Use of Cannabis sativa L. for Improving Cadmium-Contaminated Mediterranean Soils—Effect of Mycorrhizal Colonization on Phytoremediation Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Experimental Design
2.2. Artificial (Laboratory) Contamination of Soil Sample and Pot Experiment
2.3. Soil Chemical and Physical Analysis
2.4. Plant Analysis
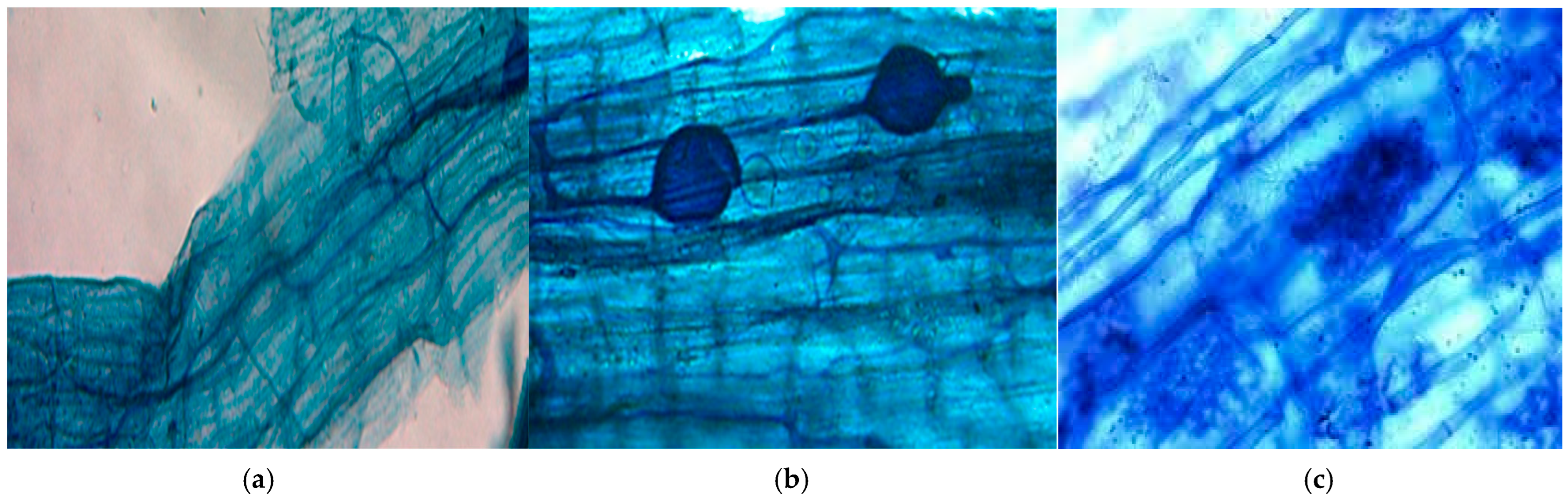
2.5. Quantification of AMF Colonization in Roots: Clearing and Staining Roots
2.6. Statistical Processing of Data
3. Results and Discussion
3.1. Soil Characterization
3.2. Pseudo-Total and Available Concentration of Cd in Soil Samples
3.3. Cd Levels in C. sativa Cultivated in the Contaminated Soil Samples
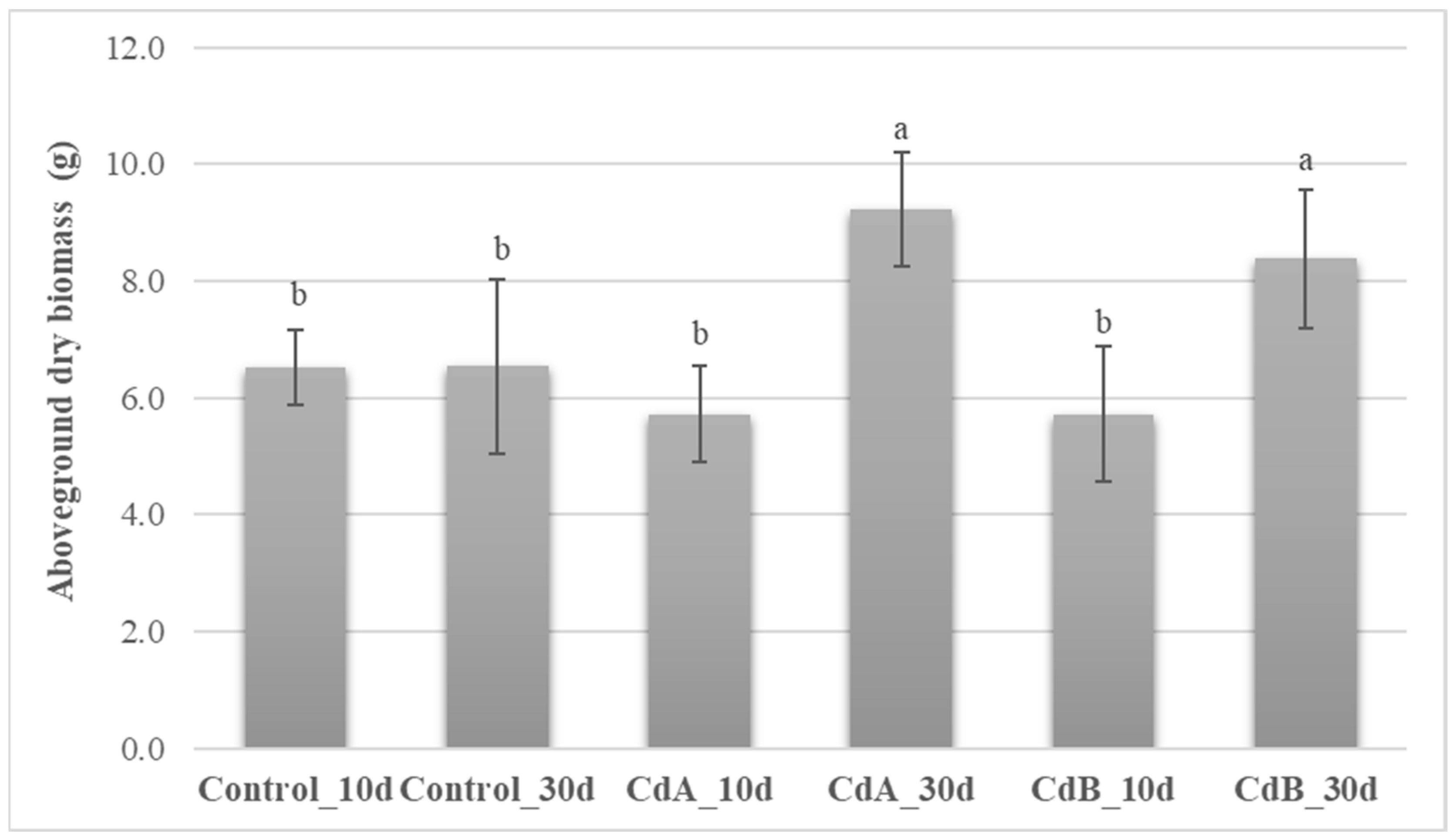
3.4. Roots and Aboveground Plant Biomass
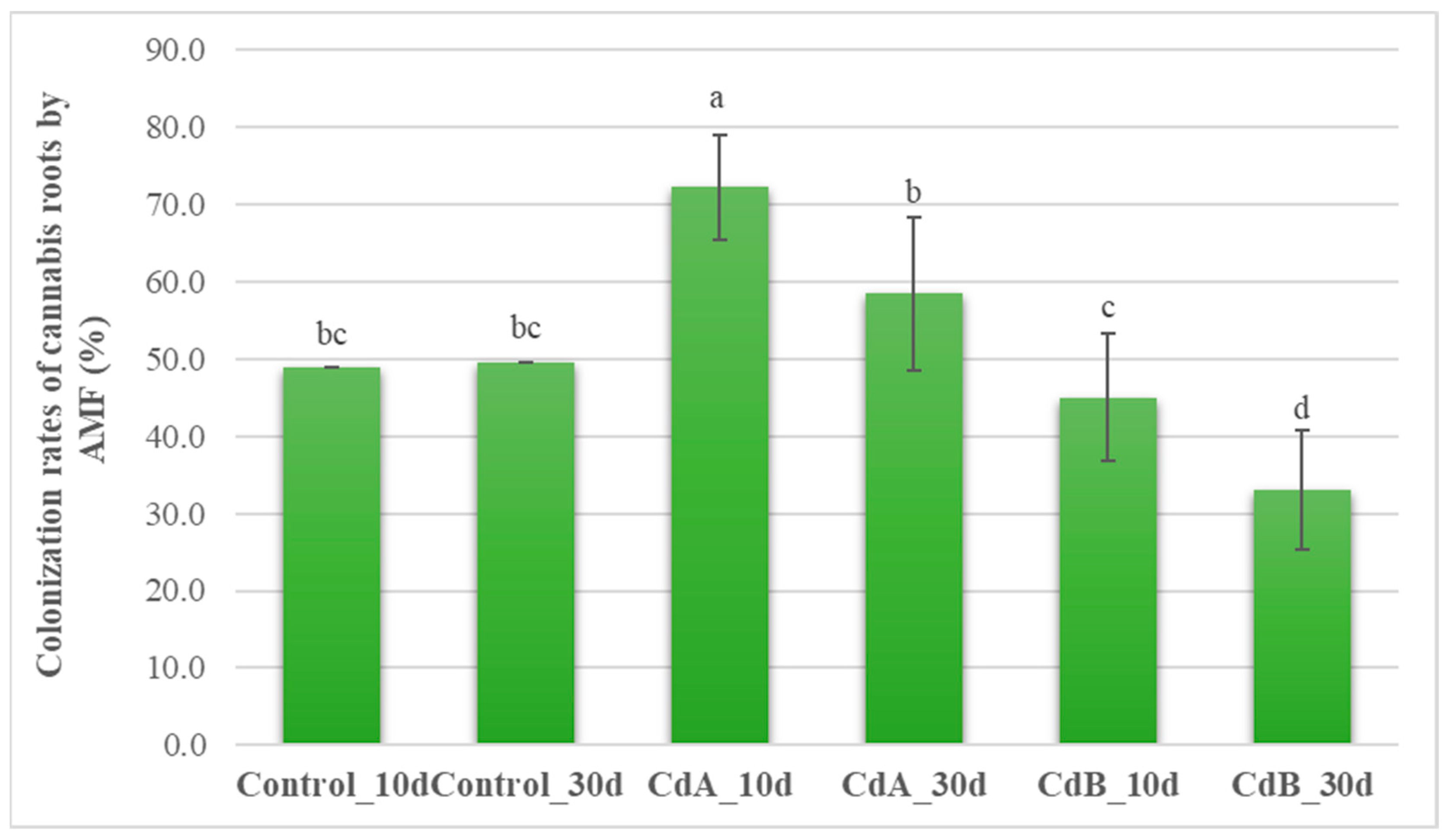
3.5. Effect of Different Levels of Cd Concentration on AMF Colonization Rates (%) at Different Incubation Times
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quagliata, G.; Celletti, S.; Coppa, E.; Mimmo, T.; Cesco, S.; Astolfi, S. Potential use of copper-contaminated soils for hemp (Cannabis sativa L.) cultivation. Environments 2021, 8, 111. [Google Scholar] [CrossRef]
- Golia, E.E.; Bethanis, J.; Ntinopoulos, N.; Kaffe, G.G.; Komnou, A.A.; Vasilou, C. Investigating the potential of heavy metal accumulation from hemp. The use of industrial hemp (Cannabis sativa L.) for phytoremediation of heavily and moderated polluted soils. Sustain. Chem. Pharm. 2023, 31, 100961. [Google Scholar] [CrossRef]
- Golia, E.E.; Angelaki, A.; Giannoulis, K.D.; Skoufogianni, E.; Bartzialis, D.; Cavalaris, C.; Vleioras, S. Evaluation of soil properties, irrigation and solid waste application levels on Cu and Zn uptake by industrial hemp. Agron. Res. 2021, 19, 92–99. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Zhu, S.; Ho, S.H.; Wu, J.; Kalita, P.K.; Ma, F. Unraveling the effects of arbuscular mycorrhizal fungus on uptake, translocation, and distribution of cadmium in Phragmites australis (Cav.) Trin. ex Steud. Ecotoxicol. Environ. Saf. 2018, 149, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Tian, D.Q. Study on the effect mechanism of Arbuscular Mycorrhiza on the absorption of heavy metal elements in soil by plants. IOP Conf. Ser. Earth Environ. Sci. 2019, 267, 052064. [Google Scholar] [CrossRef]
- Khan, S.; Hesham, A.; Qiao, M.; Rehman, S.; He, J.Z. Effects of Cd and Pb on soil microbial community structure and activities. Environ. Sci. Pollut. Res. 2010, 17, 288–296. [Google Scholar] [CrossRef]
- Hassan, W.; Akmal, M.; Muhammad, I.; Younas, M.; Zahaid, K.R.; Ali, F. Response of soil microbial biomass and enzymes activity to cadmium (Cd) toxicity under different soil textures and incubation times. Aust. J. Crop Sci. 2013, 7, 674–680. [Google Scholar]
- Turner, A. Cadmium pigments in consumer products and their health risks. Sci. Total Environ. 2019, 657, 1409–1418. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, H.; Chen, G.; Wu, X.; Zeng, F. Cadmium Accumulation in Plants: Insights from Phylogenetic Variation into the Evolution and Functions of Membrane Transporters. Sustainability 2023, 15, 12158. [Google Scholar] [CrossRef]
- Elinder, C.G. Cadmium: Uses, Occurrence, and Intake. In Cadmium and Health; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Sharma, H.; Rawal, N.; Mathew, B.B. The characteristics, toxicity and effects of cadmium. Int. J. Nanotechnol. Nanosci. 2015, 3, 1–9. [Google Scholar]
- Hu, Y.; Cheng, H.; Tao, S. The Challenges and Solutions for Cadmium-contaminated Rice in China: A Critical Review. Environ. Int. 2016, 92–93, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, L.; Xue, L.; Hu, Y.; Han, J. The Effect of Fertilizers on Soil Total and Available Cadmium in China: A Meta-Analysis. Agronomy 2024, 14, 978. [Google Scholar] [CrossRef]
- Zhou, F.; Guo, M.; Zhao, N.; Xu, Q.; Zhao, T.; Zhang, W.; Qiu, R. The effect of thermal treatment on the transformation and transportation of arsenic and cadmium in soil. J. Environ. Sci. 2024, 145, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, Z.; Agathokleous, E.; Zhu, Y.; Fan, D.; Han, J. Unveiling a New Perspective on Cadmium-Induced Hormesis in Soil Enzyme Activity: The Relative Importance of Enzymatic Reaction Kinetics and Microbial Communities. Agriculture 2024, 14, 904. [Google Scholar] [CrossRef]
- Khaliq, M.A.; James, B.; Chen, Y.H.; Ahmed Saqib, H.S.; Li, H.H.; Jayasuriya, P.; Guo, W. Uptake, translocation, and accumulation of Cd and its interaction with mineral nutrients (Fe, Zn, Ni, Ca, Mg) in upland rice. Chemosphere 2019, 215, 916–924. [Google Scholar] [CrossRef]
- Papadimou, S.G.; Golia, E.E. Green and sustainable practices for an energy plant cultivation on naturally contaminated versus spiked soils. The impact of ageing soil pollution in the circular economy framework. Environ. Res. 2024, 6, 118130. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Adriano, D.C.; Wenzel, W.W.; Vangronsveld, J.; Bolan, N.S. Role of assisted natural remediation in environmental cleanup. Geoderma 2004, 122, 121–142. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of heavy metals and metalloids in Soils. In Heavy Metals in Soils, Environmental Pollution; Alloway, B., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 22, pp. 11–50. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Xie, X.; Wu, Y.; Liang, F.; Tang, M. Arbuscular mycorrhizal fungi promote lead immobilization by increasing the polysaccharide content within pectin and inducing cell wall peroxidase activity. Chemosphere 2020, 267, 128924. [Google Scholar] [CrossRef]
- Ortas, I. The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crop. Res. 2012, 125, 35–48. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Brundrett, M. Diversity and classification of mycorrhizal associations. Biol. Rev. Camb. Philos. Soc. 2004, 79, 473–495. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.-H. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.; Antolín, M.C.; Goicoechea, N. Arbuscular mycorrhizal symbiosis as a promising resource for improving berry quality in grapevines under changing environments. Front. Plant Sci. 2018, 9, 897. [Google Scholar] [CrossRef]
- Balestrini, R.; Chitarra, W.; Antoniou, C.; Ruocco, M.; Fotopoulos, V. Improvement of plant performance under water deficit with the employment of biological and chemical priming agents. J. Agric. Sci. 2018, 156, 680–688. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Y.; Chen, S.; Polle, A.; Rennenberg, H.; Bin Luo, Z. Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ. 2019, 42, 1087–1103. [Google Scholar] [CrossRef]
- Millar, N.S.; Bennett, A.E. Stressed out symbiotes: Hypotheses for the influence of abiotic stress on arbuscular mycorrhizal fungi. Oecologia 2016, 182, 625–641. [Google Scholar] [CrossRef]
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient Exchange and Regulation in Arbuscular Mycorrhizal Symbiosis. Mol. Plant 2017, 10, 1147–1158. [Google Scholar] [CrossRef]
- Liu, L.; Gong, Z.; Zhang, Y.; Li, P. Growth, cadmium uptake and accumulation of maize (Zea mays L.) under the effects of arbuscular mycorrhizal fungi. Ecotoxicology 2014, 23, 1979–1986. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Huang, L.; Chen, B. Arbuscular mycorrhiza and plant chromium tolerance. Soil Ecol. Lett. 2019, 1, 94–104. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Oliveira, R.S.; Zhang, C.; Freitas, H. Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal contaminated saline soils. J. Hazard. Mater. 2019, 379, 120813. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Boorboori, M.R.; Zhang, H.Y. Arbuscular Mycorrhizal Fungi Are an Influential Factor in Improving the Phytoremediation of Arsenic, Cadmium, Lead, and Chromium. J. Fungi 2022, 8, 176. [Google Scholar] [CrossRef]
- Xie, X.; Huang, W.; Liu, F.; Tang, N.; Liu, Y.; Lin, H.; Zhao, B. Functional analysis of the novel mycorrhiza-specific phosphate transporter AsPT1 and PHT1 family from Astragalus sinicus during the arbuscular mycorrhizal symbiosis. New Phytol. 2013, 198, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Tu, C.; Huang, X.; Zhang, J.; Fang, H.; Huo, H.; Lin, C. Relationships between soil properties and the accumulation of heavy metals in different Brassica campestris L. growth stages in a Karst mountainous area. Ecotoxicol. Environ. Saf. 2020, 206, 111150. [Google Scholar] [CrossRef]
- Lebeau, T.; Braud, A.; J’ez´equel, K. Performance of bioaugmentation-assisted phytoextraction applied to metal contaminated soils: A review. Environ. Pollut. 2008, 153, 497–522. [Google Scholar] [CrossRef] [PubMed]
- Morar, F.; Iantovics, L.B.; Gligor, A. Analysis of Phytoremediation Potential of Crop Plants in Industrial Heavy Metal Contaminated Soil in the Upper Mures River Basin. J. Environ. Inform. 2018, 31, 1–14. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of heavy metals: Mechanisms, methods and enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Pajevic, S.; Borisev, M.; Nikolic, N.; Arsenov, D.D.; Orlovic, S.; Zupunski, M. Phytoextraction of heavy metals by fast-growing trees: A review. Phytoremediation Manag. Environ. Contam. 2016, 3, 29–64. [Google Scholar] [CrossRef]
- Miransari, M. Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnol. Adv. 2011, 29, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Bona, E.; Marsano, F.; Cavaletto, M.; Berta, G. Proteomic characterization of copper stress response in Cannabis sativa roots. Proteomics 2007, 7, 1121–1130. [Google Scholar] [CrossRef]
- Campiglia, E.; Gobbi, L.; Marucci, A.; Rapa, M.; Ruggieri, R.; Vinci, G. Hemp Seed Production: Environmental Impacts of Cannabis sativa L. Agronomic Practices by Life Cycle Assessment (LCA) and Carbon Footprint Methodologies. Sustainability 2020, 12, 6570. [Google Scholar] [CrossRef]
- Desaulniers Brousseau, V.; Goldstein, B.P.; Lachapelle, M.; Tazi, I.; Lefsrud, M. Greener green: The environmental impacts of the Canadian cannabis industry. Resour. Conserv. Recycl. 2024, 208, 107737. [Google Scholar] [CrossRef]
- Todde, G.; Carboni, G.; Marras, S.; Caria, M.; Sirca, C. Industrial hemp (Cannabis sativa L.) for phytoremediation: Energy and environmental life cycle assessment of using contaminated biomass as an energy resource. Sustain. Energy Technol. Assess. 2022, 52, 102081. [Google Scholar] [CrossRef]
- Mahmood, A.; Rashid, S.; Malik, R.N. Determination of toxic heavy metals in indigenous medicinal plants used in Rawalpindi and Islamabad cities, Pakistan. J. Ethnopharmacol. 2013, 148, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Tsaliki, E.; Kalivas, A.; Jankauskiene, Z.; Irakli, M.; Cook, C.; Grigoriadis, I.; Panoras, I.; Vasilakoglou, I.; Dhima, K. Fibre and Seed Productivity of Industrial Hemp (Cannabis sativa L.) Varieties under Mediterranean Conditions. Agronomy 2021, 11, 171. [Google Scholar] [CrossRef]
- Sun, S.; Feng, Y.; Huang, G.; Zhao, X.; Song, F. Rhizophagus irregularis enhances tolerance to cadmium stress by altering host plant hemp (Cannabis sativa L.) photosynthetic properties. Environ. Pollut. 2022, 314, 120309. [Google Scholar] [CrossRef]
- El Faiz, A.; Duponnois, R.; Winterton, P.; Ouhammou, A.; Meddich, A.; Boularbah, A.; Hafidi, M. Effect of Different Amendments on Growing of Canna indica L. Inoculated with AMF on Mining Substrate. Int. J. Phytoremediation 2014, 17, 503–513. [Google Scholar] [CrossRef]
- Citterio, S.; Santagostino, A.; Fumagalli, P.; Prato, N.; Ranalli, P.; Sgorbati, S. Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil 2003, 256, 243–252. [Google Scholar] [CrossRef]
- Ofori-Agyemang, F.; Waterlot, C.; Manu, J.; Laloge, R.; Francin, R.; Papazoglou, E.G.; Alexopoulou, E.; Lounès-Hadj Sahraoui, A.; Tisserant, B.; Mench, M.; et al. Plant testing with hemp and miscanthus to assess phytomanagement options including biostimulants and mycorrhizae on a metal contaminated soil to provide biomass for sustainable biofuel production. Sci. Total Environ. 2024, 912, 169527. [Google Scholar] [CrossRef] [PubMed]
- ISO/ISO 10390; Soil Quality, Determination of pH. International Standards Organization. ISO: Geneve, Switzerland, 2005.
- Papadimou, S.G.; Barbayiannis, Ν.; Golia, E.E. Preliminary investigation of the use of Silybum marianum (L.) Gaertn. as a Cd accumulator in contaminated Mediterranean soils: The relationships among cadmium (Cd) soil fractions and plant Cd content. Euro-Mediterr. J. Environ. Integr. 2024, 9, 405–417. [Google Scholar] [CrossRef]
- Golia, E.E. The impact of heavy metal contamination on soil quality and plant nutrition. Sustainable management of moderate contaminated agricultural and urban soils, using low cost materials and promoting circular economy. Sustain. Chem. Pharm. 2023, 33, 101046. [Google Scholar] [CrossRef]
- Dimirkou, A.; Ioannou, Z.; Golia, E.E.; Danalatos, N.; Mitsios, I.K. Sorption of cadmium and arsenic by goethite and clinoptilolite. Commun. Soil Sci. Plant Anal. 2009, 40, 259–272. [Google Scholar] [CrossRef]
- Papadimou, S.G.; Golia, E.E.; Barbayiannis, N.; Tsiropoulos, N.G. Dual role of the hyperaccumulator Silybum marianum (L.) Gaertn. in circular economy: Production of silymarin, a valuable secondary metabolite, and remediation of heavy metal contaminated soils. Sustain. Chem. Pharm. 2024, 38, 101454. [Google Scholar] [CrossRef]
- Vasilou, C.; Tsiropoulos, N.G.; Golia, E.E. Phytoremediation & Valorization of Cu-contaminated Soils Through Cannabis sativa (L.) Cultivation: A Smart Way to Produce Cannabidiol (CBD) in Mediterranean Soils. Waste Biomass Valorization 2024, 15, 1711–1724. [Google Scholar] [CrossRef]
- UPOV. International union for the protection of new varieties of plants. Guidelines for the conduct of tests for distinctness, uniformity and stability, Cannabis sativa L. Hemp, Cannabis. UPOV Code: CANNB_SAT. 2023. Available online: https://www.upov.int/edocs/mdocs/upov/en/twa_52/tg_276_2_proj_2.pdf (accessed on 13 September 2024).
- Page, A.L. Methods of Soil Analysis-Part 2: Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy, Inc. Publisher: Madison, WI, USA, 1982; Volume 9, pp. 421–422. [Google Scholar]
- ISO/DIS 11466; Environment Soil Quality. ISO Standards Compendium: Geneva, Switzerland, 1994.
- Sylvia, D.M. Vesicular-Arbuscular Mycorrhizal Fungi. In Methods of Soil Analysis: Part. 2. Microbiological and Biochemical Properties, 3rd ed.; Peter, J., Bootomley, P.J., Scott Angle, J., Weaver, R.W., Eds.; Soil Science Society of America: Madison, WI, USA, 2020; pp. 351–360. [Google Scholar]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Golia, E.E.; Dimirkou, A.; Floras, S.A. Spatial monitoring of arsenic and heavy metals in the Almyros area, Central Greece. Statistical approach for assessing the sources of contamination. Environ. Monit. Assess. 2015, 187, 399. [Google Scholar] [CrossRef]
- Baldantoni, D.; Morra, L.; Zaccardelli, M.; Alfani, A. Cadmium accumulation in leaves of leafy vegetables. Ecotoxicol. Environ. Saf. 2016, 123, 89–94. [Google Scholar] [CrossRef]
- Kwiatkowska-Malina, J. Functions of organic matter in polluted soils: The effect of organic amendments on phytoavailability of heavy metals. Appl. Soil Ecol. 2018, 123, 542–545. [Google Scholar] [CrossRef]
- Wielgusz, K.; Praczyk, M.; Irzykowska, L.; Świerk, D. Fertilization and soil pH affect seed and biomass yield, plant morphology, and cadmium uptake in hemp (Cannabis sativa L.). Ind. Crops Prod. 2022, 175, 114245. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Cadmium Uptake by Wheat (Triticum aestivum L.): An Overview. Plants 2020, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, G.A.; O’Connor, C.; Cline, G.R. Sorption of cadmium by calcareous soils: Influence of solution composition. Soil Sci. Soc. Am. J. 1984, 48, 12441247. [Google Scholar] [CrossRef]
- Napoletano, P.; Guezgouz, N.; Di Iorio, E.; Colombo, C.; Guerriero, G.; De Marco, A. Anthropic impact on soil heavy metal contamination in riparian ecosystems of Northern Algeria. Chemosphere 2023, 313, 137522. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace elements. In Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Golia, E.E.; Diakoloukas, V. Soil parameters affecting the levels of potentially harmful metals in Thessaly area, Greece: A robust quadratic regression approach of soil pollution prediction. Environ. Sci. Pollut. Res. 2022, 29, 29544–29561. [Google Scholar] [CrossRef]
- Marković, J.; Jović, M.; Smičiklas, I.; Šljivić-Ivanović, M.; Onjia, A.; Trivunac, K.; Popović, A. Cadmium retention and distribution in contaminated soil: Effects and interactions of soil properties, contamination level, aging time and in situ immobilization agents. Ecotoxicol. Environ. Saf. 2019, 174, 305–314. [Google Scholar] [CrossRef]
- Lu, N.; Wei, Y.; Zhang, Z.; Li, Y.; Li, G.; Han, J. The Response of Cd Chemical Fractions to Moisture Conditions and Incubation Time in Arable Land Soil. Sustainability 2022, 14, 6270. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Q.; Zhou, Q.; Ma, L.; Wu, Y.; Liu, Q.; Wang, S.; Feng, Y. Cadmium uptake from soil and transport by leafy vegetables: A meta-analysis. Environ. Pollut. 2020, 264, 114677. [Google Scholar] [CrossRef]
- Huang, R.; Dong, M.; Mao, P.; Zhuang, P.; Paz-Ferreiro, J.; Li, Y.; Li, Y.; Hu, X.; Netherway, P.; Li, Z. Evaluation of phytoremediation potential of five Cd (hyper)accumulators in two Cd contaminated soils. Sci. Total Environ. 2020, 721, 137581. [Google Scholar] [CrossRef]
- Marabesi, A.O. Influence of Cadmium Exposure on Industrial Hemp Grown for Cannabinoid Production (Cannabis sativa L.). Doctoral Dissertation, University of Georgia, Athens, GA, USA, 2023. [Google Scholar]
- Shi, J.; Qian, W.; Jin, Z.; Zhou, Z.; Wang, X.; Yang, X. Evaluation of soil heavy metals pollution and the phytoremediation potential of copper-nickel mine tailings ponds. PLoS ONE 2023, 18, e0277159. [Google Scholar] [CrossRef]
- Linger, P.; Ostwald, A.; Haensler, J. Cannabis sativa L. growing on heavy metal contaminated soil: Growth, cadmium uptake and photosynthesis. Biol. Plant. 2005, 49, 567–576. [Google Scholar] [CrossRef]
- Shi, G.; Cai, Q. Cadmium tolerance and accumulation in eight potential energy crops. Biotechnol. Adv. 2009, 27, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Adrees, M.; Ibrahim, M.; Tsang, D.C.W.; Zia-ur-Rehman, M.; Zahir, Z.A.; Rinklebe, J.; Tack, F.M.G.; Ok, Y.S. A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 2017, 182, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Chaney, R.L.; Malik, M.; Li, Y.M.; Brown, S.L.; Brewer, E.P.; Angle, J.S.; Baker, A.J. Phytoremediation of soil metals. Curr. Opin. Biotechnol. 1997, 8, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Alsafran, M.; Usman, K.; Ahmed, B.; Rizwan, M.; Saleem, M.H.; Al Jabri, H. Understanding the Phytoremediation Mechanisms of Potentially Toxic Elements: A Proteomic Overview of Recent Advances. Front. Plant Sci. 2022, 6, 881242. [Google Scholar] [CrossRef]
- Al-Obaidi, J.R.; Jamaludin, A.A.; Rahman, N.A.; Ahmad-Kamil, E.I. How plants respond to heavy metal contamination: A narrative review of proteomic studies and phytoremediation applications. Planta 2024, 259, 103. [Google Scholar] [CrossRef]
- Molina, A.S.; Lugo, M.A.; Pérez Chaca, M.V.; Vargas-Gil, S.; Zirulnik, F.; Leporati, J.; Ferrol, N.; Azcón-Aguilar, C. Effect of Arbuscular Mycorrhizal Colonization on Cadmium 57 Mediated Oxidative Stress in Glycine max (L.) Merr. Plants 2020, 9, 108. [Google Scholar] [CrossRef]
- Kanwal, S.; Bano, A.; Malik, R.N. Effects of arbuscular mycorrhizal fungi on wheat growth, physiology, nutrition and cadmium uptake under increasing cadmium stress. Int. J. Agron. Agric. Res. 2015, 7, 30–42. [Google Scholar]
- Vig, K. Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: A review. Adv. Environ. Res. 2003, 8, 121–135. [Google Scholar] [CrossRef]
- Sardar, K.; Qing, C.; El-Latif, H.A.; Yue, X.; He, J.Z. Soil enzymatic activities and microbial community structure with different application rates of Cd and Pb. J. Environ. Sci. 2007, 19, 834–840. [Google Scholar]
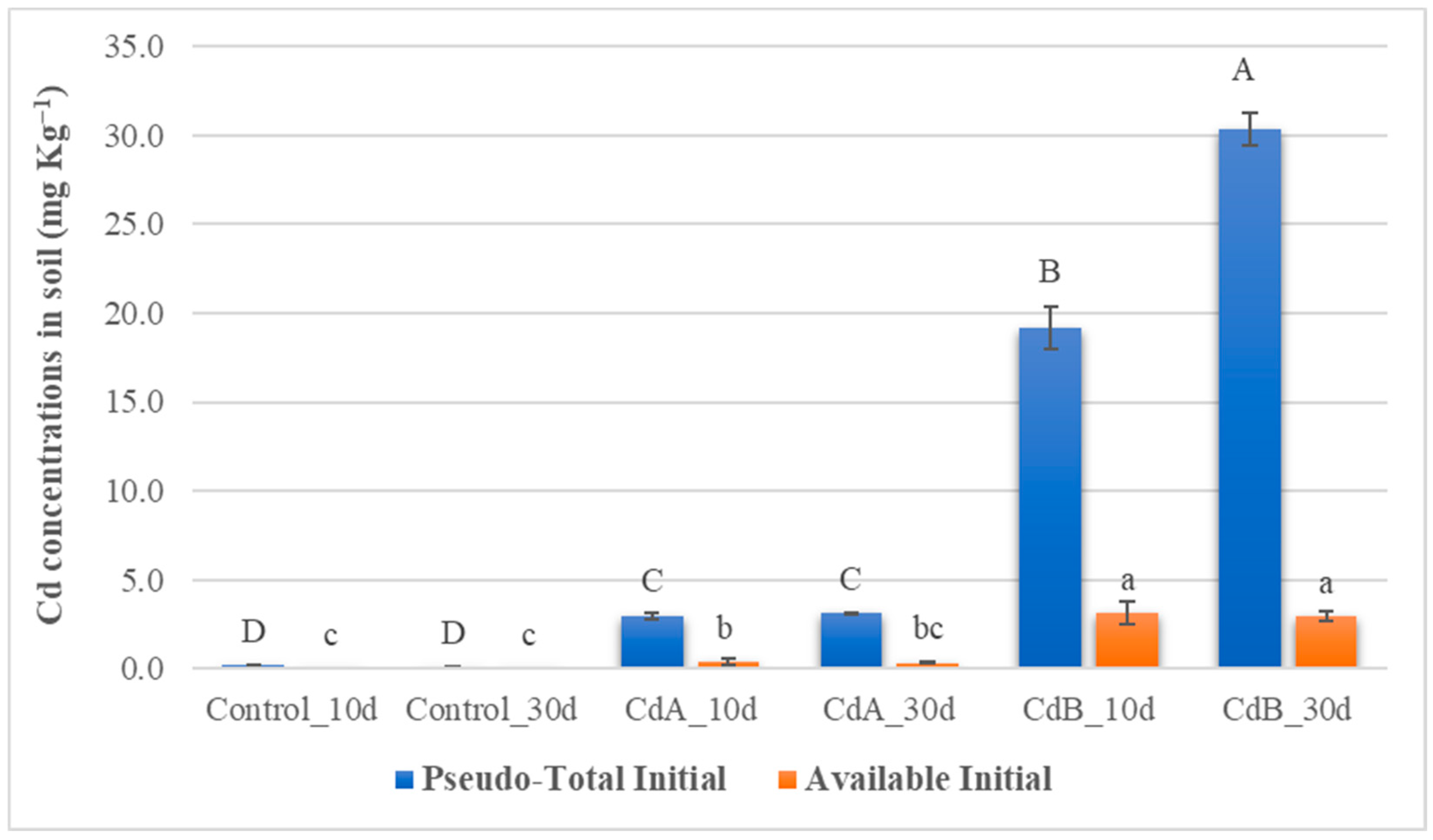

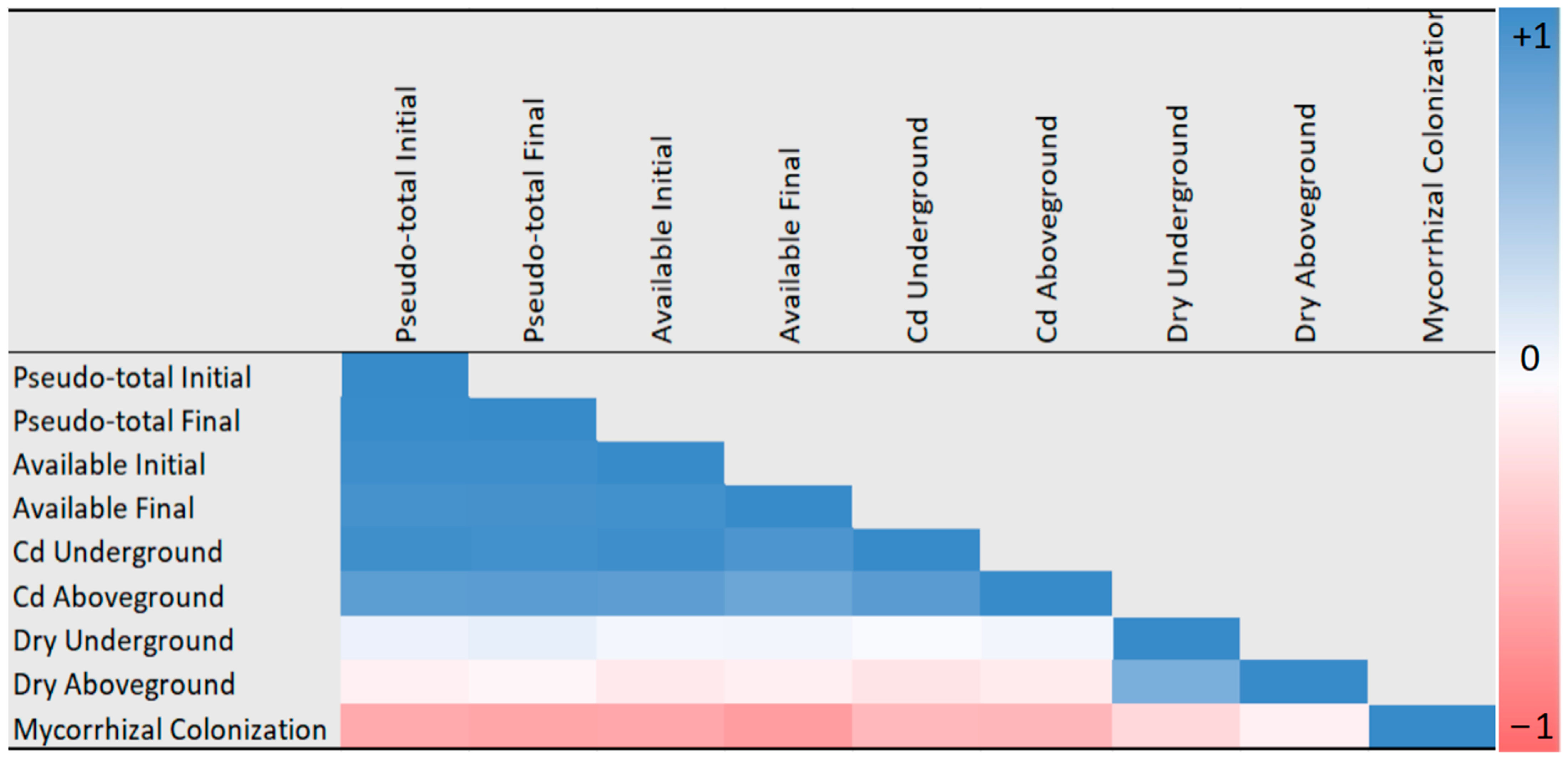
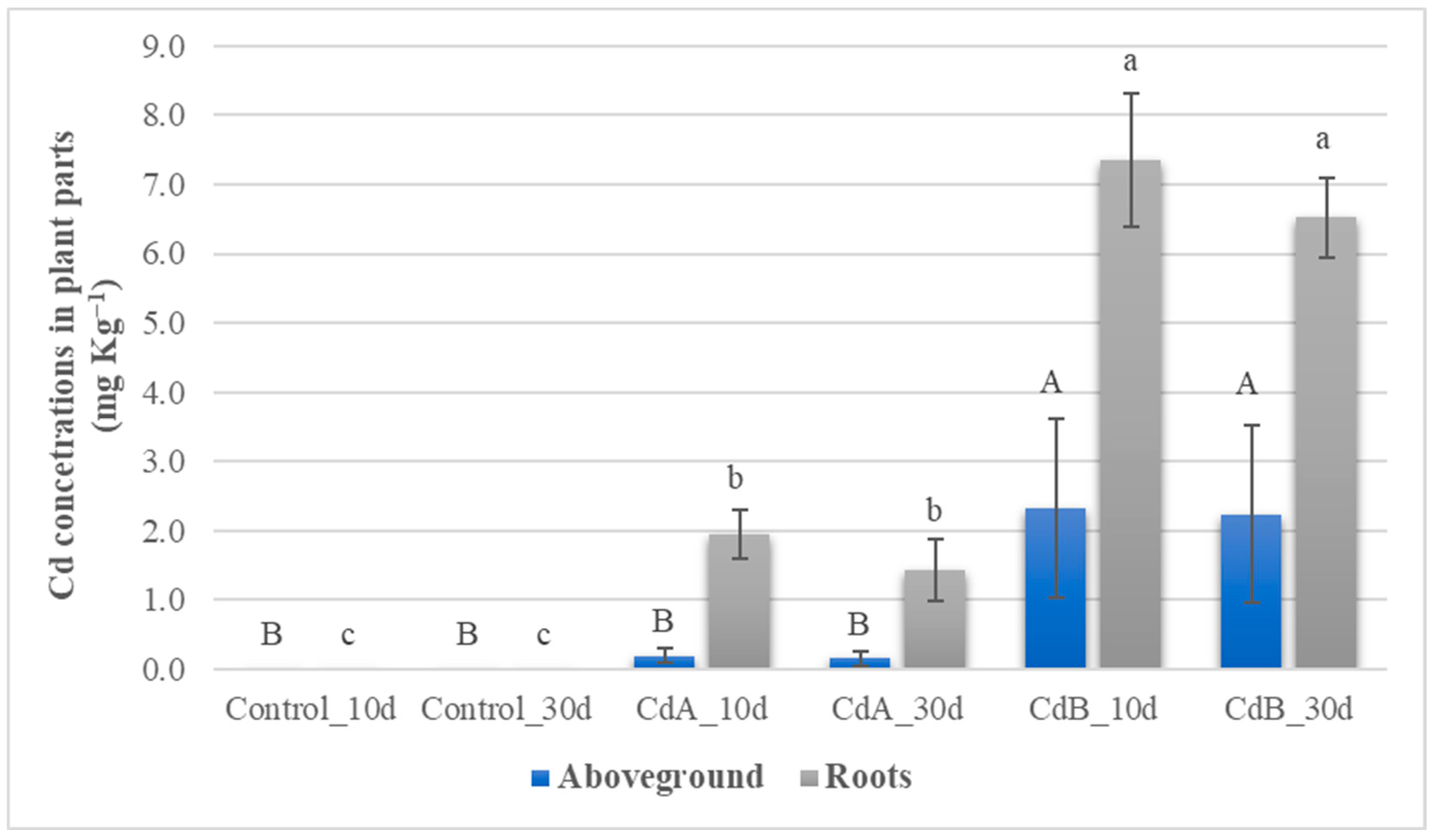
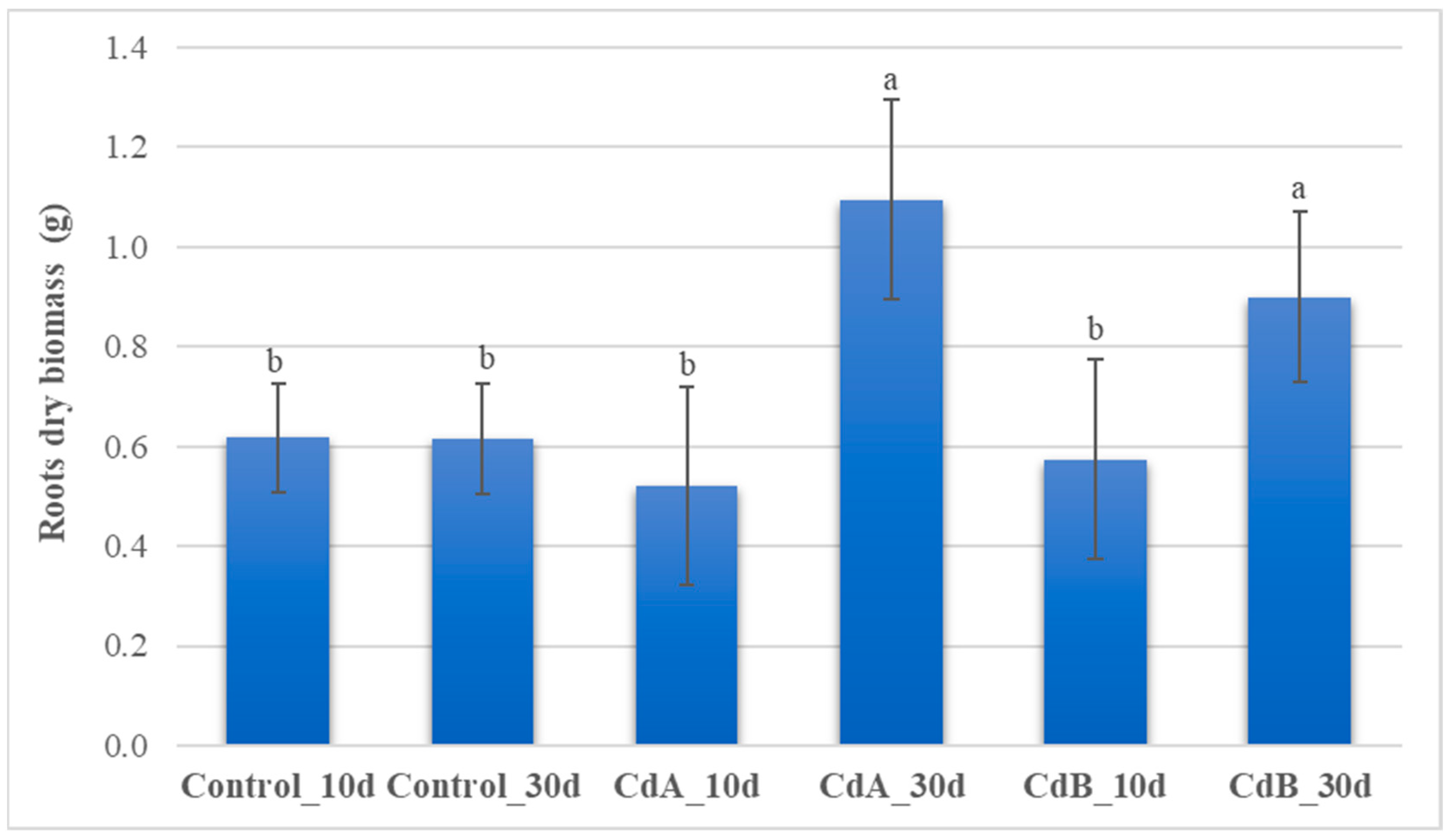
| Coding | mg Cd kg−1 | Incubation Days | Replicates |
|---|---|---|---|
| Control_10d | 0 | 10 | 10 |
| CdA_10d | 3 | 10 | 10 |
| CdB_10d | 30 | 10 | 10 |
| Control_30d | 0 | 30 | 10 |
| CdA_30d | 3 | 30 | 10 |
| CdB_30d | 30 | 30 | 10 |
| 2023 | ||||||
|---|---|---|---|---|---|---|
| April | May | June | July | August | Total | |
| Mean Temperature (°C) | 14.0 | 18 | 23.3 | 28.8 | 27.8 | - |
| Precipitation (mm) | 75.6 | 70.0 | 85.8 | 9.0 | 40.0 | 280.4 |
| Physicochemical Properties | Min | Max | Average | SD |
|---|---|---|---|---|
| pH (1:1) | 7.6 | 9.1 | 8.3 | ±0.6 |
| EC (μS/cm) | 1082.0 | 1090 | 1086 | ±3.3 |
| OM (%) | 2.3 | 3.5 | 2.9 | ±0.5 |
| CaCO3 (%) | 12.1 | 14.4 | 13.2 | ±1.0 |
| Clay (%) | 53 | 59 | 56 | ±2.4 |
| Sand (%) | 41 | 46 | 44 | ±2.0 |
| Underground | Aboveground | |||
|---|---|---|---|---|
| Treatements | Cplant/ Cpseudo-total | Cplant/ Cavailable | Cplant/ Cpseudo-total | Cplant/ Cavailable |
| Control_10d | 0.00 | 0.00 | 0.00 | 0.00 |
| Control_30d | 0.00 | 0.00 | 0.00 | 0.00 |
| CdA_10d | 0.65 | 5.06 | 0.06 | 0.49 |
| CdA_30d | 0.45 | 4.51 | 0.05 | 0.49 |
| CdB_10d | 0.25 | 2.36 | 0.08 | 0.75 |
| CdB_30d | 0.22 | 2.21 | 0.07 | 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Androudi, M.; Liava, V.; Tsaliki, E.; Ipsilantis, I.; Golia, E.E. Use of Cannabis sativa L. for Improving Cadmium-Contaminated Mediterranean Soils—Effect of Mycorrhizal Colonization on Phytoremediation Capacity. Soil Syst. 2024, 8, 100. https://doi.org/10.3390/soilsystems8030100
Androudi M, Liava V, Tsaliki E, Ipsilantis I, Golia EE. Use of Cannabis sativa L. for Improving Cadmium-Contaminated Mediterranean Soils—Effect of Mycorrhizal Colonization on Phytoremediation Capacity. Soil Systems. 2024; 8(3):100. https://doi.org/10.3390/soilsystems8030100
Chicago/Turabian StyleAndroudi, Maria, Vasiliki Liava, Eleni Tsaliki, Ioannis Ipsilantis, and Evangelia E. Golia. 2024. "Use of Cannabis sativa L. for Improving Cadmium-Contaminated Mediterranean Soils—Effect of Mycorrhizal Colonization on Phytoremediation Capacity" Soil Systems 8, no. 3: 100. https://doi.org/10.3390/soilsystems8030100
APA StyleAndroudi, M., Liava, V., Tsaliki, E., Ipsilantis, I., & Golia, E. E. (2024). Use of Cannabis sativa L. for Improving Cadmium-Contaminated Mediterranean Soils—Effect of Mycorrhizal Colonization on Phytoremediation Capacity. Soil Systems, 8(3), 100. https://doi.org/10.3390/soilsystems8030100







