Effects of Past Sulfur Deposition on the Soil Microbial Biomass at Spruce Forest Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Sites
2.2. History of Atmospheric S deposition
2.3. Sampling and Chemical Analyses
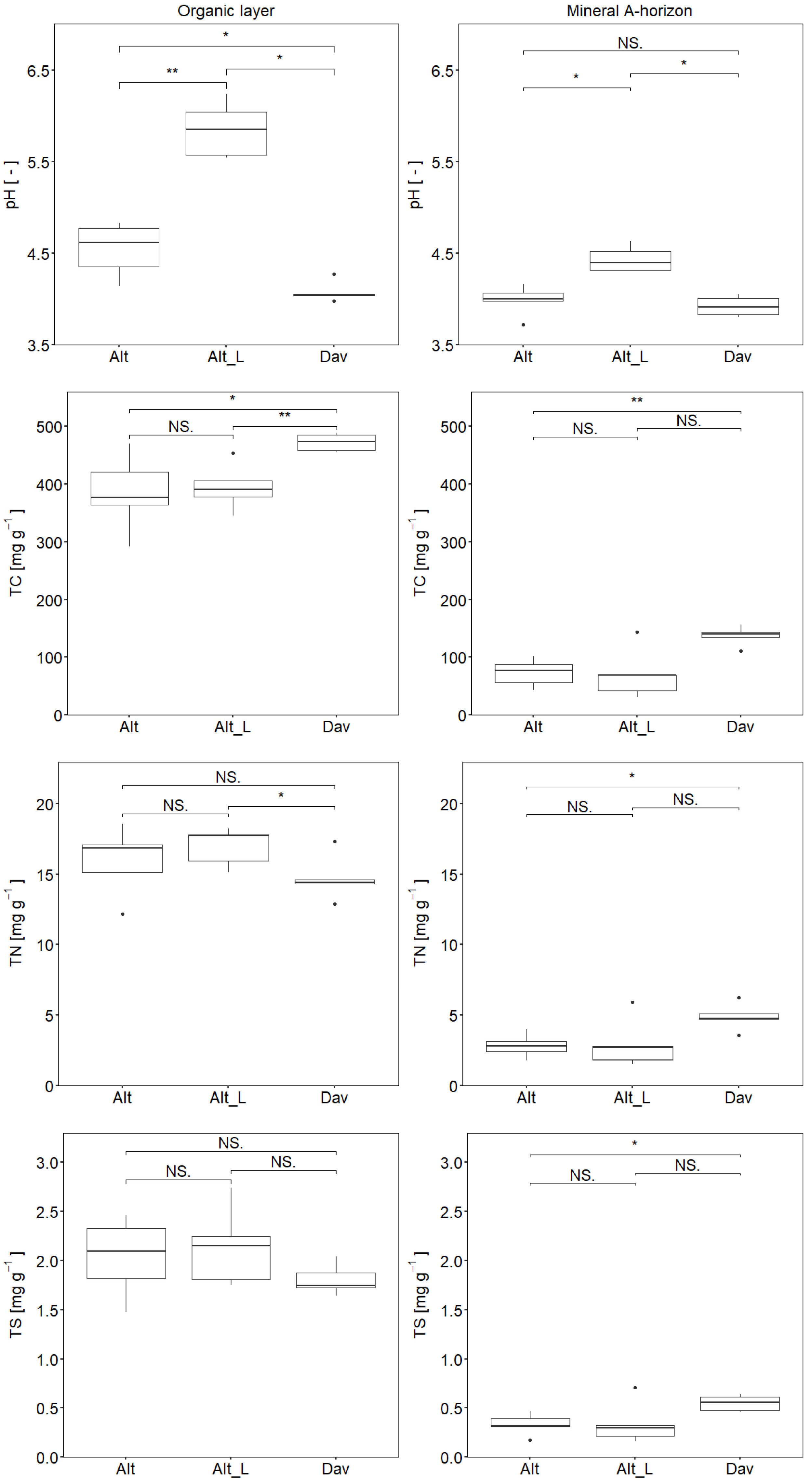
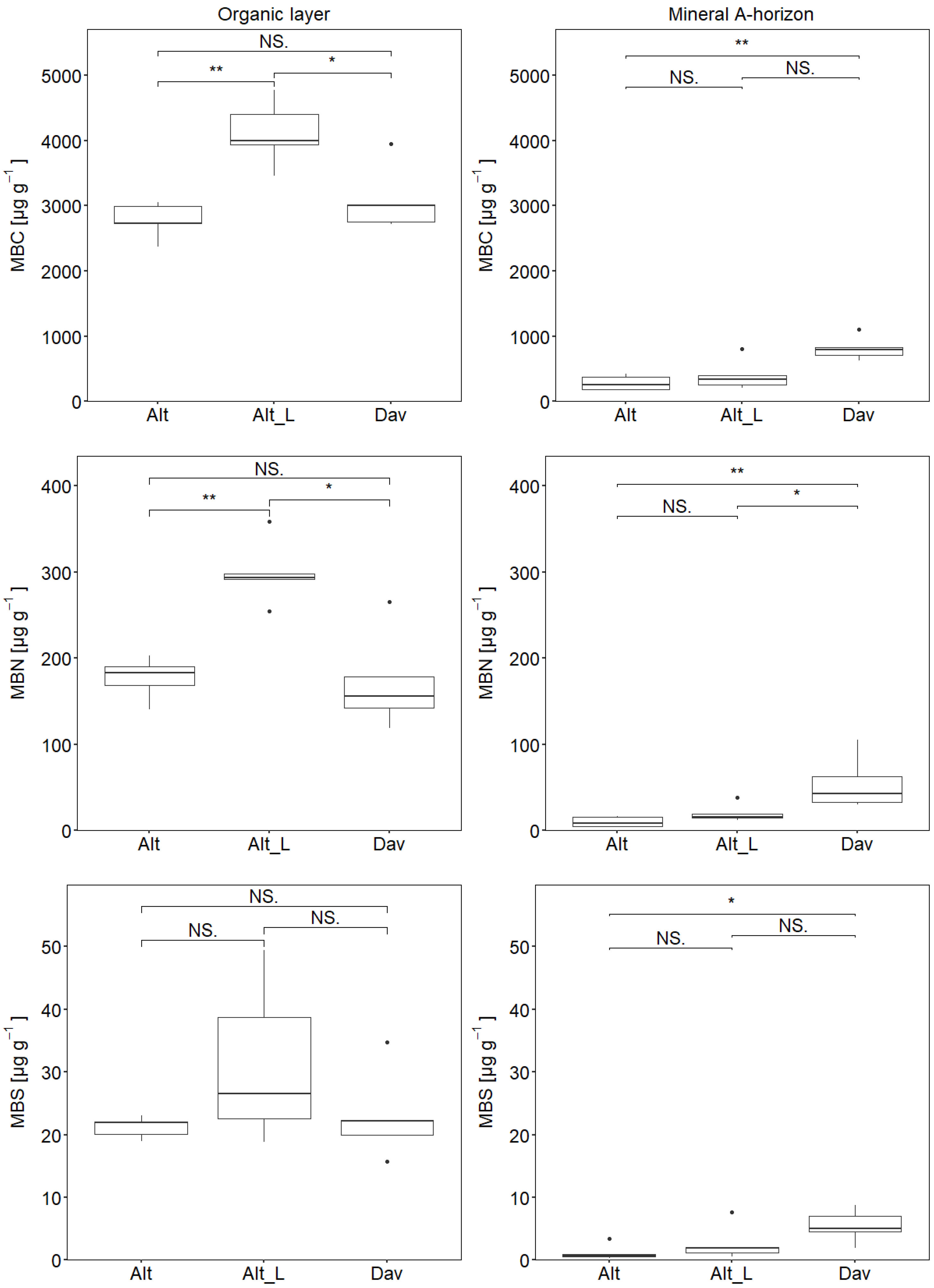
3. Results
3.1. Soil Chemical Properties
3.2. Elemental Composition in Microbial Biomass
3.3. Stoichiometry in Soil and Microbial Biomass
4. Discussion
4.1. pH Effects on Microbial Mineralization
4.2. Carbon-Dependent S Mineralization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lövland, G.; Tarrasón, L.; Torseth, K.; Dutchak, S. EMEP Assessment Report Part I—European Perspective; Norwegian Meteorological Institute: Oslo, Norway, 2004; ISBN 82-7144-032-2. [Google Scholar]
- Vanguelova, E.; Reynolds, B.; Nisbet, T.; Godbold, D. The cycling of pollutants in nonurban forested environments. In Forest Hydrology and Biogeochemistry; Ecological Studies; Springer: Dordrecht, The Netherlands, 2011; pp. 679–710. ISBN 978-94-007-1362-8. [Google Scholar]
- Engardt, M.; Simpson, D.; Schwikowski, M.; Granat, L. Deposition of sulphur and nitrogen in Europe 1900–2050. Model calculations and comparison to historical observations. Tellus B 2017, 69, 1328945. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Shortle, W.C.; Smith, K.T. Dendroecological applications in air pollution and environmental chemistry: Research Needs. Dendrochronologia 2002, 20, 133–157. [Google Scholar] [CrossRef]
- Liebold, E.; Drechsler, M. Schadenszustand und-entwicklung in den SO2-geschädigten Fichtengebieten Sachsens. AFZ DerWald 1991, 46, 492–494. [Google Scholar]
- Singh, B.R. Distribution of total and extractable S and adsorbed 35SO2−4 in some acid forest soil profiles of Southern Norway. Acta Agric. Scand. 1980, 30, 357–363. [Google Scholar] [CrossRef]
- Johnson, D.W.; Mitchell, M.J. Responses of forest ecosystems to changing sulfur inputs. In Sulfur in the Environment; Marcel Dekker: New York, NY, USA, 1998; pp. 219–262. [Google Scholar]
- Ulrich, B. The history and possible causes of forest decline in Central Europe, with particular attention to the German situation. Environ. Rev. 1995, 3, 262–276. [Google Scholar] [CrossRef]
- Zöttl, H.W.; Hüttl, R.F. Nutrient supply and forest decline in Southwest-Germany. Water Air Soil Pollut. 1986, 31, 449–462. [Google Scholar] [CrossRef]
- Wright, R.F.; Larssen, T.; Camarero, L.; Cosby, B.J.; Ferrier, R.C.; Helliwell, R.; Forsius, M.; Jenkins, A.; Kopáěek, J.; Majer, V.; et al. Recovery of acidified European surface waters. Environ. Sci. Technol. 2005, 39, 64A–72A. [Google Scholar] [CrossRef]
- Novák, M.; Kirchner, J.W.; Fottová, D.; Prechová, E.; Jăcková, I.; Krám, P.; Hruska, J. Isotopic Evidence for processes of sulfur retention/release in 13 forested catchments spanning a strong pollution gradient (Czech Republic, Central Europe). Glob. Biogeochem Cycles 2005, 19, GB4012. [Google Scholar] [CrossRef]
- Schmidt, U. Waldböden in Deutschland: Ausgewählte Ergebnisse der Zweiten Bodenzustandserhebung; Bundesministerium für Ernährung und Landwirtschaft (BMEL): Bonn, Germany, 2018. [Google Scholar]
- Alewell, C.; Manderscheid, B.; Gerstberger, P.; Matzner, E. Effects of reduced atmospheric deposition on soil solution chemistry and elemental contents of spruce needles in NE-Bavaria, Germany. J. Plant Nutr. Soil Sci. 2000, 163, 509–516. [Google Scholar] [CrossRef]
- Armbruster, M.; Abiy, M.; Feger, K.H. The biogeochemistry of two forested catchments in the Black Forest and the Eastern Ore Mountains (Germany). Biogeochemistry 2003, 65, 341–368. [Google Scholar] [CrossRef]
- Meesenburg, H.; Ahrends, B.; Fleck, S.; Wagner, M.; Fortmann, H.; Scheler, B.; Klinck, U.; Dammann, I.; Eichhorn, J.; Mindrup, M.; et al. Long-term changes of ecosystem services at Solling, Germany: Recovery from acidification, but increasing nitrogen saturation? Ecol. Indic. 2016, 65, 103–112. [Google Scholar] [CrossRef]
- Vuorenmaa, J.; Augustaitis, A.; Beudert, B.; Clarke, N.; de Wit, H.A.; Dirnböck, T.; Frey, J.; Forsius, M.; Indriksone, I.; Kleemola, S.; et al. Long-term sulphate and inorganic nitrogen mass balance budgets in European ICP Integrated Monitoring Catchments (1990–2012). Ecol. Indic. 2017, 76, 15–29. [Google Scholar] [CrossRef]
- Hartmann, K.; Lilienthal, H.; Schnug, E. Risk-mapping of potential sulphur deficiency in agriculture under actual and future climate scenarios in Germany. Asp. Appl. Biol. 2008, 88, 113–121. [Google Scholar]
- Zhao, F.; Tausz, M.; De Kok, L.J. Role of sulfur for plant production in agricultural and natural ecosystems. In Sulfur Metabolism in Phototrophic Organisms; Hell, R., Dahl, C., Knaff, D., Leustek, T., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 417–435. ISBN 978-1-4020-6863-8. [Google Scholar]
- Chalk, P.M.; Inácio, C.T.; Chen, D. Tracing S dynamics in agro-ecosystems using 34S. Soil Biol. Biochem. 2017, 114, 295–308. [Google Scholar] [CrossRef]
- Talkner, U.; Riek, W.; Dammann, I.; Kohler, M.; Göttlein, A.; Mellert, K.H.; Meiwes, K.J. Nutritional status of major forest tree species in Germany. In Status and Dynamics of Forests in Germany; Springer: Cham, Switzerland, 2019; pp. 261–293. [Google Scholar]
- Wellbrock, N.; Bolte, A.; Flessa, H. Dynamik und Räumliche Muster forstlicher Standorte in Deutschland: Ergebnisse der Bodenzustandserhebung im Wald 2006 bis 2008; Thünen Reports 43; Johann Heinrich von Thünen Institute, Federal Research Institute for Rural Areas, Forestry and Fisheries: Braunschweig, Germany, 2016. [Google Scholar]
- Göttlein, A.; Fäth, J.; Rau, M.; Mellert, K.H. Schwefel—Vom Überschuss zum Mangel? AFZ-DerWald 2020, 9, 31–33. [Google Scholar]
- Prescher, A.-K.; Schmitz, A.; Sanders, T.G.M.; Nussbaumer, A.; Pihl Karlsson, G.; Neirynck, J.; Gottardini, E.; Hansen, K.; Johnson, J.; Nieminen, T.M.; et al. Change in sulphur pools in forest ecosystems following the reduction of atmospheric sulphur dioxide. In Proceedings of EGU General Assembly Conference Abstracts; EGU General Assembly: Vienna, Austria, 2018; Volume 20, p. 9027. [Google Scholar]
- Jonard, M.; Fürst, A.; Verstraeten, A.; Thimonier, A.; Timmermann, V.; Potočić, N.; Waldner, P.; Benham, S.; Hansen, K.; Merilä, P.; et al. Tree mineral nutrition is deteriorating in Europe. Glob. Chang. Biol. 2015, 21, 418–430. [Google Scholar] [CrossRef]
- Scherer, H.W. Sulfur in Soils. J. Plant Nutr. Soil Sci. 2009, 172, 326–335. [Google Scholar] [CrossRef]
- Banerjee, M.R.; Chapman, S.J. The significance of microbial biomass sulphur in soil. Biol. Fert. Soils 1996, 22, 116–125. [Google Scholar] [CrossRef]
- Heinze, S.; Hemkemeyer, M.; Schwalb, S.A.; Khan, K.S.; Joergensen, R.G.; Wichern, F. Microbial biomass sulphur—An important yet understudied pool in soil. Agronomy 2021, 11, 1606. [Google Scholar] [CrossRef]
- Kertesz, M.A.; Mirleau, P. The role of soil microbes in plant sulphur nutrition. J. Exp. Bot. 2004, 55, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- McGill, W.; Cole, C. Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma 1981, 26, 267–286. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Kirkegaard, J.A.; Richardson, A.E.; Wade, L.J.; Blanchard, C.; Batten, G. Stable soil organic matter: A comparison of C:N:P:S ratios in Australian and other world soils. Geoderma 2011, 163, 197–208. [Google Scholar] [CrossRef]
- Chowdhury, M.A.H.; Kouno, K.; Ando, T. Correlation among microbial biomass S, soil properties, and other biomass nutrients. Soil Sci. Plant Nutr. 1999, 45, 175–186. [Google Scholar] [CrossRef]
- Wunderlich, S.; Raben, G.; Andreae, H.; Feger, K. Schwefel-Vorräte und Sulfat-Remobilisierungspotential in Böden der Level-II-Standorte Sachsens. AFZ-DerWald 2006, 60, 762–765. [Google Scholar]
- Menzer, A.; Andreae, H. Stoffbilanzen Sächsischer Level II-Flächen—Anwendung zur Qualifizierung der Bodenschutzkalkung. In Ausgleichs-und Reaktorfunktion von Waldböden im Stoff-und Wasserkreislauf; von Wilpert, K., Vögtle, B., Eds.; Berichte Freiburger Forstliche Forschung; Forstliche Versuchs- und Forschungsanstalt Baden-Württemberg: Freiburg, Germany, 2013; Volume 96, pp. 199–211. [Google Scholar]
- WSL LWF Seehornwald Davos Long-Term Research Site. Available online: https://lwf.wsl.ch/de/flaechen/davos/?tx_wslwebapps_webapps_lwfdispatcher%5Baction%5D=which&tx_wslwebapps_webapps_lwfdispatcher%5Bcontroller%5D=Lwf&cHash=8d8efd03c00049497b490abb67c5f389 (accessed on 5 January 2024).
- Grunewald, K.; Scheithauer, J. Landschaftsveränderung und Umweltbelastung im Erzgebirge. In Naturschutz im Gesellschaftlichen Kontext; Erdmann, K.-H., Bork, H.-R., Kopf, T., Eds.; Bundesamt für Naturschutz: Bonn, Germany, 2006; pp. 165–190. [Google Scholar]
- Volz, H.-A. 10 Jahre Depositionsmessungen in Fichtenbeständen: Eine Synoptische Darstellung Ausgewählter Meßreihen für Schwefel und Stickstoff im Vergleich zu den Emissionen; Schriftenreihe des Bundesministeriums für Ernährung, Landwirtschaft und Forsten—Reihe A: Angewandte Wissenschaft; Landwirtschaftsverlag: Münster, Germany, 1994; Volume 434. [Google Scholar]
- Nebe, W.; Feger, K.-H. Atmosphärische Deposition, ökosystemare Stoffbilanzen und Ernährung der Fichte bei differenzierter Immissionsbelastung–Langjährige Zeitreihen für das Osterzgebirge und den Südschwarzwald. Forstwiss. Beiträge Tharandt 2005, 22, 129. [Google Scholar]
- Andreae, H.; Gemballa, R.; Jacob, F. Leitfaden zur Forstlichen Bodenschutzkalkung in Sachsen; Staatsbetrieb Sachsenforst: Pirna, Germany, 2020; p. 102. [Google Scholar]
- Staatsbetrieb Sachsenforst; (Section Site Survey, Soil Monitoring, Laboratory, Pirna, Germany). Unpublished data 2023, personal communication, 2023.
- BUWAL. Vom Menschen verursachte Luftschadstoffemissionen in der Schweiz von 1900 bis 2010; Bundesamt für Umwelt, Wald und Landschaft (BUWAL), Schriftenreihe Umwelt: Bern, Switzerland, 1995; Volume 256, 121p. [Google Scholar]
- Augustin, S.; Achermann, B. Deposition von Luftschadstoffen in der Schweiz: Entwicklung, Aktueller Stand und Bewertung. Schweiz. Z. Fur Forstwes. 2012, 163, 323–330. [Google Scholar] [CrossRef]
- WSL Daten. Available online: https://lwf.wsl.ch/de/daten/?tx_wslwebapps_webapps_lwfdispatcher%5Baction%5D=data&tx_wslwebapps_webapps_lwfdispatcher%5Bcontroller%5D=Lwf&cHash=bca513fe05c679a167c07e65fc1a52a7 (accessed on 5 January 2024).
- Gehrig, R. Immissionssituation im Schweizer Wald. In Eidgenössische Forschungsanstalt für Wald, Schnee und Landschaft (Ed.): Waldschadenforschung in der Schweiz: Stand der Kenntnisse; Forum für Wissen; Eidgenössische Forschungsanstalt für Wald, Schnee und Landschaft: Birmensdorf, Switzerland, 1992; pp. 81–86. [Google Scholar]
- Waldner, P.; Schmitt, M.; Schaub, M.; Pannatier, E.G.; Thimonier, A. Stickstoffeintrag und Ozonbelastung im Schweizer Wald aus der Sicht der Langfristigen Waldökosystem-Forschung; Langzeitforschung für eine nachhaltige Waldnutzung; Eidgenössische Forschungsanstalt für Wald, Schnee und Landschaft WSL: Birmensdorf, Switzerland, 2009; pp. 113–124. [Google Scholar]
- DIN Deutsches Institut für Normung e.V. DIN EN ISO 14240-2:2011-09, Bodenbeschaffenheit—Bestimmung der Mikrobiellen Biomasse von Böden—Teil 2: Fumigations-Extraktionsverfahren; Beuth Verlag: Berlin, Germany, 2011. [Google Scholar]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEN value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Saggar, S.; Bettany, J.R.; Stewart, J.W.B. Measurement of microbial sulfur in soil. Soil Biol. Biochem. 1981, 13, 493–498. [Google Scholar] [CrossRef]
- Wu, J.; O’donnell, A.G.; He, Z.L.; Syers, J.K. Fumigation-extraction method for the measurement of soil microbial biomass-S. Soil Biol. Biochem. 1994, 26, 117–125. [Google Scholar] [CrossRef]
- Kandeler, E.; Stemmer, M.; Gerzabek, M.H. Role of microorganisms in carbon cycling in soils. In Microorganisms in Soils: Roles in Genesis and Functions; Varma, A., Buscot, F., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 139–157. ISBN 978-3-540-26609-9. [Google Scholar]
- Meiwes, K.J.; Khanna, P.K. Distribution and cycling of sulphur in the vegetation of two forest ecosystems in an acid rain environment. Plant Soil 1981, 60, 369–375. [Google Scholar] [CrossRef]
- Giordano, M.; Raven, J.A. Nitrogen and sulfur assimilation in plants and algae. Aquat. Bot. 2014, 118, 45–61. [Google Scholar] [CrossRef]
- Khan, K.S.; Joergensen, R.G. Stoichiometry of the soil microbial biomass in response to amendments with varying C/N/P/S ratios. Biol. Fertil. Soils 2019, 55, 265–274. [Google Scholar] [CrossRef]
- Ontman, R.; Groffman, P.M.; Driscoll, C.T.; Cheng, Z. Surprising relationships between soil pH and microbial biomass and activity in a Northern Hardwood Forest. Biogeochemistry 2023, 163, 265–277. [Google Scholar] [CrossRef]
- Thakur, S.D. Der biogeochmische Schwefelkreislauf in seiner Bedeutung für Forstökosysteme unter besonderer Berücksichtigung des ökologischen Experimentalgebietes “Wernersbach” im Tharandter Wald. Ph.D. Dissertation, TU Dresden, Tharandt, Germany, 1985. [Google Scholar]
- Edwards, P.J. Sulfur cycling, retention, and mobility in soils: A Review. Gen. Tech. Rep. 1998, 250. [Google Scholar]
- Blum, S.C.; Lehmann, J.; Solomon, D.; Caires, E.F.; Alleoni, L.R.F. Sulfur forms in organic substrates affecting S mineralization in soil. Geoderma 2013, 200–201, 156–164. [Google Scholar] [CrossRef]
| Site | Altenberg | Davos | |
|---|---|---|---|
| Abbreviation | Alt | Alt_L | Dav |
| Location | 50°47′31.99″ N, 13°43′28.99″ E | 46°48′55.2″ N, 9°51′21.3″ E | |
| Elevation [m above sea level] | 745 | 1635–1665 | |
| Annual precipitation [mm] | 1089 | 1050 | |
| Annual mean temperature [°C] | 5.4 | 3.5 | |
| Parent material | Rhyolite | Paragneiss | |
| Soil type (WRB *) | Haplic Podzol | Haplic Podzol | |
| Total liming amount [Mg ha−1] (year of treatment) | 4 (2011) | 14 (1988, 1994, 2001, 2011) | - |
| Site | Altenberg | Davos | |
|---|---|---|---|
| Abbreviation | Alt | Alt_L | Dav |
| |||
| TC (O/A) | 384.8/73.3 | 394.7/71.0 | 472.2/137.0 |
| TN (O/A) | 16.0/2.8 | 17.0/3.0 | 14.7/4.9 |
| TS (O/A) | 2.03/0.33 | 2.14/0.34 | 1.81/0.55 |
| |||
| DC (O/A) | 704.6/124.1 | 462.2/70.9 | 424.7/314.6 |
| DN (O/A) | 78.9/12.6 | 43.2/11.3 | 100.6/35.2 |
| DNH4N (O/A) | 57.7/6.4 | 49.3/7.6 | 73.1/22.4 |
| DNO3 (O/A) | <DL | <DL | <DL |
| DS (O/A) | 17.2/2.7 | 23.4/2.6 | 8.6/5.4 |
| DSO4S (O/A) | 15.8/4.1 | 23.7/4.1 | 10.4/6.7 |
| |||
| MBC (O/A) | 2774.5/282.5 | 4113.0/400.2 | 3087.6/812.2 |
| MBN (O/A) | 177.2/9.8 | 299.3/20.0 | 172.4/54.8 |
| MBS (O/A) | 21.2/1.1 | 31.2/2.7 | 23.0/2.6 |
| Site | Altenberg | Davos | |
|---|---|---|---|
| Abbreviation | Alt | Alt_L | Dav |
| |||
| TC/TN (O/A) | 24/26 | 23/24 | 32/29 |
| TC/TS (O/A) | 191/225 | 188/207 | 263/251 |
| TN/TS (O/A) | 7.9/8.7 | 8.1/8.8 | 8.2/8.9 |
| |||
| DC/DN (O/A) | 8.8/9.8 | 10.7/6.3 | 4.9/9.2 |
| DC/DS (O/A) | 40/44 | 20/28 | 60/60 |
| DN/DS (O/A) | 4.9/5.3 | 1.9/4.5 | 13.1/7.9 |
| DNH4N/DN (O/A) | 0.7/0.5 | 1.2/0.7 | 0.7/0.6 |
| DSO4S/DS (O/A) | 1.0/1.6 | 1.0/1.7 | 1.4/1.5 |
| |||
| MBC/MBN (O/A) | 16/33 | 14/20 | 19/17 |
| MBC/MBS (O/A) | 131/426 | 144/214 | 139/191 |
| MBN/MBS (O/A) | 8/13 | 10/11 | 8/16 |
| |||
| MBC/TC (O/A) | 0.7/0.4 | 1.0/0.6 | 0.7/0.6 |
| MBN/TN (O/A) | 1.1/0.3 | 1.8/0.7 | 1.2/1.1 |
| MBS/TS (O/A) | 1.0/0.4 | 1.6/0.7 | 1.3/1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julich, D.; Julich, S.; Feger, K.-H.; Klauder, T.; Zethof, J.H.T. Effects of Past Sulfur Deposition on the Soil Microbial Biomass at Spruce Forest Sites. Soil Syst. 2024, 8, 37. https://doi.org/10.3390/soilsystems8020037
Julich D, Julich S, Feger K-H, Klauder T, Zethof JHT. Effects of Past Sulfur Deposition on the Soil Microbial Biomass at Spruce Forest Sites. Soil Systems. 2024; 8(2):37. https://doi.org/10.3390/soilsystems8020037
Chicago/Turabian StyleJulich, Dorit, Stefan Julich, Karl-Heinz Feger, Tobias Klauder, and Jeroen H. T. Zethof. 2024. "Effects of Past Sulfur Deposition on the Soil Microbial Biomass at Spruce Forest Sites" Soil Systems 8, no. 2: 37. https://doi.org/10.3390/soilsystems8020037
APA StyleJulich, D., Julich, S., Feger, K.-H., Klauder, T., & Zethof, J. H. T. (2024). Effects of Past Sulfur Deposition on the Soil Microbial Biomass at Spruce Forest Sites. Soil Systems, 8(2), 37. https://doi.org/10.3390/soilsystems8020037







