Control of Soil Extracellular Enzyme Activities by Clay Minerals—Perspectives on Microbial Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Materials and Preparation
2.2. Experimental Setup
2.3. Enzyme Measurements
2.4. Statistical Analysis
3. Results
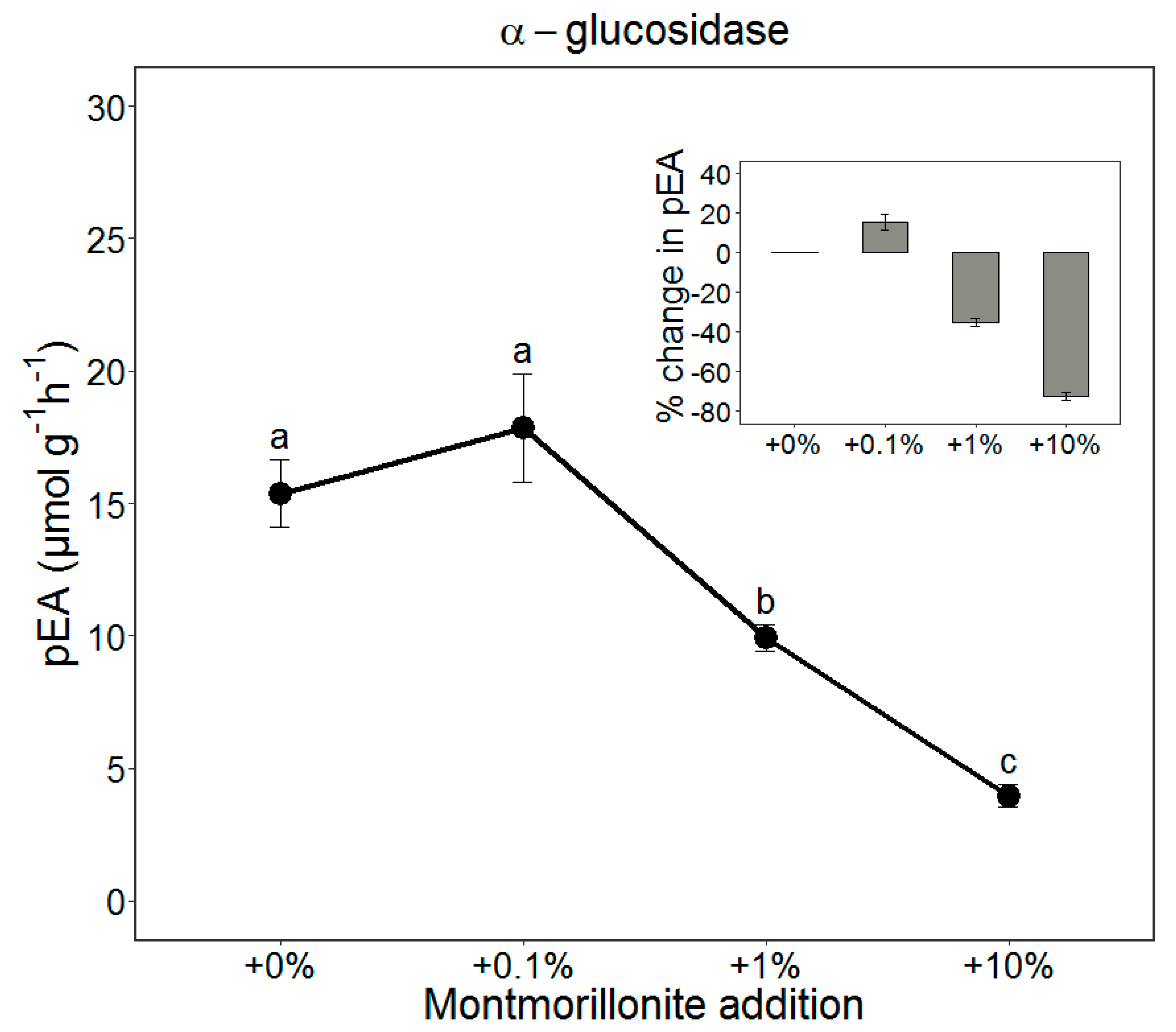
3.1. The pEA of AG Added to the Soil (Adsorption Experiment)
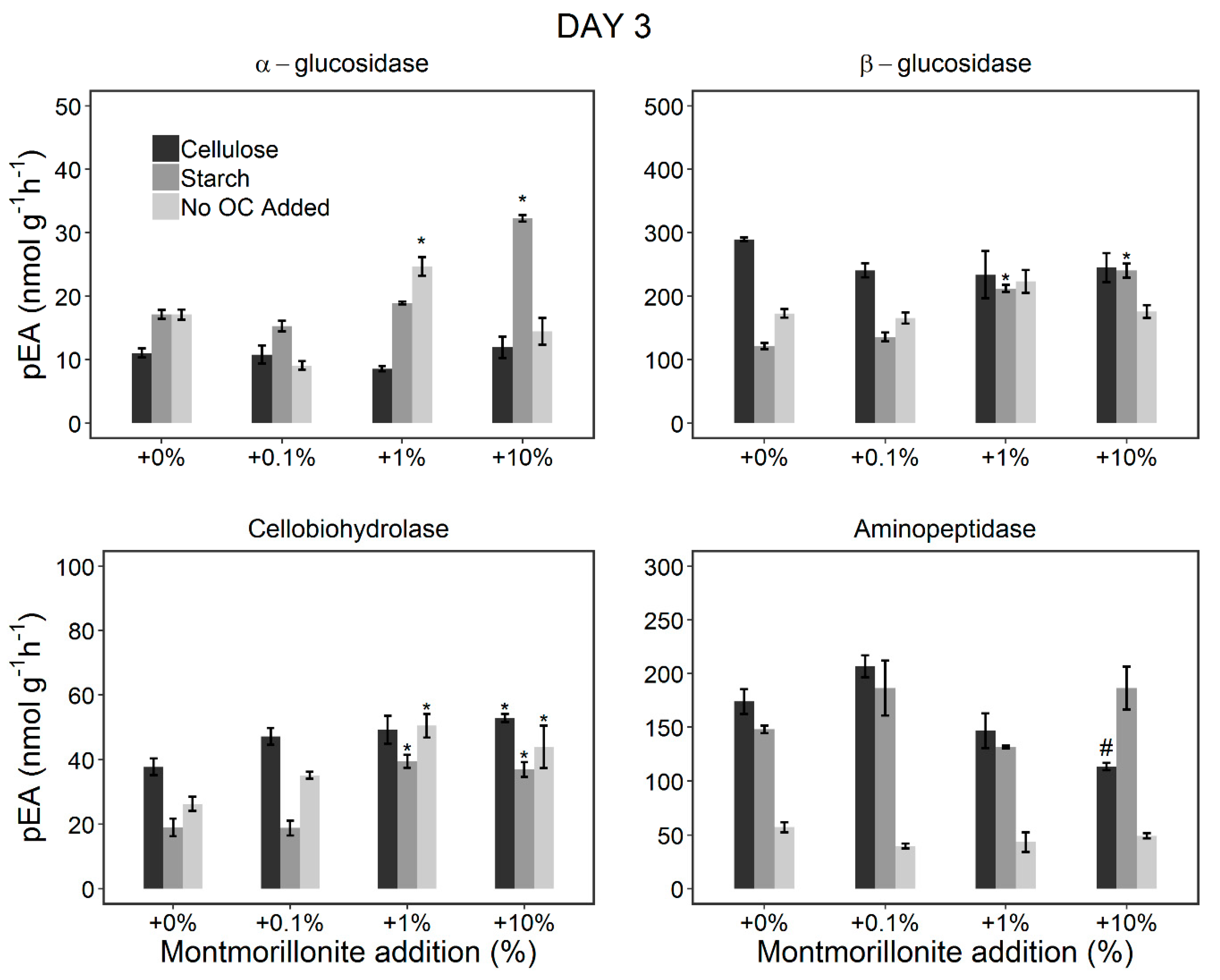
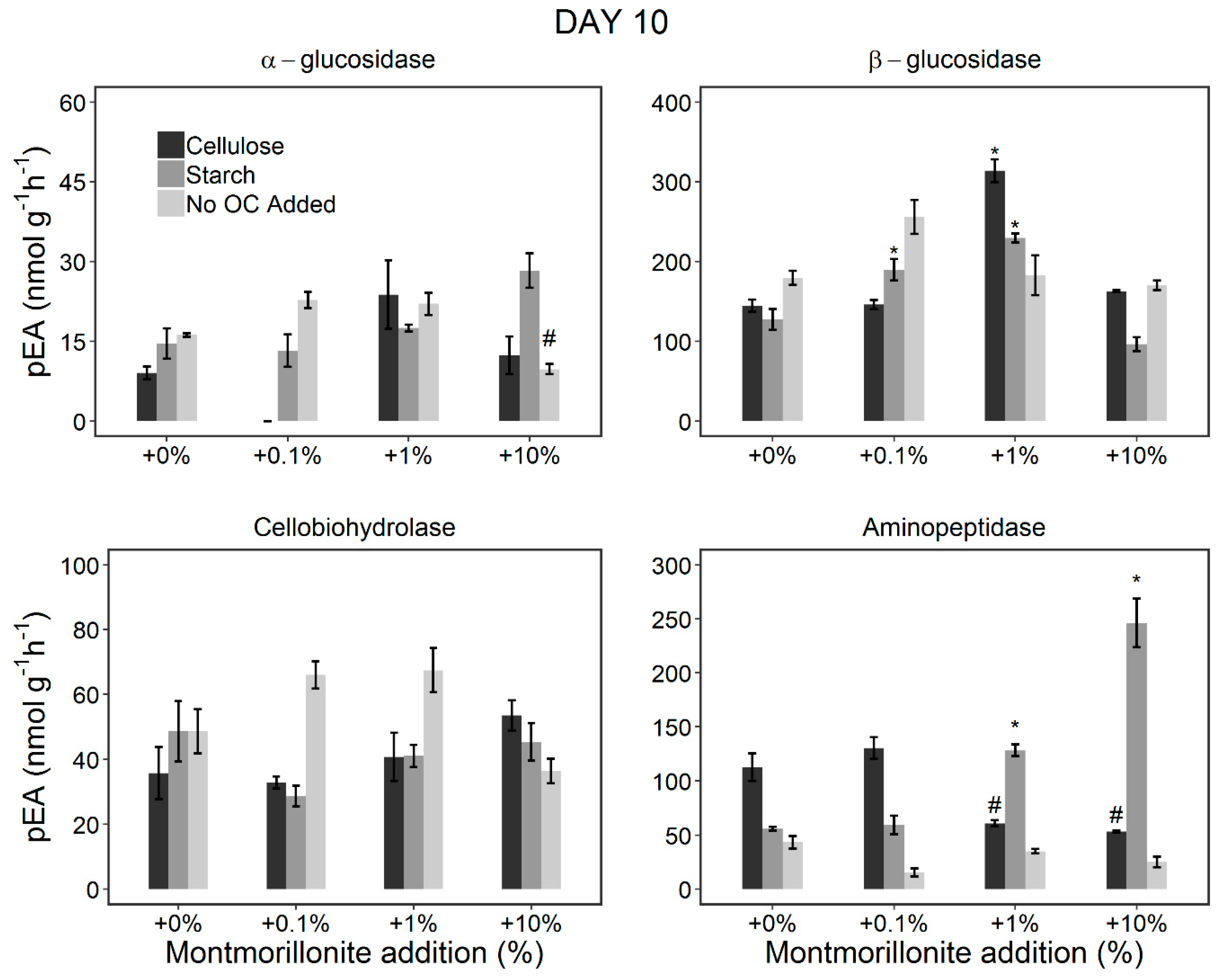
3.2. Changes in the pEA of EEs during the Incubation Period (Incubation Experiment)
3.2.1. Stimulation of the Microorganisms by OC Addition
3.2.2. Effect of Montmorillonite Addition
4. Discussion
4.1. The Inhibitory Effect of Clay Minerals on the Potential Enzyme Activity
4.2. Persistence of the EEs Due to Protection by the Clay Minerals
4.3. Potential Microbial Adaptation to Reduced Potential Enzyme Activities
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Pronk, G.J.; Heister, K.; Vogel, C.; Babin, D.; Bachmann, J.; Ding, G.-C.; Ditterich, F.; Gerzabek, M.H.; Giebler, J.; Hemkemeyer, M.; et al. Interaction of minerals, organic matter, and microorganisms during biogeochemical interface formation as shown by a series of artificial soil experiments. Biol. Fertil. Soils 2017, 53, 9–22. [Google Scholar] [CrossRef]
- Nunan, N.; Leloup, J.; Ruamps, L.S.; Pouteau, V.; Chenu, C. Effects of habitat constraints on soil microbial community function. Sci. Rep. 2017, 7, 4280. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D. Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol. Lett. 2005, 8, 626–635. [Google Scholar] [CrossRef]
- Brookes, P.C.; Chen, Y.; Chen, L.; Qiu, G.; Luo, Y.; Xu, J. Is the rate of mineralization of soil organic carbon under microbiological control? Soil Biol. Biochem. 2017, 112, 127–139. [Google Scholar] [CrossRef]
- Kaiser, C.; Franklin, O.; Richter, A.; Dieckmann, U. Social dynamics within decomposer communities lead to nitrogen retention and organic matter build-up in soils. Nat. Commun. 2015, 6, 8960. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.; Becerra, C.A.; Blankinship, J. Estimating decay dynamics for enzyme activities in soils from different ecosystems. Soil Biol. Biochem. 2017, 114, 5–11. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E.; Blagodatsky, S. Comments on the paper by Kemmitt et al. (2008) ‘Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass—A new perspective’ [Soil Biology & Biochemistry 40, 61–73]: The biology of the Regulatory Gate. Soil Biol. Biochem. 2009, 41, 435–439. [Google Scholar] [CrossRef]
- Turner, S.; Schippers, A.; Meyer-Stüve, S.; Guggenberger, G.; Gentsch, N.; Dohrmann, R.; Condron, L.M.; Eger, A.; Almond, P.C.; Peltzer, D.A.; et al. Mineralogical impact on long-term patterns of soil nitrogen and phosphorus enzyme activities. Soil Biol. Biochem. 2014, 68, 31–43. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Weintraub, M.N. Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol. Biochem. 2008, 40, 2098–2106. [Google Scholar] [CrossRef]
- Or, D.; Smets, B.F.; Wraith, J.M.; Dechesne, A.; Friedman, S.P. Physical constraints affecting bacterial habitats and activity in unsaturated porous media—A review. Adv. Water Resour. 2007, 30, 1505–1527. [Google Scholar] [CrossRef]
- Adamczyk, B.; Karonen, M.; Adamczyk, S.; Engström, M.T.; Laakso, T.; Saranpää, P.; Kitunen, V.; Smolander, A.; Simon, J. Tannins can slow-down but also speed-up soil enzymatic activity in boreal forest. Soil Biol. Biochem. 2017, 107, 60–67. [Google Scholar] [CrossRef]
- Quiquampoix, H.; Burns, R.G. Interactions between Proteins and Soil Mineral Surfaces: Environmental and Health Consequences. Elements 2007, 3, 401. [Google Scholar] [CrossRef]
- Sanjay, G.; Sugunan, S. Acid activated montmorillonite: An efficient immobilization support for improving reusability, storage stability and operational stability of enzymes. J. Porous Mater. 2008, 15, 359–367. [Google Scholar] [CrossRef]
- Servagent-Noinville, S.; Revault, M.; Quiquampoix, H.; Baron, M.H. Conformational Changes of Bovine Serum Albumin Induced by Adsorption on Different Clay Surfaces: FTIR Analysis. J. Colloid Interface Sci. 2000, 221, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.R.; Ahn, M.-Y. Organo-Mineral–Enzyme Interaction and Soil Enzyme Activity. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2011; pp. 271–292. [Google Scholar] [CrossRef]
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- Tietjen, T.; Wetzel, R.G. Extracellular enzyme-clay mineral complexes: Enzyme adsorption, alteration of enzyme activity, and protection from photodegradation. Aquat. Ecol. 2003, 37, 331–339. [Google Scholar] [CrossRef]
- Gianfreda, L.; Rao, M.A.; Violante, A. Adsorption, activity and kinetic properties of urease on montmorillonite, aluminium hydroxide and AL(OH)x-montmorillonite complexes. Soil Biol. Biochem. 1992, 24, 51–58. [Google Scholar] [CrossRef]
- Rao, M.A.; Violante, A.; Gianfreda, L. Interaction of acid phosphatase with clays, organic molecules and organo-mineral complexes: Kinetics and stability. Soil Biol. Biochem. 2000, 32, 1007–1014. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Baath, E.; Reischke, S.; Salinas, N.; Meir, P. Adaptation of soil microbial growth to temperature: Using a tropical elevation gradient to predict future changes. Glob. Chang. Biol. 2019, 25, 827–838. [Google Scholar] [CrossRef]
- Belotte, D.; Curien, J.B.; Maclean, R.C.; Bell, G. An experimental test of local adaptation in soil bacteria. Evolution 2003, 57, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Lammirato, C.; Miltner, A.; Wick, L.Y.; Kästner, M. Hydrolysis of cellobiose by β-glucosidase in the presence of soil minerals—Interactions at solid–liquid interfaces and effects on enzyme activity levels. Soil Biol. Biochem. 2010, 42, 2203–2210. [Google Scholar] [CrossRef]
- Leprince, F.; Quiquampoix, H. Extracellular enzyme activity in soil: Effect of pH and ionic strength on the interaction with montmorillonite of two acid phosphatases secreted by the ectomycorrhizal fungus Hebeloma cylindrosporum. Activité enzymatique extracellulaire dans Ie sol: Effet du pH et de la force ionique sur l’interaction avec la montmorillonite de deux phosphates acides sécrdteés par le champignon ectomycorhizien Hebeloma cylindrosporum. Eur. J. Soil Sci. 1996, 47, 511–522. [Google Scholar] [CrossRef]
- Safari Sinegani, A.A.; Emtiazi, G.; Shariatmadari, H. Sorption and immobilization of cellulase on silicate clay minerals. J. Colloid Interface Sci. 2005, 290, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Giaveno, C.; Celi, L.; Richardson, A.E.; Simpson, R.J.; Barberis, E. Interaction of phytases with minerals and availability of substrate affect the hydrolysis of inositol phosphates. Soil Biol. Biochem. 2010, 42, 491–498. [Google Scholar] [CrossRef]
- Luckert, J.; Thieke, H.U. Der Mineralbestand brandenburgischer Grundmoränen als lithostratigraphischer Indikator—erste Ergebnisse der Röntgenphasenanalyse der Tillmatrix <63 µm. Brandenburgische Geowiss. Beitr. 2000, 7, 101–113. [Google Scholar]
- Sommer, M.; Augustin, J.; Kleber, M. Feedbacks of soil erosion on SOC patterns and carbon dynamics in agricultural landscapes—The CarboZALF experiment. Soil Tillage Res. 2016, 156, 182–184. [Google Scholar] [CrossRef]
- Kirkels, F.M.S.A. The Fate of Eroded Soil Organic Carbon—Linking Terrestrial and Aquatic Carbon Cycling; University of Amsterdam: Amsterdam, The Nertherlands, 2014. [Google Scholar]
- Kaiser, M.; Ellerbrock, R.H.; Sommer, M. Separation of Coarse Organic Particles from Bulk Surface Soil Samples by Electrostatic Attraction. Soil Sci. Soc. Am. J. 2009, 73, 2118–2130. [Google Scholar] [CrossRef]
- Gianfreda, L.; Rao, M.A. Stabilizing Enzymes as Synthetic Complexes. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; pp. 319–369. [Google Scholar] [CrossRef]
- Deng, S.; Kang, H.; Freeman, C. Microplate Fluorimetric Assay of Soil Enzymes. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; pp. 311–318. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R. Relationship between carbon and nitrogen availability and extracellular enzyme activities in soil. Pedobiologia 2009, 53, 87–98. [Google Scholar] [CrossRef]
- Marx, M.C.; Kandeler, E.; Wood, M.; Wermbter, N.; Jarvis, S.C. Exploring the enzymatic landscape: Distribution and kinetics of hydrolytic enzymes in soil particle-size fractions. Soil Biol. Biochem. 2005, 37, 35–48. [Google Scholar] [CrossRef]
- Dick, W.A. Kinetics of Soil Enzyme Reactions. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; pp. 57–69. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Baldrian, P.; Šnajdr, J.; Merhautová, V.; Dobiášová, P.; Cajthaml, T.; Valášková, V. Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biol. Biochem. 2013, 56, 60–68. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 12 May 2018).
- Pflug, W. Effect of clay minerals on the activity of polysaccharide cleaving soil enzymes. Zeitschrift für Pflanzenernährung und Bodenkunde 1982, 145, 493–502. [Google Scholar] [CrossRef]
- Quiquampoix, H. A stepwise approach to the understanding of extracellular enzyme activity in soil II. Competitive effects on the adsorption of a β-d-glucosidase in mixed mineral or organo-mineral systems. Biochimie 1987, 69, 765–771. [Google Scholar] [CrossRef]
- Uddin, F. Clays, Nanoclays, and Montmorillonite Minerals. Metall. Mater. Trans. A 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- Allison, S.D. Soil minerals and humic acids alter enzyme stability: Implications for ecosystem processes. Biogeochemistry 2006, 81, 361–373. [Google Scholar] [CrossRef]
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef]
- Quiquampoix, H.; Servagent-Noinville, S.; Baron, M.-H. Enzyme Adsorption on Soil Mineral Surfaces and Consequences for the Catalytic Activity. In Enzymes in the Environment; Marcel Dekker: New York, NY, USA, 2002; pp. 285–306. [Google Scholar]
- Horton, R.; Moran, L.; Scrimgeour, G.; Perry, M.; Rawn, D. Principles of Biochemistry, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2005; Chapter 5; pp. 149–151, citeulike-article-id:7374718. [Google Scholar]
- Wei, H.; Guenet, B.; Vicca, S.; Nunan, N.; Asard, H.; AbdElgawad, H.; Shen, W.; Janssens, I.A. High clay content accelerates the decomposition of fresh organic matter in artificial soils. Soil Biol. Biochem. 2014, 77, 100–108. [Google Scholar] [CrossRef]
- Wardle, D.A. A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol. Rev. 1992, 67, 321–358. [Google Scholar] [CrossRef]
- Haider, K.; Filip, Z.; Martin, J.P. Einfluß von Montmorillonit auf die Bildung von Biomasse und Stoffwechselzwischenprodukten durch einige Mikroorganismen. Arch. für. Mikrobiol. 1970, 73, 201–215. [Google Scholar] [CrossRef]
- Stotzky, G.; Rem, L.T. Influence of clay minerals on microorganisms: I. Montmorillonite and Kaolinite on bateria. Can. J. Microbiol. 1966, 12, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In Soil Enzymology; Springer: Berlin, Germany, 2011; pp. 229–243. [Google Scholar]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Goodall, T.; Allison, S.D.; Griffiths, R.I. Soil microbial communities with greater investment in resource acquisition have lower growth yield. Soil Biol. Biochem. 2019, 132, 36–39. [Google Scholar] [CrossRef]
- Romero, M.D.; Aguado, J.; González, L.; Ladero, M. Cellulase production by Neurospora crassa on wheat straw. Enzym. Microb. Technol. 1999, 25, 244–250. [Google Scholar] [CrossRef]
- Kedi, B.; Sei, J.; Quiquampoix, H.; Staunton, S. Persistence of catalytic activity of fungal phosphatases incubated in tropical soils. Soil Biol Biochem. 2013, 56, 69–74. [Google Scholar] [CrossRef]
- Kedi, B.; Abadie, J.; Sei, J.; Quiquampoix, H.; Staunton, S. Diversity of adsorption affinity and catalytic activity of fungal phosphatases adsorbed on some tropical soils. Soil Biol. Biochem. 2013, 56, 13–20. [Google Scholar] [CrossRef]
- Hoffman, M.; Decho, A.W. Extracellular enzymes within microbial biofilms and the role of the extracellular polymer matrix. In Microbial Extracellular Polymeric Substances; Springer: Berlin, Germany, 1999; pp. 217–230. [Google Scholar]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]

| 1Vmax | 1Km | |
|---|---|---|
| * Pure enzyme | 238.1 ± 0.8 a | 2512.8 ± 326.7 a |
| +0% | 20.3 ± 2.4 b | 1877.1 ± 114.1 a |
| +0.1% | 24.3 ± 2.4 b | 1981.3 ± 118.9 a |
| +1% | 12.9 ± 1.1 c | 1791.3 ± 18.9 a |
| +10% | 5.7 ± 1.1 e | 3086.3 ± 690.5 a |
| DAY 0 | DAY 3 | DAY 10 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Df | Deviance | Residual. Deviance | p-Value | Deviance | Residual. Deviance | p-Value | Deviance | Residual. Deviance | p-Value | ||||
| AG | |||||||||||||
| NULL | 44.22 | 4.98 | 2532.60 | ||||||||||
| MT | 3 | 11.58 | (26) | 32.64 | <0.001 | 0.77 | (15) | 4.21 | <0.001 | 449.83 | (18) | 2082.78 | <0.001 |
| OC | 2 | 23.75 | (54) | 8.89 | <0.001 | 1.88 | (38) | 2.33 | <0.001 | 366.82 | (15) | 1715.96 | <0.001 |
| MT:OC | 6 | 6.28 | (14) | 2.61 | <0.001 | 1.84 | (37) | 0.49 | <0.001 | 1168.10 | (46) | 547.85 | <0.001 |
| BG | |||||||||||||
| NULL | 17.02 | 10,0343.00 | 3.68 | ||||||||||
| MT | 3 | 2.01 | (12) | 15.01 | <0.001 | 11,353.00 | (11) | 88,991.00 | 0.01 | 1.65 | (45) | 2.03 | <0.001 |
| OC | 2 | 6.69 | (39) | 8.32 | <0.001 | 41,167.00 | (41) | 47,824.00 | <0.001 | 0.56 | (15) | 1.47 | <0.001 |
| MT:OC | 6 | 7.18 | (42) | 1.14 | <0.001 | 30,748.00 | (31) | 17,076.00 | <0.001 | 1.09 | (30) | 0.38 | <0.001 |
| CB | |||||||||||||
| NULL | 29.47 | 5211.60 | 7393.80 | ||||||||||
| MT | 3 | 4.94 | (17) | 24.53 | <0.001 | 2150.65 | (41) | 3061.00 | <0.001 | 256.96 | (4) | 7136.80 | 1.00 |
| OC | 2 | 18.40 | (62) | 6.14 | <0.001 | 2003.57 | (38) | 1057.40 | <0.001 | 1518.03 | (21) | 5618.80 | <0.001 |
| MT:OC | 6 | 5.32 | (18) | 0.82 | <0.001 | 351.02 | (7) | 706.40 | 0.1 | 3153.37 | (43) | 2465.40 | <0.001 |
| AM | |||||||||||||
| NULL | 38,262.00 | 11.76 | 20.59 | ||||||||||
| MT | 3 | 1922.70 | (5) | 36,339.00 | <0.001 | 0.43 | (4) | 11.36 | 0.01 | 1.29 | (6) | 19.30 | <0.001 |
| OC | 2 | 24,226.30 | (63) | 12,113.00 | <0.001 | 9.84 | (84) | 1.52 | <0.001 | 11.27 | (55) | 8.02 | <0.001 |
| MT:OC | 6 | 7679.00 | (20) | 4434.00 | 0.05 | 0.90 | (8) | 0.62 | <0.001 | 6.82 | (33) | 1.20 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olagoke, F.K.; Kalbitz, K.; Vogel, C. Control of Soil Extracellular Enzyme Activities by Clay Minerals—Perspectives on Microbial Responses. Soil Syst. 2019, 3, 64. https://doi.org/10.3390/soilsystems3040064
Olagoke FK, Kalbitz K, Vogel C. Control of Soil Extracellular Enzyme Activities by Clay Minerals—Perspectives on Microbial Responses. Soil Systems. 2019; 3(4):64. https://doi.org/10.3390/soilsystems3040064
Chicago/Turabian StyleOlagoke, Folasade K., Karsten Kalbitz, and Cordula Vogel. 2019. "Control of Soil Extracellular Enzyme Activities by Clay Minerals—Perspectives on Microbial Responses" Soil Systems 3, no. 4: 64. https://doi.org/10.3390/soilsystems3040064
APA StyleOlagoke, F. K., Kalbitz, K., & Vogel, C. (2019). Control of Soil Extracellular Enzyme Activities by Clay Minerals—Perspectives on Microbial Responses. Soil Systems, 3(4), 64. https://doi.org/10.3390/soilsystems3040064




