Abstract
Planting nitrogen-fixing plants in post-mining sites and similar degraded areas is a common approach to speed up soil development and buildup of the nitrogen pool in soil organic matter. The aim of this study was to explore if slower growth of alder seedlings in initial post-mining sites results from adverse soil conditions or lack of microbial symbionts. To address this question, we sampled young soil (age 15 years) and more developed soil (age 70 years) from heaps after coal mining near Sokolov (Czech Republic). Soil samples were sterilized and not inoculated or inoculated with arbuscular mycorrhizal fungi (AMF) or AMF + Frankia, followed by planting with alder (Alnus glutinosa) seedlings germinated and precultured under sterile conditions. The effect of soil age on alder growth appeared to be non-significant. The only significant growth effect was seen with Frankia inoculation, implicating this inoculum as a key factor in later succession in post-mining soils. When the soil was fully inoculated, alder biomass was higher in developed soil supplied with iron (Fe) and phosphorus (P), indicating that iron and phosphorus availability may affect alder growth. In young soil, alder growth was highest with a combination of iron, phosphorus, and sulfur (S), and a positive effect of sulfur in young soil may correspond with a reduced, alkaline soil pH and increased phosphorus and iron availability.
1. Introduction
Post-mining sites often suffer from a lack of available nitrogen (N) [1]. Planting N-fixing vegetation is a common approach to speeding up soil development and buildup of the N pool in soil organic matter [2,3,4,5]. Alders are also known as pioneer trees that usually populate disturbed habitats, which are often poor in nutrients, especially N in the form of nitrates (NO3−) and ammonium ions (NH4+) [6,7]. Alder overcomes this deficiency by hosting bacteria of the genus Frankia in its root tubers/nodules, which fix atmospheric nitrogen (N2) into ammonia (NH3) using the enzyme nitrogenase.
Frankia are Gram-positive bacteria from the Actinobacteria group. Frankia alni is the only described species in this genus that lives in symbiosis with actinorhizal plants in the genus Alnus. Otherwise, the genus Frankia includes a number of strains divided into four clusters based on genome sizes [8] and on the specificity of their symbiotic relationships with plants. In the symbiosis between Frankia and plants of the Betulaceae family, intracellular infection is a characteristic feature [9]. Representatives of the genus Frankia can fix N2 from the air, both when the actual bacteria are in the root nodules of the host plants and when they are distributed freely in the soil [8]. Nitrogenase fixes atmospheric N [10] but is susceptible to the presence of oxygen. Its activity is localized in the vesicles of Frankia, which bear an oxygen-impermeable hopanoid envelope. Root nodule functions (N fixing) can consume up to 12% of the total photosynthetic production of the host plant, so symbiosis is advantageous mainly in N-poor habitats. In these habitats, however, symbiosis can be limited by a lack of Frankia inoculum.
Aside from Frankia, alders enter into symbiosis with arbuscular mycorrhizal fungi (AMF). AMF improves plant uptake of phosphorus, N, sulfur (S), and micronutrients such as zinc and copper [11,12] because their extraradical hyphae can branch more finely than plant roots [12,13]. Furthermore, AMF can absorb toxic metals before they reach the host plant and improve water uptake from the soil [11]. Root colonization by AMF can be limited because of their low dispersal abilities [14]. Soil properties such as pH, as well as plant community composition, can also affect AMF symbiotic relationships [15].
In addition, soil properties can influence N fixation and change remarkably during post-mining site development. As soil development unfolds, nutrient availability may change, which can be related to soil pH, the presence of other nutrients, and the interaction of elements. Among nutrients, iron (Fe) has many irreplaceable roles in the nitrogenases of the symbiotic Frankia bacteria [16,17]. Using specific reductases, plants obtain Fe from the soil by active sorption in the form of Fe2+ and Fe3+ ions, which are bound to oligopeptide chelates within the plant. Calcium ions (Ca2+) limit the intake of Fe [18]. Sulfur enters plants in the form of sulfates (SO42−), which are readily taken up together with nitrates and transported throughout the plant [19]. Phosphorus, which is often immobilized by clay particles [20], is actively taken up by plants in the form of hydrogen phosphates (H2PO4− or HPO42−). Plants with optimized P intake in low-nutrient soils are characterized by a dense root system or strongly developed AMF symbiosis.
In this study, we aimed to evaluate the effect of soil development that is associated with mining activity and inoculation (Frankia and AMF) on the growth (biomass production) of alder plants. For this reason, we performed an experiment with two types of soil acquired from post-mining sites in Sokolov (Czech Republic): the young and old soil types and inoculated with AMF or Frankia and their combination. Both young and old soil are part of the same chronosequence of spontaneously developing soil [21]. We cultured alder plants in the experimental pots filled with these types of soils. We measured the chemical parameters of these soils after finishing the experiments to see their effects on the biomass of alder plants. We considered root colonization of AMF in different treatments for evaluating the effect of AMF on alder growth. We hypothesized that microbial communities have significant effects on the growth of alder, and the greater growth effect of alder would be expected with Frankia inoculation. We also aim to evaluate the effect of soil properties (pH and selected micronutrients: Ca, Fe, P, and S) on the growth of inoculated plants. For this point, we performed another experiment using the above-mentioned soil types, and micronutrients were added to the experimental pots. We hypothesized that alder growth would be highest with a combination of iron, phosphorus, and sulfur, and we would expect a difference in alder growth between the two soil types.
2. Materials and Methods
We conducted two greenhouse experiments. One (Experiment 1) was completed at the Institute of Botany of the Czech Academy of Sciences in Průhonice, Czech Republic, and the other (Experiment 2) at the Institute of Soil Biology and Biogeochemistry of the Biology Centre of the Czech Academy of Sciences in České Budějovice, Czech Republic.
Heaps remaining after brown coal mining in the Sokolov area (Czech Republic) are located at 500–600 m above sea level. The average annual rainfall is 650 mm, with an average annual temperature of 6.8 °C, and the pH range is 2.1–8.5. The heaps consist mostly of alkaline tertiary substrates with a clay-like structure, which usually do not represent major limitations for the development of plants and other soil organisms. For both experiments, two types of soil were acquired from post-mining sites in Sokolov. At the time of this work, the young soil type originated in the tertiary, had a clayey texture, and was 15 years old, i.e., had undergone ecological succession for 15 years before the sampling. The developed soil type had a less clayey texture and was 70 years old at the time of the experiments.
We used the common alder (Alnus glutinosa (L.) Gaertn.) as the experimental species. Seeds were bought from Lesy České republiky Company (Czech Republic).
2.1. Experiment 1
2.1.1. Treatments and Experimental Design
Soils were sterilized by 27 kGy in the Conservation Radiation Centre of the Central Bohemia Region Museum in Roztoky u Prahy (Czech Republic). The experimental setup consisted of 700 mL pots, each containing 200 mL of expanded clay as drainage, with 450 mL of soil then added (either young or developed). Subsequently, we added either 10 mL of arbuscular mycorrhizal inoculum suspension or 10 mL of this suspension twice, sterilized by autoclaving before application to ensure the same amount of organic matter in all treatments. The inoculum itself was prepared from isolate PH5 of Rhizophagus irregularis [22] over the course of 3 months, during which the mycorrhizal fungi grew on corn (Zea mays) roots in a sterilized mixture of zeolite and river sand (1:1). The inoculum suspension was made by means of wet sieving through 250 µm and 50 µm sieves and contained spores, extraradical mycelium, and colonized root fragments.
To the respective treatments, we added 100 mL of Frankia inoculum, prepared from root nodules taken from alders growing at Sokolov post-mining sites. Finally, we added 200 mL more of sterilized soil (young or developed) and, immediately after this step, planted the alder seedlings. Seedlings were grown in sterile conditions after their planting; seeds were sterilized in 4.7% sodium hypochlorite solution and germinated in sterilized sand. Thus, both inoculums were found below the seedling roots. In total, for each soil type, we had three treatments (AMF, AMF + Frankia, and no addition of inoculum as control), and for each treatment, we had seven replicates.
After this setup was established, seedlings were left to grow in their respective pots for 5 months (May–October 2016) in the greenhouse (temperature 20–35 °C during the day, minimum 15 °C during the night) without additional lighting. Watering was conducted with deionized water, usually every day.
2.1.2. Harvest and Post-Harvest Processing
Alders were taken out of their respective pots and dissected into underground and aboveground biomass. The underground biomass was divided into roots and Frankia nodules and weighed fresh. The aboveground biomass was divided into shoots and leaves. The leaf area was measured with an LI-COR Planimeter (type LI-3100). We weighed shoots and leaves as dry biomass after drying them at 60 °C.
The soil remaining after the harvesting was subjected to an array of analyses conducted at the Laboratory of Environmental Chemistry of the Institute for Environmental Studies (Charles University in Prague, Czech Republic). From dried soil stored at room temperature in the dark, we rendered a 2 mm fraction with the help of a 2 mm sieve. From this fraction, we gained values for pH, conductivity, nitrate concentration, available P, and Fe content.
For the pH and conductivity analyses, we prepared aqueous leachate from each sample (10 g of the soil sample leached in 50 mL of deionized water). The leaching samples were left on a shaker for 1 h, then left to settle for 2 days. After this process, we filtered the samples through Vitrum V90 filter paper. The electrodes of the pH meter (SCHOTT Instrument Lab 850) and conductometer (HANNA Hi 8733 Conductivity meter) were washed thoroughly with deionized water between samples.
To measure nitrate levels in the experimental soils, we prepared leachate from each sample using the same procedure as described for the pH and conductivity analyses, with the inclusion of a blank sample consisting only of deionized water. The leached nitrates were detected via direct spectrophotometry in the UV part of the spectrum (210 nm wavelength). A 1 cm cuvette was used, and the diagnostic noise was removed by subtracting the absorbance value at 275 nm.
For measuring P concentration in the soil, a leachate was prepared of a respective soil sample (1 part) in a 1 M potassium chloride solution (5 parts). The samples in the solution were left on a shaker for 1 h and then filtered immediately. Available P was then measured spectrophotometrically using molybdenum blue. Samples from soils taken in the Sokolov area, which are very poor in phosphorus, had to be adjusted with 1 mL of a mixed agent added to 10 mL of the extract. Ten minutes after the addition, the samples were measured at 889 nm in a 5 cm flow-through cuvette against distilled water. There was no need for turbidity correction.
To gain a leachate suitable for Fe analysis, we applied the same protocol as described for P leachate. The leachates were analyzed without the need for dilution by means of flame atomic absorption spectrometry on a VARIAN apparatus (type SpectrAA 280 FS). The apparatus allowed for the calculation of the Fe concentration directly from measured absorbance. Additionally, we calculated the concentration in mg/kg in relation to the soil sample. For calibration, we used samples made in the Laboratory of Flame Atomic Absorption Spectrometry of the Faculty of Science, Charles University.
To assess the degree of root colonization by AMF, root subsamples were taken, cut into ~1 cm pieces, and stained with 0.05% trypan blue [23]. Colonization was quantified under a compound microscope (Olympus BX60) at ×200 magnification following the method of McGonigle et al. [24]. One hundred root intersections were observed using the eyepiece grid per root sample while recording the occurrence of arbuscular mycorrhizal structures (hyphae, arbuscules, and vesicles).
2.2. Experiment 2
2.2.1. Treatments and Experimental Design
For this experiment, the soils, seeds, and sand used for preparing seedlings were not sterilized. The experiment consisted of 400 mL pots filled to the top with young and developed soils. After pots were filled with the respective soil type, chemicals were added as follows for each soil type: calcium (Ca) as calcium carbonate, S, Fe + phosphate (FeP), Fe + phosphate + S (FePS), or control (no addition of chemical). There were six pots for each experimental treatment; one was used for measuring pH at the beginning of the experiment and was not used for growing alders. The pH values (two replicates for each sample) were measured in these pots.
Alders were planted in the experimental pots one week after the addition of the respective chemical(s) and left to grow for 4 months (November 2016–February 2017) in a cultivation box with a stable temperature (22 °C) and light regime (14/10 LD, 600 W·m−2 lights). The pots were watered every day, including in the first week of the experiment when there were no alder seedlings growing in them. During the first month of the experiment, we had to replace deceased seedlings with new ones in a few pots.
2.2.2. Harvest and Post-Harvest Processing
Alders were taken out of their respective pots and dissected into underground and aboveground biomass. The underground biomass was divided into roots and Frankia nodules and weighed fresh. The aboveground biomass was divided into shoots and leaves. Leaf area was measured with a LI-COR Planimeter (type LI-3100), and leaves were weighed fresh.
After the plants were harvested, we made leachates from each of the pots without drying the soil samples. We used 10 g soil samples and 50 mL deionized water for rendering each leachate. The samples were placed on a shaker for 1 h, then left to settle down overnight before being filtered through Vitrum V90 filter paper. The electrodes of a pH meter (SCHOTT Instrument Lab. 850) and conductometer (HANNA Hi 8733 Conductivity meter) were thoroughly washed with deionized water between samples.
2.3. Statistical Analyses
Data were processed in R (version 3.4.0 RC; R Core Team, [25]). We used (generalized) linear models, two-sample t-tests, and multivariate statistical methods requiring the vegan [26] and permute [27] packages. R also served as a tool for creating graphs of the results from basic exploratory analyses, requiring the ‘sciplot’ package [28]. We analyzed data with two-way analysis of variance (ANOVA) for the effects of soil type and inoculation and their interactions. A Fisher’s least significant difference post hoc test was also used for determining significant differences among treatments. We also performed a two-way ANOVA followed by a post hoc test for the effect of chemistry parameters of the soil samples in different experimental treatments and the same analysis for the effects of micronutrients on the biomass of alder plants. For these analyses, Statistica (version 13.3.0; TIBCO®, Santa Clara, CA, USA) was used.
3. Results
3.1. Experiment 1
In the first experiment, the aboveground biomass of alder plants was significantly higher in the developed compared with the young soil. Compared with the AMF and control plants, the AMF + Frankia treatment group had the highest aboveground biomass in both developed and young soils (Figure 1A). Two-way ANOVA showed significant effects of inoculum (treatment) on the aboveground biomass of alder plants (F2,36 = 12.8, p < 0.001; Figure 1A, Table S1). The effects of soil on the aboveground biomass of alder and the interaction of soil × inoculum were not significant.
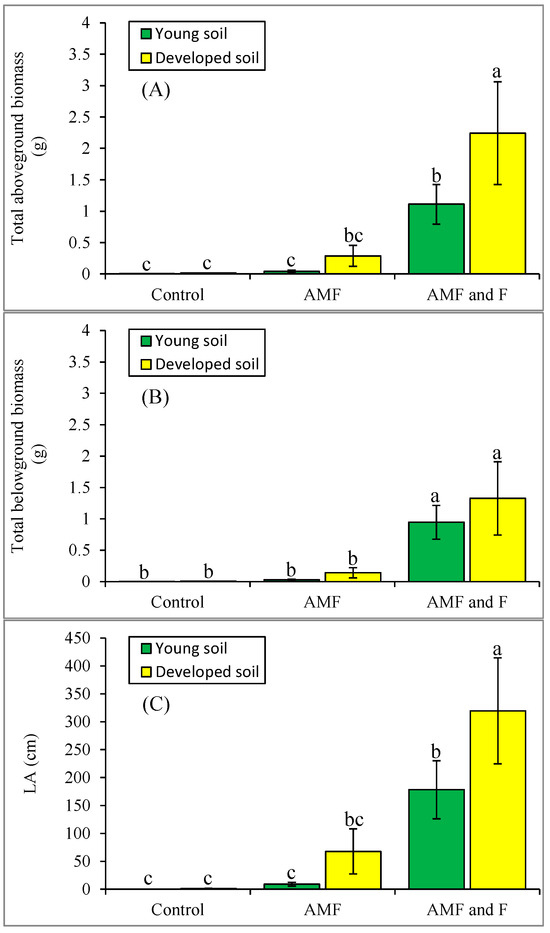
Figure 1.
Biomass of alder plants in Experiment 1 as aboveground (A) and belowground biomass (B) and leaf area (LA, cm2; (C)). The letters are based on the results of a 2-way ANOVA for biomass of alder plants (p < 0.05), followed by a Fisher’s least significant differences post hoc test (see Table S1). AMF: arbuscular mycorrhizal fungi; F: Frankia.
Belowground biomass was also higher in the developed soil compared with young soil, but not significantly so. The AMF + Frankia group had the highest belowground biomass in both developed and young soils compared with the AMF and control groups (Figure 1B). The results of a two-way ANOVA showed significant effects of inoculum on the belowground biomass of alder plants (F2,36 = 11.4, p < 0.001; Figure 1B, Table S1).
Measurements of leaf area showed significantly higher values in the developed compared with the young soil. The AMF + Frankia group had the highest leaf area in both developed and young soils compared with plants from the AMF and control groups (Figure 1C). The results of a two-way ANOVA showed significant effects of inoculum on the leaf area of alder plants (F2,36 = 16.1, p < 0.001; Figure 1C, Table S1).
pH was highest in the developed soil with AMF added, and conductivity was significantly higher for the young soil compared with the developed soil (Table 1). Conductivity was lowest with the AMF treatment and in the control condition. The effect of soil on the treatments was significant, but the effect of inoculum and the interaction of soil and inoculum were not significant for pH or conductivity (F1,36 = 7.80, p < 0.01 for pH; F1,36 = 11.4, p < 0.01 for conductivity).

Table 1.
Chemistry parameters of soil samples measured in Experiment 1.
Nitrate was lower with the young soil than with the developed soil, and the effect of soil, inoculation, and their interactions on nitrate concentrations was significant (F1,36 = 186, p < 0.001 for soil; F2,36 = 16.4, p < 0.001 for inoculum; F2,36 = 14.1, p < 0.001 for the interaction of soil and inoculum; Table 1). Iron was highest for the young soil (treatment AMF + Frankia), and the effect of soil, inoculation, and their interactions on iron concentrations was significant (F1,36 = 18.6, p < 0.001 for soil; F2,36 = 17.7, p < 0.001 for inoculum; F2,36 = 14.5, p < 0.001 for the interaction; Table 1). Phosphate was highest for all treatments with the developed soil, and the effect of soil on phosphate concentrations was significant (F1,36 = 82.5, p < 0.001; Table 1).
Figure 2 shows the rate of alder root colonization by AMF in individual treatments of the first trial, expressed as a percentage of colonized roots. Microscopically, we detected no AMF in the roots of alder trees grown in either control treatment. As Figure 2 also shows, the degree of colonization of alder roots grown with the AMF inoculum alone and with AMF + Frankia differed significantly (p < 0.05). With the inoculum of fungus alone, 60% of the roots were colonized, on average, whereas with the inoculum of fungus and bacteria, the fungus was present in an average of 82% of the roots. A statistical test of the generalized linear model, in which the presence of AMF in alder roots was explained by the mass of Frankia-containing nodules on the roots (as a marker of root colonization by Frankia bacteria), was highly significant (p < 0.001). At the same time, the linear model test, in which colonization by AMF explained the rate of colonization by Frankia, was also significant (p < 0.01).
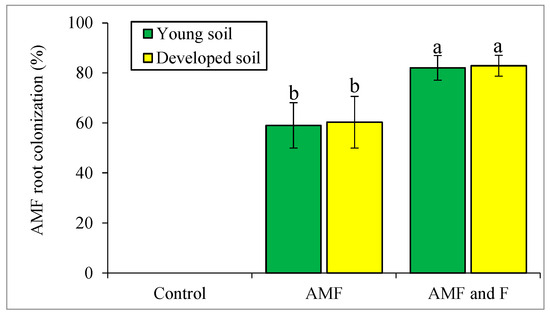
Figure 2.
The percentage of root colonization by AMF in Experiment 1. The letters are based on the results of the generalized linear model (p < 0.05), followed by Fisher’s least significant difference post hoc test. AMF: arbuscular mycorrhizal fungi; F: Frankia.
3.2. Experiment 2
In the second experiment, the aboveground biomass of alder plants was significantly higher in the developed soil compared with the young soil, except for the FePS treatment (Figure 3A). The highest aboveground biomass was observed with the FeP treatment, and plants from the Ca treatment had the lowest aboveground biomass (similar to the control plants). Two-way ANOVA showed significant effects on the aboveground alder biomass of soil, chemical addition, and the interaction of soil and chemicals (F1,40 = 11.6, p < 0.01 for soil; F4,40 = 44.4, p < 0.001 for chemicals; F4,40 = 5.76, p < 0.001 for the interaction of soil and chemicals; Figure 3A, Table S2).
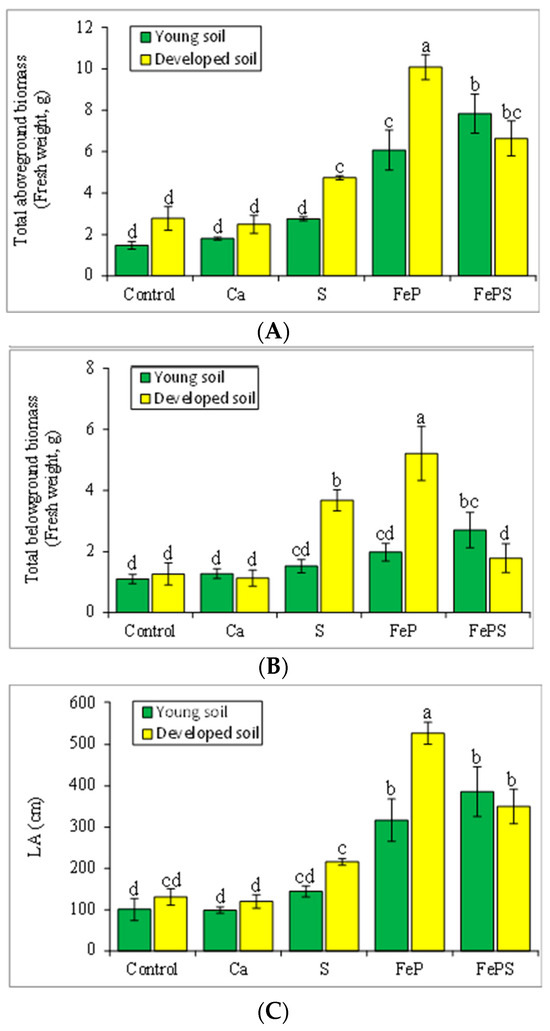
Figure 3.
Biomass of alder plants in Experiment 2, as aboveground (A) and belowground biomass (B) and leaf area (LA, cm2; (C)). The letters are based on the results of 2-way ANOVA for biomass of alder plants (p < 0.05), followed by a Fisher’s least significant differences post hoc test (see Table S2).
Belowground biomass of alder plants was significantly higher in the developed soil compared with the young soil, except for the FePS and S treatments (Figure 3B). The highest belowground biomass was observed with the FeP treatment, and the S and Ca treatments had the lowest belowground biomass (similar to the control plants). Two-way ANOVA showed significant effects on the belowground alder biomass of soil, chemical addition, and the interaction of soil and chemicals (F1,40 = 9.37, p < 0.01 for soil; F4,40 = 11.9, p < 0.001 for chemicals; F4,40 = 9.36, p < 0.001 for the interaction; Figure 3B, Table S2).
For the measurements of leaf area, values were significantly higher with the developed soil compared to the young soil, except for the FePS treatment. Plants from the FeP treatment had the highest leaf area in both developed and young soils, and the Ca treatments had lower leaf area values (Figure 3C). Two-way ANOVA showed significant effects on alder plant leaf area of soil, chemical addition, and the interaction of soil and chemicals (F1,40 = 7.75, p < 0.01 for soil; F4,40 = 38.9, p < 0.001 for chemicals; F4,40 = 4.06, p < 0.01 for the interaction; Figure 3C, Table S2). Ca did not show significant effects on aboveground biomass, belowground biomass, and leaf area (Figure 3).
pH was highest for the young soil, especially with Ca treatment (pH 8.06), and it was lowest for the developed soil, especially with the FePS treatment (6.98). The effect of soil type and chemical addition on pH levels was significant, but the interaction of soil and chemicals did not have a significant effect on pH (F1,30 = 45.7, p < 0.001 for soil; F4,30 = 7.67, p < 0.001 for chemicals; Table 2). Conductivity was significantly highest for the young soil with the S treatment, and it was lowest for the developed soil with Ca treatment, control conditions, and FeP treatment (Table 2). The effect on the conductivity of soil type, chemical addition, and their interaction was significant (F1,30 = 76.1, p < 0.001 for soil; F4,30 = 81.9, p < 0.001 for chemicals; F4,30 = 8.11, p < 0.001 for the interaction; Table 2).

Table 2.
Chemistry parameters of soil samples measured in Experiment 2.
4. Discussion
The current results indicate a non-significant effect of soil development on alder growth. The only significant effect was microbial from Frankia inoculation, suggesting that Frankia inoculum is a key factor in later succession in post-mining soils. In agreement with previous studies [29,30], our results show that Frankia inoculation is a basic precondition for successful alder growth. The implications for reclamation practice are that scarcity of Frankia inoculation in early successional stages may explain the low performance of alder plants in these initial stages [29,30,31]. This effect is also applied in well-developed soil, where nutrients, including N, are readily available, indicating that alders rely on their symbionts even in the presence of available nutrients. Of note, we found that Frankia inoculation increased not only alder growth but also AMF colonization. We speculate that this effect is indirect. Frankia-supported alders grow more vigorously, and alders thus may have more assimilates to share with AMF. We did not have a Frankia-alone treatment to distinguish the effects of Frankia and AMF on tree growth; however, previous experiments with various species of Casuarina indicate that the outcome may vary depending on the combination of host species and Frankia strain [32].
Although the two experiments from this study are not fully comparable, a negative effect of sterilization is apparent from the limited alder growth in sterilized soil (Experiment 1) compared with non-sterilized soil (Experiment 2). The substantially higher alder growth in non-sterilized soil in early succession also may suggest that in our soils, some inoculation potential exists even in initial successional stages. In contrast to the effects of Frankia, AMF colonization did not significantly increase alder growth.
The effect of soil was not significant in Experiment 1, but developed soil was associated with significantly higher alder growth in the non-sterilized soil used in Experiment 2. This finding suggests that after the Frankia colonization barrier is overcome, soil conditions are important drivers of alder growth. Our experiment showed that the addition of P and Fe increased plant growth, which may be in agreement with the positive effect of these elements on N fixation, as others have noted [16,17,33]. However, total P and presumably also Fe are quite abundant in our sites [20], and the overall availability of these elements may be a key issue. As already mentioned, overburden material is alkaline in our sites, and pH gradually decreases during site development. Moreover, P can be bound to clay minerals [20]. In our experiments, we added these elements in available form, so their increased availability could be expected to trigger higher plant growth because of their effects on N fixation. This influence also may explain why Fe and P addition had a greater effect in more developed soil with already lower pH compared to early stages. Furthermore, it could explain the positive effect of S addition in young soil, where beyond its direct effects, S can acidify alkaline soil and increase P and Fe availability [34]. When the soil was fully inoculated, alder biomass was higher in developed soil supplied with Fe and P, suggesting that Fe and P availability may limit alder growth. In young soil, alder growth was highest with the Fe, P, and S combination.
5. Conclusions
The results of the present study showed the only significant growth effect with Frankia inoculation, implicating this inoculum as a key factor in later succession in post-mining soils. When the soil was fully inoculated, alder biomass was higher in developed soil supplied with iron and phosphorus, indicating that iron and phosphorus availability may affect alder growth. In young soil, alder growth was highest with a combination of iron, phosphorus, and sulfur, and a positive effect of sulfur in young soil may correspond with a reduced, alkaline soil pH and increased phosphorus and iron availability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/soilsystems8030098/s1, Table S1: Biomass of alder plants in Experiment 1; Table S2: Biomass of alder plants in Experiment 2. Values indicate the fresh weight of samples.
Author Contributions
L.B.: methodology, investigation, formal analysis, and data curation; M.M.A.: formal analysis, writing—original draft, and writing—review and editing; J.R.: visualization, validation, and writing—review and editing; H.V.: formal analysis, validation, and methodology; J.F.: supervision, validation, funding acquisition, conceptualization, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Education, Youth, and Sports of the Czech Republic-MEYS (projects no. LM2015075, EF16_013/0001782, and 8120001-EIG CONCERT JAPAN) and Charles University (Cooperatio-Environmental and Sustainability Research, project no. 270022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All papers are available in the Web of Science database. A license might be needed to obtain the papers that are not open-access.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bradshaw, A.D. Underlying principles of restoration. Can. J. Fish. Aquat. Sci. 1996, 53, 3–9. [Google Scholar] [CrossRef]
- Batterman, S.A.; Hedin, L.O.; van Breugel, M.; Ransijn, J.; Craven, D.J.; Hall, J.S. Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature 2013, 502, 224–227. [Google Scholar] [CrossRef]
- Brookshire, E.N.J.; Wurzburger, N.; Currey, B.; Menge, D.N.L.; Oatham, M.P.; Roberts, C. Symbiotic N fixation is sufficient to support net aboveground biomass accumulation in a humid tropical. Sci. Rep. 2019, 9, 7571. [Google Scholar] [CrossRef]
- Levy-Varon, J.H.; Batterman, S.A.; Medvigy, D.; Xu, X.; Hall, J.S.; van Breugel, M.; Hedin, L.O. Tropical carbon sink accelerated by symbiotic dinitrogen fixation. Nat. Commun. 2019, 10, 5637. [Google Scholar] [CrossRef]
- Kou-Giesbrecht, S.; Menge, D.N.L. Nitrogen-fixing trees increase soil nitrous oxide emissions: A meta-analysis. Ecology 2021, 102, e03415. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Woś, B.; Pająk, M.; Wanic, T.; Krzaklewski, W.; Chodak, M. The impact of alders (Alnus spp.) on the physico-chemical properties of technosols on a lignite combustion waste disposal site. Ecol. Eng. 2018, 120, 180–186. [Google Scholar] [CrossRef]
- Perakis, S.S.; Pett-Ridge, J.C. Nitrogen-fixing red alder trees tap rock-derived nutrients. Proc. Natl. Acad. Sci. USA 2019, 116, 5009–5014. [Google Scholar] [CrossRef]
- Sellstedt, A.; Richau, K.H. Aspects of nitrogen-fixing Actinobacteria, in particular free-living and symbiotic Frankia. FEMS Microbiol. Lett. 2013, 342, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.B.; Seefeldt, L.C.; Peters, J.W. Insights into nucleotide signal transduction in nitrogenase: Structure of an iron protein with MgADP bound. Biochemistry 2000, 39, 14745–14752. [Google Scholar] [CrossRef]
- Newsham, K.K.; Fitter, A.H.; Watkinson, A.R. Arbuscular mycorrhiza protect an annual grass from root pathogenic fungi in the field. J. Ecol. 1995, 83, 991. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Douds, D.D.; Bécard, G.; Shachar-Hill, Y. Carbon uptake and the metabolism and transport of lipids in and arbuscular mycorrhiza. Plant Physiol. 1999, 120, 587–598. [Google Scholar] [CrossRef]
- Bolan, N.S. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 1991, 34, 189–207. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T.; Rohr, J.R.; Aldrich-Wolfe, L.; Morton, J.B. Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J. Ecol. 2007, 95, 95–105. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Emery, S.M.; Rudgers, J.A. Fungal symbionts alter plant responses to global change. Am. J. Bot. 2013, 100, 1445–1457. [Google Scholar] [CrossRef]
- Brear, E.; David, D.A.; Smith, P.M.C. Iron: An essential micronutrient for the legume-rhizobium symbiosis. Front. Plant Sci. 2013, 4, 359. [Google Scholar] [CrossRef]
- Larson, C.A.; Mirza, B.; Mazza Rodrigues, J.L.; Passy, S.I. Iron limitation effects on nitrogen-fixing organisms with possible implications for cyanobacterial blooms. FEMS Microbiol. Ecol. 2018, 94, fiy046. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; de Kok, L.J. Managing sulphur metabolism in plants. Plant Cell Environ. 2006, 29, 382–395. [Google Scholar] [CrossRef]
- Šourková, M.; Frouz, J.; Šantrůčková, H. Accumulation of carbon, nitrogen, and phosphorus during soil formation on alder spoil heaps after brown-coal mining, near Sokolov (Czech Republic). Geoderma 2005, 124, 203–214. [Google Scholar] [CrossRef]
- Frouz, J.; Prach, K.; Pižl, V.; Háněl, L. Interactions between soil development, vegetation and soil fauna during spontaneous succession in post mining sites. Eur. J. Soil Biol. 2008, 44, 109–121. [Google Scholar] [CrossRef]
- Schüßler, A.; Walker, C. The Glomeromycota: A Species List with New Families and New Genera; The Royal Botanic Garden Kew, Botanische Staatssammlung Munich, and Oregon State University: Gloucester, UK, 2010. [Google Scholar]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA-mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. 2017. Available online: https://CRAN.R-project.org/package=vegan (accessed on 17 January 2017).
- Simpson, G.L. Permute: Functions for Generating Restricted Permutations of Data. 2022. Available online: https://cran.r-project.org/web/packages/permute/permute.pdf (accessed on 20 August 2024).
- Morales, M. Package ‘sciplot’: Scientific Graphing Functions for Factorial Designs; R Package Version 1.2-0; Montreal. 2022. Available online: https://cran.r-project.org/web/packages/sciplot/sciplot.pdf (accessed on 20 August 2024).
- Seeds, J.D.; Bishop, J.G. Low Frankia inoculation potentials in primary successional sites at Mount St. Helens, Washington, USA. Plant Soil 2009, 323, 225–233. [Google Scholar] [CrossRef]
- Pourhassan, N.; Wichard, T.; Roy, S.; Bellenger, J.P. Impact of elevated CO2 on metal homeostasis and the actinorhizal symbiosis in early successional alder shrubs. Environ. Exp. Bot. 2015, 109, 168–176. [Google Scholar] [CrossRef]
- Parker, M.A. Mutualism as a constraint on invasion success for legumes and rhizobia. Divers. Distrib. 2001, 7, 125–136. [Google Scholar] [CrossRef]
- Wheeler, C.T.; Tilak, M.; Scrimgeour, C.M.; Hooker, J.E.; Handley, L.L. Effects of symbiosis with Frankia and arbuscular mycorrhizal fungus on the natural abundance of 15N in four species of Casuarina. J. Exp. Bot. 2000, 51, 287–297. [Google Scholar] [CrossRef][Green Version]
- Míguez-Montero, M.A.; Valentine, A.; Pérez-Fernández, M.A. Regulatory effect of phosphorus and nitrogen on nodulation and plant performance of leguminous shrubs. AoB PLANTS 2020, 12, plz047. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of the Soil, 15th ed.; Pearson: New York, NY, USA, 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).