Soil Contamination by Heavy Metals and Radionuclides and Related Bioremediation Techniques: A Review
Abstract
1. Introduction
- Review of the state of ecosystems contaminated with heavy metals and radionuclides.
- Identification of the advantages and disadvantages of using biosorption technologies for the joint fixation of heavy metals and radionuclides.
- Substantiation of the possibility of using phosphogypsum for soil bioremediation.
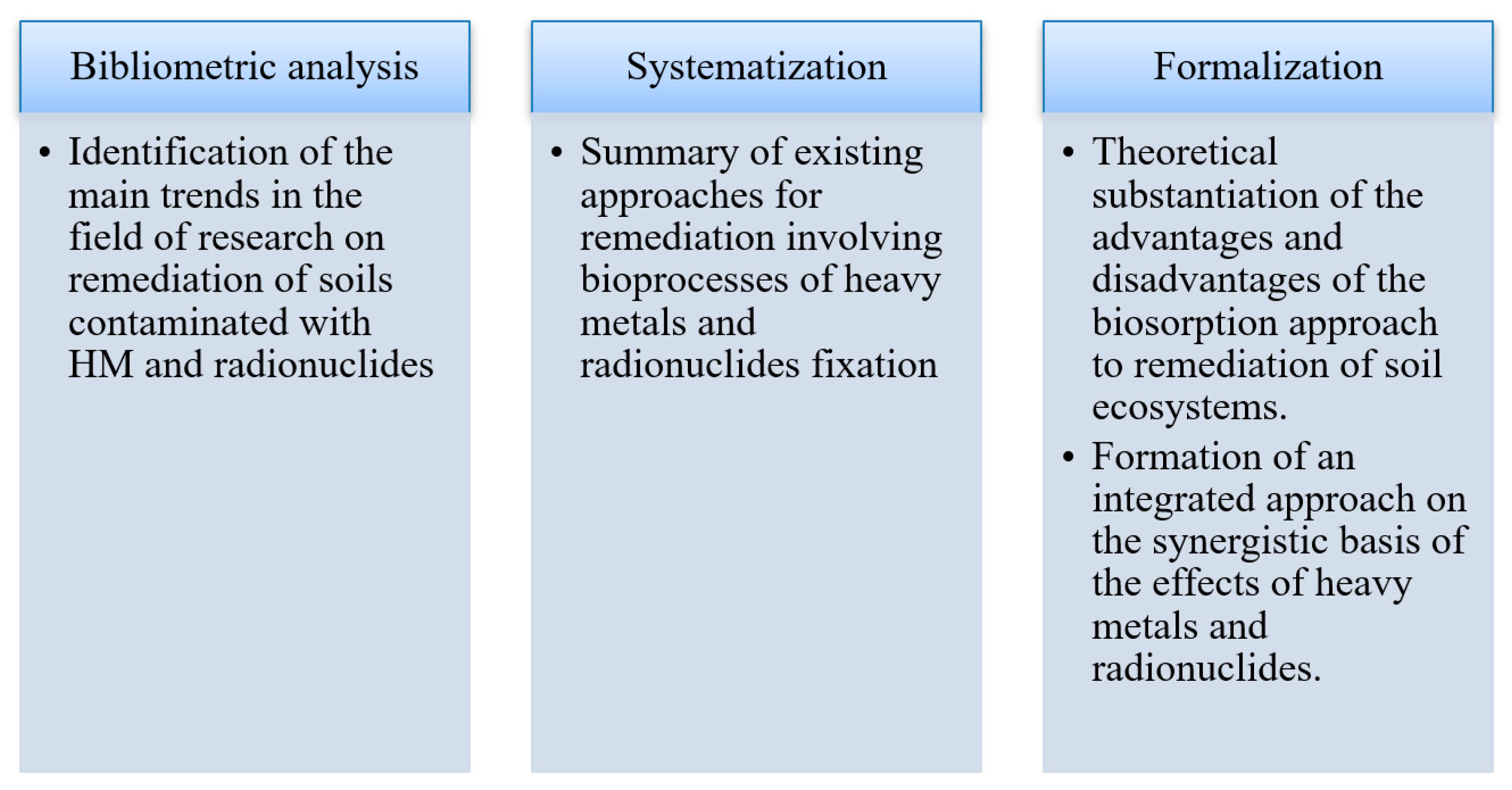
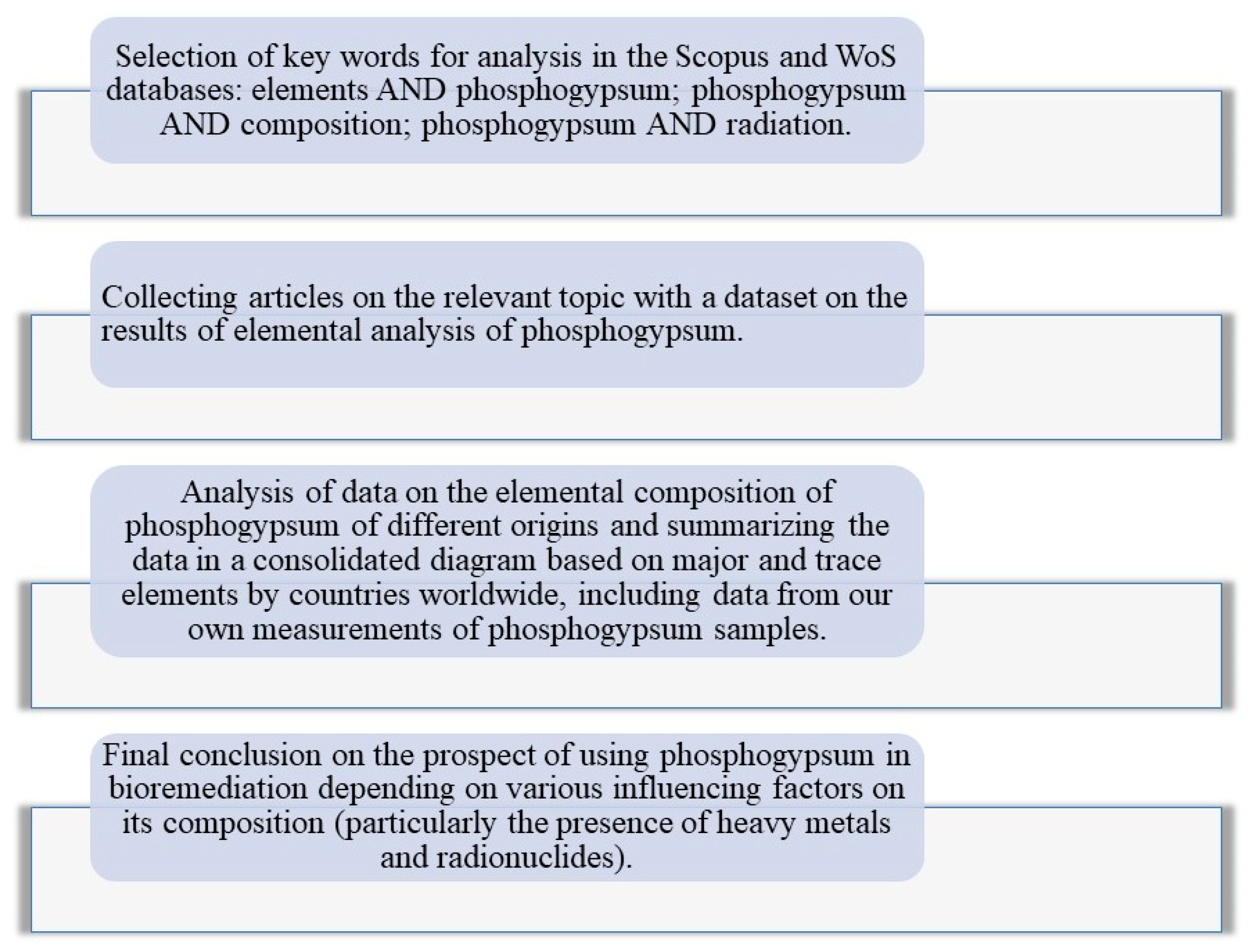
2. Methodological Approach
3. Review of the State of Ecosystems Contaminated with Heavy Metals and Radionuclides
3.1. Sources of Radionuclides and Heavy Metals in the Ecosystem
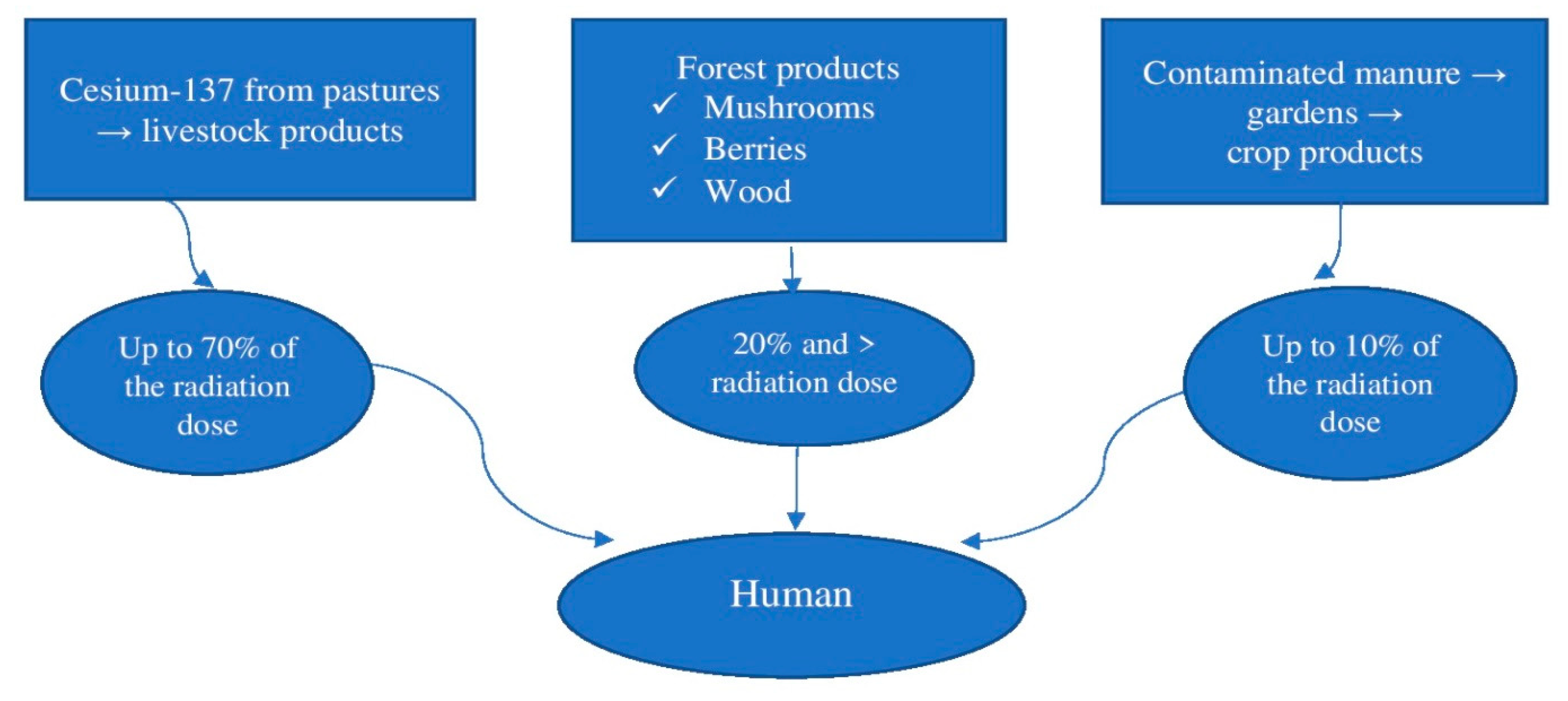
3.2. Monitoring of Radionuclides and Heavy Metals in Ecosystems and Impact on Humans: Ukraine CASE Study
- -
- Availability of sufficient areas that are subject to minimal anthropogenic impact (for example, biosphere reserves, nature reserves, and national nature parks);
- -
- Selection of background monitoring criteria that would take into account the prevalence of individual substances in nature, their migration in the natural environment, and the presence of potential sources of their anthropogenic intake;
- -
- Selection of effective methods for monitoring the state parameters of environmental objects.
- -
- Natural autorehabilitation (radioactive decay, and fixation and redistribution of radionuclides in the soil);
- -
- Strengthening of biogeochemical barriers to fix radionuclides in soils, reducing the risk of radiation contamination of food;
- -
- Strengthening the radioecological monitoring of soils and agricultural products, radiological control, and compliance with recommendations for agricultural production.
4. Biotechnologies for Integrated Fixation of Heavy Metals and Radionuclides: Identification of Advantages and Disadvantages
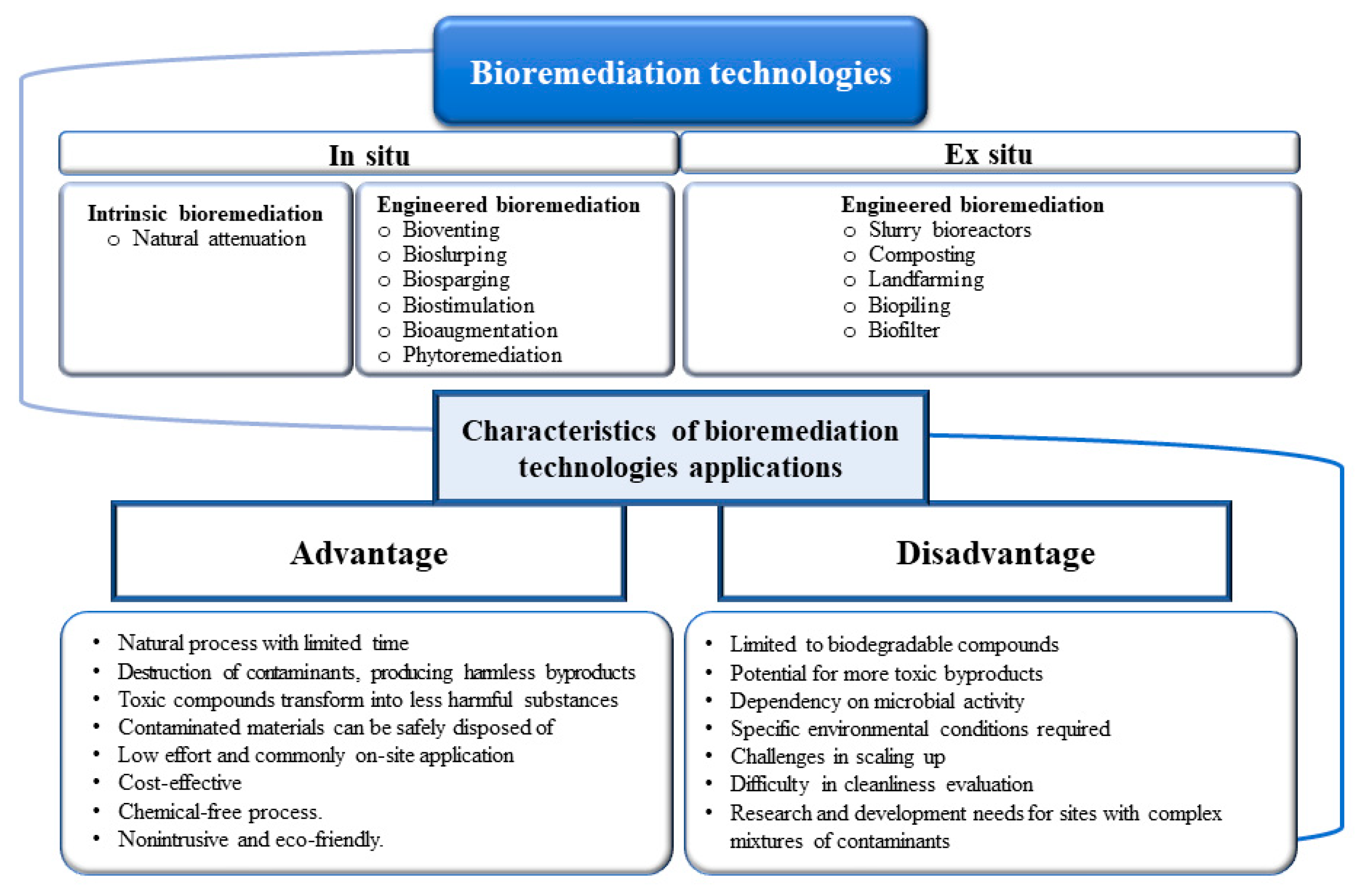
4.1. Soil Bioremediation Methods
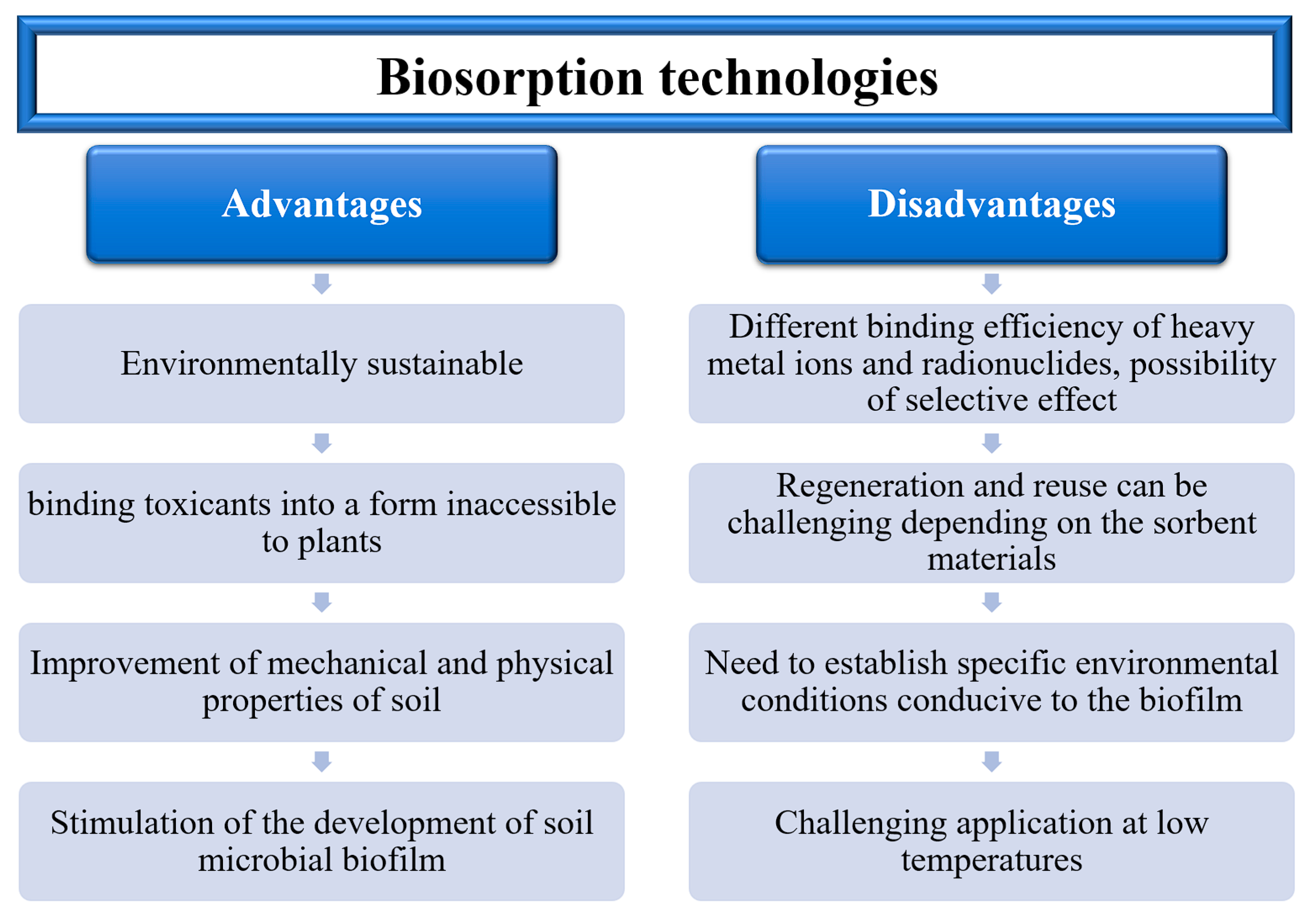
4.2. Biosorption Technologies and Their Aspects of Realisation
- -
- Studies of microorganisms of different physiological groups (including the use of genetically modified strains) on the ability to sorb and transform soluble forms of heavy metals and radioactive elements into insoluble ones;
- -
- Bacterial reduction processes of technetium, chromium, and uranium when used as final electron acceptors in bacterial energy metabolism for the purpose of their detoxification in systems with neutral, acidic, and alkaline pH values;
- -
- Determining the products of bacterial transformation of radionuclides and heavy metals formed under different conditions;
- -
- Possibilities of reducing the toxic effects of heavy metals and radionuclides on soil microorganisms;
- -
- Development of nanobioremediation technology.
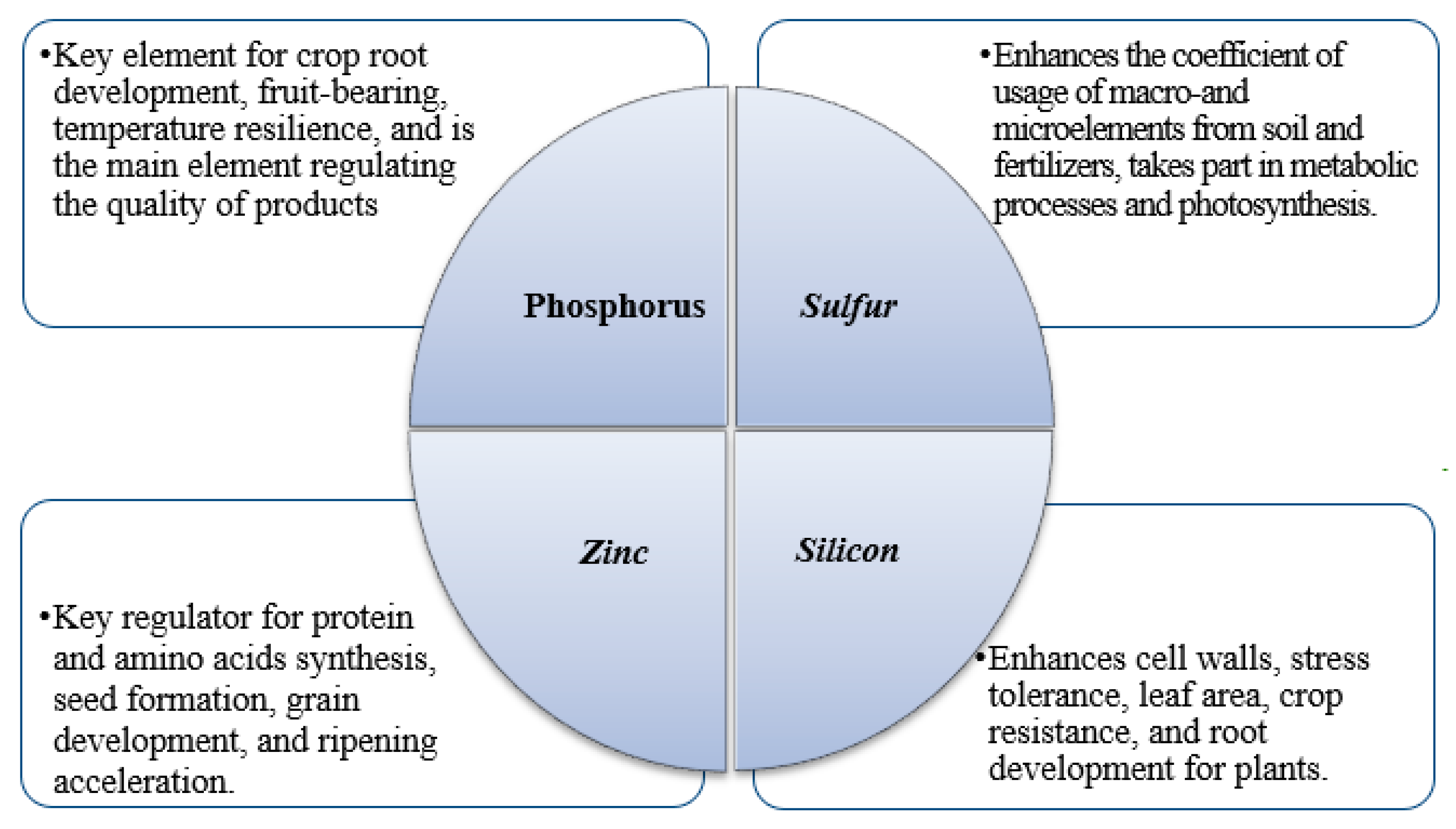
5. Possibility of Using Phosphogypsum for Soil Bioremediation
- -
- Heavy metals (e.g., As, Pb, and Cr) have lower concentrations in phosphogypsum from the Sumy region than in phosphogypsum from China, Spain, the USA, and Brazil;
- -
- Some rare earth elements (such as La, Ce, Pr, and Y) are represented in phosphogypsum from the Sumy region (Ukraine) and less represented in phosphogypsum from other regions of the world.
| wt.% | Ukraine a | China b | United States c | Spain d | Brazil e | India f | Morocco g | Poland h | Tunisia i | France j | Greece k |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | 22.9–31.4 | 31.6–43.3 | 22.7–39.4 | 17.7–32.6 | 31–36 | 30.9–38.9 | 32.2–35 | 29.6–42.7 | 30.7–37.2 | 31.3–33.4 | 34.30 |
| SO3 | 29.8–36 | 34–49 | 22.9–51.9 | 30.7–46 | 44.5 | 44.2–52.9 | 17–45.1 | 42.1–56.5 | 37.5–47 | n.m. | 41.50 |
| SiO2 | 13.1–24.7 | 3.6–15.3 | 3.2–51.3 | n.m. | 0.8 | 0.5–4.3 | 0.3–9.7 | 0.4–1.8 | 1.0–3.8 | 0.6–1.5 | n.m. |
| Al2O3 | 0.96–2.52 | 0.08–2.59 | 0.069–1.14 | n.m. | 0.11–0.2 | 0.1–0.77 | 0.13–0.77 | 0.18–1.7 | 0.04–0.11 | 0.11–0.31 | n.m. |
| P2O5 | 0.63–0.79 | 0.68–1.82 | 0.5–3.8 | 0.49–1.18 | 0.07–1.29 | 0.82–1.04 | 0.59–1.62 | 1.5 | 0.8–1.69 | 0.36–0.69 | n.m. |
| Fe2O3 | 0.41–0.94 | 0.05–1.95 | 0,13–1.15 | n.m. | 0.25–0.77 | 0.1–0.56 | 0.15–0.83 | 0.06–0.20 | 0.03–0.13 | n.m. | 0.84 |
| K2O | 0.1–0.32 | 0.17–0.33 | 0.02–0.9 | 0.02 | 0.04 | 0.03 | 0.05–0.4 | n.m. | 0.01–0.03 | n.m. | n.m. |
| TiO2 | 0.05–0.17 | 0.04–0.27 | 0.03–0.46 | n.m. | 0.18–0.52 | 0.02–0.05 | 0.01–0.03 | n.m. | n.m. | n.m. | n.m. |
| Na2O | 0.02–0.07 | 0.05 | 0.11–1.42 | 0.02 | 0.02–0.09 | 0.03–0.11 | 0.14–0.55 | n.m. | 0.05–0.29 | 0.02–0.19 | n.m. |
| MnO | 0.01 | 0.08–0.18 | 0.06–0.07 | n.m. | 0.004–0.017 | n.m. | 0.01 | n.m. | n.m. | 0.0002–0.0004 | n.m. |
| MgO | 0.01 | 0.01–0.23 | 0.03–0.13 | n.m. | 0.02–0.76 | 0.02–0.56 | 0.21–0.54 | n.m. | 0.01–0.07 | n.m. | 0.13 |
| ppm | Ukraine a | China b | United States c | Spain d | Brazil e | India f | Morocco g | Poland h | Tunisia i | France j | Greece k |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | 3.6–7.0 | 27.6 | 2.5–35.1 | 2.5–11 | 6.3–9 | n.m. | 1.5–2.9 | 3.39 | 6–9.6 | 5.4–17.5 | 13 |
| As | <4.96 | 7.15 | 0.77–20.1 | 0.6–8.56 | n.m. | n.m. | 1.84–1.94 | 8.05 | 1 | n.m. | 0.61–17 |
| Pb | 4.6–4.7 | 28.15 | 2.06–11.4 | 1.99–10.8 | 7.2–31 | 0.07 | 0.17–1.7 | 10.4 | 0.9 | 1.68–4.57 | 11 |
| Zn | 3.2–19.7 | 37.5 | 1.19–32.1 | 1.92–13.1 | 4.4–85.1 | n.m. | 3–28 | n.m. | 9–137 | n.m. | 12–123 |
| Cr | 4.6–11.9 | 37 | 1.69–20.2 | 3.59–20.3 | 11.1–14.7 | 2.73 | 5.85–11 | 5.9 | 6–13 | n.m. | 15.8–153 |
| Ni | 1.4–1.7 | 16.6 | 0.21–17.79 | 0.87–2.67 | 5.4–11 | 14.48 | 1.2–300 | 3.6 | 0.94–4.1 | n.m. | 21 |
| Cd | 1.19–6.36 | 0.48 | 0.28–10.8 | 1.39–2.83 | <0.1 | n.m. | 0.8–7.38 | 1.7 | 8–17.7 | 1.2–2.1 | 0.98–6.67 |
| V | 1.6–2.2 | 27.5 | 0.38–10.7 | 2.9–12.8 | 6.9–9.2 | n.m. | 1.94–5 | n.m. | 2–3 | 1.43–3.91 | n.m. |
| Ga | 0.49–0.78 | n.m. | n.m. | n.m. | 9–10.4 | n.m. | n.m. | n.m. | 0.87 | n.m. | n.m. |
| Sr | 981 | n.m. | 1.05–899 | 360–596 | 4884.9–6179.1 | n.m. | 530–778 | n.m. | n.m. | 813.2–1275 | 172–470 |
| Ba | 20.5–27.2 | 215 | 30.3–88.9 | 37 | 767.1–6104 | n.m. | 23–63.3 | n.m. | 10 | 92.36–215.6 | 38.3–331 |
| Y | 197.2–148.8 | 74 | 43.36 | 106–142 | 90–105.3 | n.m. | 127 | n.m. | 53.2 | 34.65–100.7 | n.m. |
| La | 195.3–137.1 | 36.5–46 | 36.38 | n.m. | 921.1–1969 | n.m. | 60.7 | 40 | 46.3 | 12.96–43.35 | 24.9–30.5 |
| Ce | 282.1–200 | 30.6–32 | 63.84 | 19.5–81.2 | 2109.1–3547 | n.m. | 39 | 53 | 74.4 | 6.53–18.72 | 19.2–60.7 |
| Pr | 46.7–33.4 | 5 | 5.01 | n.m. | 256.1–276.2 | n.m. | 11 | 8 | n.m. | 1.9–6.9 | n.m. |
| Eu | 0.98 | n.m. | 1.4 | n.m. | 23.7–25.9 | n.m. | 2.48 | 2 | n.m. | 0.49–1.7 | 0.85–1.08 |
| Cs | 0.38 | n.m. | n.m. | n.m. | <0.1 | n.m. | n.m. | n.m. | 0.05 | n.m. | 0.09–4.82 |
| Th | 3.3–5.8 | n.m. | n.m. | 1.1 | 67.2–81 | n.m. | 3.04–3.27 | n.m. | 0.74 | 0.22–1.39 | 0.59–10.1 |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Chen, Q.; Tian, L.; Shi, K.; Wu, M. Environmental-Friendly Remediation Technology and Its Application in Heavy Metal Polluted Soil. Mater. Rep. 2023, 37, 21030018-11. [Google Scholar]
- Bhaduri, D.; Sihi, D.; Bhowmik, A.; Verma, B.C.; Munda, S.; Dari, B. A Review on Effective Soil Health Bio-Indicators for Ecosystem Restoration and Sustainability. Front. Microbiol. 2022, 13, 938481. [Google Scholar] [CrossRef] [PubMed]
- Chornobyl Radiation Ecological Biosphere Reserve. History of Creation. Available online: https://zapovidnyk.org.ua/index.php?fn=istor (accessed on 22 January 2023).
- Morooka, K.; Kurihara, E.; Takehara, M.; Takami, R.; Fueda, K.; Horie, K.; Takehara, M.; Yamasaki, S.; Ohnuki, T.; Grambow, B.; et al. New Highly Radioactive Particles Derived from Fukushima Daiichi Reactor Unit 1: Properties and Environmental Impacts. Sci. Total Environ. 2021, 773, 145639. [Google Scholar] [CrossRef] [PubMed]
- Akbay, C.; Aytop, H.; Dikici, H. Evaluation of Radioactive and Heavy Metal Pollution in Agricultural Soil Surrounding the Lignite-Fired Thermal Power Plant Using Pollution Indices. Int. J. Environ. Health Res. 2023, 33, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Melnychuk, A.O.; Tarariko, M.Y. Eco-Energy and Economic Efficiency of Alternative Fertilization Systems on Radioactively Contaminated Soils of Polissya of Ukraine. Agroecol. J. Sci. Theor. 2015, 1, 121–125. [Google Scholar] [CrossRef]
- Bulyhin, S.Y.; Vitvitskyi, S.V.; Bulanyi, O.V.; Tonkha, O.L. Monitoring of Quality of Soils; Publishing House of the National University of Life and Environmental Sciences of Ukraine: Kyiv, Ukraine, 2019; 421p. [Google Scholar]
- Feng, G.; Yong, J.; Liu, Q.; Chen, H.; Mao, P. Response of Soil Microbial Communities to Natural Radionuclides along Specific-Activity Gradients. Ecotoxicol. Environ. Saf. 2022, 246, 114156. [Google Scholar] [CrossRef] [PubMed]
- Horvath, M.; Heltai, G.; Várhegyi, A.; Mbokazi, L.A. Study on the Possible Relationship between Physico-Chemical Properties of the Covering Soil and the Mobility of Radionuclides and Potentially Toxic Elements in a Recultivated Spoil Bank. Minerals 2022, 12, 1534. [Google Scholar] [CrossRef]
- Dinis, M.D.L.; Fiúza, A.; Góis, J.; de Carvalho, J.S.; Meira Castro, A.C. Assessment of Natural Radioactivity, Heavy Metals, and Particulate Matter in Air and Soil around a Coal-Fired Power Plant—An Integrated Approach. Atmosphere 2021, 12, 1433. [Google Scholar] [CrossRef]
- Kim, J.H.; Anwer, H.; Kim, Y.S.; Park, J.-W. Decontamination of Radioactive Cesium-Contaminated Soil/Concrete with Washing and Washing Supernatant—Critical Review. Chemosphere 2021, 280, 130419. [Google Scholar] [CrossRef]
- Yang, L.; Fan, L.; Huang, B.; Xin, J. Efficiency and mechanisms of fermented horse manure, vermicompost, bamboo biochar, and fly ash on Cd accumulation in rice. Environ. Sci. Pollut. Res. Int. 2020, 27, 27859–27869. [Google Scholar] [CrossRef]
- Yin, A.; Shen, C.; Huang, Y.; Yue, M.; Huang, B.; Xin, J. Reduction of Cd accumulation in Se-biofortified rice by using fermented manure and fly ash. Environ. Sci. Pollut. Res. Int. 2020, 27, 39391–39401. [Google Scholar] [CrossRef]
- Shen, C.; Fu, H.; Huang, B.; Liao, Q.; Huang, Y.; Wang, Y.; Wang, Y.; Xin, J. Physiological and molecular mechanisms of boron in alleviating cadmium toxicity in Capsicum annuum. Sci. Total Environ. 2023, 903, 166264. [Google Scholar] [CrossRef]
- Huang, B.; Liao, Q.; Fu, H.; Ye, Z.; Mao, Y.; Luo, J.; Wang, Y.; Yuan, H.; Xin, J. Effect of potassium intake on cadmium transporters and root cell wall biosynthesis in sweet potato. Ecotoxicol. Environm. Saf. 2023, 250, 114501. [Google Scholar] [CrossRef]
- Selim, H.M. (Ed.) Phosphate in Soils; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Pozzebon, E.A.; Seifert, L. Emerging Environmental Health Risks Associated with the Land Application of Biosolids: A Scoping Review. Environ. Health 2023, 22, 57. [Google Scholar] [CrossRef]
- Chernysh, Y.; Balintova, M.; Shtepa, V.; Skvortsova, P.; Skydanenko, M.; Fukui, M. Integration of Processes of Radionuclide-Contaminated Territories Decontamination in the Framework of Their Ecological-Socio-Economic Rehabilitation. Ecol. Eng. Environ. Technol. 2022, 23, 110–124. [Google Scholar] [CrossRef]
- Awasthi, G.; Nagar, V.; Mandzhieva, S.; Minkina, T.; Sankhla, M.S.; Pandit, P.P.; Aseri, V.; Awasthi, K.K.; Rajput, V.D.; Bauer, T.; et al. Sustainable Amelioration of Heavy Metals in Soil Ecosystem: Existing Developments to Emerging Trends. Minerals 2022, 12, 1. [Google Scholar] [CrossRef]
- Basu, S.; Banerjee, P.; Banerjee, S.; Ghosh, B.; Bhattacharjee, A.; Roy, D.; Singh, P.; Kumar, A.A. Bioremediation Strategies to Overcome Heavy Metals and Radionuclides from the Environment. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 287–302. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; He, Z. The Impact of Heavy Metal Contamination on Soil Health. In Managing Soil Health for Sustainable Agriculture; Reicosky, D., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; Volume 2, pp. 1–36. [Google Scholar]
- Nyiramigisha, P.; Komariah, A.; Sajidan, M. Harmful Impacts of Heavy Metal Contamination in the Soil and Crops Grown Around Dumpsites. Rev. Agric. Sci. 2021, 9, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Somani, M.; Datta, M.; Gupta, S.K.; Sreekrishnan, T.R.; Ramana, G.V. Comprehensive Assessment of the Leachate Quality and Its Pollution Potential from Six Municipal Waste Dumpsites of India. Bioresour. Technol. Rep. 2019, 6, 198–206. [Google Scholar] [CrossRef]
- Ramelli, G.P.; Taddeo, I.; Herrmann, U.; Weber, P. Toxicological Profile for Cadmium: U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry. Eur. J. Paediatr. Neurol. 2012, 13, 105–180. [Google Scholar] [CrossRef]
- Rezapour, S.; Samadi, A.; Kalavrouziotis, I.K.; Ghaemian, N. Impact of the Uncontrolled Leakage of Leachate from a Municipal Solid Waste Landfill on Soil in a Cultivated-Calcareous Environment. Waste Manag. 2018, 82, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The Uptake and Bioaccumulation of Heavy Metals by Food Plants, Their Effects on Plants Nutrients, and Associated Health Risk: A Review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Ayub, M.; Ullah, Z.; Ali, A.; Sardar, T.; Iqbal, J.; Gao, X.; Bundschuh, J.; Li, C.; Khattak, S.A.; et al. Groundwater Quality, Health Risk Assessment, and Source Distribution of Heavy Metals Contamination around Chromite Mines: Application of GIS, Sustainable Groundwater Management, Geostatistics, PCAMLR, and PMF Receptor Model. Int. J. Environ. Res. Public Health 2023, 20, 2113. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Sikarwar, V.S.; He, J.; Dastyar, W.; Dionysiou, D.D.; Wang, W.; Zhao, M. Opportunities and Challenges in Sustainable Treatment and Resource Reuse of Sewage Sludge: A Review. Chem. Eng. J. 2018, 337, 616–641. [Google Scholar] [CrossRef]
- Beschkov, V. Control of Pollution in the Non-Ferrous Metals Industry. In: Control of Pollution in the Non-ferrous Metals Industry. Available online: http://www.eolss.net/sample-chapters/c09/e4-14-04-05.pdf (accessed on 19 January 2023).
- Pan, X.; Zhang, S.; Zhong, Q.; Gong, G.; Wang, G.; Guo, X.; Xu, X. Effects of soil chemical properties and fractions of Pb, Cd, and Zn on bacterial and fungal communities. Sci. Total Environ. 2020, 715, 136904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, X.; Deng, J.; Li, G.; Li, Z.; Jiang, J.; Wu, Q.; Duan, L. Emission characteristics of heavy metals from a typical copper smelting plant. J. Hazard. Mater. 2021, 424, 127311. [Google Scholar] [CrossRef] [PubMed]
- Fekiacova, Z.; Cornu, S.; Pichat, S. Tracing contamination sources in soils with Cu and Zn isotopic ratios. Sci. Total Environ. 2015, 517, 96–105. [Google Scholar] [CrossRef]
- Müller, A.K.; Westergaard, K.; Christensen, S.; Sørensen, S.J. The effect of long-term mercury pollution on the soil microbial community. FEMS Microbiol. Ecol. 2001, 36, 11–19. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Sundseth, K.; Pacyna, E.G. Sources and Fluxes of Harmful Metals. In Environmental Determinants of Human Health. Molecular and Integrative Toxicology; Pacyna, J., Pacyna, E., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Environment Agency. Ferrous and Non-Ferrous Metals: Pollution Inventory Reporting. Available online: https://www.gov.uk/government/publications/pollution-inventory-reporting-guidance-notes/ferrous-and-non-ferrous-metals-pollution-inventory-reporting (accessed on 20 December 2023).
- Qi, M.; Wu, Y.; Zhang, S.; Li, G.; An, T. Pollution Profiles, Source Identification and Health Risk Assessment of Heavy Metals in Soil near a Non-Ferrous Metal Smelting Plant. Int. J. Environ. Res. Public. Health 2023, 20, 1004. [Google Scholar] [CrossRef]
- Non-Ferryytrrous Metals—AR4 WGIII Chapter 7: Industry. IPCC—Intergovernmental Panel on Climate Change. Available online: https://archive.ipcc.ch/publications_and_data/ar4/wg3/en/ch7s7-4-2.html (accessed on 20 December 2023).
- United Nations Environment Programme. UNEP Global Mercury Partnership Study Report on Mercury from Non-Ferrous Metals Mining and Smelting. 2021. Available online: https://www.unep.org/globalmercurypartnership/resources/report/mercury-non-ferrous-metals-mining-and-smelting (accessed on 21 December 2023).
- Emission Control for Non-Ferrous Industry. GEA Engineering for a Better World. Available online: https://www.gea.com/en/chemical/emission-control/non-ferrous.jsp (accessed on 20 December 2023).
- Basta, N.T.; Ryan, J.A.; Chaney, R.L. Trace Element Chemistry in Residual-Treated Soil: Key Concepts and Metal Bioavailability. J. Environ. Qual. 2005, 34, 49–63. [Google Scholar] [CrossRef]
- Rosen, V.; Chen, Y. Effects of Compost Application on Soil Vulnerability to Heavy Metal Pollution. Environ. Sci. Pollut. Res. 2018, 25, 35221–35231. [Google Scholar] [CrossRef]
- Raymond, A.W.; Felix, E.O. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks, and Best Available Strategies for Remediation. Int. Sch. Res. Netw. ISRN Ecol. 2012, 2011, 402647. [Google Scholar] [CrossRef]
- Symkanych, O.I.; Sukharev, S.M.; Delehan-Kokayko, S.V.; Maslyuk, V.T.; Svatyuk, A.N.I. Distribution of Heavy Metals and Radionuclides in the Protected Areas of Transcarpathia. Uzhhorod University Scientific Herald. Series Physics 2015, 3, 145–152. [Google Scholar]
- Savchuk, I.M.; Romanchuk, L.D.; Yashchuk, I.V.; Kovalyova, S.P.; Bondarchuk, L.V. Monitoring of Heavy Metals in Fodder and Animal Husbandry Products of the Polissia Zone of Ukraine. Sci. Horiz. 2022, 25, 45–54. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, W.; Wu, W.; Ma, Z.; Li, H.; Zhang, L. Study on Remediation Effect of Radioactive-Heavy Metal Contaminated Soil in Stone Coal Mines by Chemical Elution. Coal Sci. Technol. 2022, 50, 261–266. [Google Scholar]
- Mohuba, S.C.; Abiye, T.A.; Demlie, M.B.; Nhleko, S. Natural Radioactivity and Metal Concentration in the Thyspunt Area, Eastern Cape Province, South Africa. Environ. Monit. Assess. 2022, 194, 112. [Google Scholar] [CrossRef]
- Baghdady, A.; Awad, S.; Gad, A. Assessment of Metal Contamination and Natural Radiation Hazards in Different Soil Types Near Iron Ore Mines, Bahariya Oasis, Egypt. Arab. J. Geosci. 2018, 11, 506. [Google Scholar] [CrossRef]
- Mitrovic, B.; Vranjes, B.; Kostic, O.; Perovic, V.; Mitrovic, M.; Pavlovic, P. Radionuclides and Heavy Metals in Soil, Vegetables, and Medicinal Plants in Suburban Areas of the Cities of Belgrade and Pancevo, Serbia. Nucl. Technol. Radiat. Prot. 2019, 34, 278–284. [Google Scholar] [CrossRef]
- Cuenca, R.H.; Hagimoto, Y.; Moghaddam, M. Three-and-a-half Decades of Progress in Monitoring Soils and Soil Hydraulic Properties. Procedia Environ. Sci. 2013, 19, 384–393. [Google Scholar] [CrossRef][Green Version]
- Lee, C.; Park, S.-W.; Kim, A.H.R. Development of Mobile Scanning System for Effective In-Situ Spatial Prediction of Radioactive Contamination at Decommissioning Sites. Nucl. Instrum. Methods Phys. Res. 2020, 966, 163833. [Google Scholar] [CrossRef]
- Bida, P.I.; Rudko, O.M.; Malimon, S.S.; Kushniruk, A.O.M. Introduction of Drainage and Sorption Systems on Radioactively Contaminated Peat Soils of Polissya of Ukraine. Environ. Sci. 2020, 5, 36–40. [Google Scholar] [CrossRef]
- Chobotko, H.M.; Landin, V.P.; Yaskovets, I.I.; Raichuk, L.A.; Shvydenko, I.K. Radiologically Critical Ecosystems and Their Role in the Formation of Contamination of Agricultural Products. Ahroekolohichnyi Zhurnal 2018, 4, 29–35. [Google Scholar] [CrossRef]
- Kovalyova, S.P.; Mozharivska, I.A. Heavy Metal Concentration in Soils while Growing Energy Crops in the Radioactively Contaminated Territory. Sci. Horiz. 2020, 3, 121–126. [Google Scholar] [CrossRef]
- Yoon, I.-H.; Park, C.W.; Kim, I.; Yang, H.-M.; Kim, S.-M.; Kim, J.-H. Characteristic and Remediation of Radioactive Soil in Nuclear Facility Sites: A Critical Review. Environ. Sci. Pollut. Res. 2021, 28, 67990–68005. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, L.; Mishchuk, N.; Kovalchuk, V. Basic Principles and Problems in Decontamination of Natural Disperse Systems. Electrokinet. Treat. Soils. Adv. Colloid. Interface Sci. 2022, 310, 102798. [Google Scholar] [CrossRef] [PubMed]
- Impens, N.R.E.N.; Jensen, K.A.; Skipperud, L.; Gompel, A.V.; Vanhoudt, N. In-Depth Understanding of Local. Soil. Chemistry Reveals That Addition of Ca May Counteract the Mobilisation of 226Ra and Other Pollutants before Wetland Creation on the Grote Nete River Banks. Sci. Total Environ. 2022, 823, 153703. [Google Scholar] [CrossRef] [PubMed]
- Salbu, B.; Lind, O.C. Analytical Techniques for Characterizing Radioactive Particles Deposited in the Environment. J. Environ. Radioact. 2020, 211, 106078. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.; Khosla, A.B. Conventional and Contemporary Techniques for Removal of Heavy Metals from Soil. In Biodegradation Technology of Organic and Inorganic Pollutants, 2nd ed.; Mendes, K.F., Sousa, R.N., Mielke, K.C., Eds.; IntechOpen: Rijeka, Croatia, 2021; Volume 3, pp. 154–196. [Google Scholar] [CrossRef]
- Groudeva, V.I.; Doycheva, A.; Krumova, K.; Groudev, S.N. Bioremediation In Situ of an Alkaline Soil Polluted with Heavy Metals. Adv. Mater. Res. 2007, 20–21, 287–290. [Google Scholar] [CrossRef]
- Marcon, L.; Oliveras, J.; Puntes, V.F. In Situ Nanoremediation of Soils and Groundwaters from the Nanoparticle’s Standpoint: A Review. Sci. Total Environ. 2021, 791, 148324. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.; Desai, S.; Wagh, N.S.; Lakkakula, J. New Bioremediation Technologies to Remove Heavy Metals and Radionuclides. In Industrial Wastewater Reuse; Springer Nature Singapore: Singapore, 2023; pp. 267–316. [Google Scholar]
- Zhang, H.; Chen, Y.; Liu, S.X.; Jachimowicz, A.E.; Li, A. Big Data Research on Agricultural Soil Contamination by Zeolite Application. J. Elem. 2022, 27, 265–287. [Google Scholar] [CrossRef]
- Kornilovich, B.; Mishchuk, N.; Abbruzzese, K.; Pshinko, G.; Klishchenko, R. Enhanced Electrokinetic Remediation of Metals-Contaminated Clay. Colloids Surf. A Physicochem. Eng. Asp. 2005, 265, 114–123. [Google Scholar] [CrossRef]
- Megharaj, M.; Venkateswarlu, K.; Naidu, R. Bioremediation. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 485–489. [Google Scholar] [CrossRef]
- Nadaf, M.; Jadav, K.D.; Gingine, V. Decontamination of Soil by Electro Kinetic Treatment; Lecture Notes in Civil Engineering; Springer: Singapore, 2021; pp. 91–103. [Google Scholar] [CrossRef]
- Olodovskii, P. Theory of the Effect of Inhibition of the Transfer of Radionuclides and Heavy Metals from Soil to Plants by an Ameliorant. V. Calculation of the Binding Energy of Exchange Ions in Disperse Systems with Low pH. J. Eng. Phys. Thermophys. 2001, 74, 243–249. [Google Scholar] [CrossRef]
- Sethi, S. Holistic Approach to Remediate Heavy Metals and Radionuclides. In Industrial Wastewater Reuse; Springer Nature: Singapore, 2023; pp. 113–132. [Google Scholar] [CrossRef]
- Chandra, D.; General, T.; Nisha; Chandra, S. Microorganisms: An Asset for Decontamination of Soil. In Smart Bioremediation Technologies; Academic Press: Cambridge, MA, USA, 2019; pp. 319–345. [Google Scholar]
- Gadd, G.M. Heavy Metal Pollutants: Environmental and Biotechnological Aspects. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Mishra, M.; Mohan, D. Bioremediation of Contaminated Soils: An Overview. In Adaptive Soil Management: From Theory to Practices; Springer: Singapore, 2017; pp. 323–337. [Google Scholar]
- Singh, B.S.M.; Singh, D.; Dhal, N.K. Enhanced Phytoremediation Strategy for Sustainable Management of Heavy Metals and Radionuclides. Case Stud. Chem. Environ. Eng. 2022, 5, 100176. [Google Scholar] [CrossRef]
- Devedee, A.K.; Sahoo, M.; Choudhary, K.; Singh, M.; Ghanshyam. Bioremediation of Soil: An Overview. In Microbes and Microbial Biotechnology for Green Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 13–27. [Google Scholar]
- Phian, S.; Nagar, S.; Kaur, J.; Rawat, C.D. Emerging Issues and Challenges for Microbes-Assisted Remediation. In Microbes and Microbial Biotechnology for Green Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 47–89. [Google Scholar]
- Xing, Y.; Tan, S.; Liu, S.; Xu, S.; Wan, W.; Huang, Q.; Chen, W. Effective Immobilization of Heavy Metals via Reactive Barrier by Rhizosphere Bacteria and Their Biofilms. Environ. Res. 2022, 207, 112080. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Sun, G.-X. Evaluation of Water Management on Arsenic Methylation and Volatilization in Arsenic-Contaminated Soils Strengthened by Bioaugmentation and Biostimulation. J. Environ. Sci. 2024, 137, 515–526. [Google Scholar] [CrossRef]
- Khalid, M.; Liu, X.; ur Rahman, S.; Rehman, A.; Zhao, C.; Li, X.; Yucheng, B.; Hui, N. Responses of Microbial Communities in Rhizocompartments of King Grass to Phytoremediation of Cadmium-Contaminated Soil. Sci. Total Environ. 2023, 904, 167226. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, X.; Xie, J. Biomineralization Technology for Solidification/Stabilization of Heavy Metals in Ecosystem: Status and Perspective. Front. Ecol. Evol. 2023, 11, 1189356. [Google Scholar] [CrossRef]
- Mendoza-Burguete, Y.; de la Luz Pérez-Rea, M.; Ledesma-García, J.; Campos-Guillén, J.; Ramos-López, M.A.; Guzmán, C.; Rodríguez-Morales, J.A. Global Situation of Bioremediation of Leachate-Contaminated Soils by Treatment with Microorganisms: A Systematic Review. Microorganisms 2023, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.-J.; Ding, X.-Z.; Cui, M.-J.; Zheng, J.-J.; Chen, Z.-B.; Pei, J.-L.; Zhang, J.-W. Mechanisms and Influencing Factors of Biomineralization Based Heavy Metal Remediation: A Review. Biogeotechnics 2023, 1, 100039. [Google Scholar] [CrossRef]
- Li, M.; Cheng, X.; Guo, H. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Lopez-Fernandez, M.; Jroundi, F.; Ruiz-Fresneda, M.A.; Merroun, M.L. Microbial interaction with and tolerance of radionuclides: Underlying mechanisms and biotechnological applications. Microb. Biotechnol. 2020, 14, 810–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Xu, R.; Qin, H.; Liu, H.; Zhao, K. Heavy metal bioremediation using microbially induced carbonate precipitation: Key factors and enhancement strategies. Front. Microbiol. 2023, 14, 1116970. [Google Scholar] [CrossRef]
- Renshaw, J.; Mackay, R.; Macaskie, L. Immobilization of Metals and Radionuclides by Microbial Biomineralization Processes. Available online: https://www.birmingham.ac.uk/Documents/college-les/gees/biomineralizationprocesses.pdf (accessed on 21 December 2023).
- Priya, A.K.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of Heavy Metals by Microorganisms: Evaluation of Different Underlying Mechanisms. Chemosphere 2022, 307, 135957. [Google Scholar] [CrossRef]
- Vasconcellos, S.; Paganotti, A.; Vital, V.G.; Santos Lima, L.M.; Paiva, G.M.S.; de Lima, L.F.; Moreira, E.; Sousa, L.O.; Guerini, G.G.; Santos, V.T.; et al. Biotransformation of Metal-Rich Effluents and Potential Recycle Applications. In Bioremediation for Global Environmental Conservation; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- White, C.; Wilkinson, S.C.; Gadd, G.M. The role of microorganisms in biosorption of toxic metals and radionuclides. Int. Biodeterior. Biodegrad. 1995, 35, 17–40. [Google Scholar] [CrossRef]
- Gavrilescu, M. Removal of heavy metals from the environment by biosorption. Eng. Life Sci. 2004, 4, 219–232. [Google Scholar] [CrossRef]
- Das, N. Remediation of Radionuclide Pollutants through Biosorption—An Overview. Clean. Soil. Air Water 2012, 40, 16–23. [Google Scholar] [CrossRef]
- Kotrba, P. Microbial Biosorption of Metals—General Introduction. In Microbial Biosorption of Metals; Kotrba, P., Mackova, M., Macek, T., Eds.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Zabochnicka-Świątek, M.; Krzywonos, M. Potentials of Biosorption and Bioaccumulation Processes for Heavy Metal Removal. Pol. J. Environ. Studies. 2014, 23, 551–561. [Google Scholar]
- Mathew, A.T.; Saravanakumar, M.P. Removal of Bisphenol A and Methylene Blue by α -MnO2 Nanorods: Impact of Ultrasonication, Mechanism, Isotherm, and Kinetic Models. J. Hazard. Toxic Radioact. Waste 2021, 25, 04021005. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Janyasuthwiong, S.; Rene, E.R. Bioprecipitation—A Promising Technique for Heavy Metal Removal and Recovery from Contaminated Wastewater Streams. MOJ Civil. Eng. 2017, 2, 191–193. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, S.; Roh, Y. Effect of Divalent Cations (Cu, Zn, Pb, Cd, and Sr) on Microbially Induced Calcium Carbonate Precipitation and Mineralogical Properties. Front. Microbiol. 2021, 12, 646748. [Google Scholar] [CrossRef]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial Interventions in Bioremediation of Heavy Metal Contaminants in Agroecosystem. Front. Microbiol. 2022, 13, 824084. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wu, B.; Chai, X. In Situ Remediation Technology for Heavy Metal Contaminated Sediment: A Review. Int. J. Environ. Res. Public Health 2022, 19, 16767. [Google Scholar] [CrossRef] [PubMed]
- Mugwar, A. Bioprecipitation of Heavy Metals and Radionuclides with Calcium Carbonate in Aqueous Solutions and Particulate Media. Cardiff University. 2015. Available online: https://www.semanticscholar.org/paper/Bioprecipitation-of-heavy-metals-andradionuclides-Mugwar/a0070cd67b0c051e674db74c8257ad955d79c308 (accessed on 21 December 2023).
- Kumari, D.; Qian, X.Y.; Pan, X.; Achal, V.; Li, Q.; Gadd, G.M. Microbially-induced Carbonate Precipitation for Immobilization of Toxic Metals. Adv. Appl. Microbiol. 2016, 94, 79–108. [Google Scholar] [CrossRef] [PubMed]
- Nnaji, N.D.; Onyeaka, H.; Miri, T.; Ugwa, C. Bioaccumulation for Heavy Metal Removal: A Review. SN Appl. Sci. 2023, 5, 125. [Google Scholar] [CrossRef]
- Rahmat, M.A.; Ismail, A.F.; Rodzi, N.D.; Aziman, E.S.; Idris, W.M.R.; Lihan, T. Assessment of natural radionuclides and heavy metals contamination to the environment: Case study of Malaysian unregulated tin-tailing processing industry. Nucl. Eng. Technol. 2022, 54, 2230–2243. [Google Scholar] [CrossRef]
- Zalewska, T.; Saniewski, M. Bioaccumulation of gamma emitting radionuclides in red algae from the Baltic Sea under laboratory conditions. Oceanologia 2011, 53, 631–650. [Google Scholar] [CrossRef]
- Abdelkarim, M.S.; Imam, N. Radiation hazards and extremophiles bioaccumulation of radionuclides from hypersaline lakes and hot springs. Int. J. Environ. Sci. Technol. 2023, 21, 3021–3036. [Google Scholar] [CrossRef]
- Borghei, S.M.; Arjmandi, R.; Moogouei, R. Bioaccumulation of Radionuclide Metals in Plants: A Case Study of Cesium. In Radionuclide Contamination and Remediation Through Plants; Springer International Publishing: Cham, Switzerland, 2014; pp. 177–195. [Google Scholar] [CrossRef]
- Srisuksawad, K.; Prasertchiewchan, N. Experimental Studies on the Bioaccumulation of Selected Heavy Metals and Radionuclides in the Blood Cockle Anadara granosa of the Bang Pakong Estuary. Environ. Bioindic. 2007, 2, 253–263. [Google Scholar] [CrossRef]
- Tykva, R. Sources of Environmental Radionuclides and Recent Results in Analyses of Bioaccumulation. A review. Nukleonika 2004, 49. Available online: https://bibliotekanauki.pl/articles/147281 (accessed on 22 December 2023).
- Diaz-Bone, R.; Van de Wiele, T. Biotransformation of metal(loid)s by intestinal microorganisms. Pure Appl. Chemistry. 2010, 82, 409–427. [Google Scholar] [CrossRef]
- Jabbar, T.; Wallner, G. Biotransformation of Radionuclides: Trends and Challenges. In Radionuclides in the Environment; Walther, C., Gupta, D., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Lovley, D.R. Microbial detoxification of metals and radionuclides. Curr. Opin. Biotechnol. 2001, 12, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Francis, A. Microbial Transformations of Radionuclides and Environmental Restoration through Bioremediation. Symposium on “Emerging Trends in Separation Science and Technology” SESTEC 2006 Bhabha Atomic Research Center (BARC), Trombay, Mumbai. Brookhaven National Laboratory. 2006, pp. 1–15. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=fdce929bc2c9995a1567fc17853c0b6b443cd3c8 (accessed on 22 December 2023).
- Mani, D.; Kumar, C. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: An overview with special reference to phytoremediation. Int. J. Environ. Sci. Technol. 2014, 11, 843–872. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomicrobiology of Metal and Mineral Transformations in the Environment. Extremophiles. Encyclopedia of Life Support Systems. Available online: https://www.eolss.net/sample-chapters/c03/E6-38-18.pdf (accessed on 22 December 2023).
- Thakare, M.; Sarma, H.; Datar, S.; Roy, A.; Pawar, P.; Gupta, K.; Pandit, S.; Prasad, R. Understanding the Holistic Approach to Plant-Microbe Remediation Technologies for Removing Heavy Metals and Radionuclides from Soil. Curr. Res. Biotechnol. 2021, 3, 84–98. [Google Scholar] [CrossRef]
- Harher, Y.K.; Voitsekhovych, O.V. Twenty-Five Years since the Chornobyl Disaster. Security of the Future; National Report of Ukraine; KIM: Kyiv, Ukraine, 2011; pp. 39–42. [Google Scholar]
- Chibuike, G.U.; Obiora, S.C. Heavy Metal Polluted Soils: Effect on Plants and Bioremediation Methods. Appl. Environ. Soil. Sci. 2014, 752708. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Saravanan, A.; Vo, D.-V.N. Advances in Biosorbents for Removal of Environmental Pollutants: A Review on Pretreatment, Removal Mechanism, and Future Outlook. J. Hazard. Mater. 2021, 420, 126596. [Google Scholar] [CrossRef]
- Kumar, M.; Seth, A.; Singh, A.K.; Rajput, M.S.; Sikandar, A.M. Remediation Strategies for Heavy Metals Contaminated Ecosystem: A Review. J. Environ. Sustain. 2021, 12, 100155. [Google Scholar] [CrossRef]
- Priyadarshanee, M.; Das, S. Biosorption and Removal of Toxic Heavy Metals by Metal Tolerating Bacteria for Bioremediation of Metal Contamination: A Comprehensive Review. J. Environ. Chem. Eng. 2021, 9, 104686. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Meng, J.; Cai, Y.; Cheng, M.; Wu, S.; Li, Z. Characterization of Modified Rice Straw Biochar in Immobilizing Bacillus subtilis 168 and Evaluation on Its Role as a Novel Agent for Zearalenone-Removal Delivery. J. Hazard. Mater. 2023, 453, 131424. [Google Scholar] [CrossRef]
- Li, M.; Yao, J.; Sunahara, G.; Hawari, J.; Duran, R.; Liu, J.; Liu, B.; Cao, Y.; Pang, W.; Li, H.; et al. Novel Microbial Consortia Facilitate Metalliferous Immobilization in Non-Ferrous Metal(loid)s Contaminated Smelter Soil: Efficiency and Mechanisms. Environ. Pollut. 2022, 313, 120042. [Google Scholar] [CrossRef]
- Zhang, Y.; Majeed, Z.; Tian, M.; Xie, Y.; Zheng, K.; Luo, Z.; Li, C.; Zhao, C. Application of Hydrogen-Bonded Organic Frameworks in Environmental Remediation. Separations 2023, 10, 196. [Google Scholar] [CrossRef]
- Qi, X.; Xiao, S.; Chen, X.; Ali, I.; Gou, J.; Wang, D.; Zhu, B.; Zhu, W.; Shang, R.; Han, M. Biochar-Based Microbial Agent Reduces U and Cd Accumulation in Vegetables and Improves Rhizosphere Microecology. J. Hazard. Mater. 2022, 436, 129147. [Google Scholar] [CrossRef]
- Oziegbe, O.; Oluduro, A.O.; Oziegbe, E.J.; Ahuekwe, E.F.; Olorunsola, S.J. Assessment of Heavy Metal Bioremediation Potential of Bacterial Isolates from Landfill Soils. Saudi J. Biol. Sci. 2021, 28, 3948–3956. [Google Scholar] [CrossRef]
- Maqsood, Q.; Sumrin, A.; Waseem, R.; Hussain, M.; Imtiaz, M.; Hussain, N. Bioengineered Microbial Strains for Detoxification of Toxic Environmental Pollutants. Environ. Res. 2023, 227, 115665. [Google Scholar] [CrossRef]
- Salah-Tazdaït, R.; Tazdaït, D. Phyto and Microbial Remediation of Heavy Metals and Radionuclides in the Environment; Routledge: London, UK, 2022. [Google Scholar]
- Singha, S.; Chatterjee, S. Soil Pollution by Industrial Effluents, Solid Wastes, and Reclamation Strategies by Microorganisms. In Microbes and Microbial Biotechnology for Green Remediation; Springer International Publishing: Cham, Switzerland, 2022; pp. 471–488. [Google Scholar]
- Chen, Z.; Li, Q.; Yang, Y.; Sun, J.; Li, G.; Liu, X.; Shu, S.; Li, X.; Liao, H. Uranium Removal from a Radioactive Contaminated Soil by Defined Bioleaching Bacteria. J. Radioanal. Nucl. Chem. 2022, 331, 439–449. [Google Scholar] [CrossRef]
- Bryukhanov, A.L.; Khijniak, T.V. The Application of Sulfate-Reducing Bacteria in the Bioremediation of Heavy Metals and Metalloids. Appl. Biochem. Microbiol. 2022, 58 (Suppl. S1), S1–S15. [Google Scholar] [CrossRef]
- Aslam, F.; Mazhar, S. Nano-Bioremediation of Heavy Metals from Environment Using a Green Synthesis Approach. Int. J. Adv. Appl. Sci. 2023, 12, 7. [Google Scholar] [CrossRef]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitraş, D.-G.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, E.-L.; et al. Phosphogypsum Circular Economy Considerations: A Critical Review from More than 65 Storage Sites Worldwide. J. Clean. Prod. 2023, 414, 137561. [Google Scholar] [CrossRef]
- Diwa, R.R.; Tabora, E.U.; Palattao, B.L.; Haneklaus, N.H.; Vargas, E.P.; Reyes, R.Y.; Ramirez, J.D. Evaluating radiation risks and resource opportunities associated with phosphogypsum in the Philippines. J. Radioanal. Nucl. Chem. 2022, 331, 967–974. [Google Scholar] [CrossRef]
- TENORM: Fertilizer and Fertilizer Production Wastes. U.S. Environmental Protection Agency. Available online: https://www.epa.gov/radiation/tenorm-fertilizer-and-fertilizer-production-wastes (accessed on 22 December 2023).
- Plyatsuk, L.; Balintova, M.; Chernysh, Y.; Demcak, S.; Holub, M.; Yakhnenko, E. Influence of Phosphogypsum Dump on the Soil Ecosystem in the Sumy region (Ukraine). Appl. Sci. 2019, 9, 5559. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ghoneim, A.M.; Seleem, M.; Zuhair, R.; El-Refaey, A.; Khalafallah, N. Phosphogypsum and Poultry Manure Enhance Diversity of Soil Fauna, Soil Fertility, and Barley (Hordeum aestivum L.) Grown in Calcareous Soils. Sci. Rep. 2023, 13, 9944. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhu, H.; Zhou, P.; Wang, X.; Wang, Z.; Yang, S.; Yang, D.; Li, B. Application of Phosphogypsum in Soilization: A Review. Int. J. Environ. Sci. Technol. 2023, 20, 10449–10464. [Google Scholar] [CrossRef]
- Li, C.; Dong, Y.; Yi, Y.; Tian, J.; Xuan, C.; Wang, Y.; Wen, Y.; Cao, J. Effects of Phosphogypsum on Enzyme Activity and Microbial Community in Acid Soil. Sci. Rep. 2023, 13, 6189. [Google Scholar] [CrossRef] [PubMed]
- Ben Mefteh, A.; Bouket, L.; Daoud, A.; Luptakova, L.; Alenezi, F.N.; Gharsallah, N.; Belbahri, L. Metagenomic Insights and Genomic Analysis of Phosphogypsum and Its Associated Plant Endophytic Microbiomes Reveals Valuable Actors for Waste Bioremediation. Microorganisms 2019, 7, 382. [Google Scholar] [CrossRef]
- Lei, L.; Gu, J.; Wang, X.; Song, Z.; Wang, J.; Yu, J.; Hu, T.; Dai, X.; Xie, J.; Zhao, W. Microbial Succession and Molecular Ecological Networks Response to the Addition of Superphosphate and Phosphogypsum during Swine Manure Composting. J. Environ. Manag. 2021, 279, 111560. [Google Scholar] [CrossRef] [PubMed]
- Trifi, H.; Najjari, A.; Achouak, W.; Barakat, M.; Ghedira, K.; Mrad, F.; Saidi, M.; Sghaier, H. Metataxonomics of Tunisian Phosphogypsum Based on Five Bioinformatics Pipelines: Insights for Bioremediation. Genomics 2020, 112, 981–989. [Google Scholar] [CrossRef]
- Chernysh, Y.; Hasegawa, K. Improvement of the Model System to Develop Eco-Friendly Bio-Utilization of Phosphogypsum; Lecture Notes in Mechanical Engineering; Springer: Cham, Switzerland, 2020; pp. 357–366. [Google Scholar]
- Ulianchuk-Martyniuk, O.V.; Michuta, O.R.; Ivanchuk, N.V. Finite Element Analysis of the Diffusion Model of the Bioclogging of the Geobarrier. Eurasian J. Math. Comput. Appl. 2021, 9, 100–111. [Google Scholar] [CrossRef]
- Yimer, A.M.; Assen, A.H.; Mghaimimi, I.E.L.; Lakbita, O.; Adil, K.; Belmabkhout, Y. Unlocking the potential of phosphogypsum waste: Unified synthesis of functional metal-organic frameworks and zeolite via a sustainable valorization route. Chem. Eng. J. 2024, 479, 147902. [Google Scholar] [CrossRef]
- Ait Brahim, J.; Merroune, A.; Mazouz, H.; Beniazza, R. Recovery of rare earth elements and sulfuric acid solution from phosphate byproducts via hydrofluoric acid conversion. J. Ind. Eng. Chem. 2023, 127, 446–453. [Google Scholar] [CrossRef]
- Akfas, F.; Elghali, A.; Aboulaich, A.; Munoz, M.; Benzaazoua, M.; Bodinier, J.-L. Exploring the potential reuse of phosphogypsum: A waste or a resource? Sci. Total Environ. 2024, 908, 168196. [Google Scholar] [CrossRef]
- Wei, Z.; Deng, Z. Research hotspots and trends of comprehensive utilization of phosphogypsum: Bibliometric analysis. J. Environ. Radioact. 2022, 242, 106778. [Google Scholar] [CrossRef]
- Wang, J.; Dong, F.; Wang, Z.; Yang, F.; Du, M.; Fu, K.; Wang, Z. A novel method for purification of phosphogypsum. Physicochem. Probl. Miner. Process. 2020, 56, 975–983. [Google Scholar] [CrossRef]
- Zou, C.; Shi, Z.; Yang, Y.; Zhang, J.; Hou, Y.; Zhang, N. The Characteristics, Enrichment, and Migration Mechanism of Cadmium in Phosphate Rock and Phosphogypsum of the Qingping Phosphate Deposit, Southwest China. Minerals 2023, 13, 107. [Google Scholar] [CrossRef]
- Guan, Q.; Sui, Y.; Liu, C.; Wang, Y.; Zeng, C.; Yu, W.; Gao, Z.; Zang, Z.; Chi, R.-A. Characterization and Leaching Kinetics of Rare Earth Elements from Phosphogypsum in Hydrochloric Acid. Minerals 2022, 12, 703. [Google Scholar] [CrossRef]
- Zhou, B.; Zhu, H.; Xu, S.; Du, G.; Shi, S.; Liu, M.; Xing, F.; Ren, J. Effect of phosphogypsum on the properties of magnesium phosphate cement paste with low magnesium-to-phosphate ratio. Sci. Total Environ. 2021, 798, 149262. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, S.; Qu, G.; Chen, B.; Zhao, C.; Liu, L.; Li, J.; Ren, Y. Highly targeted solidification behavior of hazardous components in phosphogypsum. Chem. Eng. J. Adv. 2022, 9, 100227. [Google Scholar] [CrossRef]
- Weiksnar, K.D.; Clavier, K.A.; Robey, N.M.; Townsend, T.G. Changes in trace metal concentrations throughout the phosphogypsum lifecycle. Sci. Total Environ. 2022, 851, 158163. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare earths recovery and gypsum upgrade from Florida phosphogypsum. Miner. Metall. Process. 2017, 34, 201–206. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Zhang, P. REE extraction from phosphoric acid, phosphoric acid sludge, and phosphogypsum. Miner. Process. Extr. Metall. 2015, 124, 143–150. [Google Scholar] [CrossRef]
- Romero-Hermida, M.I.; Flores-Alés, V.; Hurtado-Bermúdez, S.J.; Santos, A.; Esquivias, L. Environmental Impact of Phosphogypsum-Derived Building Materials. Int. J. Environ. Res. Public Health 2020, 17, 4248. [Google Scholar] [CrossRef]
- Pérez-López, R.; Nieto, J.M.; López-Coto, I.; Aguado, J.L.; Bolívar, J.P.; Santisteban, M. Dynamics of contaminants in phosphogypsum of the fertilizer industry of Huelva (SW Spain): From phosphate rock ore to the environment. Appl. Geochem. 2010, 25, 705–715. [Google Scholar] [CrossRef]
- Costa, R.P.; de Medeiros, M.H.G.; Rodriguez Martinez, E.D.; Quarcioni, V.A.; Suzuki, S.; Kirchheim, A.P. Effect of soluble phosphate, fluoride, and pH in Brazilian phosphogypsum used as setting retarder on Portland cement hydration. Case Stud. Constr. Mater. 2022, 17, e01413. [Google Scholar] [CrossRef]
- Calado, B.; Tassinari, C. Geochemistry of the upper estuarine sediments of the Santos estuary: Provenance and anthropogenic pollution. J. Geol. Surv. Braz. 2020, 3, 189–209. [Google Scholar] [CrossRef]
- Lütke, S.F.; Oliveira, M.L.S.; Silva, L.F.O.; Cadaval, T.R.S.; Dotto, G.L. Nanominerals assemblages and hazardous elements assessment in phosphogypsum from an abandoned phosphate fertilizer industry. Chemosphere 2020, 256, 127138. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Puthiyaveetil Othayoth, S.; Pania, R.; Parikh, S.; Vaishnav, P. Efficient Recovery of Trapped Phosphorus from Waste Phosphogypsum of a Phosphoric Acid Plant. Chem. Sci. Rev. Lett. 2022, 11, 340–348. [Google Scholar] [CrossRef]
- Raut, S.P.; Patil, U.S.; Madurwar, M.V. Utilization of phosphogypsum and rice husk to develop sustainable bricks. Mater. Today Proc. 2022, 60, 595–601. [Google Scholar] [CrossRef]
- Muthukumar, P.; Shewale, M.; Asalkar, S.; Shinde, N.; Korke, P.; Anitha, M.; Gobinath, R.; Anuradha, R. Experimental study on lightweight panel using phosphogypsum. Mater. Today Proc. 2022, 49, 1852–1856. [Google Scholar] [CrossRef]
- Yassine, I.; Joudi, M.; Hafdi, H.; Hatimi, B.; Mouldar, J.; Bensemlali, M.; Nasrellah, H.; El Mahammedi, M.A.; Bakasse, M. Synthesis of Brushite from Phosphogypsum Industrial Waste. Biointerface Res. Appl. Chem. 2021, 12, 6580–6588. [Google Scholar] [CrossRef]
- Ennaciri, Y.; Bettach, M.; El Alaoui-Belghiti, H. Recovery of nano-calcium fluoride and ammonium bisulphate from phosphogypsum waste. Int. J. Environ. Stud. 2020, 77, 297–306. [Google Scholar] [CrossRef]
- Arhouni, F.E.; Hakkar, M.; Ouakkas, S.; Haneklaus, N.; Boukhair, A.; Nourreddine, A.; Benjelloun, M. Evaluation of the physicochemical, heavy metal and radiological contamination from phosphogypsum discharges of the phosphoric acid production unit on the coast of El Jadida Province in Morocco. J. Radioanal. Nucl. Chem. 2023, 332, 4019–4028. [Google Scholar] [CrossRef]
- Akfas, F.; Elghali, A.; Bodinier, J.-L.; Parat, F.; Muñoz, M. Geochemical and mineralogical characterization of phosphogypsum and leaching tests for the prediction of the mobility of trace elements. Environ. Sci. Pollut. Res. 2023, 30, 43778–43794. [Google Scholar] [CrossRef]
- Abouloifa, W.; Belbsir, H.; Ettaki, M.; Mounir, S.H.; El-Hami, K. Moroccan Phosphogypsum: Complete Physico-Chemical Characterization and Rheological Study of Phosphogypsum-Slurry. Chem. Afr. 2023, 6, 1605–1618. [Google Scholar] [CrossRef]
- Szajerski, P.; Bogobowicz, A.; Bem, H.; Gasiorowski, A. Quantitative evaluation and leaching behavior of cobalt immobilized in sulfur polymer concrete composites based on lignite fly ash, slag and phosphogypsum. J. Clean. Prod. 2019, 222, 90–102. [Google Scholar] [CrossRef]
- Grabas, K.; Pawełczyk, A.; Stręk, W.; Szełęg, E.; Stręk, S. Study on the Properties of Waste Apatite Phosphogypsum as a Raw Material of Prospective Applications. Waste Biomass Valor. 2019, 10, 3143–3155. [Google Scholar] [CrossRef]
- Gijbels, K.; Nguyen, H.; Kinnunen, P.; Samyn, P.; Schroeyers, W.; Pontikes, Y.; Schreurs, S.; Illikainen, M. Radiological and leaching assessment of an ettringite-based mortar from ladle slag and phosphogypsum. Cem. Concr. Res. 2020, 128, 105954. [Google Scholar] [CrossRef]
- Myka, A.; Łyszczek, R.; Zdunek, A.; Rusek, P. Thermal analysis of materials based on calcium sulphate derived from various sources. J. Therm. Anal. Calorim. 2022, 147, 9923–9934. [Google Scholar] [CrossRef]
- The Possibility of Obtaining Rare Earth Elements from Potential Sources in Poland. Warszawa: Institute of Nuclear Chemistry and Technology. 2019. Available online: http://www.ichtj.waw.pl/ichtj/publ/annual/anrep18.pdf#page=47 (accessed on 29 December 2023).
- El Zrelli, R.; Rabaoui, L.; Daghbouj, N.; Abda, H.; Castet, S.; Josse, C.; van Beek, P.; Souhaut, M.; Michel, S.; Bejaoui, N.; et al. Characterization of phosphate rock and phosphogypsum from Gabes phosphate fertilizer factories (SE Tunisia): High mining potential and implications for environmental protection. Environ. Sci. Pollut. Res. 2018, 25, 14690–14702. [Google Scholar] [CrossRef]
- Antar, K.; Jemal, M. A thermogravimetric study into the effects of additives and water vapor on the reduction of gypsum and Tunisian phosphogypsum with graphite or coke in a nitrogen atmosphere. J. Therm. Anal. Calorim. 2018, 132, 113–125. [Google Scholar] [CrossRef]
- Jalali, J.; Magdich, S.; Jarboui, R.; Loungou, M.; Ammar, E. Phosphogypsum biotransformation by aerobic bacterial flora and isolated Trichoderma asperellum from Tunisian storage piles. J. Hazard. Mater. 2016, 308, 362–373. [Google Scholar] [CrossRef]
- Moalla, R.; Gargouri, M.; Khmiri, F.; Kamoun, L.; Zairi, M. Phosphogypsum purification for plaster production: A process optimization using full factorial design. Environ. Eng. Res. 2017, 23, 36–45. [Google Scholar] [CrossRef]
- Bisone, S.; Gautier, M.; Chatain, V.; Blanc, D. Spatial distribution and leaching behavior of pollutants from phosphogypsum stocked in a gypstack: Geochemical characterization and modeling. J. Environ. Manag. 2017, 193, 567–575. [Google Scholar] [CrossRef]
- Gaidajis, G.; Anagnostopoulos, A.; Garidi, A.; Mylona, E.; Zevgolis, I.E. Laboratory evaluation of phosphogypsum for alternative uses. Environ. Geotech. 2018, 5, 310–323. [Google Scholar] [CrossRef]
- Noli, F.; Sidirelli, M.; Tsamos, P. Dispersion of radionuclides and heavy metals from phosphogypsum stacks in soil and plants at Northwestern Greece. J. Radioanal. Nucl. Chem. 2023, 332, 4213–4221. [Google Scholar] [CrossRef]
- Kalinitchenko, V.P.; Glinushkin, A.P.; Minkina, T.M.; Mandzhieva, S.S.; Sushkova, S.N.; Sukovatov, V.A.; Il’ina, L.P.; Makarenkov, D.A. Chemical Soil-Biological Engineering Theoretical Foundations, Technical Means, and Technology for Safe Intrasoil Waste Recycling and Long-Term Higher Soil Productivity. ACS Omega 2020, 5, 17553–17564. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, U.; Kasimov, I.; Mukhamedgaliev, B. Contemporary Generation Additives for Modification of Cements and Other Knitting Building Materials. Int. J. Sci. Technol. Res. 2020, 9, 1191–1192. [Google Scholar]



| Step | Description |
|---|---|
| 1 Drying | Over-drying at 40 °C for 24 h |
| 2 Milling | Fraction size smaller than 1 mm |
| 3 Digestion | Ethos 1 (MLS GmbH, Leutkirch im Allgäu, Germany) microwave-assisted wet digestion system for 35 min at 210 °C |
| 4 Measurement | Inductively coupled plasma-atomic emission spectrometry (ICP-OES, Agilent 720, Agilent Technologies Inc., Santa Clara, CA, USA) |
| HM | Sources | Effects on Soil | References |
|---|---|---|---|
| Cd | Non-ferrous metal extraction, production of phosphate fertilisers, burning of fossil fuels, waste incineration, tannery industry, electroplating, and battery disposal. | The disruption of metabolic functions hinders enzyme activities, reducing the availability of N and S in the soil for crops. | [20,21,23,24,25] |
| Pb | Emissions from power generation, metallurgy, mechanical engineering, metalworking, electrical engineering, chemistry and petrochemistry, woodworking and pulp and paper industries, food industry, and construction-material production, as well as automotive transport. | Organisms’ metabolic abnormalities affect soil enzymes and interrupt nutrient balance, reducing soil productivity. | [19,21,23,26,27,28] |
| Zn | Emissions from non-ferrous metallurgy, waste incineration plants, coal combustion, and tyre wear. | Phytotoxic effects on soil fertility, diminishing microbial biomass N; and lacking essential soil macronutrients, such as phosphorus. | [9,21,26,29,30] |
| Cu | Emissions of non-ferrous metallurgy enterprises; combustion of leaded gasoline, municipal incinerators, and copper mining residue. | Limited amounts of soil N and S hinder crop production. Inhibit β-glycosidase more than cellulose. Diminish microbial biomass N. | [21,26,27,31,32] |
| Hg | Emissions from non-ferrous metallurgy, fossil fuel burning, steel production, metal smelting. | Disruption of metabolic function in organisms. | [21,26,33,34] |
| As | Burning of fuel, emissions from power generation, production of construction materials, pharmaceutical and textile industry. As used in herbicides, insecticides, and desiccants. | Disruption of metabolic function in organisms. | [21,22,26,27] |
| Cr | Emissions from ferrous and non-ferrous metallurgy (alloying additives, alloys, and refractories) and mechanical engineering (electroplating). | Disruption of metabolic function in organisms. | [21,26,35,36] |
| Ni | Emissions from non-ferrous metallurgy, burning of fuel, waste incineration, and chemical industries. | Disruption of metabolic function in organisms. | [21,26,37,38,39] |
| Method | Brief Definition | Process Features Considering Their Limitations | References |
|---|---|---|---|
| Biomineralisation | Deposition of heavy metals as insoluble compounds. It includes two primary methods: microbiological carbonate precipitation and enzymatic carbonate precipitation. | It is considered an environmentally friendly bioremediation method that is not less effective than chemical methods. However, limitations related to microorganism strains, pollutant concentrations, and soil properties must be taken into account. Further research on soils treated with biomineralization, the solidification and stabilisation (S/S) of toxicants, is necessary to understand the patterns of strength change in polluted soils treated with biomineralization. Additionally, it is important to investigate changes in the rate of heavy metal fixation and the mechanical properties of contaminated soil. | [77,78,79,80,81,82,83] |
| Biosorption | This is a physicochemical and metabolically independent process that relies on various mechanisms, including absorption, adsorption, ion exchange, surface complexation, and precipitation. | Advantages include low cost and significantly higher efficiency in removing metals from diluted solutions. Heavy metal adsorption and removal can be performed using biomass, which can generate income for businesses that do not use biomass, such as organic waste. Various environmental parameters, such as temperature, metal type and concentration, metal oxidation state, microbe type, metal removal method, and biosorbent concentration, can influence the ability of microorganisms to bind metals. This may have a negative impact on biosorption efficiency. | [84,85,86,87,88,89,90,91,92] |
| Bioprecipitation | In the process of bioprecipitation, the formed metabolites react with metals present in the groundwater, resulting in the precipitation of metals, i.e., the transformation of metals from the aqueous phase to the solid phase. | Bioprecipitation is more effective in treating wastewater than soils; however, the profitability of recycling or selling recovered metals can vary depending on the investments in infrastructure of the investments in infrastructure of a company. It is recommended to use it in conjunction with other biological methods. | [78,93,94,95,96,97,98] |
| Bioaccumulation | Active uptake of heavy metals into cells involves the binding of toxic metals or chemical compounds inside the cellular structure. | This method not only is cost-effective but also helps minimise the environmental impact of pollution. Metal bioaccumulation is particularly useful as an impact indicator, as metals are not metabolised. Metal ions initially attach to the cell surface and are later transported into the cell. This process can lead to a temporary reduction in metal ion concentration. However, it can be utilised to synthesise metal-rich nanoparticles, provided that the processing is performed in specialised bioreactors rather than in situ. | [85,99,100,101,102,103,104,105] |
| Biotransformation | Breakdown of heavy metal compounds into less toxic forms or their conversion into less toxic forms (associated with biodegradation). | Photoautotrophic microbes are capable of biotransforming heavy metals into relatively biologically inaccessible and insoluble metal sulphides. By characterising the role of sulphur assimilation pathways in the biotransformation of heavy metals, we can develop more effective processes for heavy metal bioremediation. The use of additional sulphate nutrition can enhance the rate of biotransformation in aerobic microbes. | [78,85,106,107,108,109,110,111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernysh, Y.; Chubur, V.; Ablieieva, I.; Skvortsova, P.; Yakhnenko, O.; Skydanenko, M.; Plyatsuk, L.; Roubík, H. Soil Contamination by Heavy Metals and Radionuclides and Related Bioremediation Techniques: A Review. Soil Syst. 2024, 8, 36. https://doi.org/10.3390/soilsystems8020036
Chernysh Y, Chubur V, Ablieieva I, Skvortsova P, Yakhnenko O, Skydanenko M, Plyatsuk L, Roubík H. Soil Contamination by Heavy Metals and Radionuclides and Related Bioremediation Techniques: A Review. Soil Systems. 2024; 8(2):36. https://doi.org/10.3390/soilsystems8020036
Chicago/Turabian StyleChernysh, Yelizaveta, Viktoriia Chubur, Iryna Ablieieva, Polina Skvortsova, Olena Yakhnenko, Maksym Skydanenko, Leonid Plyatsuk, and Hynek Roubík. 2024. "Soil Contamination by Heavy Metals and Radionuclides and Related Bioremediation Techniques: A Review" Soil Systems 8, no. 2: 36. https://doi.org/10.3390/soilsystems8020036
APA StyleChernysh, Y., Chubur, V., Ablieieva, I., Skvortsova, P., Yakhnenko, O., Skydanenko, M., Plyatsuk, L., & Roubík, H. (2024). Soil Contamination by Heavy Metals and Radionuclides and Related Bioremediation Techniques: A Review. Soil Systems, 8(2), 36. https://doi.org/10.3390/soilsystems8020036









