Determining Drought and Salinity Stress Response Function for Garlic
Abstract
1. Introduction
2. Materials and Methods
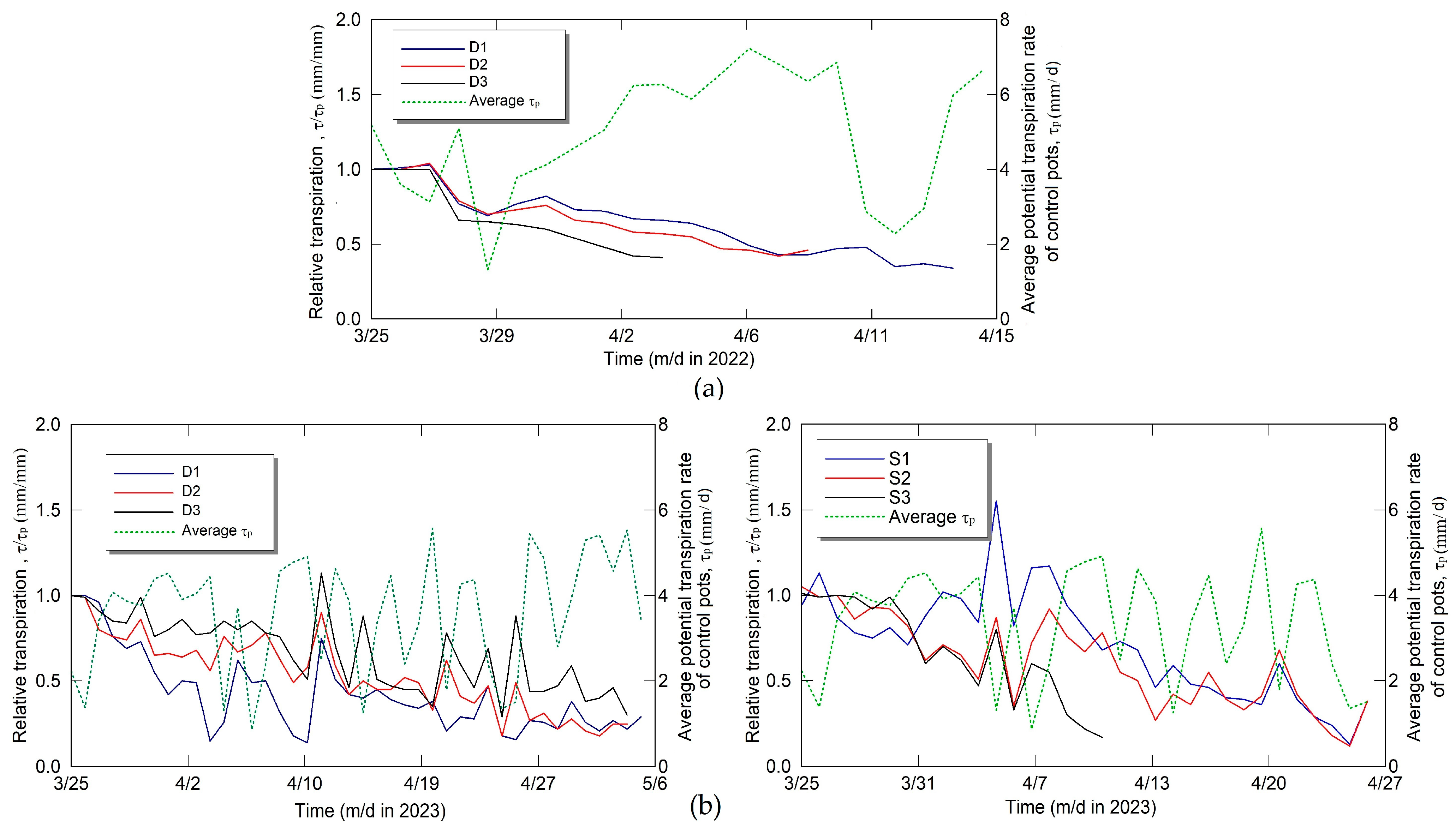
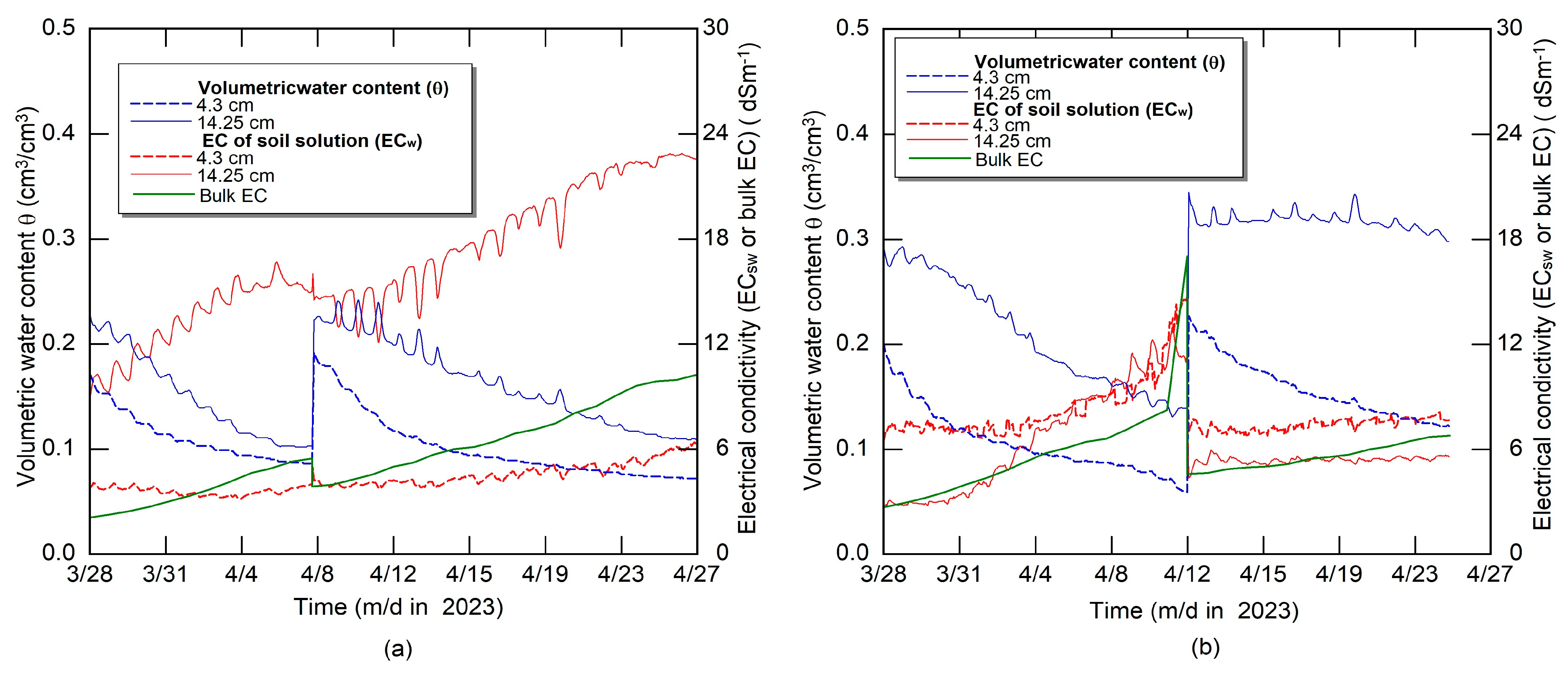
2.1. Description of Pot Experiments
2.2. Plant Root Water Uptake
2.2.1. Theorical Expression
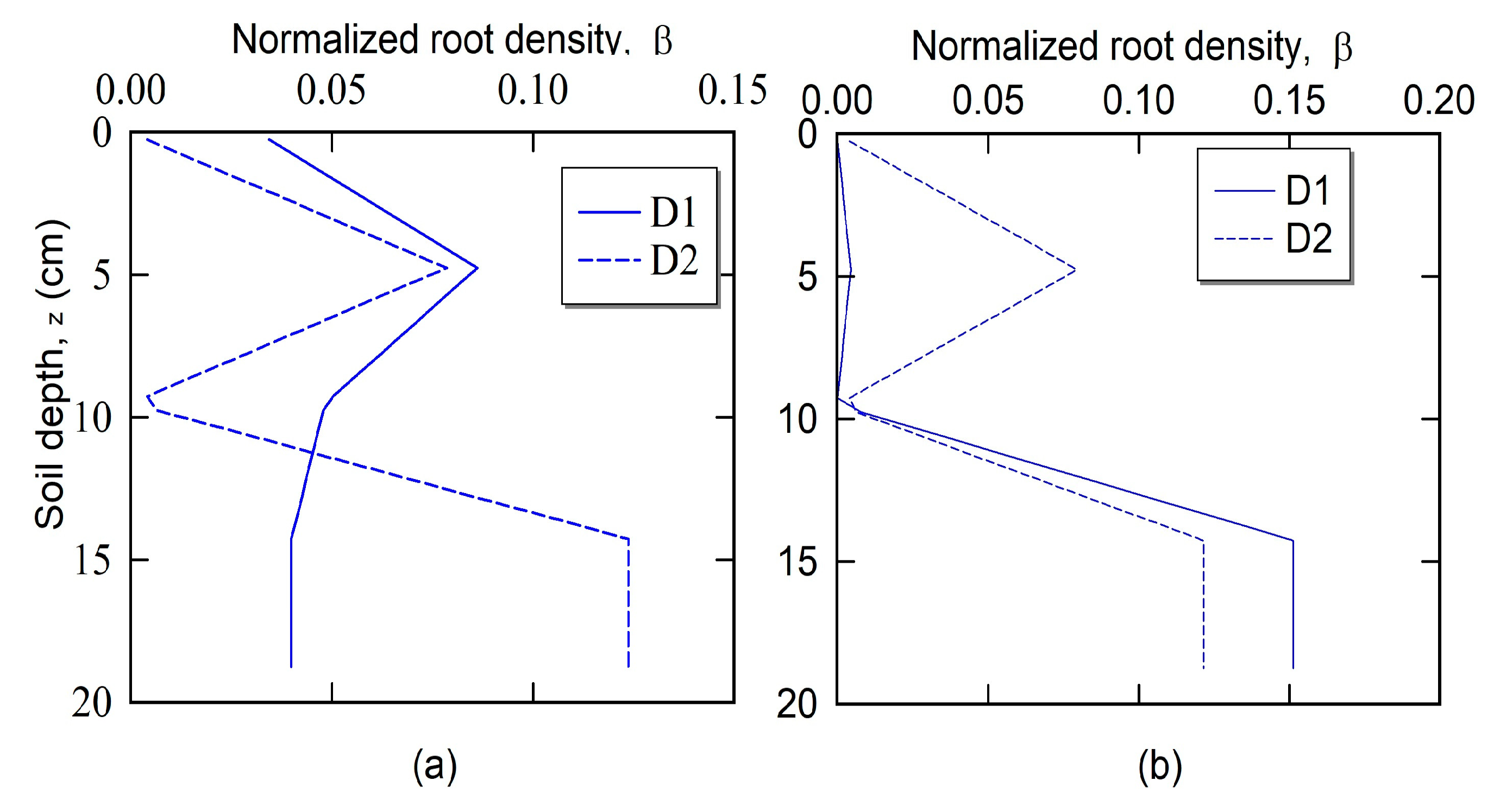
2.2.2. Survey for Root Length Distribution
2.3. Bulk Method
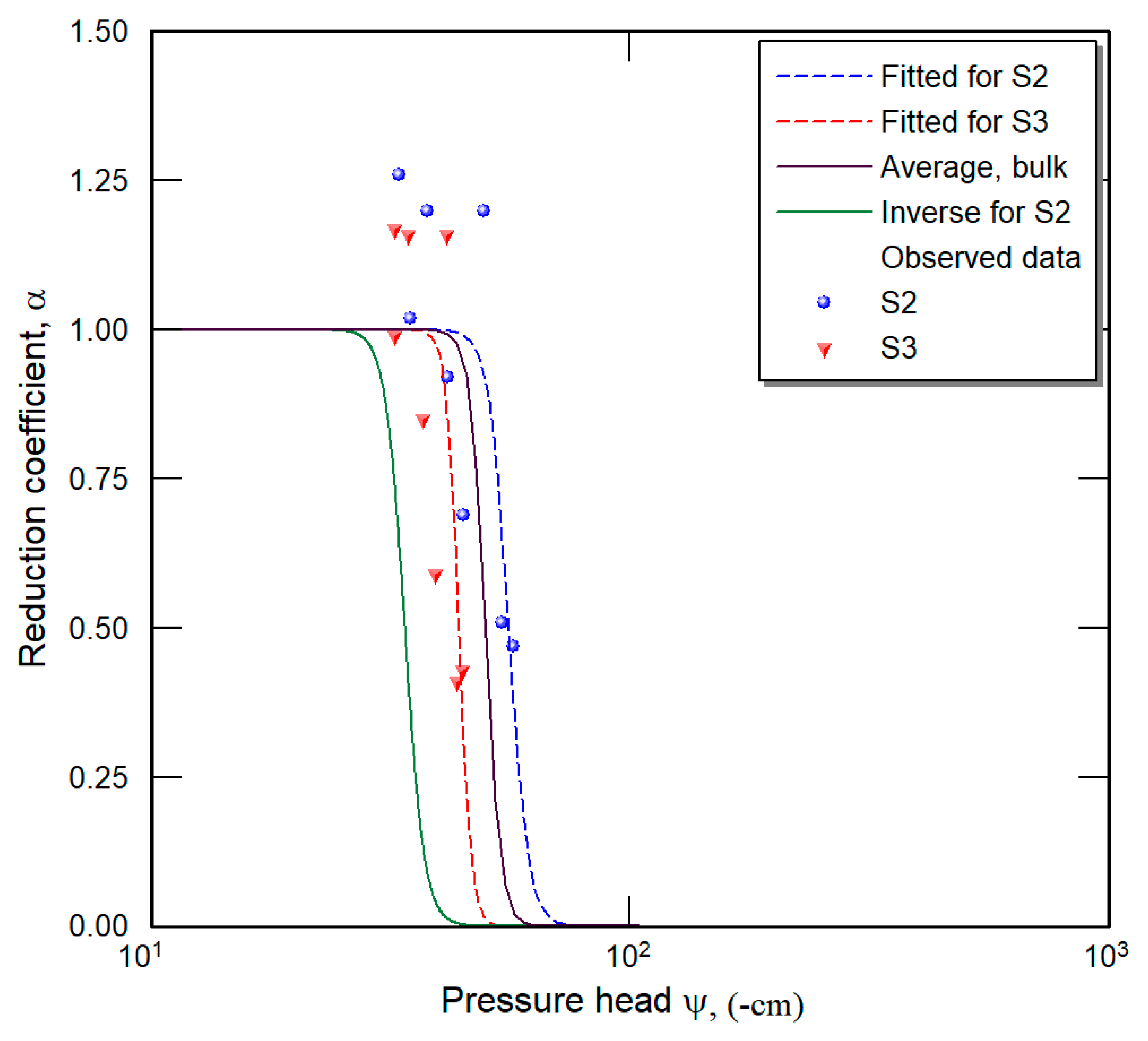
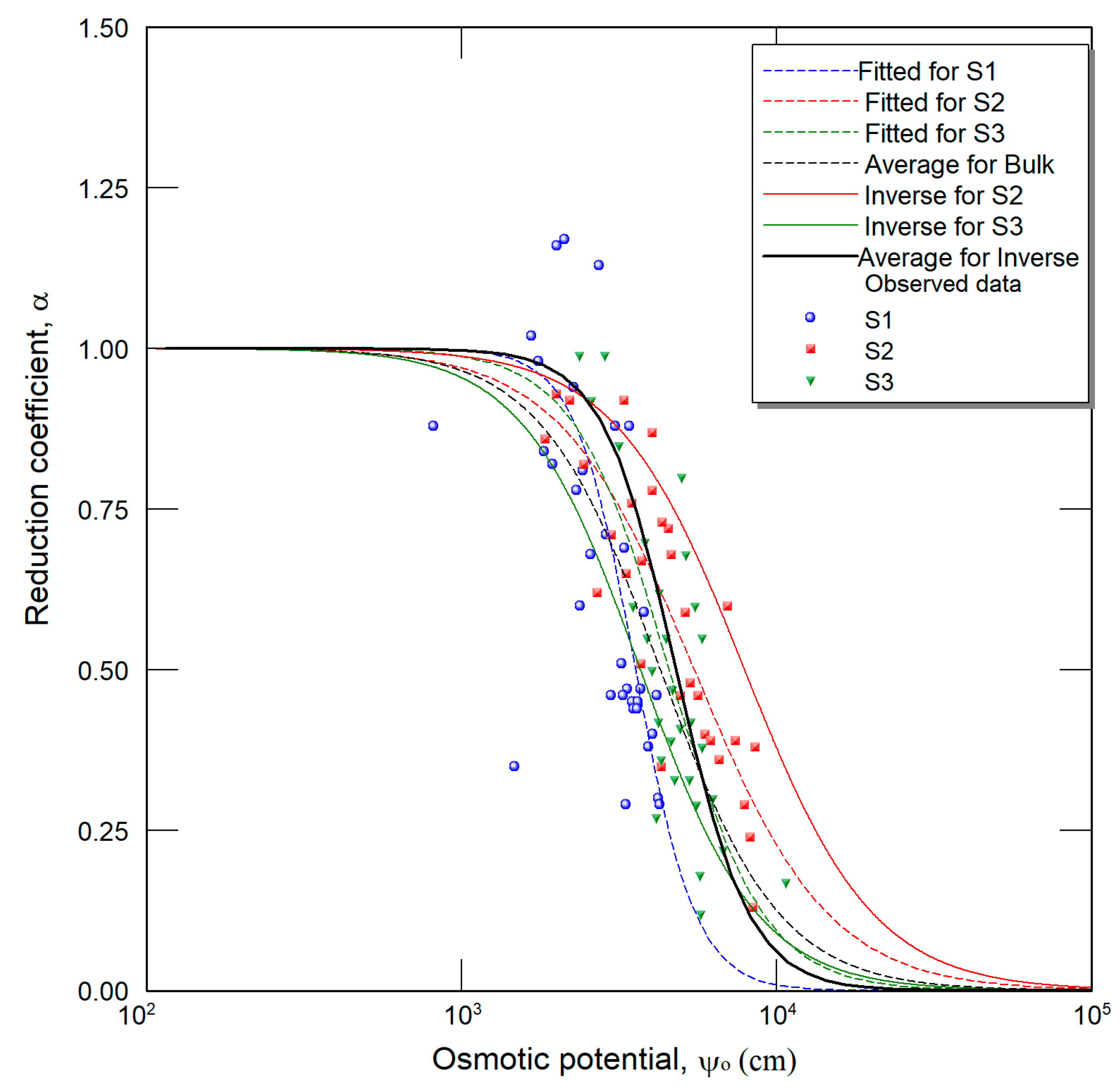
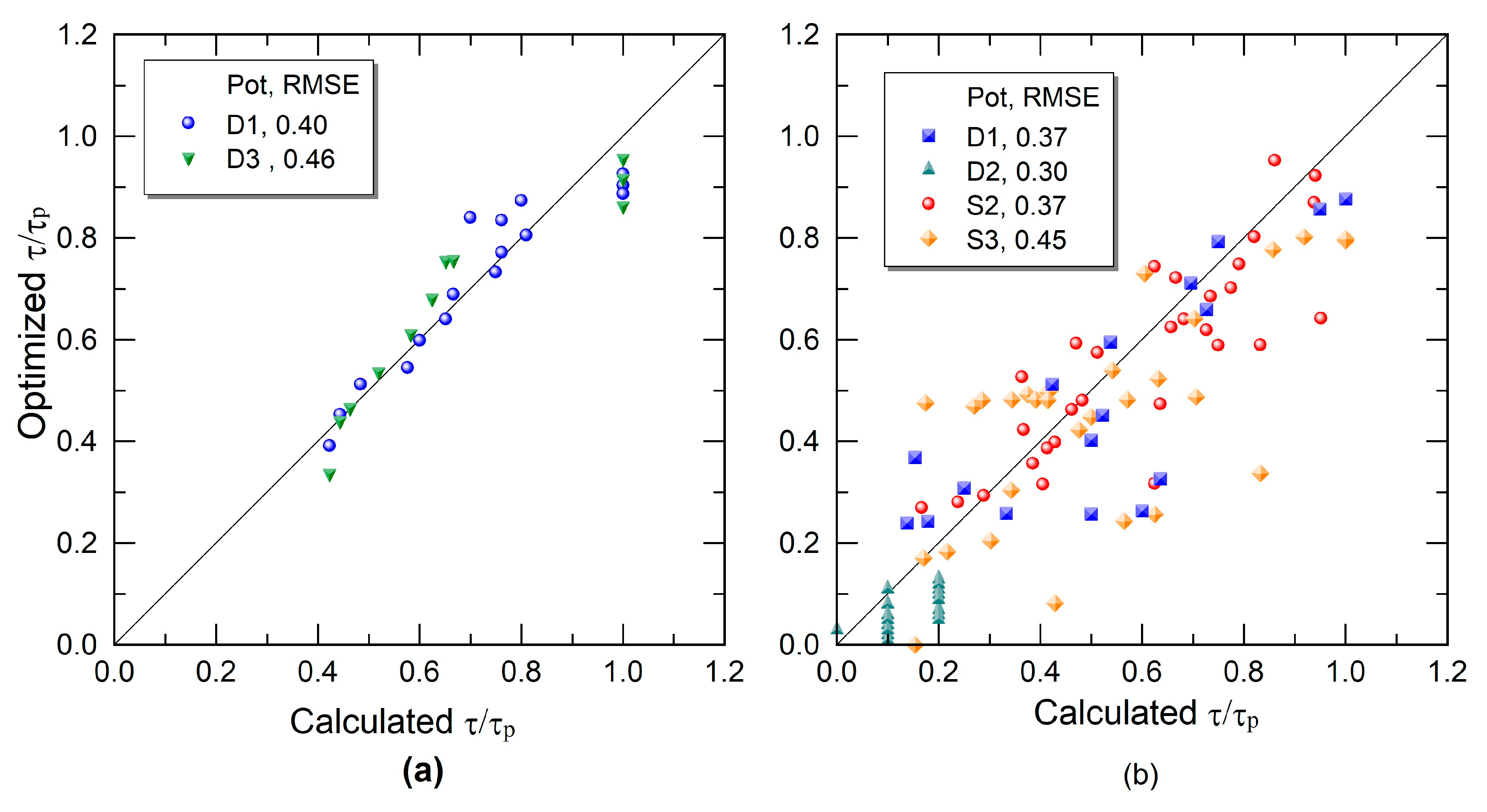
2.4. Inverse Method
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Vinocur, B.; Altman, A. Plant Responses to Drought, Salinity and Extreme Temperatures: Towards Genetic Engineering for Stress Tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Singh, A. Soil Salinity: A Global Threat to Sustainable Development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Shaygan, M.; Baumgartl, T. Reclamation of Salt-Affected Land: A Review. Soil Syst. 2022, 6, 61. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, Salinity and Drought Stresses: An Overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Thu, N.B.A.; Nguyen, Q.T.; Hoang, X.L.T.; Thao, N.P.; Tran, L.S.P. Evaluation of Drought Tolerance of the Vietnamese Soybean Cultivars Provides Potential Resources for Soybean Production and Genetic Engineering. BioMed Res. Int. 2014, 2014, 809736. [Google Scholar] [CrossRef]
- Šimůnek, J.; Hopmans, J.W. Modeling Compensated Root Water and Nutrient Uptake. Ecol. Model. 2009, 220, 505–521. [Google Scholar] [CrossRef]
- Ren, D.; Xu, X.; Hao, Y.; Huang, G. Modeling and Assessing Field Irrigation Water Use in a Canal System of Hetao, Upper Yellow River Basin: Application to Maize, Sunflower and Watermelon. J. Hydrol. 2016, 532, 122–139. [Google Scholar] [CrossRef]
- De Melo, M.L.A.; de Jong van Lier, Q. Revisiting the Feddes Reduction Function for Modeling Root Water Uptake and Crop Transpiration. J. Hydrol. 2021, 603, 126952. [Google Scholar] [CrossRef]
- McGeorge, W.T. Diagnosis and Improvement of Saline and Alkaline Soils. Agric. Handb. U Dept Agric. 1954, 18, 348. [Google Scholar] [CrossRef]
- Šimůnek, J.; Genuchten, M.T.v.; Šejna, M. The HYDRUS Software Package for Simulating the Two- and Three-Dimensional Movement of Water, Heat, and Multiple Solutes in Variably-Saturated Media; PC Progress: Prague, Czech Republic, 2006. [Google Scholar]
- Van Dam, J.C.; Huygen, J.; Wesseling, J.G.; Feddes, R.A.; Kabat, P.; Van Walsum, P.E.V.; Van Diepen, C.A. Theory of SWAP, Version 2.0: Simulation of Water Flow, Solute Transport and Plant Growth in the Soil-Water-Atmosphere-Plant Environment; DLO Winand Staring Centre: Wageningen, The Netherlands, 1997; p. 71. [Google Scholar]
- Fujimaki, H.; Tokumoto, I.; Saito, T.; Inoue, M.; Shibata, M.; Okazaki, T.; El-Mokh, F. Determination of Irrigation Depths Using a Numerical Model and Quantitative Weather Forecasts and Comparison with an Experiment. In Practical Applications of Agricultural System Models to Optimize the Use of Limited Water; Lascano, R.J., Ed.; Acsess: Madison, WI, USA, 2014. [Google Scholar] [CrossRef]
- Zhou, X.; Condori-Apfata, J.A.; Liu, X.; Condori-Pacsi, S.J.; Valencia, M.V.; Zhang, C. Transcriptomic Changes Induced by Drought Stress in Hardneck Garlic during the Bolting/Bulbing Stage. Agronomy 2021, 11, 246. [Google Scholar] [CrossRef]
- Francois, L.E. Yield and Quality Response of Salt-Stressed Garlic. HortScience 1994, 29, 1314–1317. [Google Scholar] [CrossRef]
- Chauhan, C.P.S.; Shisodia, P.K.; Minhas, P.S.; Chauhan, R.S. Response of Onion (Allium cepa) and Garlic (Allium sativum) to Irrigation with Different Salinity Waters with or without Mitigating Salinity Stress at Seedling Establishment Stage. Indian J. Agric. Sci. 2007, 77, 483–485. [Google Scholar]
- Astaneh, R.K.; Bolandnazar, S.; Nahandi, F.Z.; Oustan, S. The Effects of Selenium on Some Physiological Traits and K, Na Concentration of Garlic (Allium sativum L.) under NaCl Stress. Inf. Proces. Agric. 2018, 5, 156–161. [Google Scholar] [CrossRef]
- Yanagawa, A.; Fujimaki, H. Tolerance of Canola to Drought and Salinity Stresses in Terms of Root Water Uptake Model Parameters. J. Hydrol. Hydromech. 2013, 61, 73–80. [Google Scholar] [CrossRef]
- Fujimaki, H.; Ando, Y.; Cui, Y.; Mitsuhiro, I. Parameter Estimation of a Root Water Uptake Model under Salinity Stress. Vadose Zone J. 2008, 7, 31. [Google Scholar] [CrossRef]
- Ebrahimian, H.; Fujimaki, H.; Toderich, K. Parameterization of the Response Function of Sesame to Drought and Salinity Stresses. Agric. Switz. 2023, 13, 1516. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A Numerical Model for Water and Solute Movement in and below the Root Zone. United States Department of Agriculture Agricultural Research Service US Salinity Laboratory. J. Geosci. Environ. Prot. 1987, 5, 3. [Google Scholar]
- Homaee, M.; Feddes, R.A.; Dirksen, C. A Macroscopic Water Extraction Model for Nonuniform Transient Salinity and Water Stress. Soil Sci. Soc. Am. J. 2002, 66, 1764–1772. [Google Scholar] [CrossRef]
- Newman, E. A Method of Estimating the Total Length of Root in a Sample. J. Appl. Ecol. 1996, 3, 139. [Google Scholar] [CrossRef]
- Kalra, A.; Goel, S.; Elias, A.A. Understanding Role of Roots in Plant Response to Drought: Way Forward to Climate-Resilient Crops. Plant Genome 2023, 17, e20395. [Google Scholar] [CrossRef] [PubMed]
- Homaee, M.; Feddes, R.A.; Dirksen, C. Simulation of Root Water Uptake II. Non-Uniform Transient Water Stress Using Different Reduction Functions. Agric. Water Manag. 2002, 57, 111–126. [Google Scholar] [CrossRef]




| Soil | θsat | θr | αv | n |
|---|---|---|---|---|
| cm3 cm−3 | cm3 cm−3 | cm−1 | ||
| Tottori sand | 0.42 | 0.03 | 0.018 | 4.0 |
| Kanto loam | 0.64 | 0 | 0.0025 | 1.26 |
| Method | Replicate | 2022 | 2023 | ||
|---|---|---|---|---|---|
| h50 (-cm) | p50 | ||||
| D1 | 5592 | 1.25 | 5884 | 1.73 | |
| Bulk | D2 | 7312 | 1.8 | 1974 | 1.34 |
| D3 | 4839 | 0.92 | 2812 | 1.43 | |
| Mean ± STD | 5914 ± 1035 | 1.32 ± 0.36 | 3557 ± 1681 | 1.50 ± 0.17 | |
| D1 | 4589 | 1.43 | 6623 | 1.49 | |
| Inverse | D2 | - | - | 1180 | 0.81 |
| D3 | 4738 | 1.48 | - | - | |
| Mean ± STD | 4664 ± 75 | 1.46 ± 0.03 | 3902 ± 2722 | 1.15 ± 0.34 | |
| Method | Replicate | h50 (-cm) | p50 | h050 (-cm) | p050 |
|---|---|---|---|---|---|
| S1 | - | - | 3516 | 2.42 | |
| Bulk | S2 | 56 | 21.32 | 5509 | 2.08 |
| S3 | 44 | 33.67 | 4589 | 2.99 | |
| Mean ± STD | 50 ± 6 | 27.5 ± 6.18 | 4538 ± 814 | 2.5 ± 0.37 | |
| S1 | - | - | - | - | |
| Inverse | S2 | 34 | 21.3 | 7621 | 5.82 |
| S3 | - | - | 2020 | 1.68 | |
| Mean ± STD | - | - | 4821 ± 2801 | 3.8 ± 2.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nana, J.B.; Abd El Baki, H.M.; Fujimaki, H. Determining Drought and Salinity Stress Response Function for Garlic. Soil Syst. 2024, 8, 59. https://doi.org/10.3390/soilsystems8020059
Nana JB, Abd El Baki HM, Fujimaki H. Determining Drought and Salinity Stress Response Function for Garlic. Soil Systems. 2024; 8(2):59. https://doi.org/10.3390/soilsystems8020059
Chicago/Turabian StyleNana, Jean Bosco, Hassan M. Abd El Baki, and Haruyuki Fujimaki. 2024. "Determining Drought and Salinity Stress Response Function for Garlic" Soil Systems 8, no. 2: 59. https://doi.org/10.3390/soilsystems8020059
APA StyleNana, J. B., Abd El Baki, H. M., & Fujimaki, H. (2024). Determining Drought and Salinity Stress Response Function for Garlic. Soil Systems, 8(2), 59. https://doi.org/10.3390/soilsystems8020059







