Complex Speciation and Distribution of Iron, Sulfur, and Trace Metals in Coal Mine Soils Reflect Grain- and Sub-Grain-Scale Heterogeneity during Pyrite Oxidative Dissolution
Abstract
1. Introduction
2. Materials and Methods
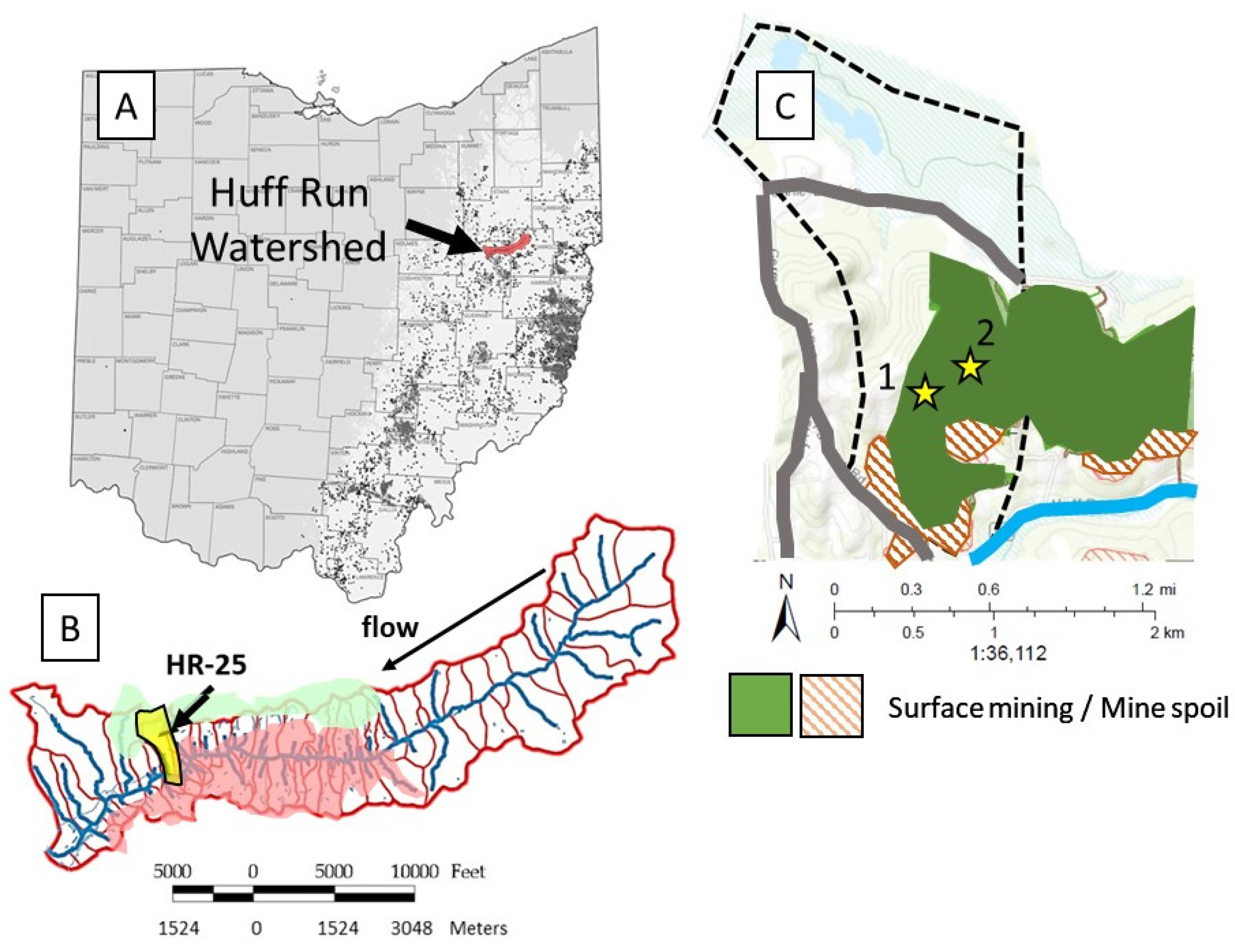
2.1. Site Description
2.2. Soil Sample Collection, Thin-Section Preparation, and Grain-Scale Characterization
2.3. Characterization of Fe, S, and Trace Metal Speciation and Distribution
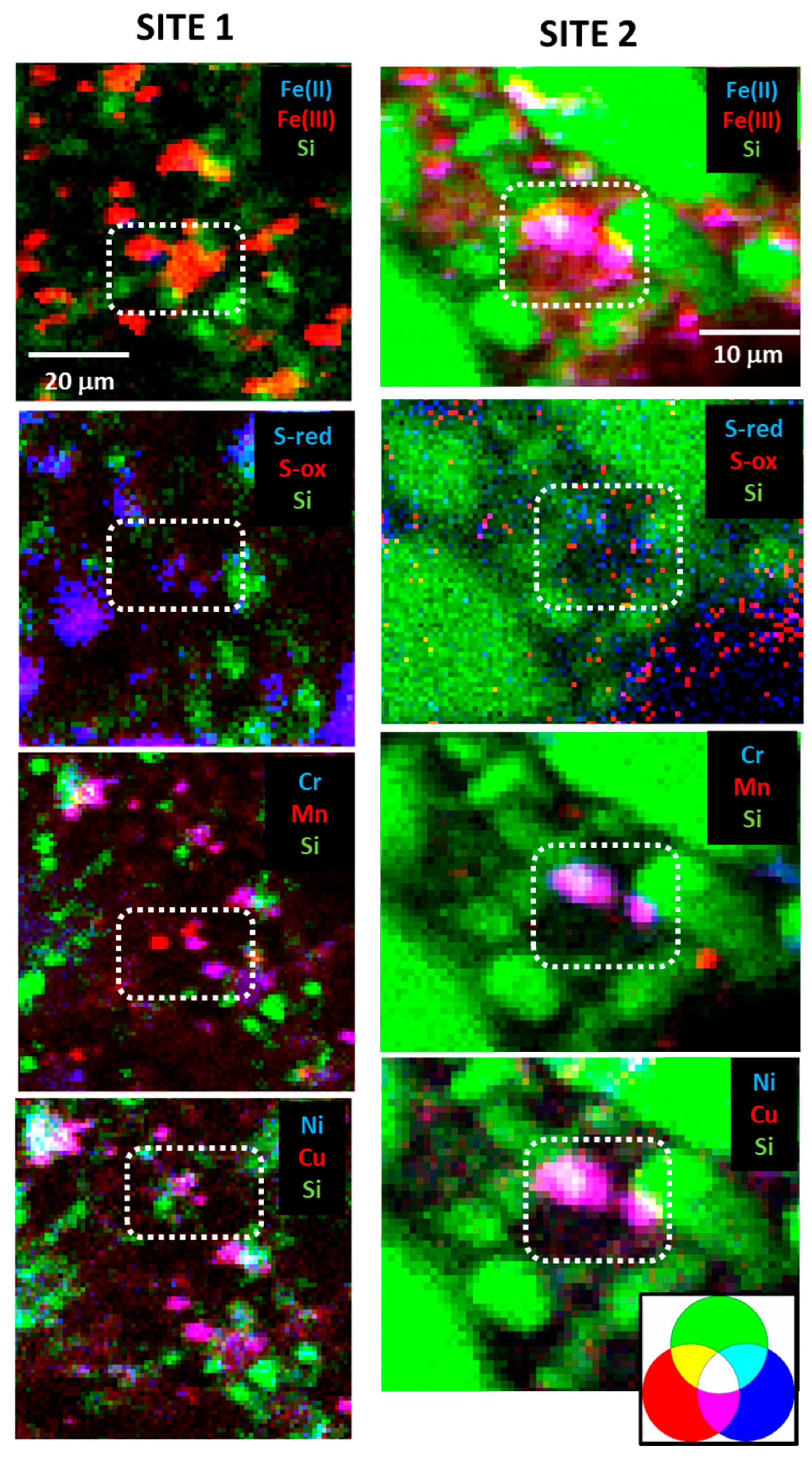
3. Results
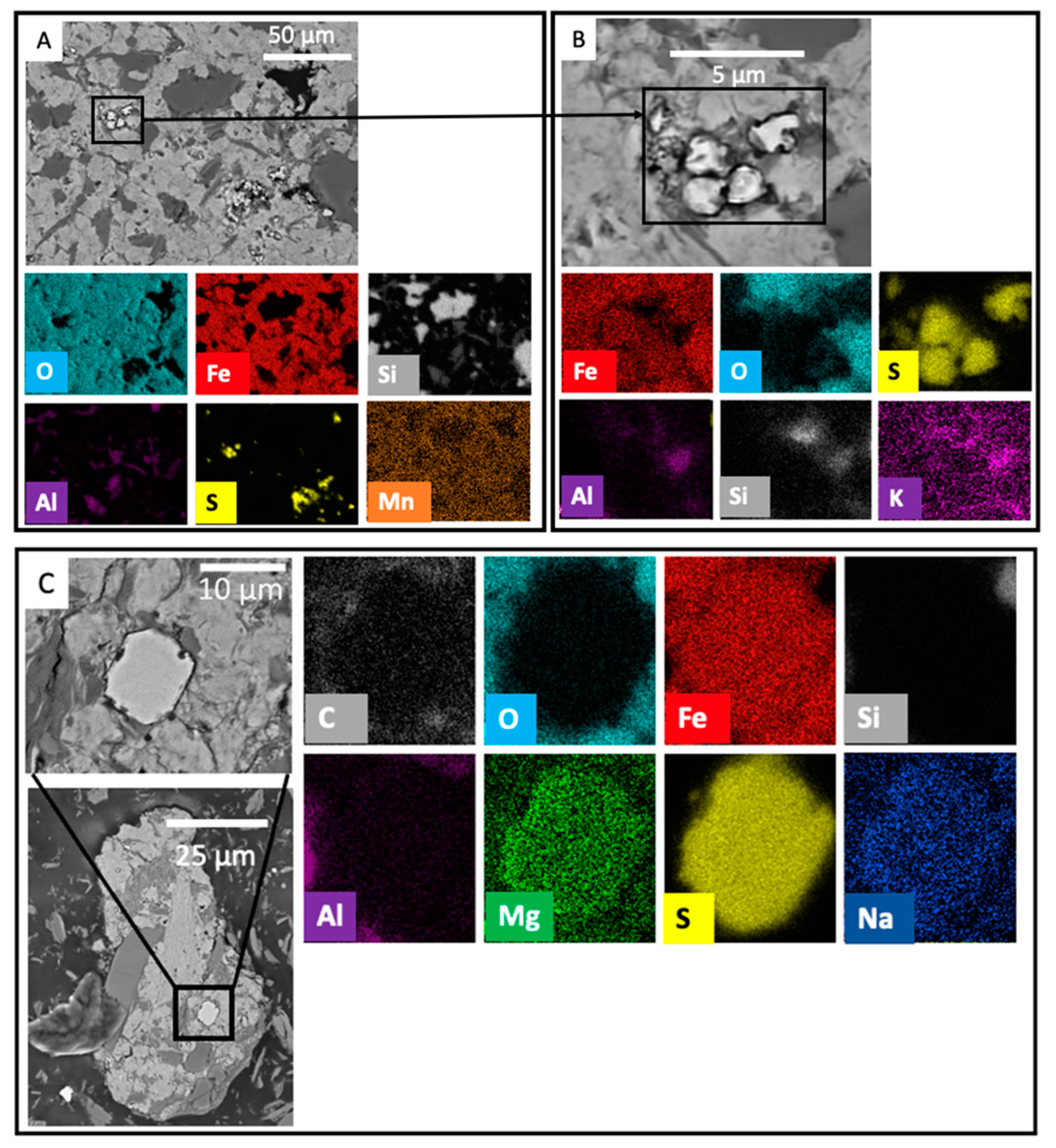
3.1. Unweathered to Partially Weathered Pyrite
3.2. Partial to Complete Pseudomorphic Replacement of Pyrite
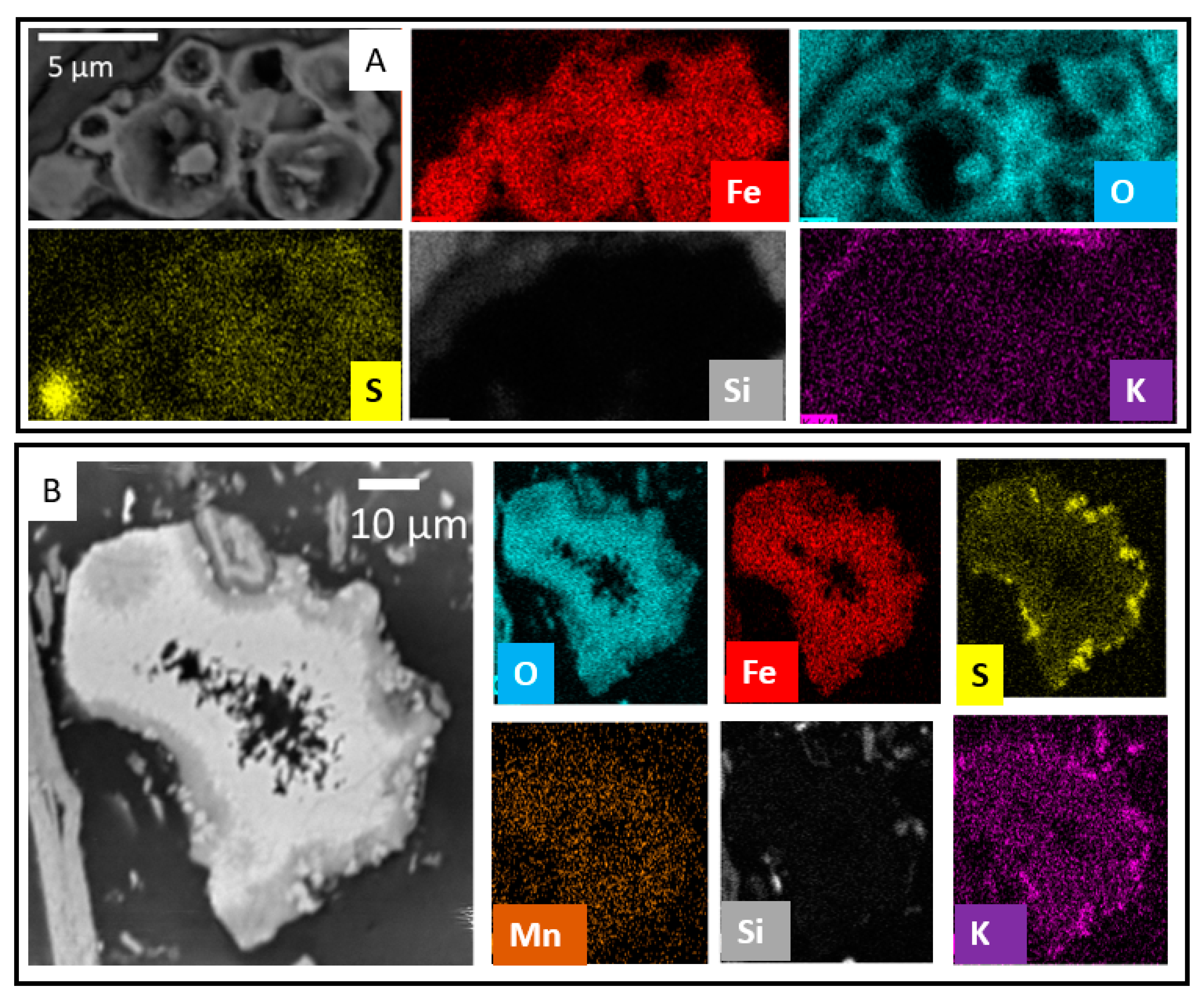
3.3. Fe Oxide Secondary Mineral Surface Coatings
3.4. Discrete Secondary Fe-Bearing Phases
4. Discussion
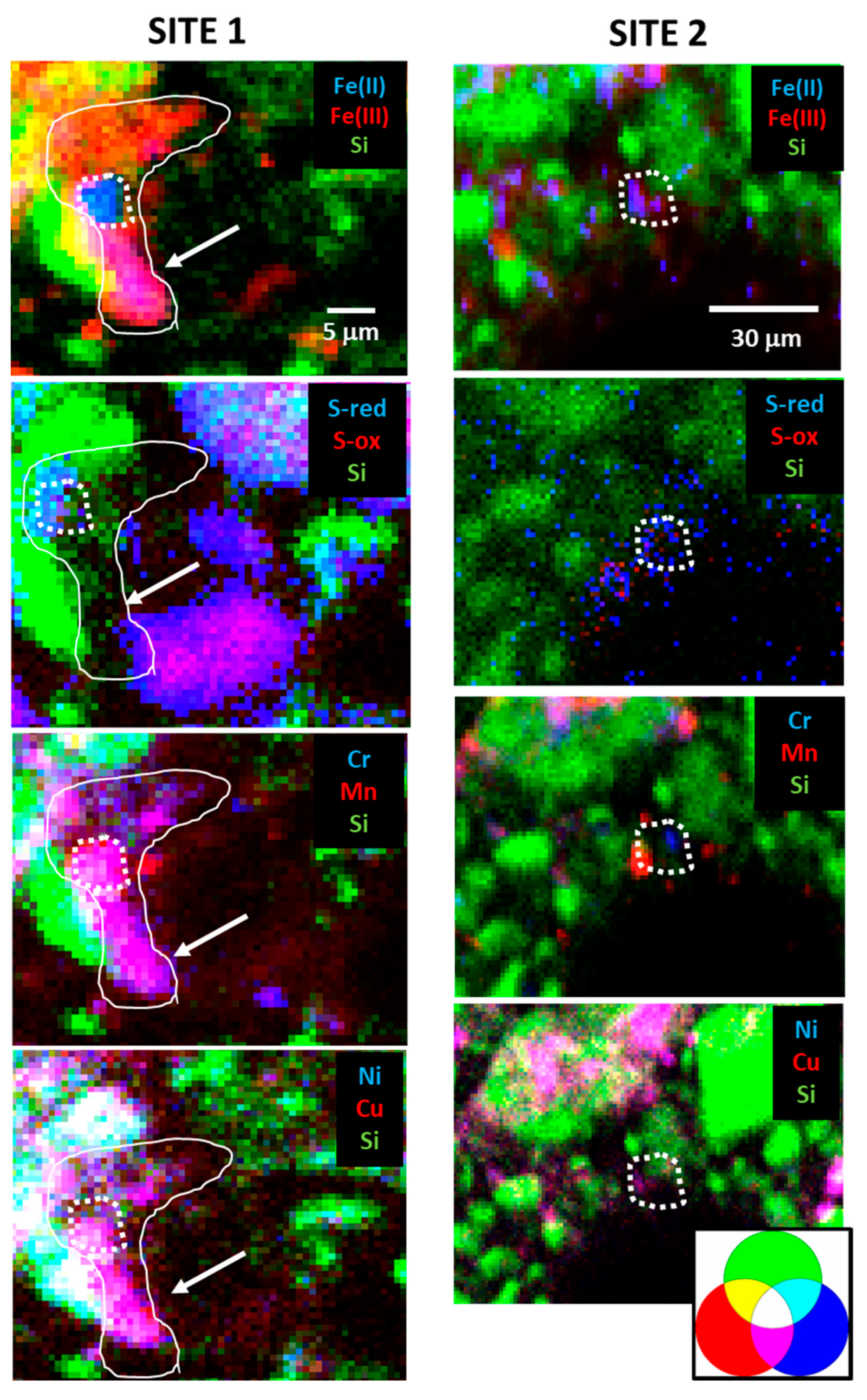
4.1. Factors That Control Pyrite Weathering, Potential Fe Transport, and Re-Precipitation
4.2. Fate of Trace Metals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouhani, A.; Skousen, J.; Tack, F.M.G. An Overview of Soil Pollution and Remediation Strategies in Coal Mining Regions. Minerals 2023, 13, 1064. [Google Scholar] [CrossRef]
- Skousen, J.; Daniels, W.L.; Zipper, C.E. Soils on Appalachian coal-mined lands. In Appalachia’s Coal-Mined Landscapes: Resources and Communities in a New Energy Era; Springer: Cham, Germany, 2021; pp. 85–109. [Google Scholar]
- Clark, E.V.; Daniels, W.L.; Zipper, C.E.; Eriksson, K. Mineralogical influences on water quality from weathering of surface coal mine spoils. Appl. Geochem. 2018, 91, 97–106. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Bai, Z.; Reading, L. Effects of surface coal mining and land reclamation on soil properties: A review. Earth-Sci. Rev. 2019, 191, 12–25. [Google Scholar] [CrossRef]
- Singer, D.M.; Herndon, E.; Cole, K.; Koval, J.; Perdrial, N. Formation of secondary mineral coatings and the persistence of reduced metal-bearing phases in soils developing on historic coal mine spoil. Appl. Geochem. 2020, 121, 104711. [Google Scholar] [CrossRef]
- Maiti, S.K. Ecorestoration of the Coalmine Degraded Lands; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Kirby, B.; Vengadajellum, C.; Burton, S.; Cowan, D. Coal, coal mines and spoil heaps. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Shrestha, R.K.; Lal, R. Ecosystem carbon budgeting and soil carbon sequestration in reclaimed mine soil. Environ. Int. 2006, 32, 781–796. [Google Scholar] [CrossRef]
- Large, R.R.; Halpin, J.A.; Danyushevsky, L.V.; Maslennikov, V.V.; Bull, S.W.; Long, J.A.; Gregory, D.D.; Lounejeva, E.; Lyons, T.W.; Sack, P.J.; et al. Trace element content of sedimentary pyrite as a new proxy for deep-time ocean–atmosphere evolution. Earth Planet. Sci. Lett. 2014, 389, 209–220. [Google Scholar] [CrossRef]
- Dang, Z.; Liu, C.; Haigh, M.J. Mobility of heavy metals associated with the natural weathering of coal mine spoils. Environ. Pollut. 2002, 118, 419–426. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy Metal Contamination: An Alarming Threat to Environment and Human Health. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2019; pp. 103–125. [Google Scholar] [CrossRef]
- Neculita, C.M.; Rosa, E. A review of the implications and challenges of manganese removal from mine drainage. Chemosphere 2019, 214, 491–510. [Google Scholar] [CrossRef]
- Lindsay, M.B.; Condon, P.D.; Jambor, J.L.; Lear, K.G.; Blowes, D.W.; Ptacek, C.J. Mineralogical, geochemical, and microbial investigation of a sulfide-rich tailings deposit characterized by neutral drainage. Appl. Geochem. 2009, 24, 2212–2221. [Google Scholar] [CrossRef]
- Cravotta, C.A., III. Abandoned Mine Drainage in the Swatara Creek Basin, Southern Anthracite Coalfield, Pennsylvania, USA: 2. Performance of Treatment Systems. Mine Water Environ. 2010, 29, 200–216. [Google Scholar] [CrossRef][Green Version]
- Ross, M.R.V.; Nippgen, F.; Hassett, B.A.; McGlynn, B.L.; Bernhardt, E.S. Pyrite Oxidation Drives Exceptionally High Weathering Rates and Geologic CO2 Release in Mountaintop-Mined Landscapes. Glob. Biogeochem. Cycles 2018, 32, 1182–1194. [Google Scholar] [CrossRef]
- Cravotta, C.A. Dissolved metals and associated constituents in abandoned coal-mine discharges, Pennsylvania, USA. Part 1: Constituent quantities and correlations. Appl. Geochem. 2008, 23, 166–202. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of ferrihydrite into goethite or hematite, revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Gillham, R.; Reardon, E. Pyrite oxidation in carbonate-buffered solution: 2. Rate control by oxide coatings. Geochim. et Cosmochim. Acta 1990, 54, 395–402. [Google Scholar] [CrossRef]
- Gu, X.; Heaney, P.J.; Reis, F.D.A.A.; Brantley, S.L. Deep abiotic weathering of pyrite. Science 2020, 370, eabb8092. [Google Scholar] [CrossRef]
- Von der Heyden, B.P.; Roychoudhury, A.N. Application, chemical interaction and fate of iron minerals in polluted sediment and soils. Curr. Pollut. Rep. 2015, 1, 265–279. [Google Scholar] [CrossRef]
- Ruiz-Agudo, C.; Putnis, C.V.; Ruiz-Agudo, E.; Putnis, A. The influence of pH on barite nucleation and growth. Chem. Geol. 2015, 391, 7–18. [Google Scholar] [CrossRef]
- Buerge-Weirich, D.; Hari, R.; Xue, H.; Behra, P.; Sigg, L. Adsorption of Cu, Cd, and Ni on Goethite in the Presence of Natural Groundwater Ligands. Environ. Sci. Technol. 2002, 36, 328–336. [Google Scholar] [CrossRef]
- Karapınar, N. Removal of Heavy Metal Ions by Ferrihydrite: An Opportunity to the Treatment of Acid Mine Drainage. Water Air Soil Pollut. 2016, 227, 1–8. [Google Scholar] [CrossRef]
- Marescotti, P.; Carbone, C.; Comodi, P.; Frondini, F.; Lucchetti, G. Mineralogical and chemical evolution of ochreous precipitates from the Libiola Fe–Cu-sulfide mine (Eastern Liguria, Italy). Appl. Geochem. 2012, 27, 577–589. [Google Scholar] [CrossRef]
- Wise, M. Huff Run Watershed Plan; Huff Run Watershed Restoration Partnership, Inc.: Mineral City, OH, USA, 2005. [Google Scholar]
- Wood, D.L.; Cole, K.A.; Herndon, E.M.; Singer, D.M. Lime slurry treatment of soils developing on abandoned coal mine spoil: Linking contaminant transport from the micrometer to pedon-scale. Appl. Geochem. 2023, 151, 105617. [Google Scholar] [CrossRef]
- Smart, K.E.; Singer, D.M. Surface Coal Mine Soils: Evidence for Chronosequence Development. Soil Syst. 2023, 7, 59. [Google Scholar] [CrossRef]
- Singer, D.; Herndon, E.; Zemanek, L.; Cole, K.; Sanda, T.; Senko, J.; Perdrial, N. Biogeochemical Controls on the Potential for Long-Term Contaminant Leaching from Soils Developing on Historic Coal Mine Spoil. Soil Syst. 2021, 5, 3. [Google Scholar] [CrossRef]
- Chowdhury, A.R.; Singer, D.M.; Herndon, E. Colloidal metal transport in soils developing on historic coal mine spoil. Appl. Geochem. 2021, 128, 104933. [Google Scholar] [CrossRef]
- Chowdhury, M.A.R.; Singer, D.M. Trace Metal Enrichment in the Colloidal Fraction in Soils Developing on Abandoned Mine Spoils. Minerals 2022, 12, 1290. [Google Scholar] [CrossRef]
- ODNR. Economic Impact Analysis of the Ohio Abandoned Mine Land Program. In Ohio Department of Natural Resources Division of Mineral Resources Management; Ohio University’s Voinovich School of Leadership and Public Affairs: Athens, OH, USA, 2014. [Google Scholar]
- ODNR. Huff Run Watershed Acid Mine Drainage Abatement and Treatment Plan; Prepared for Ohio DNR by Gannett Fleming. Available online: watersheddata.com (accessed on 7 December 2014).
- Newville, M.; Sutton, S.R.; Rivers, M.L.; Eng, P. Micro-beam X-ray absorption and fluorescence spectroscopies at GSECARS: APS beamline 13ID. J. Synchrotron Radiat. 1999, 6, 353–355. [Google Scholar] [CrossRef]
- Newville, M. Larch: An Analysis Package for XAFS and Related Spectroscopies. J. Phys. Conf. Ser. 2013, 430, 012007. [Google Scholar] [CrossRef]
- Yazbek, L.D.; Cole, K.A.; Shedleski, A.; Singer, D.; Herndon, E.M. Hydrogeochemical Processes Limiting Aqueous and Colloidal Fe Export in a Headwater Stream Impaired by Acid Mine Drainage. ACS ES&T Water 2020, 1, 68–78. [Google Scholar] [CrossRef]
- Singer, D.M.; Herndon, E.; Cole, K.; Burkey, M.; Morisson, S.; Cahill, M.; Bartucci, M.A. Micron-scale distribution controls metal(loid) release during simulated weathering of a Pennsylvanian coal shale. Geochim. Cosmochim. Acta 2020, 269, 117–135. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Ewing, R.C.; Hough, R.; Walshe, J. Trace metal nanoparticles in pyrite. Ore Geol. Rev. 2011, 42, 32–46. [Google Scholar] [CrossRef]
- Chiriță, P.; Schlegel, M.L. Pyrite oxidation in air-equilibrated solutions: An electrochemical study. Chem. Geol. 2017, 470, 67–74. [Google Scholar] [CrossRef]
- Holmes, P.R.; Crundwell, F.K. The kinetics of the oxidation of pyrite by ferric ions and dissolved oxygen: An electrochemical study. Geochim. et Cosmochim. Acta 2000, 64, 263–274. [Google Scholar] [CrossRef]
- Ruppert, L.F.; Hower, J.C.; Eble, C.F. Arsenic-bearing pyrite and marcasite in the Fire Clay coal bed, Middle Pennsylvanian Breathitt Formation, eastern Kentucky. Int. J. Coal Geol. 2005, 63, 27–35. [Google Scholar] [CrossRef]
- Diehl, S.; Goldhaber, M.; Koenig, A.; Lowers, H.; Ruppert, L. Distribution of arsenic, selenium, and other trace elements in high pyrite Appalachian coals: Evidence for multiple episodes of pyrite formation. Int. J. Coal Geol. 2012, 94, 238–249. [Google Scholar] [CrossRef]
- Gu, X.; Rempe, D.M.; Dietrich, W.E.; West, A.J.; Lin, T.-C.; Jin, L.; Brantley, S.L. Chemical reactions, porosity, and microfracturing in shale during weathering: The effect of erosion rate. Geochim. Cosmochim. Acta 2020, 269, 63–100. [Google Scholar] [CrossRef]
- Huminicki, D.M.; Rimstidt, J.D. Iron oxyhydroxide coating of pyrite for acid mine drainage control. Appl. Geochem. 2009, 24, 1626–1634. [Google Scholar] [CrossRef]
- Rusch, B.; Hanna, K.; Humbert, B. Coating of quartz silica with iron oxides: Characterization and surface reactivity of iron coating phases. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 172–180. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, Y. Schwertmannite transformation to goethite and the related mobility of trace metals in acid mine drainage. Chemosphere 2021, 269, 128720. [Google Scholar] [CrossRef]
- Fitzpatrick, R.; Mosley, L.; Raven; Shand, P. Schwertmannite formation and properties in acidic drain environments following exposure and oxidation of acid sulfate soils in irrigation areas during extreme drought. Geoderma 2017, 308, 235–251. [Google Scholar] [CrossRef]
- Chen, Q.; Cohen, D.R.; Andersen, M.S.; Robertson, A.M.; Jones, D.R. Stability and trace element composition of natural schwertmannite precipitated from acid mine drainage. Appl. Geochem. 2022, 143, 105370. [Google Scholar] [CrossRef]
- Bigham, J.; Schwertmann, U.; Traina, S.; Winland, R.; Wolf, M. Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim. Cosmochim. Acta 1996, 60, 2111–2121. [Google Scholar] [CrossRef]
- Uzarowicz, Ł.; Skiba, S. Technogenic soils developed on mine spoils containing iron sulphides: Mineral transformations as an indicator of pedogenesis. Geoderma 2011, 163, 95–108. [Google Scholar] [CrossRef]
- Duiker, S.W.; Rhoton, F.E.; Torrent, J.; Smeck, N.E.; Lal, R. Iron (hydr) oxide crystallinity effects on soil aggregation. Soil Sci. Soc. Am. J. 2003, 67, 606–611. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, X.-Y.; Xian, Q.-S.; Weisbrod, N.; Yang, J.E.; Wang, H.-L. A field study of colloid transport in surface and subsurface flows. J. Hydrol. 2016, 542, 101–114. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Navarre-Sitchler, A.; Steefel, C.I.; Yang, L.; Tomutsa, L.; Brantley, S.L. Evolution of porosity and diffusivity associated with chemical weathering of a basalt clast. J. Geophys. Res. 2009, 114, 100. [Google Scholar] [CrossRef]
- Bigham, J.M.; Nordstrom, D.K. Iron and Aluminum Hydroxysulfates from Acid Sulfate Waters. Rev. Miner. Geochem. 2000, 40, 351–403. [Google Scholar] [CrossRef]
- Filipek, L.H.; Nordstrom, D.K.; Ficklin, W.H. Interaction of acid mine drainage with waters and sediments of West Squaw Creek in the West Shasta Mining District, California. Environ. Sci. Technol. 1987, 21, 388–396. [Google Scholar] [CrossRef]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metal-sulfate salts from sulfide mineral oxidation. Rev. Mineral. Geochem. 2000, 40, 303–350. [Google Scholar] [CrossRef]
- Li, H.; Ji, H.; Shi, C.; Gao, Y.; Zhang, Y.; Xu, X.; Ding, H.; Tang, L.; Xing, Y. Distribution of heavy metals and metalloids in bulk and particle size fractions of soils from coal-mine brownfield and implications on human health. Chemosphere 2017, 172, 505–515. [Google Scholar] [CrossRef]
- Strawn, D.G.; Hickey, P.J.; McDaniel, P.A.; Baker, L.L. Distribution of As, Cd, Pb, and Zn in redox features of mine-waste impacted wetland soils. J. Soils Sediments 2012, 12, 1100–1110. [Google Scholar] [CrossRef]
- Lanzirotti, A.; Tappero, R.; Schulze, D.G. Chapter 2-Practical Application of Synchrotron-Based Hard X-Ray Microprobes in Soil Sciences. In Developments in Soil Science; Singh, B., Gräfe, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 27–72. [Google Scholar]
- Horowitz, A.J.; Elrick, K.A. The relation of stream sediment surface area, grain size and composition to trace element chemistry. Appl. Geochem. 1987, 2, 437–451. [Google Scholar] [CrossRef]
- Luoma, S.N. Processes affecting metal concentrations in estuarine and coastal marine sediments. In Heavy Metals in the Marine Environment; CRC Press: Boca Raton, FL, USA, 2018; pp. 51–66. [Google Scholar]
- Acero, P.; Ayora, C.; Torrentó, C.; Nieto, J.-M. The behavior of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite. Geochim. Cosmochim. Acta 2006, 70, 4130–4139. [Google Scholar] [CrossRef]
- Sidenko, N.V.; Sherriff, B.L. The attenuation of Ni, Zn and Cu, by secondary Fe phases of different crystallinity from surface and ground water of two sulfide mine tailings in Manitoba, Canada. Appl. Geochem. 2005, 20, 1180–1194. [Google Scholar] [CrossRef]
- Herndon, E.; Yarger, B.; Frederick, H.; Singer, D. Iron and Manganese Biogeochemistry in Forested Coal Mine Spoil. Soil Syst. 2019, 3, 13. [Google Scholar] [CrossRef]
- Ghosh, A.; Pal, M.; Biswas, K.; Ghosh, U.C.; Manna, B. Manganese oxide incorporated ferric oxide nanocomposites (MIFN): A novel adsorbent for effective removal of Cr(VI) from contaminated water. J. Water Process. Eng. 2015, 7, 176–186. [Google Scholar] [CrossRef]
- Clark, E.V.; Zipper, C.E.; Daniels, W.L.; Keefe, M.J. Appalachian coal mine spoil elemental release patterns and depletion. Appl. Geochem. 2018, 98, 109–120. [Google Scholar] [CrossRef]
- Gao, X.; Xu, M.; Hu, Q.; Wang, Y. Leaching behavior of trace elements in coal spoils from Yangquan coal mine, Northern China. J. Earth Sci. 2016, 27, 891–900. [Google Scholar] [CrossRef]
- Beauchemin, S.; Langley, S.; MacKinnon, T. Geochemical properties of 40-year old forested pyrrhotite tailings and impact of organic acids on metal cycling. Appl. Geochem. 2019, 110, 104437. [Google Scholar] [CrossRef]
- Adams, R.; Ahlfeld, D.; Sengupta, A. Investigating the Potential for Ongoing Pollution from an Abandoned Pyrite Mine. Mine Water Environ. 2007, 26, 2–13. [Google Scholar] [CrossRef]
- Hammond, C.M.; Root, R.A.; Maier, R.M.; Chorover, J. Metal Lability and Mass Transfer Response to Direct-Planting Phytostabilization of Pyritic Mine Tailings. Minerals 2022, 12, 757. [Google Scholar] [CrossRef]
- Larsen, O.; Postma, D. Kinetics of reductive bulk dissolution of lepidocrocite, ferrihydrite, and goethite. Geochim. et Cosmochim. Acta 2001, 65, 1367–1379. [Google Scholar] [CrossRef]
- Anschutz, A.J.; Penn, R.L. Reduction of crystalline iron(III) oxyhydroxides using hydroquinone: Influence of phase and particle size. Geochem. Trans. 2005, 6, 60. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, M.A.R.; Singer, D.M. Complex Speciation and Distribution of Iron, Sulfur, and Trace Metals in Coal Mine Soils Reflect Grain- and Sub-Grain-Scale Heterogeneity during Pyrite Oxidative Dissolution. Soil Syst. 2024, 8, 2. https://doi.org/10.3390/soilsystems8010002
Chowdhury MAR, Singer DM. Complex Speciation and Distribution of Iron, Sulfur, and Trace Metals in Coal Mine Soils Reflect Grain- and Sub-Grain-Scale Heterogeneity during Pyrite Oxidative Dissolution. Soil Systems. 2024; 8(1):2. https://doi.org/10.3390/soilsystems8010002
Chicago/Turabian StyleChowdhury, Md Abu Raihan, and David M. Singer. 2024. "Complex Speciation and Distribution of Iron, Sulfur, and Trace Metals in Coal Mine Soils Reflect Grain- and Sub-Grain-Scale Heterogeneity during Pyrite Oxidative Dissolution" Soil Systems 8, no. 1: 2. https://doi.org/10.3390/soilsystems8010002
APA StyleChowdhury, M. A. R., & Singer, D. M. (2024). Complex Speciation and Distribution of Iron, Sulfur, and Trace Metals in Coal Mine Soils Reflect Grain- and Sub-Grain-Scale Heterogeneity during Pyrite Oxidative Dissolution. Soil Systems, 8(1), 2. https://doi.org/10.3390/soilsystems8010002




