Abstract
Hulls and shells are an abundant by-product from almond production with potential as an organic matter amendment (OMA). A combination of incubation study and field research was conducted in 2019–2021 to evaluate the impacts of three practices in combination on orchard soils’ C and N cycling, including a 210-day period of laboratory incubation with hulls and shells, and field sampling of orchard soils with and without historic applications of green waste compost as an OMA; with hulls and shells and with and without off-ground harvest where orchard soils remain undisturbed year round. Hulls and shells increased microbial biomass carbon in the field study by 248 μg g−1 dry soil after one year (p < 0.001) and during incubation, and increased cumulative respiration in soils with and without historic OMA (p < 0.001). Historic OMA resulted in double the total soil organic carbon (SOC) and total nitrogen (TN) compared to soil without resulting in significantly higher respiration and N mineralization when amended with hulls and shells. The decomposition of hull and shell biomass following surface application progressed at similar rates in the laboratory and field (1.7 g kg−1 d−1 during incubation (R2 = 0.84) and 1.3 g kg−1 d−1 in the field trial (R2 = 0.91). Our results highlight the suitability of hulls and shells as a by-product source of OMA for improving soil health in orchards with historic OMA and transitioning to organic matter management.
1. Introduction
With over 2.2 million metric tons of hulls and shells being generated each year [1] by nearly 650,000 hectares of almonds in California [2], diversifying the uses of these by-products would benefit industry resource use. Nutrients released from hulls and shells following soil application are valuable inputs to supply the annual nutrient demand of the almond crop, specifically for potassium (K) [3,4]. Recycling these agricultural by-products can reduce grower dependence on fertilizers, while leveraging carbon inputs for orchard soil health [4,5,6,7] and overall sustainability of food systems.
Trials in orchards with organic matter amendments (OMAs) like hulls and shells are limited, especially when compared to annual crops [6]. Two trials with hulls and shells in orchards showed improvements in water retention and weed suppression [8,9]. A ten-year study on organic avocado showed that surface application of hulls and shells coupled with no-till management created an organic litter layer more similar to forest ecosystems than orchards [10]. These plots amended with hulls and shells showed increases in soil organic C (SOC), Kjeldahl N, and available phosphorus (P) and matched or exceeded yields compared to trees without amendment [10]. In another avocado system, hulls and shells increased the Shannon diversity index of the rhizosphere microbiome and created a disease-suppressive effect attributed to changes in the soil microbiome [11,12]. These results generally agree with other orchard soil studies with OMA [13,14,15].
Understanding the impact of stacking multiple soil health practices is needed to support field-scale decision making about OMA use. Growers often apply multiple materials sequentially, which is underrepresented in empirical studies. Furthermore, studies exploring how historic OMA impacts response to subsequent application of different OMA sources are of particular value. Several studies concluded there was either no effect or a small effect of low relevance following OMA use on soils with a divergent history of management [16,17,18]. The effect on decomposition of the new OMA source can be difficult to demonstrate given the high rates of mineralization from SOC and TN pools in historically amended soils [19,20,21]. One study by Mallory and Griffin [22] was an exception where historically amended soil resulted in lower soil nitrate mineralization from an OMA source than an unamended soil, which the authors attributed to immobilization. However, there remains a knowledge gap of understanding C and N cycling in soils with historic OMA use, as well as subsequent effects on the turnover of different OMA sources.
In orchards where the practice of no-till OMA management is common, minimizing disturbance of the OMA layer may more rapidly result in benefits to soil health. In almonds, off-ground harvest, also known as catch-frame harvest, is an emerging technology that allows for full utilization of surface-applied OMA because of minimal soil disturbance. Conventional on-ground harvest in almonds requires soils to be bare, and disturbance by repeated machinery passes generates significant losses and amounts of dust, a serious air quality hazard that negatively affects human health [23]. Off-ground harvest uses fewer machinery passes and does not require bare soil, thus reducing top soil loss, and enabling the accumulation of litter layers from surface-applied OMA over time.
In this work, we paired a 210-day period of soil incubation and a field study in a commercial almond orchard to explore how soil respiration, N mineralization and soil microbial biomass C and N were affected by hulls and shells with and without a historic application of green waste compost as an OMA and off-ground harvest. In the incubation study, we hypothesized that hulls and shells would increase C and N pools in the historic OMA soil compared to the soil without previous OMA use. Furthermore, the addition of hulls and shells in soil without historic OMA use would increase microbial biomass leading to N immobilization. In the field trial, we hypothesized that hulls and shells combined with off-ground harvest would increase SOC, TN, mineral N and soil microbial biomass compared to unamended and conventional on-ground harvest soils.
2. Materials and Methods
2.1. Laboratory Incubation
The effect of soil management history on C and N mineralization under a subsequent amendment with almond hulls and shells was examined in soil that had received an application of green waste compost as an OMA for three years (2015–2017) [6]. This soil was collected from an almond (Prunus dulcis) orchard located near Escalon, CA, in San Joaquin County, USA (37°49′33″ N 121°6′45″ W), where the soil was a Manteca fine sandy loam (coarse-loamy, mixed, superactive, thermic Haplic Durixeroll). Soil samples were taken in October 2020 from a depth of 0–10 cm. Four randomized plots had received surface-applied green waste compost while four had served as a control in the previous study [6]. These two soils were incubated with and without hull and shells, resulting in four treatments. Soils were aggregated by treatment, air-dried, sieved to 2 mm, and weighed into 96 1-quart glass mason jars in aliquots of 615 g, reaching a depth of 7.6 cm equal to a dry bulk density of 1.0 g cm−3. The 96 jars were arranged in a completely randomized design, with 48 receiving the control soil and 48 receiving the historically amended soil. Almond hulls and shells were obtained from Mariani Nut Company in Winters, CA, oven-dried, and partially ground by hand using a mortar and pestle. Next, 24.3 g of oven dry hulls and shells was added to 24 jars of each soil, separated from the soil surface by a thin, flexible mesh layer allowing for separation during sampling (Figure 1). On each of six sampling dates over 210 days, four replicates of each treatment were destructively harvested.

Figure 1.
Diagram showing soil and amendment placement for the incubation study.
On Day 1 of the incubation, 120 mL of de-ionized (DI) water was added to each jar to re-wet the soil up to field capacity, which had been determined to be ~19% volumetric water content in a previous study [24]. Water was added a few mL at a time to avoid preferential flow. An additional 15 mL of DI water was added to amended jars to re-wet the amendment layer to its capacity, which was determined by wetting samples of oven-dry amendment, letting them drain, and recording the weight of water held. The amount of water added to the amendment layer was adjusted throughout the incubation based on dry masses, while the amount of water added to the soil was kept constant. DI water was added weekly by weight to bring the jars to field capacity and timed to occur four days before sampling dates to ensure a uniform water content at each sampling. Jars were stored without lids and maintained in a temperature (20 °C) and humidity-controlled (60%) dark room located in the UC Davis Postharvest Facility for the study duration.
2.2. Field Study
The field study was established in 2019 at a mature almond orchard located in Woodland, CA, in Yolo County, USA (38°40′12″ N–121°53′40” W), on a San Ysidro clay loam (fine, smectitic, thermic Typic Palexeroll) using a randomized complete block design with four treatments and four blocks. Almond hulls and shells procured from Mariani Nut Company in Winters, CA, were applied to treatment plots at a rate of approximately 16.8 metric tons ha−1 dry weight (18 metric tons ha−1 fresh weight) in fall of 2020. The amount of hulls and shells used in the incubation trial was calculated to match this application rate, and the hulls and shells used in both trials were from the same shipment and source. The C:N ratio of the amendment was approximately 52:1, consisting of 44.5% carbon and 0.80% nitrogen on average. Almond harvest took place in early August of 2021 and both off-ground and on-ground harvest machinery were used in their respective plots. Irrigation and fertilization followed grower best management practices. Litter bags (made of 0.79 mm mesh and each containing 67.0 g of dry hull and shell mix) were used in amendment plots to measure decomposition by net mass loss over time. On Day 60, 4.5 metric tons ha−1 of compost was applied across the entire orchard, which landed on top of the litter bags, causing a small error by slightly increasing the mass of the litter bags.
2.3. Sample Collection and Analysis
For the incubation study, measurements were performed on Days 0, 15, 30, 45, 60, 120, and 210. Four jars per treatment were destructively sampled and hull and shell residue was removed by lifting the 62.2 cm2 mesh out of the jar and analyzed separately. Soil was emptied onto a metal tray, homogenized and subsamples were air-dried for SOC and TN, oven-dried for moisture content or refrigerated prior to processing for microbial biomass, dissolved organic C, and mineral N. For the field trial, soil was sampled in September 2020 and October 2021, with amendment residue collection from the litter bags. Soil samples were taken from a depth of 0–10 cm using a Dutch auger at three points in each treatment row, aggregated, stored on ice until returned to the lab where they were sieved to 2 mm.
Carbon dioxide (CO2) samples for determination of microbial respiration were taken from each jar at Days 15, 30, 60, 90, 120, and 210. Jars were sealed with metal lids with septa. At 0, 30, and 60 min, a syringe was used to mix headspace gas and transfer 20 mL of it into evacuated exetainers. Gas samples were analyzed using a LI-COR LI-6251 CO2 Analyzer. One mL of each gas sample was injected into the analyzer using a syringe and the peak reading was recorded. A set of three standards were run every 16 samples. Raw readings were transformed to parts per million using standard curves, which were unique for each run and were fit to all standard values from that run. Values were converted to a per gram of dry soil basis based on 615 g dry soil per jar. Cumulative CO2 efflux was calculated as a rate of CO2 increase over the hour measured and extrapolated between measurement dates. These rates were used to calculate cumulative CO2 efflux over time piecewise using trapezoidal areas.
Soil subsamples from the baseline and final sampling dates of the incubation and from Days 0, 200, and 365 of the field trial were sent to the UC Davis Analytical Lab for quantitative determination of SOC [25] and TN [26]. Amendment residues recovered from the incubation as described above were oven-dried and aggregated by their sampling date. For the field study, hull and shell residue was collected from litter bags in the off-ground harvest treatment rows at 30, 60, 120, 150, 240, 293, 365 days of decomposition and oven-dried. Amendment decomposition rates were determined by the mass of oven-dry samples. Subsamples from litter layer residues were sent to the UC Davis Analytical Lab for quantitative determination of total C and N [27].
To obtain ammonium and nitrate N, and microbial biomass C (MBC) and N (MBN), 6 g of fresh soil was kept at a controlled temperature and processed within one week. For microbial biomass measurements, one subsample was fumigated with chloroform for 24 h while a second remained unfumigated [28]. Both fumigated and unfumigated subsamples were then extracted by adding 30 mL 0.5 M K2SO4, shaking for one hour, and filtering through Fisher brand Q5 filter paper [28,29]. Soil extracts were frozen until analysis with a Shimadzu TOC-Vcsh TOC Analyzer to obtain non-purgeable organic C and total extractable N. Standards were included with runs and readings were transformed using standard curves. A check standard was run every 12 samples. The resulting values were blank-adjusted. Microbial biomass was calculated as the difference between fumigated and unfumigated samples. A conversion factor of 1/0.45 was used for MBC and 1/0.54 for MBN [30]. Values were converted to a per gram of dry soil basis. Dissolved organic C (DOC) was also obtained using the same methods as MBC and MBN.
For the incubation trial, soil extracts were analyzed for ammonium and nitrate concentrations using colorimetric assays [31,32]. Microcuvettes were run in duplicate on a Thermo Scientific Genesys 10S UV-Vis Spectrophotometer. Standards and blanks were included and readings were transformed using standard curves that were unique for each run. Values were blank-adjusted and converted to a per gram of dry soil basis. Since ammonium concentrations were low, we report the combined concentration of ammonium and nitrate as mineral N. Regression lines for the rates of observed mineral N increases were fitted for each replicate. Fits with an R2 value of greater than 0.85 were accepted.
2.4. Statistical Analysis
Data formatting and linear regressions on amendment decomposition rates were performed in Microsoft Excel and statistical analysis was performed using R 4.1.1 (R Foundation for Statistical Computing). Analysis of variance (ANOVA) was used with fixed effects (history, amendment, and time or harvest, amendment, and time) and all interaction terms to assess the effect of amendment with or without hulls and shells, historic OMA use and time for the incubation trial, and amendment with or without hulls and shells, off-ground or on-ground harvest and time for the field trial. Time was treated as a categorical variable because measurements were taken from different jars at each time point and therefore, there was no correlated variance structure. Linear models were created and a three-way ANOVA on each model was used to test for significant differences of fixed effects. Residuals were tested for homogeneity and normality using Quantile-Quantile Plots and Scale-Location Plots. Multiple pairwise comparisons of least square means were performed using the emmeans package, which applied the Tukey method of p-value adjustment with a significance level of 0.05. For amendment layer data only, p-values were adjusted using the Dunnett method or were not adjusted, as appropriate. To calculate rates of mineral nitrogen accumulation, regressions were performed on mineral nitrogen over time and lines with R2 values above 0.85 were accepted.
3. Results
3.1. Total Soil Organic C and N
Values of SOC and TN for the soil with historic OMA were significantly greater (p < 0.001) than soil without historic OMA at the beginning of the incubation (Table 1). After 210 days, SOC and TN remained similar with slight increases that were not significant. In the field trial, SOC and TN were not significantly different between plots with and without hull and shell amendment.

Table 1.
Initial (i) and final (f) values of total soil organic carbon (SOC) and nitrogen (TN) after 210 days of incubation for soils with and without historic OMA, and bulk field soil with and without hull and shells. Letters represent significant differences (p < 0.05) between treatments at each time.
3.2. Decomposition and Nutrient Release from the Amendment Layer
Hull and shell decomposition was similar in both studies. Under laboratory conditions, the amendment lost 37.8% of its dry mass after 210 days (Figure 2). Decomposition fit a linear decline (R2 = 0.84) of 0.17% per day (1.7 g kg−1 d−1) and aligned well with the field study where the amendment lost 42.4% of its dry mass after 240 days, and 55.2% after one year, and fit a linear decline (R2 = 0.90) of 0.13% per day (1.3 g kg−1 d−1).
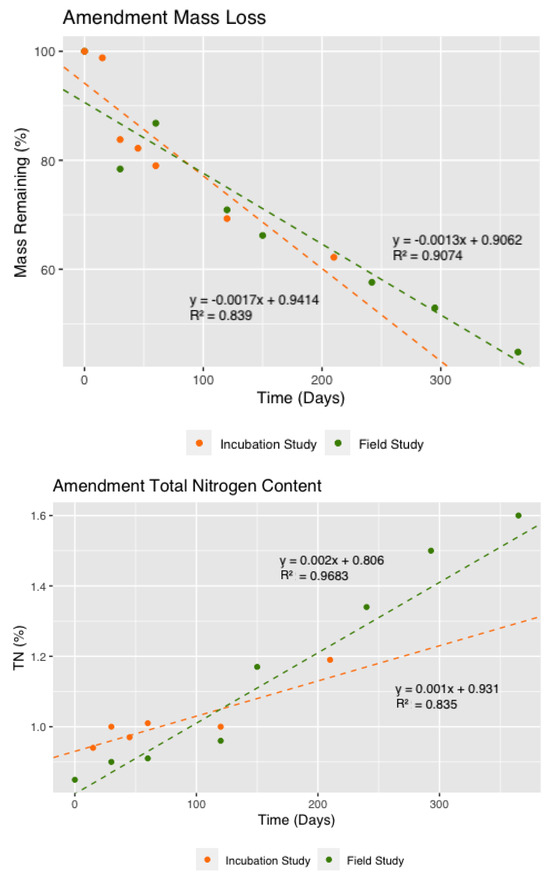
Figure 2.
Amendment mass loss (above) and total nitrogen (TN) content (below) of hull and shell amendment residue for incubation and field studies. Dotted lines represent linear regressions with the equations and R2 values given.
The total carbon (TC) concentration of the amendment layer in the field was not significantly different between 0 and 365 days, and remained stable over time during incubation (Figure 2). By contrast, the TN concentration of the amendment layer in the incubation study increased up until Day 210 to 1.19%. We observed a similar trend in the field trail, where TN of the amendment layer increased significantly over time at 150 days (p < 0.01), and by 365 days, TN in the field trial had increased significantly from 0.85 to 1.60% (p < 0.001).
3.3. Dissolved Organic Carbon and Microbial Biomass
Historic OMA use led to increases in dissolved organic carbon (DOC) as the incubation progressed. At Day 210, DOC in soils with historic OMA use and hull and shells were higher than unamended soils, and significantly different for soils without historic OMA applications (Figure 3). The baseline MBC for the field study ranged from 175 to 215 μg C g−1 dry soil with no significant differences prior to experimentation. After one year, there was a significant increase from the hull and shell amendment (p < 0.001) with no difference between soil treated with off and onground harvest. There was a limited effect on MBN and no significant differences were detected.
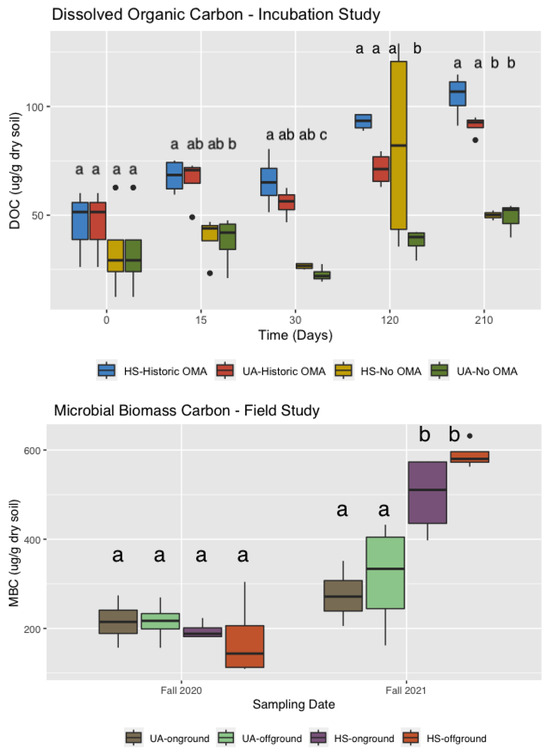
Figure 3.
Dissolved organic carbon (DOC) in the incubation study (above) and microbial biomass carbon (MBC) in the field study (below). Treatments are hulls and shells (HS) and unamended (UA) on soils with and without historic OMA, and field soil followed by off or onground harvest. Different letters are significant differences (p < 0.05) at each time.
3.4. Cumulative Respiration
Cumulative CO2 efflux from microbial respiration was significantly affected by both historic OMA use and hull and shell amendment in the incubation study, with a large impact of hulls and shells. Hulls and shells increased the final cumulative CO2 efflux by 5.62 mg CO2 g−1 dry soil and 4.19 mg CO2 g−1 dry soil on soil with and without historic OMA use (p < 0.001), respectively. (Figure 4). There was also a significant interaction between soil history and hulls and shells (p < 0.001). Historic OMA use increased final cumulative CO2 evolution by an estimated 2.23 mg CO2 g−1 dry soil when amendment was present (p < 0.001) and 0.81 mg CO2 g−1 dry soil without amendment (p < 0.001). Hulls and shells more than tripled microbial respiration in soils with historic OMA use, and more than quadrupled microbial respiration in soils without historic OMA use.
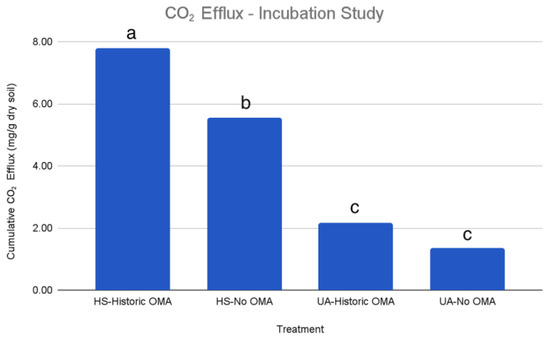
Figure 4.
Cumulative CO2 efflux in the incubation study. Treatments are hulls and shells (HS) and unamended on soils with and without historic OMA. Different letters represent significant differences (p < 0.05) between treatments.
3.5. Mineral Nitrogen
Mineral N accumulation in the incubation study ranged from 65 to 103 mg kg−1 dry soil and peaked at Day 210. An ANOVA showed significant effects of both hulls and shells and amendment (p < 0.001) and soil management history (p < 0.01) on the amount of mineral N. The experimental conditions resulted in net N mineralization for all treatments, but the rates in soils without hulls and shells were higher. Differences among treatments were significant at Days 45, 120, and 210 (Figure 5). The interaction effect between hulls and shells, and historic OMA use (p < 0.005) is of particular interest. Peak mineral N, at Day 210 was the lowest in the treatment with hulls and shells, but without historic OMA use, and significantly lower than the three other treatments (p < 0.001).
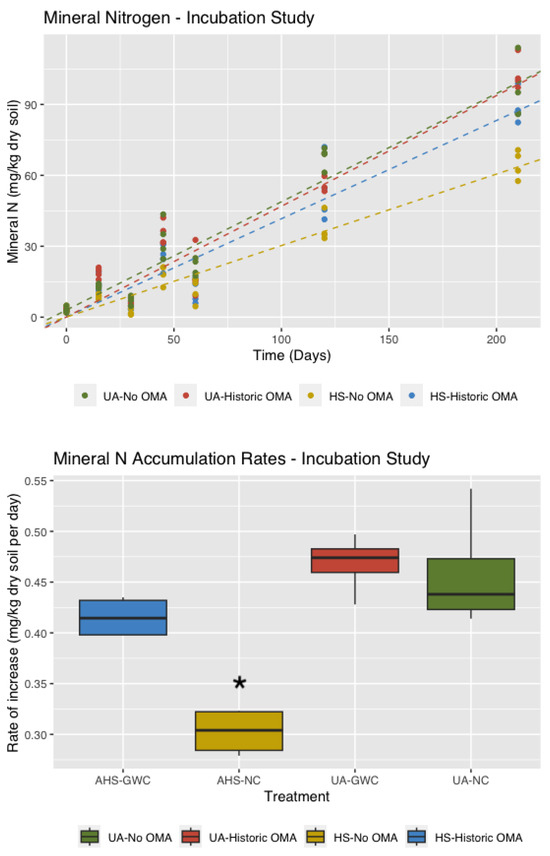
Figure 5.
Values of mineral nitrogen with lines (above) and the average net N mineralization rates (below). Treatments are hulls and shells (HS) and unamended on soils with and without historic OMA. The asterisk shows significant differences at p < 0.05.
N mineralization in soils with historic OMA use was significantly lower with hulls and shells than without (p < 0.01) and similar to soils without hulls and shells and historic OMA use (p = 0.36) (Figure 5). Incubation of hulls and shells with orchard soil with lower organic matter significantly reduced net N mineralization compared to hulls and shells on higher organic matter or unamended soils with low or high organic matter. The N mineralization rate for the soils with hulls and shells without historic OMA use was significantly lower (p < 0.01) than the other treatments (Figure 5). This result demonstrates that over the period of measurement, soil receiving hulls and shells for the first time may see reduced net N mineralization compared to soil with a history of OMA use.
4. Discussion
4.1. Impact of Soil History Highlights Long-Term Effects on C and N Cycling
The impact of soil management history was apparent before the incubation began, as soil organic C and N stores reflected past management decisions. We did not see significant short-term changes to the SOC and TN pools from hulls and shells over the 210 days of incubation, suggesting that changes in these soil pools require more than 210 days. By contrast, a field study by López [10] demonstrated that hulls and shells can significantly increase soil C and N, observing a 110% increase in SOC and a 65% increase in Kjeldahl N over ten years in an avocado orchard. Plant-driven mechanisms regulating C and N cycling such as the presence of living roots and labile C exudates were absent in our incubation.
While we did not observe changes in TN of the bulk soil over the length of our incubation, we did observe an increase in TN within the amendment residue in both trials. Higher C:N residues can lead to the immobilization of mineral N through microbial activity within the litter layer, likely to support microbial biomass growth in the amendment layer itself, an overlooked and understudied aspect of surface-applied OMA [4]. With a C:N ratio of 52 for hulls and shells, we would expect N to be preferentially assimilated by microbes in the litter layer during microbial biomass growth, with a typical C:N range from 5.0 to 15 [33]. If those microbial cells were located in the litter layer rather than in the soil below, they would be unaccounted for in soil TN. During the incubation of straw OMAs, researchers noted small increases in straw TN and attributed this to microbial biomass [34]. Microbial N assimilation in the litter layer has been observed in forests and other non-agronomic systems, where accumulation is believed to reach a threshold before transitioning to mineralization [33,35]. Prescott and Vesterdal [35] emphasize that litter residue in forest ecosystems is a combination of both plant residue and microbial products. Thus, we deduce that N mineralization in our incubation is attributed predominantly to soil organic N pools rather than the decomposition of added hulls and shells.
4.2. Mineral N Accumulation Is Affected by Both History and Amendments
All incubation treatments led to a net N mineralization, meaning that N immobilization through microbial N uptake was less than gross N mineralization under laboratory conditions. The mineral N values observed here were comparable to what might be seen in fertilized almond orchards, which can range from 0 to 50 mg N kg−1 dry soil [36]. While the net N mineralization in our incubation is attributed to soil organic N pools rather than added nutrients, it is relevant that the scale of measurement is comparable to a field setting. In our field trial, where trees received the grower’s standard application of N fertilizer, no deficiencies were found during leaf nutrient analysis (data not shown), indicating in the short-term that there was no effect of N immobilization on tree N uptake.
Our results are contrary to previous findings by Mallory and Griffin [21], who observed that during a 282-day period of incubation, there was lower net N mineralization in soils with historic OMA use than without. However, more research may be needed. Mallory and Griffin [21] saw an interaction between soil history and the new OMA applied, implying that the effect may vary based on the amendment used, fibrous C concentration [21], or lignin content. Our hulls and shells had a higher C:N ratio than either of the amendments previously tested (52:1 compared to 31:1 and 7:1) and was applied at a much higher rate, i.e., 18,000 kg/ha compared to 100 kg/ha. Although the soils in both studies were well-drained, their site was not an orchard, instead hosting annual rotations of potatoes and a variety of cover crops and subject to higher levels of disturbance by tillage.
4.3. Microbial Biomass and Respiration Rapidly Respond to Amendments
In our field study, soil MBC significantly increased after one year as a result of the hull and shell amendment. Based on the established long-term effects of OMAs [9,37], we anticipate that microbial biomass in our field soils with hull and shell amendment will increase further compared to those unamended, especially when retaining the litter layer. Since the change to offground harvest occurred in August, shortly before our one-year sampling date, the effects of the harvest strategy may not have been detectable. A reduction in soil disturbance is likely to lead to changes in soil microbial biomass over time.
The incubation study resulted in a strong effect of hulls and shells on CO2 efflux, one measure of microbial activity. Soil CO2 efflux has long been used as an indicator of soil quality and fertility and a tool for predicting C, N, and P mineralization [38]. The higher respiration from the historic OMA soil reflects higher total C and N compared to the unamended soil. The presence of a C source in the form of hulls and shells dramatically elevated microbial respiration, especially in the soil with historic OMA use. Microbial respiration rates in the amended treatments showed a decline from a high initial rate upon rewetting on Day 1, followed by a second peak at 120 days and a second decline. This two-part decomposition pattern could indicate that microorganisms depleted the labile C pool and moved on to a more recalcitrant one [35]. Generalizing across a broad range of soil ecosystems, Grandy and Neff [39] assert that carbohydrates and proteins are selectively degraded from plant residues, leaving behind less labile materials. Many papers have used two-part or double-exponential models to describe microbial mineralization of a labile and recalcitrant nutrient pool [40,41,42]. The multi-stage decomposition process suggested by a two-pool model characterizes this amendment as a slow-release option for returning C and N to the soil, potentially avoiding losses of nitrate via leaching. The similar decomposition rates seen in our field and laboratory studies suggest amendment characteristics may be useful for predicting decomposition rates across conditions.
On average, the amended jars lost 0.82 g of applied C, or about 7.6%, as CO2. Some loss to microbial respiration is always necessary to store carbon in soil over the long term [43], but moisture, temperature, and application method may influence the amount. A study on straw OMA performed under laboratory conditions similar to ours (20 °C, 60% relative humidity, and frequent watering) found that 13% of the carbon from surface-applied straw was lost as CO2 under continuously moist conditions and only 3% was lost when the soil was allowed to dry to below the permanent wilting point before watering [34]. Curtin et al. [34] found that the amount of C lost as CO2 was much higher when straw OMA was incorporated into the soil, as opposed to surface-applied, which is typical in orchard conditions for many different OMA sources.
Greater CO2 efflux may not correlate with an increase in microbial biomass, as other factors can impact the microbial C use efficiency, defined as the ratio of carbon assimilated to carbon respired [44], and the metabolic quotient, defined as the rate of respiration per unit of microbial biomass (qCO2) [45]. Manzoni et al. [33] discuss this phenomenon in litter decomposition, arguing that decomposers lower their C use efficiency when faced with a N-limited residue leading to higher respiration. Kallenbach et al. [46] notes that there are conflicting results regarding whether higher C:N ratio amendments lead to higher or lower CUE. Since high C:N amendments may promote more C-efficient microbes [44], they may improve overall C use efficiency. However, a meta-analysis [47] found that both microbial respiration and qCO2 were higher when the C:N ratio of the litter layer was higher, hypothesizing that a N-limited microbial community cannot build up as much biomass as the C concentration, leading to excess respiration. A trial in an apple orchard similarly recorded greater cumulative CO2 efflux during a 6-week incubation period of the top 6 cm of soil from plots that had received a bark mulch (C:N ratio = 85) compared to chicken manure (C:N ratio = 3.2) [12]. Many different interpretations of qCO2 have been proposed including C use efficiency [48], fungal:bacterial ratios [47], ecosystem development and/or disturbance [49], or system stress due to the pH [50].
We were unable to detect any differences in soil MBC or MBN in our incubation study (data not shown). The relationship between microbial biomass and C and N mineralization and immobilization is not straightforward. In one study of plant material incorporated into soils with differing starting values of microbial biomass, higher microbial biomass led to faster C and N mineralization initially, but no increase in cumulative net C and N mineralization, as the lower biomass treatments seemed to catch up over the two-month period [42]. Bonde et al. [51] examined the amount of potentially mineralizable N attributable to microbial biomass and noted that several studies concluded conflicting results. The presence of living roots and their associations with microbial functional groups can further regulate outcomes in field settings. Salazar-Villegas et al. [52] recommend using active microbial biomass instead of total microbial biomass because of the high levels of microbial dormancy in some context. These uncertainties about how soil microbial biomass relates to other common soil measurements underscore its complexity and areas for future research. Our findings also indicate the importance of understanding the litter layer microbial biomass pool as it relates to the below-ground pool when using surface-applied OMA. Future research could characterize the size and composition of these microbial communities in undisturbed litter layers and the mineral soil beneath.
5. Conclusions
Our research supports the use of hulls and shells as a surface-applied OMA in almond orchards. Hull and shell amendments increased microbial respiration under laboratory conditions and microbial biomass under field conditions. Our characterization of hull and shell decomposition rates can inform best practices for the timing and quantity of hull and shell applications. Furthermore, we did not find evidence for the risk of N immobilization with high C:N OMAs like hulls and shells. The observed higher N mineralization with historic applications of OMA soil offers evidence of the effectiveness of stacking soil health practice by integrating compost and hull and shell amendments. Hull and shell amendments offer an organic matter management strategy to increase microbial activity while supporting N mineralization, and recycling by-products within orchard agroecosystems.
Author Contributions
Conceptualization and funding acquisition: E.M.A., P.H.B. and S.D.S.K.; Experimental Design: L.W.H., E.M.A., A.C.M.G. and S.D.S.K.; Investigation: L.W.H., E.M.A. and E.G.G.; Data analysis and visualization: L.W.H. and S.D.S.K.; Writing: L.W.H. and S.D.S.K.; Editing: L.W.H., E.M.A., A.C.M.G., P.H.B. and S.D.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Western Sustainable Agriculture Research and Education Grant, grant number SW20-912, and the Foundation for Food and Agriculture Research Seedling Solutions Program, grant number CA18-SS-0000000260.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Contact the corresponding author for data requests.
Acknowledgments
The authors wish to extend their gratitude to Kirk Pumphrey at Westwind Farms for their assistance and the Almond Board of California for ongoing support of our work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fernandez-Bayo, J.D.; Shea, E.A.; Parr, A.E.; Achmon, Y.; Stapleton, J.J.; VanderGheynst, J.S.; Hodson, A.K.; Simmons, C.W. Almond Processing Residues as a Source of Organic Acid Biopesticides during Biosolarization. Waste Manag. 2020, 101, 74–82. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. National Agricultural Statistics Service 2020 California Almond Acreage Report; Pacific Region: Sacramento, CA, USA, 2021.
- Muhammad, S.; Sanden, B.; Lampinen, B.; Saa, S.; Siddiqui, M.; Smart, D.; Olivos-Del Rio, A.; Shackel, K.; Dejong, T.; Brown, P. Seasonal Changes in Nutrient Content and Concentrations in a Mature Deciduous Tree Species: Studies in Almond (Prunus dulcis (Mill.) D. A. Webb). Europ. J. Agron. 2015, 65, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.M.; Rivers, D.J.; Gaudin, A.C.M.; Geisseler, D.; Brown, P.H.; Khalsa, S.D.S. In a Nutshell: Almond Hull and Shell Organic Matter Amendments Increase Soil and Tree Potassium Status. Plant Soil 2024, 495, 699–722. [Google Scholar] [CrossRef]
- Jahanzad, E.; Holtz, B.A.; Zuber, C.A.; Doll, D.; Brewer, K.M.; Hogan, S.; Gaudin, A.C.M. Orchard Recycling Improves Climate Change Adaptation and Mitigation Potential of Almond Production Systems. PLoS ONE 2020, 15, e0229588. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, S.D.S.; Hart, S.C.; Brown, P.H. Nutrient Dynamics from Surface-Applied Organic Matter Amendments on No-till Orchard Soil. Soil Use Manag. 2022, 38, 649–662. [Google Scholar] [CrossRef]
- Villa, Y.B.; Khalsa, S.D.S.; Ryals, R.; Duncan, R.A.; Brown, P.H.; Hart, S.C. Organic Matter Amendments Improve Soil Fertility in Almond Orchards of Contrasting Soil Texture. Nutr. Cycl. Agroecosyst. 2021, 120, 343–361. [Google Scholar] [CrossRef]
- Jafari, M.; Haghighi, J.A.P.; Zare, H. Mulching Impact on Plant Growth and Production of Rainfed Fig Orchards under Drought Conditions. J. Food Agric. AMP Environ. 2012, 10, 428–433. [Google Scholar] [CrossRef]
- Verdú, A.M.; Mas, M.T. Mulching as an Alternative Technique for Weed Management in Mandarin Orchard Tree Rows. Agron. Sustain. Dev. 2007, 27, 367–375. [Google Scholar] [CrossRef]
- López, R.; Burgos, P.; Hermoso, J.M.; Hormaza, J.I.; González-Fernández, J.J. Long Term Changes in Soil Properties and Enzyme Activities after Almond Shell Mulching in Avocado Organic Production. Soil Tillage Res. 2014, 143, 155–163. [Google Scholar] [CrossRef]
- Bonilla, N.; Vida, C.; Martínez-Alonso, M.; Landa, B.B.; Gaju, N.; Cazorla, F.M.; de Vicente, A. Organic Amendments to Avocado Crops Induce Suppressiveness and Influence the Composition and Activity of Soil Microbial Communities. Appl. Environ. Microbiol. 2015, 81, 3405–3418. [Google Scholar] [CrossRef]
- Vida, C.; Bonilla, N.; de Vicente, A.; Cazorla, F.M. Microbial Profiling of a Suppressiveness-Induced Agricultural Soil Amended with Composted Almond Shells. Front. Microbiol. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Peck, G.M.; Merwin, I.A.; Thies, J.E.; Schindelbeck, R.R.; Brown, M.G. Soil Properties Change during the Transition to Integrated and Organic Apple Production in a New York Orchard. Appl. Soil Ecol. 2011, 48, 18–30. [Google Scholar] [CrossRef]
- Walsh, B.D.; MacKenzie, A.F.; Salmins, S.; Buszard, D.J. Impact of Soil Management Systems on Organic Dwarf Apple Orchards and Soil Aggregate Stability, Bulk Density, Temperature and Water Content. Can. J. Soil Sci. 1996, 76, 203–209. [Google Scholar] [CrossRef]
- Baldi, E.; Toselli, M.; Marcolini, G.; Quartieri, M.; Cirillo, E.; Innocenti, A.; Marangoni, B. Compost Can Successfully Replace Mineral Fertilizers in the Nutrient Management of Commercial Peach Orchard. Soil Use Manag. 2010, 26, 346–353. [Google Scholar] [CrossRef]
- Hadas, A.; Kautsky, L.; Portnoy, R. Mineralization of Composted Manure and Microbial Dynamics in Soil as Affected by Long-Term Nitrogen Management. Soil Biol. Biochem. 1996, 28, 733–738. [Google Scholar] [CrossRef]
- Sanchez, J.E.; Willson, T.C.; Kizilkaya, K.; Parker, E.; Harwood, R.R. Enhancing the Mineralizable Nitrogen Pool through Substrate Diversity in Long Term Cropping Systems. Soil Sci. Soc. Am. J. 2001, 65, 1442–1447. [Google Scholar] [CrossRef]
- Stark, C.H.; Condron, L.M.; O’Callaghan, M.; Stewart, A.; Di, H.J. Differences in Soil Enzyme Activities, Microbial Community Structure and Short-Term Nitrogen Mineralisation Resulting from Farm Management History and Organic Matter Amendments. Soil Biol. Biochem. 2008, 40, 1352–1363. [Google Scholar] [CrossRef]
- Langmeier, M.; Frossard, E.; Kreuzer, M.; Mäder, P.; Dubois, D.; Oberson, A. Nitrogen Fertilizer Value of Cattle Manure Applied on Soils Originating from Organic and Conventional Farming Systems. Agronomy 2002, 22, 789–800. [Google Scholar] [CrossRef]
- Lazicki, P.; Geisseler, D.; Lloyd, M. Nitrogen Mineralization from Organic Amendments Is Variable but Predictable. J. Environ. Qual. 2020, 49, 483–495. [Google Scholar] [CrossRef]
- Nett, L.; Ruppel, S.; Ruehlmann, J.; George, E.; Fink, M. Influence of Soil Amendment History on Decomposition of Recently Applied Organic Amendments. Soil Sci. Soc. Am. J. 2012, 76, 1290–1300. [Google Scholar] [CrossRef]
- Mallory, E.B.; Griffin, T.S. Impacts of Soil Amendment History on Nitrogen Availability from Manure and Fertilizer. Soil Sci. Soc. Am. J. 2007, 71, 964–973. [Google Scholar] [CrossRef]
- Schenker, M.B.; Pinkerton, K.E.; Mitchell, D.; Vallyathan, V.; Elvine-Kreis, B.; Green, F.H.Y. Pneumoconiosis from Agricultural Dust Exposure among Young California Farmworkers. Environ. Health Perspect. 2009, 117, 988–994. [Google Scholar] [CrossRef]
- Lepsch, H.C.; Brown, P.H.; Peterson, C.A.; Gaudin, A.C.M.; Khalsa, S.D.S. Impact of Organic Matter Amendments on Soil and Tree Water Status in a California Orchard. Agric. Water Manag. 2019, 222, 204–212. [Google Scholar] [CrossRef]
- Total Organic Carbon—Combustion Method. Available online: https://anlab.ucdavis.edu/analysis/Soils/322 (accessed on 2 May 2022).
- Total Nitrogen and Carbon—Combustion Method. Available online: https://anlab.ucdavis.edu/analysis/Soils/320 (accessed on 7 April 2022).
- Total Nitrogen and Carbon—Combustion Method. Available online: https://anlab.ucdavis.edu/analysis/Plant/522 (accessed on 2 May 2022).
- Horwath, W.R.; Paul, E.A. Microbial Biomass. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1994; pp. 753–773. ISBN 978-0-89118-865-0. [Google Scholar]
- Mulvaney, R.L. Nitrogen—Inorganic Forms. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 1123–1184. ISBN 978-0-89118-866-7. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform Fumigation and the Release of Soil Nitrogen: A Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Doane, T.A.; Horwath, W.R. Spectrophotometric Determination of Nitrate with a Single Reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Verdouw, H.; Van Echteld, C.J.A.; Dekkers, E.M.J. Ammonia Determination Based on Indophenol Formation with Sodium Salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The Global Stoichiometry of Litter Nitrogen Mineralization. Science 2008, 321, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Curtin, D.; Selles, F.; Wang, H.; Biederbeck, V.O.; Campbell, C.A. Carbon Dioxide Emissions and Transformation of Soil Carbon and Nitrogen during Wheat Straw Decomposition. Soil Sci. Soc. Am. J. 1998, 62, 1035–1041. [Google Scholar] [CrossRef]
- Prescott, C.E.; Vesterdal, L. Decomposition and Transformations along the Continuum from Litter to Soil Organic Matter in Forest Soils. For. Ecol. Manag. 2021, 498, 119522. [Google Scholar] [CrossRef]
- Schellenberg, D.L.; Alsina, M.M.; Muhammad, S.; Stockert, C.M.; Wolff, M.W.; Sanden, B.L.; Brown, P.H.; Smart, D.R. Yield-Scaled Global Warming Potential from N2O Emissions and CH4 Oxidation for Almond (Prunus Dulcis) Irrigated with Nitrogen Fertilizers on Arid Land. Agric. Ecosyst. Environ. 2012, 155, 7–15. [Google Scholar] [CrossRef]
- Volpiano, C.G.; Lisboa, B.B.; José, J.F.B.d.S.; Beneduzi, A.; Granada, C.E.; Vargas, L.K. Soil-Plant-Microbiota Interactions to Enhance Plant Growth. Rev. Bras. Ciênc. Solo 2022, 46, e0210098. [Google Scholar] [CrossRef]
- Haney, R.L.; Brinton, W.H.; Evans, E. Estimating Soil Carbon, Nitrogen, and Phosphorus Mineralization from Short-term Carbon Dioxide Respiration. Commun. Soil Sci. Plant Anal. 2008, 39, 2706–2720. [Google Scholar] [CrossRef]
- Grandy, A.S.; Neff, J.C. Molecular C Dynamics Downstream: The Biochemical Decomposition Sequence and Its Impact on Soil Organic Matter Structure and Function. Sci. Total Environ. 2008, 404, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.P.; Navarro, A.F.; Sánchez-Monedero, M.A.; Roig, A.; Cegarra, J. Influence of Sewage Sludge Compost Stability and Maturity on Carbon and Nitrogen Mineralization. Soil Biol. Biochem. 1998, 30, 305–313. [Google Scholar] [CrossRef]
- Deans, J.R.; Molina, J.A.E.; Clapp, C.E. Models for Predicting Potentially Mineralizable Nitrogen and Decomposition Rate Constants. Soil Sci. Soc. Am. J. 1986, 50, 323–326. [Google Scholar] [CrossRef]
- Franzluebbers, K.; Weaver, R.W.; Juo, A.S.R.; Franzluebbers, A.J. Mineralization of Carbon and Nitrogen from Cowpea Leaves Decomposing in Soils with Different Levels of Microbial Biomass. Biol. Fertil. Soils 1995, 19, 100–102. [Google Scholar] [CrossRef]
- Janzen, H.H. The Soil Carbon Dilemma: Shall We Hoard It or Use It? Soil Biol. Biochem. 2006, 38, 419–424. [Google Scholar] [CrossRef]
- Andrews, E.M.; Kassama, S.; Smith, E.E.; Brown, P.H.; Khalsa, S.D.S. A Review of Potassium-Rich Crop Residues Used as Organic Matter Amendments in Tree Crop Agroecosystems. Agriculture 2021, 11, 580. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. Application of Eco-Physiological Quotients (qCO2 and qD) on Microbial Biomasses from Soils of Different Cropping Histories. Soil Biol. Biochem. 1990, 22, 251–255. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Wallenstein, M.D.; Schipanksi, M.E.; Grandy, A.S. Managing Agroecosystems for Soil Microbial Carbon Use Efficiency: Ecological Unknowns, Potential Outcomes, and a Path Forward. Front. Microbiol. 2019, 10, 1146. [Google Scholar] [CrossRef]
- Sakamoto, K.; Oba, Y. Effect of Fungal to Bacterial Biomass Ratio on the Relationship between COz Evolution and Total Soil Microbial Biomass. Biol. Fertil. Soils 1994, 17, 39–44. [Google Scholar] [CrossRef]
- Spohn, M. Microbial Respiration per Unit Microbial Biomass Depends on Litter Layer Carbon-to-Nitrogen Ratio. Biogeosciences 2015, 12, 817–823. [Google Scholar] [CrossRef]
- Wardle, D.A.; Ghani, A. A Critique of the Microbial Metabolic Quotient (qCO2) as a Bioindicator of Disturbance and Ecosystem Development. Soil Biol. Biochem. 1995, 27, 1601–1610. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. The Metabolic Quotient for CO2 (qCO2) as a Specific Activity Parameter to Assess the Effects of Environmental Conditions, Such as pH, on the Microbial Biomass of Forest Soils. Soil Biol. Biochem. 1993, 23, 393–395. [Google Scholar] [CrossRef]
- Bonde, T.; Schnurer, J.; Rosswall, T. Microbial Biomass as a Fraction of Potentially Mineralizable Nitrogen in Soils from Long-Term Field Experiments. Soil Biol. Biochem. 1988, 20, 447–452. [Google Scholar] [CrossRef]
- Salazar-Villegas, A.; Blagodatskaya, E.; Dukes, J.S. Changes in the Size of the Active Microbial Pool Explain Short-Term Soil Respiratory Responses to Temperature and Moisture. Front. Microbiol. 2016, 7, 524. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).