika Kft.: Budapest, Hungary, 2001. [Google Scholar]
- Stewart, A. The potential for domestication and seed propagation of native New Zealand grasses for turf. In Greening the City: Bringing Diversity Back into the Urban Environment; Royal New Zealand Institute of Horticulture: Lincoln, New Zealand, 2005; pp. 277–284. [Google Scholar]
- Nawrocki, A.; Popek, R.; Przybysz, A. Where Trees Cannot Grow—Herbaceous Plants as Filters in Air Purification from PM. 2024. Available online: https://doi.org/10.24326/ICDSUPL2.E026 (accessed on 21 May 2024).
- Souri, M.K. Influence of N-forms and changes in nutrient solution pH on growth of Palisadegrass plants. J. Plant Nutr. 2022, 45, 1827–1836. [Google Scholar] [CrossRef]
- Török, P.; Schmidt, D.; Bátori, Z.; Aradi, E.; Kelemen, A.; Hábenczyus, A.A.; Diaz, C.P.; Tölgyesi, C.; Pál, R.W.; Balogh, N.; et al. Invasion of the North American sand dropseed (Sporobolus cryptandrus)—A new pest in Eurasian sand areas? Glob. Ecol. Conserv. 2021, 32, e01942. [Google Scholar] [CrossRef]
- Tilley, D.; St. John, L.; Ogle, D. Plant Guide for Sand Dropseed (Sporobolus cryptandrus); USDA Natural Resources Conservation Service, Idaho Plant Materials Center: Aberdeen, ID, USA, 2009.
- Penksza, K.; Engloner, A. Taxonomic study of Festuca wagneri (Degen Thaisz et Flatt) Degen, Thaisz et Flatt. 1905. Acta Bot. Acad. Sci. Hung. 1999/2000, 42, 257–264. [Google Scholar]
- Korneck, D.; Gregor, T. Festuca tomanii sp. nov., ein Dünen-Schwingel des nördlichen oberrhein-, des mittleren main- und des böhmischen Elbetales. Kochia 2015, 9, 37–58. [Google Scholar] [CrossRef]
- Wilson, B.L.; Darris, D.C.; Fiegener, R.; Johnson, R.; Horning, M.E.; Kuykendall, K. Seed transfer zones for a native grass (Festuca roemeri): Genecological evidence. Nativ. Plants J. 2008, 9, 287–303. [Google Scholar] [CrossRef]
- Ushey, K.; Allaire, J.; Tang, Y. Reticulate: Interface to ‘Python’. R Package Version 1.38.0. 2024. Available online: https://github.com/rstudio/reticulate (accessed on 13 May 2024).
- Virtanen, P.; Reddy, T.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, J.N.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Waskom, M. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin, Germany, 2016. [Google Scholar]
- Tarnawa, Á.; Kende, Z.; Sghaier, A.H.; Kovács, G.P.; Gyuricza, C.; Khaeim, H. Effect of Abiotic Stresses from Drought, Temperature, and Density on Germination and Seedling Growth of Barley (Hordeum vulgare L.). Plants 2023, 12, 1792. [Google Scholar] [CrossRef] [PubMed]
- Meixue, Q.; Duan, W.; Chen, L. The role of cryptogams in soil property regulation and vascular plant regeneration A review. Appl. Sci. 2024, 14, 2. [Google Scholar] [CrossRef]
- Kitir, N.; Yildirim, E.; Şahin, Ü.; Turan, M.; Ekinci, M.; Ors, S.; Kul, R.; Ünlü, H.; Ünlü, H. Peat use in horticulture. In Peat; IntechOpen: London, UK, 2018; pp. 75–90. [Google Scholar] [CrossRef]
- Zhong, Z.; Bian, F.; Zhang, X. Testing composted bamboo residues with and without added effective microorganisms as a renewable alternative to peat in horticultural production. Ind. Crops Prod. 2018, 112, 602–607. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed Germination and Dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef]
- Larsen, S.U.; Bailly, C.; Côme, D.; Corbineau, F. Use of the hydrothermal time model to analyse interacting effects of water and temperature on germination of three grass species. Seed Sci. Res. 2004, 14, 35–50. [Google Scholar] [CrossRef]
- Pócs, T. Die Vegetation des “Akademischen Waldes” in Rákoskeresztúr. Bot. Közlemények 1954, 45, 283–294. [Google Scholar]
- Csáky, P.A. Important plant species of the northern part of Turjánvidék from a floristic point of view. In Nature Conservation and Research in the Northern Part of Turjánvidék Rosalia; Duna–Ipoly Nemzeti Park Igazgatóság: Budapest, Hungary, 2018; Volume 10, pp. 145–252. [Google Scholar]
- Nimbalkar, M.S.; Pawar, N.V.; Pai, S.R.; Dixit, G.B. Synchronized variations in levels of essential amino acids during germination in grain Amaranth. Braz. J. Bot. 2020, 43, 481–491. [Google Scholar] [CrossRef]
- Aldana, S.; López, D.R.; López, M.V.; Arana, D.; Batlla Marchelli, B. Germination response to water availability in populations of Festuca pallescens along a Patagonian rainfall gradient based on hydrotime model parameters. Sci. Rep. 2021, 11, 10653. [Google Scholar] [CrossRef]
- Eshghi, S.; Bahadoran, M.; Salehi, H. Growth of tall fescue (Festuca arundinacea Schreb.) seedling sown in soil mixed with nitrogen and natural zeolite. Adv. Hortic. Sci. 2014, 28, 20–24. [Google Scholar] [CrossRef]
- Vivanco, P.; Oliveira, J.A.; Martín, I. Optimal germination conditions for monitoring seed viability in wild populations of fescues. Span. J. Agric. Res. 2021, 19, e0804. [Google Scholar] [CrossRef]
- Gregorie, G. Effects of Organic Fertilizers on Turfgrass Quality and Growth. Master’s Thesis, The University of Guelph, Ottawa, ON, Canada, 2004. [Google Scholar]
- Liu, Y.; Zhang, S.; De Boeck, H.J.; Hou, F. Effects of Temperature and Salinity on Seed Germination of Three Common Grass Species. Plant Sci. Sec. Funct. Plant Ecol. 2021, 12, 731433. [Google Scholar] [CrossRef] [PubMed]
- Bewley, D.J.; Bradford, K.J.; Hilhorst, K.J.; Henk, W.M.; Nonogaki, H. Seeds, Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Zhang, T.; Liu, M.; Huang, X.; Hu, W.; Qiao, N.; Song, H.; Zhang, B.; Zhang, R.; Yang, Z.; Liu, Y.; et al. Direct effects of nitrogen addition on seed germination of eight semi-arid grassland species. Ecol. Evol. 2020, 10, 8793–8800. [Google Scholar] [CrossRef]
- Shiade, S.R.G.; Boelt, B. Seed germination and seedling growth parameters in nine tall fescue varieties under salinity stress. Acta Agric. Scand. Sestion B. Soil Plant Sci. 2020, 70, 485–494. [Google Scholar] [CrossRef]
- Ali, A.H.; Marghany, M.R.; Atito, E.; BaruÇular, C.; Kamel, N.M.; Mohamed, M.M.; Ahmed, M.M.; El-Sayed, M.A. Desert plants seeds morphology and germination strategy. Int. J. Conserv. Sci. 2022, 13, 1249–1260. [Google Scholar]
- Stanisavljević, R.S.; Vucković, S.M.; Simić, A.S.; Marković, J.P.; Lakić, Z.P.; Terzić, D.V.; Dokić, D.J. Acid and Temperature Treatments Result in Increased Germination of Seeds of Three Fescue Species. Not. Bot. Horti Agrobot. Cluj Napoca 2012, 40, 220–226. [Google Scholar] [CrossRef][Green Version]
- Nematollahi, F.; Tehranifara, A.; Nematia, S.H.; Kazemia, F.; Gazanchianb, G.A. Improving early growing stage of Festuca arundinacea Schreb. using media amendments under water stress conditions. Desert 2018, 23, 295–306. [Google Scholar]
- Danielson, H.R.; Toole, V.K. Action of Temperature and Light on the Control of Seed Germination in Alta Tall Fescue (Festuca arundinacea Schreb.). Crop Sci. 1976, 16, 296–300. [Google Scholar] [CrossRef]
- Al-Qahtani Alhajhoj, M.R. Effect of Addition of Sand and Soil Amendments to Loam and Brick Grit Media on the Growth of Two Turf Grass Species (Lolium perenne and Festuca rubra). J. Appl. Sci. 2009, 9, 2485–2489. [Google Scholar] [CrossRef][Green Version]
- Çelikler, S.; Güleryüz, G.; Bilaloğlu, R. Germination Responses to GA3 and Stratification of Threatened Festuca L. Species from Eastern Mediterranean. Z. Naturforschung C 2006, 61, 372–376. [Google Scholar] [CrossRef]
- Zargar Shooshtari, F.; Souri, M.K.; Hasandokht, M.R.; Jari, S.K. Glycine mitigates fertilizer requirements of agricultural crops Case study with cucumber as a high fertilizer demanding crop. Chem. Biol. Technol. Agric. 2020, 7, 19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).