Abstract
Soil CO2 efflux (Fsoil) is a major component of the ecosystem carbon balance. Globally expansive semiarid ecosystems have been shown to influence the trend and interannual variability of the terrestrial carbon sink. Modeling Fsoil in water-limited ecosystems remains relatively difficult due to high spatial and temporal variability associated with dynamics in moisture availability and biological activity. Measurements of the processes underlying variability in Fsoil can help evaluate Fsoil models for water-limited ecosystems. Here we combine automated soil chamber and flux tower data with models to investigate how soil temperature (Ts), soil moisture (θ), and gross ecosystem photosynthesis (GEP) control Fsoil in semiarid ecosystems with similar climates and different vegetation types. Across grassland, shrubland, and savanna sites, θ regulated the relationship between Fsoil and Ts, and GEP influenced Fsoil magnitude. Thus, the combination of Ts, θ, and GEP controlled rates and patterns of Fsoil. In a root exclusion experiment at the grassland, we found that growing season autotrophic respiration accounted for 45% of Fsoil. Our modeling results indicate that a combination of Ts, θ, and GEP terms is required to model spatial and temporal dynamics in Fsoil, particularly in deeper-rooted shrublands and savannas where coupling between GEP and shallow θ is weaker than in grasslands. Together, these results highlight that including θ and GEP in Fsoil models can help reduce uncertainty in semiarid ecosystem carbon dynamics.
1. Introduction
Semiarid ecosystems have been shown to impact global carbon dynamics [1,2]. Ecosystem respiration strongly influences net carbon balance [3] and contributes significantly to variability in the net carbon exchange of semiarid ecosystems [4,5]. Soil carbon dioxide (CO2) efflux (Fsoil) represents CO2 efflux due to belowground plant and microbial respiration and biogeochemical processes, and is a major component of total ecosystem respiration [6,7]. Increased understanding of the processes underlying Fsoil variation in globally expansive semiarid ecosystems is necessary to reduce uncertainty in terrestrial carbon dynamics.
While controls on respiration processes in more mesic regions are well documented [8,9], Fsoil in water-limited ecosystems exhibits spatial and temporal variability associated with dynamics in moisture availability and biological activity [10,11,12,13,14,15,16]. Compared to mesic sites, Fsoil estimates in water-limited ecosystems are more uncertain, partially due to relatively sparse data in drylands despite the recent increase in measurements of Fsoil globally [17]. Limitations in available data inhibit the development and evaluation of new Fsoil models for application in water-limited ecosystems. Measurements that examine the processes underlying variability in Fsoil across a variety of environmental and biological conditions would be useful to develop and evaluate models that recognize the role of temperature, moisture, and substrate limitation on carbon exchange [16,18], particularly in globally extensive drylands projected to expand in response to global change [19,20].
Multivariate models can be useful to represent how dynamics in substrate availability and environmental factors contribute to pulses and seasonality in the metabolic activity of water-limited ecosystems [21,22,23,24]. However, existing biogeochemical models largely represent respiration processes with static temperature sensitivity equations and empirical moisture functions predominantly developed in mesic regions [8,14,25,26]. In water limited-ecosystems, dynamics in soil moisture and photosynthesis strongly regulate Fsoil over sub-seasonal to interannual timescales [9,27]. Soil moisture availability can influence the magnitude, temperature response, and seasonality of Fsoil and can cause hysteresis between Fsoil and its drivers [11,15,16,18,24,28,29,30,31]. Vegetation structure and function characteristics—including root distribution, hydraulic redistribution, root respiration, photosynthate exudation, and effects on microclimate—can influence the factors that control spatial and temporal variability in Fsoil [16,22,32,33,34,35].
Previous studies in water-limited ecosystems have illustrated how Fsoil varies with interacting environmental and vegetative factors. Variation in plant and soil characteristics modify the response of Fsoil to changes in water availability and temperature [13,36], and vegetation structure impacts the timescales over which environmental and vegetative factors influence Fsoil in mixed-vegetation ecosystems [34,37]. Despite advances in our understanding of dryland Fsoil, representing how these interacting factors impact Fsoil in heterogeneous ecosystems remains a modeling challenge.
Models are beginning to capture the effects of moisture availability and vegetation activity on the temperature dependency of Fsoil [31]. Such model structures impose moisture constraints on Fsoil [38] and assume that respiration processes are stimulated by canopy photosynthesis [39]. However, it is not well known if these new models can capture dynamics in Fsoil associated with interacting environmental and vegetative factors across structurally diverse semiarid ecosystems.
Multisite measurements targeted to investigate the complex interactions between these drivers can help us determine if new Fsoil models are broadly applicable in semiarid ecosystems. Trenched-plot experiments can help isolate the interacting effects of environmental and vegetative factors on Fsoil [6,40,41,42]. Even if trenched-plots are unavailable, measurements from plots that differ in their distance from patchy vegetation can be used to assess how plants may be influencing Fsoil through effects on microclimate and root activity [13,34,37].
In this study, we integrated data and modeling to investigate how environmental and vegetative factors influence Fsoil across three semiarid sites. These sites were similar in climate forcing but differed in stand structure, with major differences in the amounts of grass, shrubs, and trees. The objectives of this study were to (1) combine automated soil chamber and flux tower data to investigate how soil temperature (Ts), soil moisture (θ), and gross ecosystem photosynthesis (GEP) regulate Fsoil in semiarid grassland, shrubland, and savanna ecosystems; and (2) assess the ability of data-informed models to predict temporal variability in Fsoil across three structurally diverse semiarid ecosystems. To achieve these objectives, we combined data from a grassland trenched-plot experiment with measurements from intercanopy and under-canopy plots in shrubland and savanna sites that differed in their proximity to vegetation. We then tested model performance at each site to determine if the mechanisms underlying variation in Fsoil were broadly consistent across these ecosystems. We hypothesize that the combination of Ts, θ, and GEP control Fsoil at each site, and that the relative explanatory value of model drivers varies among sites due to differences in how vegetation structure impacts coupling between shallow soil moisture and carbon exchange. Based on this hypothesis, we predict that the relative explanatory value of θ and GEP will differ the most at sites with deeper rooting depths (savanna > shrubland > grassland) where GEP is less coupled to shallow soil moisture.
2. Materials and Methods
2.1. Site Description
This study was conducted at three AmeriFlux sites in southeast Arizona, USA. Kendall Grassland (grassland; AmeriFlux site ID: US-Wkg) is a warm-season, semiarid grassland dominated by perennial bunchgrasses (mainly, Eragrostis lehmannia). Lucky Hills Shrubland (shrubland; site ID: US-Whs) is a shrubland composed of a variety of Chihuahuan desert shrubs (Larrea tridentata, Parthenium incanum, Acacia constricta). Both sites are located within the USDA Agricultural Research Service Walnut Gulch Experimental Watershed. Santa Rita Mesquite Savanna (savanna; site ID: US-SRM) is a semiarid grassland that has experienced encroachment by velvet mesquite trees (Prosopis velutina). The savanna site is located in the Santa Rita Experimental Range, roughly 80 km west of the other sites. A detailed description of the sites can be found in a previous study [43]. The sites experience similar mean annual temperature (~17 °C) and mean annual precipitation (320–384 mm) but differ in their vegetative structure and productivity (Table 1 and Figure 1). Grass covers 37% of the grassland, whereas woody cover dominates the shrubland (40%) and savanna (35%). Canopy height and mean annual leaf area index increase from lowest to highest for the shrubland, grassland, and savanna. Roughly 60% of annual precipitation occurs in July–September, associated with the North American Monsoon.

Table 1.
Description of the study sites.
Figure 1.
Webcam images depicting the vegetation type of the grassland (a), shrubland (b), and mesquite savanna (c) sites.
2.2. Soil CO2 Efflux and Environmental Measurements
The net efflux of carbon dioxide (CO2) at the soil-atmosphere interface (Fsoil) was measured using an infrared gas analyzer coupled with automated soil chambers (LI-8100, LI-COR, Lincoln, NE, USA). Automated chambers were deployed at each site in plots near vegetation. Soil collars were inserted to a depth of 8–9 cm, leaving 2–3 cm of the collars exposed. We used the FV8100 Data File Viewer (LI-COR) to estimate Fsoil by fitting an exponential regression to the rate of increase in CO2 molar fraction over each 120 s measurement interval. We excluded Fsoil estimates from fits with R2 < 0.90 and values of Fsoil < −1 or >15 µmol CO2 m−2 s−1.
In 2017, Fsoil was measured twice per hour at the grassland using four chambers adjacent to patches of perennial bunchgrass (Eragrostis lehmanniana, “grass”). To exclude the effects of vegetation activity on Fsoil, we added four additional chambers in bare plots and trenched each plot’s perimeter on 22 June 2017 prior to the summer rainy season, hereafter referred to as “trenched”. Trenches were dug to ~30 cm depth and lined with ground cover fabric to prevent root growth back into the plot. Roughly ~84% of the grass roots at this site are within the top 30 cm of soil [44]. We regularly weeded the trenched plots to ensure the soil was bare throughout the growing season. We assume CO2 efflux measured in trenched plots represents heterotrophic respiration (Rh), while total Fsoil measured in grass plots includes Rh and belowground autotrophic respiration (Ra). We define Ra as the difference between grass Fsoil and trenched Rh. Supplementary measurements of Ts and θ were measured at a depth of 5 cm with a LI-COR temperature probe and a soil moisture probe (EC-5, Decagon, now METER Group, Washington, DC, USA), respectively. Beginning in June 2017, ECH2O 5TM probes (METER Group) were used to measure 5 cm Ts and θ for all chambers at the site.
To extend our investigation across sites with differing vegetation structure, we also measured Fsoil, Ts, and θ in the shrubland and savanna. In the shrubland in 2012, Fsoil was measured every two hours using four chambers under creosote bush shrubs (Larrea tridentata) and four chambers located between the sparsely separated shrubs (~2 m from canopy drip lines). In the savanna in 2015, hourly Fsoil was measured using three chambers installed halfway between the tree bole and drip line of velvet mesquite trees (Prosopis velutina) and from three chambers ~5 m from trees in the intercanopy space. A malfunctioning chamber at the savanna site was excluded from analysis, which reduced the number of tree plots to two. We measured 5 cm Ts and θ at the shrubland and savanna using LI-COR temperature probes and ECH2O probes, respectively. Importantly, the intercanopy plots at the shrubland and savanna sites were not trenched and therefore were likely influenced by root activity.
2.3. Ecosystem Photosynthesis
To quantify how vegetation activity influences Fsoil, ecosystem-scale carbon fluxes were measured using the eddy covariance technique. Details of the instrumentation and methods used at each site have been described previously [45]. Briefly, 30 min average net ecosystem exchange of CO2 (NEE) was partitioned into gross ecosystem photosynthesis (GEP; hereafter referred to as photosynthesis) and ecosystem respiration (Reco; [43]). An exponential function was fit to friction velocity-filtered nighttime NEE and air temperature over a ~5 day moving window to determine Reco [46]. GEP was calculated as the difference between Reco and NEE, with the sign convention of positive values for GEP and Reco. A previous comparison of Reco and Fsoil at the savanna site [47] showed that integrated Fsoil was greater than Reco over the course of a growing season. This indicates that Fsoil is systematically overestimated, or Reco is underestimated, as Reco should also account for aboveground respiration. If Reco is underestimated, this would result in an underestimate of GEP. However, this systematic bias should not have a large impact on our modeling results so long as GEP and Fsoil capture the temporal variability in these processes. This is because the empirical model coefficients described below are optimized to fit the data.
2.4. Data Analysis
At each site, plot means were calculated as the average of replicates. Missing data and outliers were replaced with the mean of replicates. Hourly means were used to examine the impact of θ on the relationship between Fsoil and Ts. To investigate how water availability influenced the temperature response of Fsoil at the grassland, we fit Equation (1) to data binned by θ quantiles in 10% increments.
Daily means were calculated from sub-daily measurements to account for differences in sampling rates among sites. The response of Fsoil to recent carbon inputs was determined by regressing daily mean Fsoil against daily mean GEP, and we used the Student’s t-test to evaluate differences in regression parameters [48]. Daily means were used to investigate seasonality in carbon fluxes and environmental variables and to examine relationships between Fsoil and GEP. We used the paired t-test to test for differences in daily mean Fsoil and drivers between plots that varied in their distance from vegetation. At the grassland, we tested for differences between plots in the basal rate and temperature sensitivity of Fsoil by testing for overlap in the 95% confidence intervals of coefficients determined by fitting Equation (1) described below.
2.5. Model Development
We used a modeling framework to investigate how the inclusion of environmental and vegetative terms influenced predicted spatial and temporal variation in Fsoil. All models were based on an exponential temperature function [8]:
where Fsoil is soil CO2 efflux (µmol CO2 m−2 s−1), Fref is the basal Fsoil when Ts is 0 °C (µmol CO2 m−2 s−1), and b is the temperature sensitivity of Fsoil. We supplemented temperature-based models with θ and GEP terms to represent the effects of moisture availability and vegetation activity on Fsoil [31]. Moisture effects were incorporated into Equation (1) using a quadratic structure that reflects how excessively high or low θ suppresses Fsoil [38,49,50] as:
where θopt is the optimum θ value for which Fsoil is greatest and c represents the sensitivity of Fsoil to θ by controlling the slope of the exponential curve (higher values of c indicate stronger effects of θ). At each site we determined θopt by examining the response of daily mean Fsoil to θ and visually estimating the value of θ associated with maximum Fsoil. Following reference [31], we added a photosynthesis term to Equation (1) to represent the effects of Ts and GEP on Fsoil using:
where n represents the degree to which GEP drives Fsoil relative to heterotrophic processes (n = 0 indicates strong GEP effect on Fsoil) and GEPmax is the maximum value of GEP. The combined effects of temperature, moisture, and photosynthesis were represented by
Models were fit using nonlinear least squares regression in which the coefficients were estimated using an iterative method based on starting values in Matlab (Mathworks, Inc., Natick, MA, USA). To account for differences in model complexity, model performance was assessed using the coefficient of determination (R2), Akaike Information Criterion (AIC; [51]), and root mean squared error (RMSE). We used cross-correlation to test for lags between daily mean Fsoil and daily mean GEP. For sites with significant lag, we re-fit Equations (3) and (4) with optimum lag and assessed changes in model performance.
3. Results
3.1. Seasonality of Soil CO2 Efflux
Across all sites, daily mean Fsoil and GEP followed seasonal dynamics of changes in water availability (Figure 2a,c,d; Figures S1 and S2). At the grassland site with grass and trenched plots, a brief and limited spring growing season (DOY 75–100) was followed by low Fsoil, GEP, and θ, despite increasing Ts (Figure 2a–d). Average pre-summer monsoon (DOY 0–175) daily mean Fsoil for the grass plot was 0.52 µmol CO2 m−2 s−1. During the monsoon (DOY 175–250), θ was high and average daily mean Fsoil for the grass plot increased significantly to an average 2.3 µmol CO2 m−2 s−1 (p < 0.01). Rates of GEP responded gradually to the onset of monsoon precipitation, whereas Fsoil increased rapidly with θ. (Figure 2a,c,d). Post-monsoon (DOY 250–365) rates of Fsoil and GEP decreased following seasonal decreases in GEP, θ and Ts (Figure 2a–d).
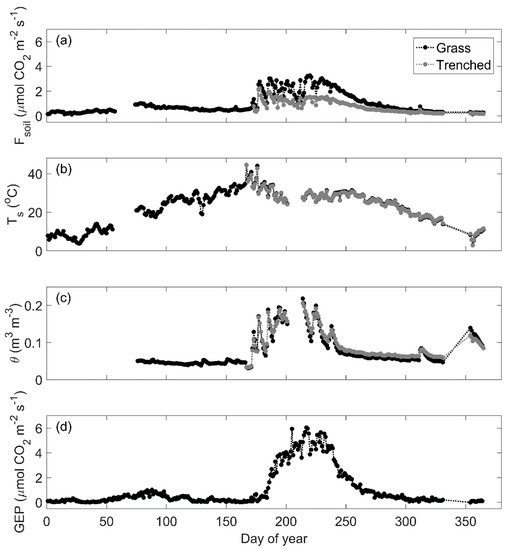
Figure 2.
Seasonal pattern in 2017 of observed (a) daily mean soil CO2 efflux (Fsoil), (b) soil temperature (Ts), and (c) volumetric soil moisture (θ) for soil near patches of grass (black) and in bare, trenched intercanopy space (gray) from the grassland. Also shown is (d) daily mean gross ecosystem photosynthesis (GEP).
3.2. Environmental Controls on Soil CO2 Efflux
Median Fsoil increased with θ (Figures S3–S5), and daily mean θ contributed to 51–77% of the variation in daily mean Fsoil at all sites. (Figures S6–S8). Ts explained significant variation in Fsoil for high θ, but Ts and Fsoil were weakly coupled when θ was low (Figure 3a). Since the amount of variation in Fsoil explained by Equation (1) varied significantly with θ, we used the quantile fit results (Figure 3a) and re-fit Equation (1) into wet (grass: θ > 7th quantile; trenched: θ > 6th quantile) and dry (grass: θ < 8th quantile; trenched: θ < 7th quantile) conditions. Rates of Fsoil were generally low for dry conditions, despite increasing Ts, whereas Fsoil increased strongly with Ts when the soil was wet (Figure 3b,c). Basal soil CO2 efflux (Fref) and the temperature sensitivity of Fsoil (b) varied significantly with θ and differed between the grass and trenched plots (Figure 3b,c; p < 0.01). Fref was 54% and 64% greater for wet than dry conditions at the grass and trenched plots, respectively. Between plots, grass Fref was 63% and 73% greater than trenched Fref for wet and dry conditions, respectively. Wet conditions were associated with greater b than dry conditions, and this difference was more pronounced in vegetated plots than in trenched ones. Similar to the grassland, wet conditions at the savanna corresponded with high Fref and b; however, the temperature response of Fsoil at the savanna was more variable than at the grassland (Figure S9). Unexpectedly, Fsoil did not show a clear response to Ts at the shrubland (Figure S10). For all sites, we found that Ts alone was not the only driver of Fsoil and that even when accounting for variation in θ, significant variation in Fsoil remained unexplained (Figure 3, Figures S9 and S10).
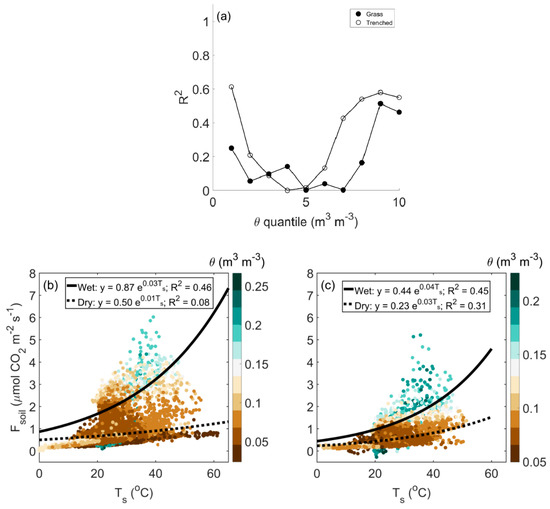
Figure 3.
(a) Coefficient of determination (R2) values from an exponential fit of soil CO2 efflux (Fsoil) to soil temperature (Ts), Equation (1), for different 10% quantiles of volumetric soil moisture (θ) at the grassland site. The influence of θ (color) on the temperature response of Fsoil for grass (b) and trenched plots (c) with curves fit for wet and dry θ conditions.
3.3. Physiological Controls on Soil CO2 Efflux
Although the seasonal pattern of Fsoil at the grassland was similar for the grass and trenched plots, there were significant differences in the magnitude of daily mean Fsoil between plots (Figure 2a) that strongly correlated with daily mean GEP (R2 = 0.66). Daily mean Fsoil during the monsoon was significantly greater for the grass (2.3 µmol CO2 m−2 s−1) than trenched plots (1.2 µmol CO2 m−2 s−1; p < 0.01), despite similar Ts and θ (p > 0.1). Using the difference in Fsoil between grass and trenched plots in the grassland, we estimate that belowground autotrophic (Ra) and heterotrophic (Rh) respiration accounted for 44% and 56% of cumulative growing season Fsoil, respectively (Figure 4).
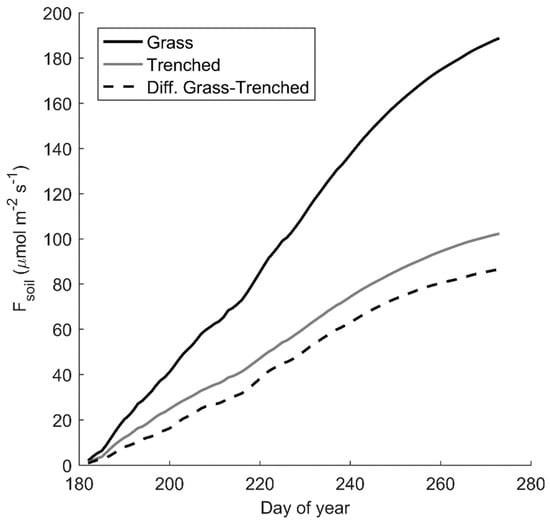
Figure 4.
Cumulative daily mean soil CO2 efflux (Fsoil) for grass (black) and bare, trenched intercanopy soil (gray) during the 2017 experiment comparison period at the grassland site. The grass-trenched difference (dashed) is an estimate of belowground autotrophic respiration.
To investigate how plant activity influenced Fsoil across sites with varying vegetation type and productivity, we examined the relationship between Fsoil and GEP. Daily mean Fsoil increased with daily mean GEP at all sites, and the rate of increase was greater for plots near vegetation (Figure 5; p < 0.01). At all sites, Fsoil was 35–59% greater for plots near vegetation compared to plots that were either trenched (grassland only) or located further from vegetation (~2–5 m).
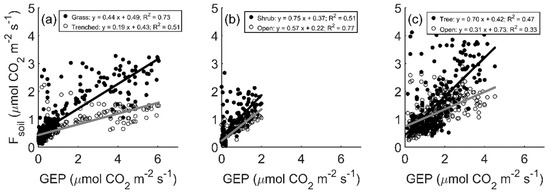
Figure 5.
Linear relationship between daily mean soil CO2 efflux (Fsoil) and gross ecosystem photosynthesis (GEP) at the grassland (a), shrubland (b), and savanna (c) sites. Closed circles indicate plots near or under vegetation canopies, and open circles are for plots either trenched (a) or between shrub/tree canopies (b,c).
3.4. Model Performance
To integrate these varied environmental controls on Fsoil we used a multivariate modeling approach by sequentially adding Ts, θ, and GEP as explanatory variables. For the grassland, we tested the ability of data-informed models to predict temporal variability in Fsoil. The model based solely on Ts (Equation (1)) explained less than 40% of the variation in observed daily mean Fsoil for the grass and trenched plots (Table 2). As shown in Section 3.2 and Section 3.3, the temperature response of Fsoil varied strongly with θ and the magnitude of Fsoil was related to GEP. Models that represented these observed effects of θ (Equation (2)) and GEP (Equation (3)) on Fsoil outperformed Equation (1), as indicated by higher R2 lower AIC, and lower RMSE (Table 2). For the grass plots, adding either a moisture or photosynthesis term to Equation (1) increased R2 to a similar degree. Conversely, in the trenched plots that were manipulated to exclude root activity associated with photosynthesis, goodness of fit metrics show that the model with a moisture term (Equation (2)) was better than the model with a photosynthesis term (Equation (3)). The complete model—which included temperature, moisture, and photosynthesis terms (Equation (4))—outperformed less complex models in the grass plots. However, in the trenched plots that were uninfluenced by GEP, Equations (2) and (4) explained a similar amount of variation in Fsoil but Equation (4) had lower AIC.

Table 2.
Fitted model (Equations (1)–(4)) parameters and the coefficient of determination (R2), Akaike information criterion (AIC), and root mean squared error (RMSE, µmol CO2 m−2 s−1) used to assess model performance at the grassland, shrubland, and savanna sites. Bold numbers indicate best performance among model groups (highest R2; lowest AIC; lowest RMSE).
We also tested the models at the shrubland and savanna to determine if the trend in performance was consistent across sites with different vegetation. As in the grassland, temperature alone was a poor predictor of variation in Fsoil, and adding moisture (Equation (2)) or photosynthesis (Equation (3)) terms strongly improved model performance (Table 2). Adding a moisture term to the temperature-based model explained more variation in Fsoil than did adding a photosynthesis term (Table 2). To test if this difference in relative explanatory power was related to the timing of photosynthesis relative to microbial respiration of root exudates, we used cross-correlation analysis to investigate lags between Fsoil and GEP. Correlation was maximized when Fsoil was lagged relative to GEP by zero days in the grassland, one day in the shrubland, and two days in the savanna (Table 3). Applying these lags and re-fitting the models increased the amount of variation in Fsoil explained by Equation (3) to be comparable to Equation (2) at the shrubland and savanna. The complete model (Equation (4)) performed best in the shrubland and savanna. As indicated by lower AIC, Equation (4) improved model performance most in the savanna—which is also where we observed the weakest coupling between daily mean GEP and θ and the largest Fsoil–GEP lag among sites (Table 3). We found that the relative explanatory value of model drivers varied among sites (Table 2).

Table 3.
Lag times for maximum cross-correlation between daily means of gross ecosystem photosynthesis (GEP) and soil CO2 efflux (Fsoil). Also shown is the coefficient of determination (R2) of linear regressions between un-lagged and lagged daily mean θ and GEP for the grassland, shrubland, and savanna sites.
Importantly, model drivers influenced temporal dynamics in predicted Fsoil (Figure 6 and Figure 7). Equation (1) (Ts) failed to reproduce the seasonality observed in Fsoil, generally over-predicting Fsoil during the dry pre-monsoon period and under-predicting Fsoil during the growing season (Figure 6, Figures S11 and S12). Adding a moisture term to the temperature-based model improved predicted seasonality in Fsoil because it better captured variability in observed Fsoil and predicted high Fsoil immediately following rain events at the monsoon onset. However, Equation (2) tended to underestimate growing season Fsoil since it did not represent the stimulating effect of GEP on Fsoil (Figure 4). Adding a photosynthesis term (Equation (3)) better predicted the magnitude of growing season Fsoil but was delayed relative to observations due to lags between the onset of high θ and GEP upregulation. Thus, the inclusion of both moisture and photosynthesis terms in Equation (4) was required to model the seasonality, magnitude, and variability in Fsoil at all sites (Figure 7).
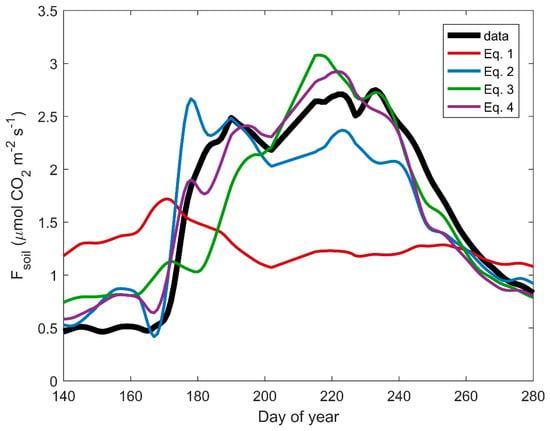
Figure 6.
Loess smoothed temporal dynamics in daily mean observed (black) and predicted (color) soil CO2 efflux (Fsoil) at the grassland site. Equation (1) is based on an exponential relationship between Fsoil and soil temperature (Ts), whereas Equations (2) and (3) combine Equation (1) with volumetric soil moisture (θ) and gross ecosystem photosynthesis (GEP) terms, respectively. Equation (4) is the complete model with Ts, θ, and GEP terms.
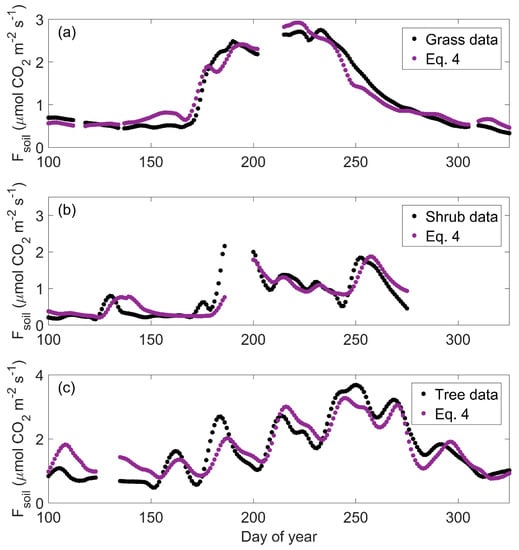
Figure 7.
Loess smoothed temporal dynamics in daily mean observed (black) and predicted (purple) soil CO2 efflux (Fsoil) at the grassland (a), shrubland (b), and savanna (c) sites. Equation (4) is the complete model with soil temperature (Ts), volumetric soil moisture (θ) and gross ecosystem photosynthesis (GEP) terms.
4. Discussion
In three semiarid ecosystems we found that Ts, θ, and GEP influenced the dynamics, magnitude, and variability of Fsoil. Water availability strongly influenced Fsoil rates and patterns, whereas GEP stimulated Fsoil, particularly for plots near vegetation. These results provide additional evidence that moisture availability regulates temporal variation in Fsoil, and biological factors impact spatial variation in Fsoil [10,11,13,52]. The complete model (Equation (4)) integrated Ts, θ, and GEP controls and better captured temporal dynamics in observed Fsoil than less complex models. Testing the model across sites with similar climate forcing indicated that the explanatory value of model drivers varied with vegetation structure and productivity. Together, these results show that models that account for Ts, θ, and GEP can represent how Fsoil responds to changes in water availability and vegetation activity in ecosystems with varying structure.
4.1. Water Availability Limits Autotrophic and Heterotrophic Respiration
The temperature response of Fsoil was conditional on θ (Figure 3). Modeling these interactions is important since warming-driven reductions in θ can suppress Fsoil despite higher Ts [23]. In the grassland, high θ enhanced the temperature sensitivity of Fsoil, whereas low θ suppressed Fsoil in the grass and trenched plots (Figure 3), which indicates that water availability constrains both Ra and Rh. Low soil moisture can inhibit Rh by decreasing substrate availability, microbial activity, solute transport, or some combination of these drivers [23,29,53,54]. Moisture limitation may also suppress Ra through reductions in root exudates due to decreased rates of photosynthesis and phloem transport in water-stressed plants [23,55]. Thus, changes in moisture availability can cause seasonal variation in the magnitude and temperature response of Fsoil in semiarid ecosystems, as previously found in mesic forests [38]. Models that account for interactions between Ts and θ are more apt to capture seasonality and pulsed dynamics in Fsoil that impact the carbon balance of semiarid ecosystems [7,56]. The need to represent water stress effects on Fsoil is likely to apply beyond semiarid ecosystems since most regions already experience periods of water limitation [57] and drylands are projected to expand [19].
4.2. Ecosystem Photosynthesis Stimulates Soil CO2 Efflux
The link between GEP and Fsoil contributes to spatial variation in Fsoil rates (Figure 5). We found that recent photosynthesis impacts Fsoil likely through enhanced root respiration and the stimulating effect of root exudation on microbial respiration (Figure 5, Table 2 and Table 3), which has been reported across a variety of ecosystems [11,35,37,39,58]. Even when θ was similar, growing season rates of Fsoil were greater for plots near vegetation (Figure 2 and Figure 3). Even though the shrubland and savanna did not have trenched plots, Fsoil closer to the vegetation was higher and responded more strongly to photosynthesis variation. Despite the uncertainty in GEP due to NEE measurement and partitioning bias, and differences in measurement scale, GEP was correlated with Fsoil and was important to predict temporal dynamics in Fsoil (Table 3; [13]). Note that since GEP used here is an ecosystem-scale flux, and photosynthetic inputs are likely to vary across space (Figure 3 and Figure 4), predictive models of Fsoil could be improved if new tools to disaggregate ecosystem flux measurements were used to determine the spatial distribution of GEP [59].
The effects of GEP on Fsoil are illustrated by seasonal changes in Fsoil partitioning. We found that the difference in Fsoil between plots increased as the growing season progressed (Figure 2, Figures S1 and S2), and Fsoil was greater and more sensitive to GEP for plots near vegetation (Figure 5), as previously reported in this region [13]. These dynamics are likely linked to plant physiology and phenology, which have been shown to affect the magnitude and partitioning of Fsoil in temperate forests [41,60] and California grasslands [11]. Applying the model at the grassland showed that GEP had a stronger effect on Fsoil for the grass plots (Table 2; low n: strong GEP effect on Fsoil) than the trenched plots (high n: weak GEP effect on Fsoil). At the grassland, our estimate of Ra (difference in Fsoil between the grass and trenched plots) correlated strongly with GEP (R2 = 0.66) and was a considerable fraction (44%) of total growing season Fsoil (Figure 4). While we did not have trenched plots at the shrubland and savanna to partition Fsoil, differences in the seasonal pattern of Fsoil for plots that differed in their proximity to vegetation indicate that Ra is likely also a considerable fraction of Fsoil at these sites (Figure 5, Figures S1 and S2). Our estimate of the growing season Ra:Fsoil ratio for the grassland is lower than the mean value reported in a review of grass and crop ecosystems (60.4%; [61]) but similar to results from a mesic grassland (48–52%; [62]). Since Ra can be a significant component of Fsoil in ecosystems that span a wide range of water availability, refined understanding of controls on Ra is necessary to reduce uncertainty in Fsoil.
4.3. Moisture and Photosynthesis Terms Improve Modeled Carbon-Water Dynamics
Model predictions based solely on temperature do not accurately reflect Fsoil in water-limited ecosystems. However, by explicitly representing the combined effects of Ts, θ, and GEP, the full model (Equation (4)) predicted temporal variation in observed Fsoil associated with dynamics in moisture availability and vegetation activity across structurally diverse sites (Figure 7). At the shrubland in particular, Equation (4) explained 74% of the variation in daily mean Fsoil even though we did not observe a relationship between Fsoil and Ts (Table 2). Decoupling between Fsoil and Ts when moisture was limiting (Figure 3) likely explains why the temperature-only model (Equation (1)) did not capture seasonal dynamics in Fsoil (Figure 6). By adding θ to a temperature-based model, predicted Fsoil was suppressed when water was limiting and enhanced when θ was optimal (Figure 6). Together, Ts and θ explained more than 50% of the observed variability across sites (Table 2). The superior performance of Equation (2) over Equations (1) and (3) across sites with different vegetation structure underscores that water availability is a key control on respiration processes in semiarid ecosystems (Table 2).
While supplementing a temperature-based model with either θ or GEP terms increased the amount of explained variation in Fsoil to a similar degree, the combination of Ts, θ, and GEP was required to maximize model performance and predict seasonality in Fsoil. Models that accounted for θ captured the pulsed increase in metabolic activity at the monsoon onset characteristic of semiarid ecosystems [12,21,63], whereas GEP terms improved the prediction of Fsoil magnitude and seasonality by reflecting the stimulating effect of photosynthesis on basal Fsoil ([39]; Figure 6). We found that Fsoil increased rapidly in response to increased θ at the beginning of the monsoon, whereas GEP increased more gradually (Figure 2), which reflect differences in the timing of ecosystem responses to rainfall pulses [56]. While these results indicate that this model captures Fsoil dynamics in these subtropical, warm-season ecosystems, future studies should test this model in cool-season desert ecosystems. We also found that predictions from GEP-based models lagged observed Fsoil. Previous research has documented lags between GEP and Fsoil ranging from hourly to daily timescales depending on the vegetative cover and time of year [22,31,37,64]. Consistent with previous research, no lag was detected at the grassland [65]. However, applying one or two days of lag between GEP and Fsoil improved model performance at the shrubland and savanna (Table 2). These results provide additional evidence in support of incorporating lag information in semi-empirical Fsoil models [65]. Together, our findings indicate that models with Ts, θ, and GEP terms can better capture rainfall-driven pulses in carbon dynamics than simpler models [56].
4.4. Vegetation Activity and Structure Influence the Relative Importance of Soil CO2 Efflux Drivers
The degree of vegetation activity influences the relative importance of Fsoil controls. In the grassland, Fsoil for grass plots was more sensitive to GEP (Table 2; low n), whereas Fsoil for trenched plots was more sensitive to θ (high c). This result is consistent with our expectation that θ would more strongly regulate Fsoil from plots manipulated to exclude the effects of GEP on belowground activity. Similarly, Fsoil was more sensitive to θ than GEP (Table 2; high c, high n) at sites with low cumulative GEP (shrubland, grassland trenched plots). We observed unexpected differences between sites in how θ influenced the relationship between Fsoil and Ts. Contrary to the grassland, the effect of θ on the relationship between Fsoil and Ts was weaker in the savanna and was not observed in the shrubland (Figure 3, Figures S9 and S10). Previous work in this region found that the temperature sensitivity of Fsoil was lower during wet conditions in grass plots but not influenced by moisture in plots near mesquite trees [37]. The complete model captured how vegetation modulated the effects of environmental controls on Fsoil and therefore may be applicable in various ecosystems subject to water limitation.
Interactions between vegetation structure and carbon-water coupling can help explain site differences in the explanatory power of Fsoil controls. Vegetation can cause decoupling and lags between Fsoil and its controls [16,34] due to plant structure and rooting characteristics. Previous work in this region found that Fsoil was more sensitive to antecedent photosynthesis rates in mesquite plots, whereas Fsoil near grass plots was more influenced by same-day photosynthesis [37]. Similarly, we found greater lag between GEP and Fsoil (Table 3) at the savanna (two days) and shrubland (one day) than the grassland (zero days), perhaps due to larger structure and longer phloem transport distance for the woody plants [22,37]. While θ and GEP controls were interchangeable for the grass plots, θ had greater explanatory power than unlagged GEP in the shrubland and savanna. Lagging GEP made its explanatory power comparable to θ (Table 2). Thus, we suggest that future semi-empirical models include terms that account for lag. Differences in lags may be related to variation in rooting characteristics between sites. In the short-rooted grassland, strong coupling between shallow θ and GEP leads to covariation which makes either term suitable to explain variation in Fsoil. Conversely, trees and shrubs generally have a higher proportion of roots in deep soil than grasses [44], and GEP is more coupled to deeper θ [4,64], leading to more of a disconnect between GEP and shallow θ and their effects on Fsoil [32,56,66].
Fsoil drivers suggest that ecosystem composition likely alters the magnitude and spatial variability of Fsoil. Woody encroachment has been shown to increase Fsoil variation in semiarid grassland [13] and savanna ecosystems [34]. Vegetation structure and functioning contributes to spatial variation in Fsoil (Figure 5) and modifies the response of Fsoil to environmental controls (Figure 3 and Table 2). The strong performance of the complete model across ecosystems with a differing structure indicates that simple models with environmental and vegetative controls [31] may be useful to investigate how changes in ecosystem composition will impact Fsoil. To increase the utility of this model, further research should focus on how to represent differences in θ-GEP coupling between woody ecosystems and grasslands [67].
5. Conclusions
Temperature, moisture, and photosynthesis were each important controls on Fsoil in grassland, shrubland, and savanna ecosystems. While models relying on Ts erroneously predicted high Fsoil before the growing season, those with θ and GEP controls captured variation in Fsoil associated with dynamics in moisture availability and vegetation activity. This study is novel in that it is the first to test if a Fsoil model driven by daily Ts, θ, and GEP can capture temporal dynamics and variability in Fsoil across semiarid sites with similar climate forcing but differing vegetation structure. While the mechanism governing the relative importance of Ts, θ, and GEP controls across sites remains unclear, it is likely related to vegetation characteristics associated with productivity and water use. Our results indicate that this simple model structure can capture Fsoil dynamics associated with transitions from process-rate limitation to substrate constraints [22]. This study builds upon recent modeling advances [31] and indicates that this type of modeling approach can capture spatial and temporal variation in Fsoil across structurally diverse, semiarid ecosystems, particularly if time series data of plant function is available. Combining this modeling approach with increased monitoring of Fsoil at flux tower sites could help investigate connections between plot and ecosystem-scale carbon exchange [47]. Future studies should test this model in cool-season ecosystems, which tend to be temperature-limited when soil moisture is non-limiting [24]. It is reasonable to infer that the relative importance of model drivers would differ between cool-season ecosystems and the warm-season ecosystems examined herein. Accounting for the interactive effects of Ts, θ, and GEP on Fsoil will be important to determine the response of water-limited ecosystems to changes in climate and land cover.
Supplementary Materials
The following are available online: http://www.mdpi.com/2571-8789/3/1/6/s1. Figure S1: Time series of carbon fluxes and controls at the shrubland, Figure S2: Time series of carbon fluxes and controls at the savanna, Figure S3: Relationship between soil moisture and soil CO2 efflux at the grassland, Figure S4: Relationship between soil moisture and soil CO2 efflux at the shrubland, Figure S5: Relationship between soil moisture and soil CO2 efflux at the savanna, Figure S6: Controls on soil CO2 efflux at the grassland, Figure S7: Controls on soil CO2 efflux at the shrubland, Figure S8: Controls on soil CO2 efflux at the savanna, Figure S9: Temperature response of soil CO2 efflux at the savanna, Figure S10: Temperature response of soil CO2 efflux at the shrubland, Figure S11: Predicted temporal dynamics of soil CO2 efflux at the shrubland, Figure S12: Predicted temporal dynamics of soil CO2 efflux at the savanna.
Author Contributions
R.L.S. designed the experiment with assistance from G.A.B.-G., E.P.H., M.C.R. and D.J.P.M.; M.C.R. and R.L.S. performed the experiment; M.C.R., R.L.S. and D.J.P.M. analyzed the data. M.C.R. wrote the manuscript with input from all coauthors.
Funding
This work was supported by USDA-ARS and funding for these AmeriFlux Core Sites (US-SRM, US-Wkg, and US-Whs) was also provided by the U.S. Department of Energy Berkeley National Labs.
Acknowledgments
The soil CO2 efflux data used in this study are available upon request to the corresponding author. Eddy covariance flux data used in this paper are available at the AmeriFlux Data Repository (http://ameriflux.lbl.gov/) or upon request to the corresponding author. Phenocam imagery is available at http://https://phenocam.sr.unh.edu. This work was supported by USDA-ARS and funding for these AmeriFlux Core Sites (US-SRM, US-Wkg, and US-Whs) was also provided by the U.S. Department of Energy Berkeley National Labs. We thank R. Bryant for his expert technical assistance in maintaining the instrumentation at the sites. M.C.R. thanks R. Canales for assistance with data analysis. USDA-ARS is an equal opportunity employer.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Poulter, B.; Frank, D.; Ciais, P.; Myneni, R.B.; Andela, N.; Bi, J.; Broquet, G.; Canadell, J.G.; Chevallier, F.; Liu, Y.Y.; Running, S.W.; et al. Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 2014, 509, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Ahlström, A. The dominant role of semiarid ecosystems in the trend and variability of the land CO2 sink. Science 2015, 348, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Valentini, R.; Matteucci, G.; Dolman, A.J.; Schulze, E.; Rebmann, C.; Moors, E.J.; Granier, A.; Gross, P.; Jensen, N.O. Respiration as the main determininant of carbon balance in European forests. Nature 2000, 404, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.L.; Hamerlynck, E.P.; Jenerette, G.D.; Moran, M.S.; Gafford, G.A.B. Carbon dioxide exchange in a semidesert grassland through drought-induced vegetation change. J. Geophys. Res. 2010, 115, 1–12. [Google Scholar] [CrossRef]
- Hamerlynck, E.P.; Scott, R.L.; Sánchez-Cañete, E.P.; Barron-Gafford, G.A. Nocturnal soil CO2 uptake and its relationship to subsurface soil and ecosystem carbon fluxes in a Chihuahuan Desert shrubland. J. Geophys. Res. Biogeosci. 2013, 118, 1593–1603. [Google Scholar] [CrossRef]
- Ryan, M.G.; Law, B.E. Interpreting, measuring, and modeling soil respiration. Biogeochemistry 2005, 73, 3–27. [Google Scholar] [CrossRef]
- Sánchez-Cañete, E.P.; Scott, R.L.; van Haren, J.; Barron-Gafford, G.A. Improving the accuracy of the gradient method for determining soil carbon dioxide efflux: Accurate Long-Term Fsoil Based on the GM. J. Geophys. Res. Biogeosci. 2017, 122, 50–64. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J.A. On the temperature dependence of soil respiration. Source Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent of confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Xu, M.; Qi, Y. Soil surface CO2 efflux and its spatial and temporal variations in a young ponderosa pine plantation in northern California. Glob. Chang. Biol. 2001, 7, 667–677. [Google Scholar] [CrossRef]
- Tang, J.; Baldocchi, D.D. Spatial–temporal variation in soil respiration in an oak–grass savanna ecosystem in California and its partitioning into autotrophic and heterotrophic components. Biogeochemistry 2005, 73, 183–207. [Google Scholar] [CrossRef]
- Sponseller, R.A. Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Glob. Chang. Biol. 2007, 13, 426–436. [Google Scholar] [CrossRef]
- Cable, J.M.; Barron-Gafford, G.A.; Ogle, K.; Pavao-Zuckerman, M.; Scott, R.L.; Williams, D.G.; Huxman, T.E. Shrub encroachment alters sensitivity of soil respiration to temperature and moisture. J. Geophys. Res. Biogeosci. 2012, 117. [Google Scholar] [CrossRef]
- Reichstein, M.; Rey, A.; Freibauer, A.; Tenhunen, J.; Valentini, R.; Banza, J.; Casals, P.; Cheng, Y.; Grünzweig, J.M.; Irvine, J.; et al. Modeling temporal and large-scale spatial variability of soil respiration from soil water availability, temperature and vegetation productivity indices. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Wang, B.; Zha, T.S.; Jia, X.; Gong, J.N.; Bourque, C.; Feng, W.; Tian, Y.; Wu, B.; Qing Zhang, Y.; Peltola, H. Soil water regulates the control of photosynthesis on diel hysteresis between soil respiration and temperature in a desert shrubland. Biogeosciences 2017, 14, 3899–3908. [Google Scholar] [CrossRef]
- Vargas, R.; Allen, M.F. Environmental controls and the influence of vegetation type, fine roots and rhizomorphs on diel and seasonal variation in soil respiration. New Phytol. 2008, 179, 460–471. [Google Scholar] [CrossRef]
- Xu, M.; Shang, H. Contribution of soil respiration to the global carbon equation. J. Plant Physiol. 2016, 203, 16–28. [Google Scholar] [CrossRef]
- Xu, L.; Baldocchi, D.D.; Tang, J. How soil moisture, rain pulses, and growth alter the response of ecosystem respiration to temperature. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Feng, S.; Fu, Q. Expansion of global drylands under a warming climate. Atmos. Chem. Phys. 2013, 13, 10081–10094. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated dryland expansion under climate change. Nat. Clim. Chang. 2016, 6, 166. [Google Scholar] [CrossRef]
- Jenerette, G.D.; Scott, R.L.; Huxman, T.E. Whole ecosystem metabolic pulses following precipitation events. Funct. Ecol. 2008, 22, 924–930. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Gavrichkova, O. Time lag between photosynthesis and carbon dioxide efflux from soil: A review of mechanisms and controls. Glob. Chang. Biol. 2010, 16, 3386–3406. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Wan, S. Predominant role of water in regulating soil and microbial respiration and their responses to climate change in a semiarid grassland. Glob. Chang. Biol. 2009, 15, 184–195. [Google Scholar] [CrossRef]
- Cable, J.M.; Ogle, K.; Lucas, R.W.; Huxman, T.E.; Loik, M.E.; Smith, S.D.; Tissue, D.T.; Ewers, B.E.; Pendall, E.; Welker, J.M.; et al. The temperature responses of soil respiration in deserts: A seven desert synthesis. Biogeochemistry 2011, 103, 71–90. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. A global database of soil respiration data. Biogeosciences 2010, 7, 1915–1926. [Google Scholar] [CrossRef]
- Yan, Z.; Bond-Lamberty, B.; Todd-Brown, K.E.; Bailey, V.L.; Li, S.; Liu, C.; Liu, C. A moisture function of soil heterotrophic respiration that incorporates microscale processes. Nat. Commun. 2018, 9, 2562. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Baldocchi, D.D.; Xu, L. Tree photosynthesis modulates soil respiration on a diurnal time scale. Glob. Chang. Biol. 2005, 11, 1298–1304. [Google Scholar] [CrossRef]
- Conant, R.T.; Klopatek, J.M.; Klopatek, C.C. Environmental factors controlling soil respiration in three semiarid ecosystems. Soil Sci. Soc. Am. J. 2000, 64, 383–390. [Google Scholar] [CrossRef]
- Jassal, R.S.; Black, T.A.; Novak, M.D.; Gaumont-Guay, D.; Nesic, Z. Effect of soil water stress on soil respiration and its temperature sensitivity in an 18-year-old temperate Douglas-fir stand. Glob. Chang. Biol. 2008, 14, 1305–1318. [Google Scholar] [CrossRef]
- Luo, Y.; Ahlström, A.; Allison, S.D.; Batjes, N.H.; Brovkin, V.; Carvalhais, N.; Chappell, A.; Ciais, P.; Davidson, E.A.; Finzi, A.; et al. Toward more realistic projections of soil carbon dynamics by Earth system models. Glob. Biogeochem. Cycles 2016, 30, 40–56. [Google Scholar] [CrossRef]
- Zhang, Q.; Phillips, R.P.; Manzoni, S.; Scott, R.L.; Oishi, A.C.; Finzi, A.; Daly, E.; Vargas, R.; Novick, K.A. Changes in photosynthesis and soil moisture drive the seasonal soil respiration-temperature hysteresis relationship. Agric. For. Meteorol. 2018, 259, 184–195. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Nadezhdina, N.; David, T.S.; David, J.S.; Ferreira, M.I.; Dohnal, M.; Tesař, M.; Gartner, K.; Leitgeb, E.; Nadezhdin, V.; Cermak, J.; et al. Trees never rest: The multiple facets of hydraulic redistribution. Ecohydrology 2010, 3, 431–444. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Scott, R.L.; Jenerette, G.D.; Huxman, T.E. The relative controls of temperature, soil moisture, and plant functional group on soil CO2 efflux at diel, seasonal, and annual scales. J. Geophys. Res. Biogeosci. 2011, 116. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Baldocchi, D.D.; Gershenson, A.; Goldstein, A.; Misson, L.; Wong, S. Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Glob. Chang. Biol. 2007, 13, 2018–2035. [Google Scholar] [CrossRef]
- Cable, J.M.; Ogle, K.; Williams, D.G.; Weltzin, J.F.; Huxman, T.E. Soil texture drives responses of soil respiration to precipitation pulses in the sonoran desert: Implications for climate change. Ecosystems 2008, 11, 961–979. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Cable, J.M.; Bentley, L.P.; Scott, R.L.; Huxman, T.E.; Jenerette, G.D.; Ogle, K. Quantifying the timescales over which exogenous and endogenous conditions affect soil respiration. New Phytol. 2014, 202, 442–454. [Google Scholar] [CrossRef]
- Suseela, V.; Conant, R.T.; Wallenstein, M.D.; Dukes, J.S. Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob. Chang. Biol. 2012, 18, 336–348. [Google Scholar] [CrossRef]
- Sampson, D.A.; Janssens, I.A.; Curiel Yuste, J.; Ceulemans, R. Basal rates of soil respiration are correlated with photosynthesis in a mixed temperate forest. Glob. Chang. Biol. 2007, 13, 2008–2017. [Google Scholar] [CrossRef]
- Scott-Denton, L. Spatial and temporal controls of soil respiration rate in a high-elevation, subalpine forest. Soil Biol. Biochem. 2003, 35, 525–534. [Google Scholar] [CrossRef]
- Moore, D.J.P.; Trahan, N.A.; Wilkes, P.; Quaife, T.; Stephens, B.B.; Elder, K.; Desai, A.R.; Negron, J.; Monson, R.K. Persistent reduced ecosystem respiration after insect disturbance in high elevation forests. Ecol. Lett. 2013, 16, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Trahan, N.A.; Dynes, E.L.; Pugh, E.; Moore, D.J.P.; Monson, R.K. Changes in soil biogeochemistry following disturbance by girdling and mountain pine beetles in subalpine forests. Oecologia 2015, 177, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.L.; Biederman, J.A.; Hamerlynck, E.P.; Barron-gafford, G.A. The carbon balance pivot point of southwestern U.S. semiarid ecosystems: Insights from the 21st century drought. J. Geophys. Res. Biogeosci. 2015, 120, 2612–2624. [Google Scholar] [CrossRef]
- Cox, J.R.; Frasier, G.W.; Renard, K.G. Biomass distribution at grassland and shrubland sites. Rangelands 1986, 8, 67–68. [Google Scholar]
- Scott, R.L.; Jenerette, G.D.; Potts, D.L.; Huxman, T.E. Effects of seasonal drought on net carbon dioxide exchange from a woody-plant-encroached semiarid grassland. J. Geophys. Res. Biogeosci. 2009, 114. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Chang. Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Barba, J.; Cueva, A.; Bahn, M.; Barron-Gafford, G.A.; Bond-Lamberty, B.; Hanson, P.J.; Jaimes, A.; Kulmala, L.; Pumpanen, J.; Scott, R.L.; et al. Comparing ecosystem and soil respiration: Review and key challenges of tower-based and soil measurements. Agric. For. Meteorol. 2018, 249, 434–443. [Google Scholar] [CrossRef]
- Andrade, J.M.; Estévez-Pérez, M.G. Statistical comparison of the slopes of two regression lines: A tutorial. Anal. Chim. Acta 2014, 838, 1–12. [Google Scholar] [CrossRef]
- Savage, K.; Davidson, E.A.; Richardson, A.D.; Hollinger, D.Y. Three scales of temporal resolution from automated soil respiration measurements. Agric. For. Meteorol. 2009, 149, 2012–2021. [Google Scholar] [CrossRef]
- Yan, L.; Chen, S.; Huang, J.; Lin, G. Differential responses of auto- and heterotrophic soil respiration to water and nitrogen addition in a semiarid temperate steppe. Glob. Chang. Biol. 2010, 16, 2345–2357. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar]
- Zeng, X.; Song, Y.; Zhang, W.; He, S. Spatio-temporal variation of soil respiration and its driving factors in semi-arid regions of North China. Chin. Geogr. Sci. 2018, 28, 12–24. [Google Scholar] [CrossRef]
- Davidson, E.A.; Richardson, A.D.; Savage, K.E.; Hollinger, D.Y. A distinct seasonal pattern of the ratio of soil respiration to total ecosystem respiration in a spruce-dominated forest. Glob. Chang. Biol. 2006, 12, 230–239. [Google Scholar] [CrossRef]
- Moyano, F.E.; Manzoni, S.; Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 2013, 59, 72–85. [Google Scholar] [CrossRef]
- Wang, B.; Zha, T.S.; Jia, X.; Wu, B.; Zhang, Y.Q.; Qin, S.G. Soil moisture modifies the response of soil respiration to temperature in a desert shrub ecosystem. Biogeosciences 2014, 11, 259–268. [Google Scholar] [CrossRef]
- Huxman, T.E.; Snyder, K.A.; Tissue, D.; Leffler, A.J.; Ogle, K.; Pockman, W.T.; Sandquist, D.R.; Potts, D.L. Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 2004, 141, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Jenerette, G.D.; Barron-Gafford, G.A.; Guswa, A.J.; McDonnell, J.J.; Villegas, J.C. Organization of complexity in water limited ecohydrology. Ecohydrology 2012, 5, 184–199. [Google Scholar] [CrossRef]
- Högberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.S.; Ekblad, A.; Högberg, M.N.; Nyberg, G.; Ottosson-Löfvenius, M.; Read, D.J. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–792. [Google Scholar] [CrossRef]
- Xu, K.; Metzger, S.; Desai, A.R. Upscaling tower-observed turbulent exchange at fine spatio-temporal resolution using environmental response functions. Agric. For. Meteorol. 2017, 232, 10–22. [Google Scholar] [CrossRef]
- Savage, K.; Davidson, E.A.; Tang, J. Diel patterns of autotrophic and heterotrophic respiration among phenological stages. Glob. Chang. Biol. 2013, 19, 1151–1159. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garrten, C.T.; Andrews, J.A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Gomez-Casanovas, N.; Matamala, R.; Cook, D.R.; Gonzalez-Meler, M.A. Net ecosystem exchange modifies the relationship between the autotrophic and heterotrophic components of soil respiration with abiotic factors in prairie grasslands. Glob. Chang. Biol. 2012, 18, 2532–2545. [Google Scholar] [CrossRef]
- Vargas, R.; Sánchez-Cañete, P.E.; Serrano-Ortiz, P.; Curiel Yuste, J.; Domingo, F.; López-Ballesteros, A.; Oyonarte, C. Hot-moments of soil CO2 efflux in a water-limited grassland. Soil Syst. 2018, 2, 47. [Google Scholar] [CrossRef]
- Baldocchi, D.; Tang, J.; Xu, L. How switches and lags in biophysical regulators affect spatial-temporal variation of soil respiration in an oak-grass savanna. J. Geophys. Res. Biogeosci. 2006, 111. [Google Scholar] [CrossRef]
- Vargas, R.; Baldocchi, D.D.; Allen, M.F.; Bahn, M.; Black, T.A.; Collins, S.L.; Yuste, J.C.; Hirano, T.; Jassal, R.S.; Pumpanen, J.; et al. Looking deeper into the soil: Biophysical controls and seasonal lags of soil CO2 production and efflux. Ecol. Appl. 2010, 20, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Kurc, S.A.; Small, E.E. Soil moisture variations and ecosystem-scale fluxes of water and carbon in semiarid grassland and shrubland. Water Resour. Res. 2007, 43. [Google Scholar] [CrossRef]
- Jenerette, G.D.; Scott, R.L.; Barron-Gafford, G.A.; Huxman, T.E. Gross primary production variability associated with meteorology, physiology, leaf area, and water supply in contrasting woodland and grassland semiarid riparian ecosystems. J. Geophys. Res. Biogeosci. 2009, 114. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).