Immune Checkpoint Inhibitor-Induced Ocular Toxicity: A Case of Pembrolizumab-Associated Corneal Ulceration and Evisceration
Abstract
1. Introduction and Clinical Significance
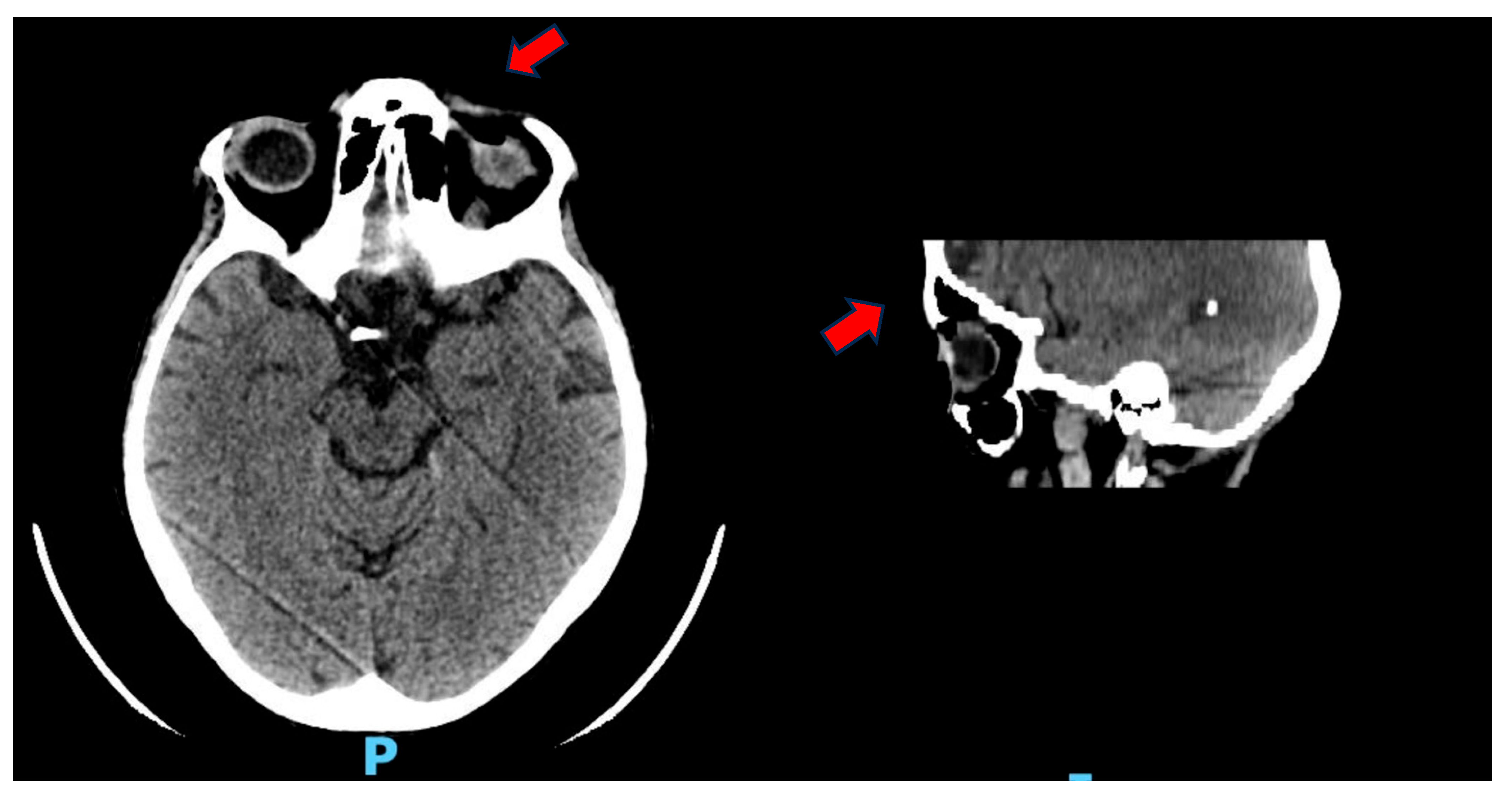
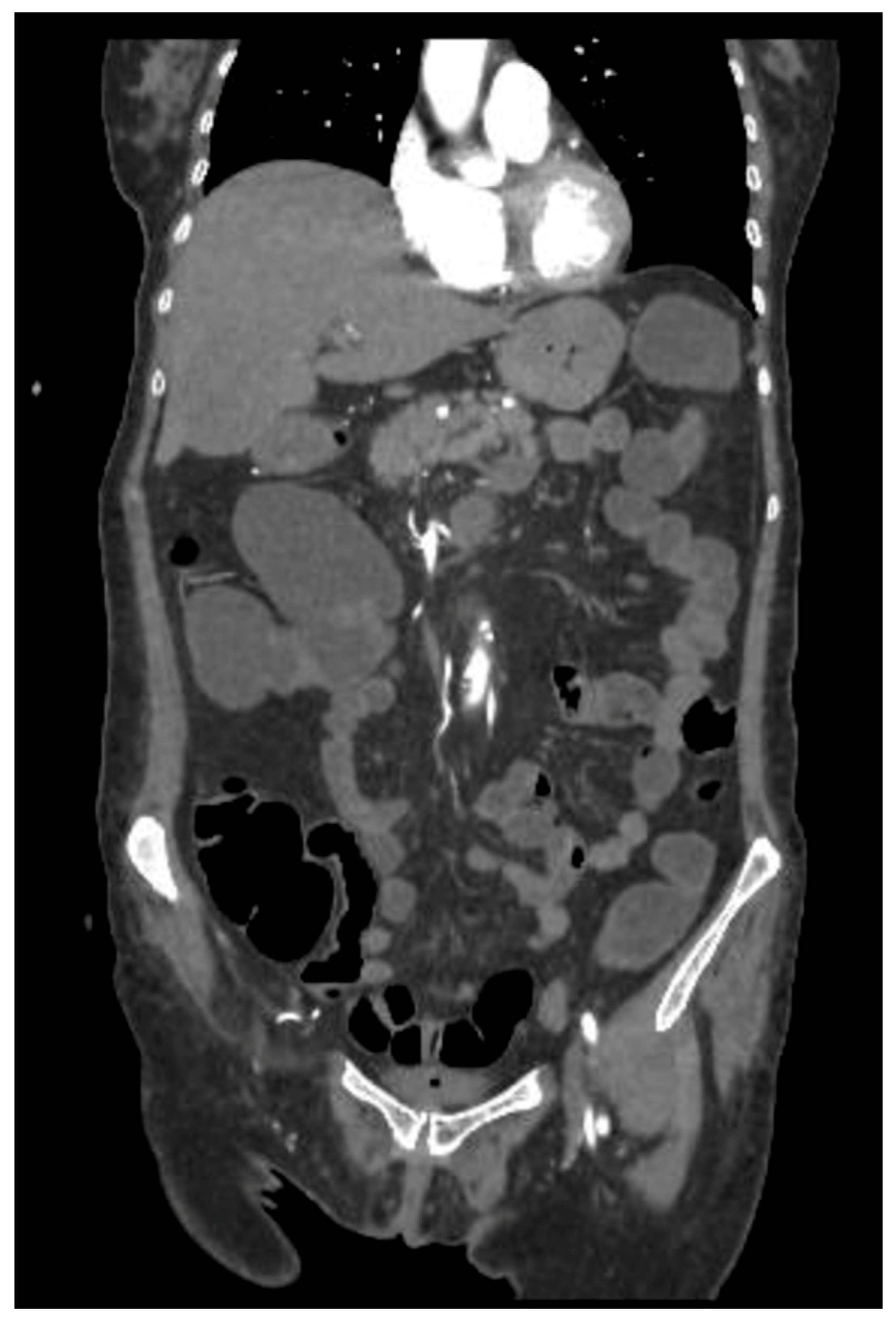
2. Case Presentation
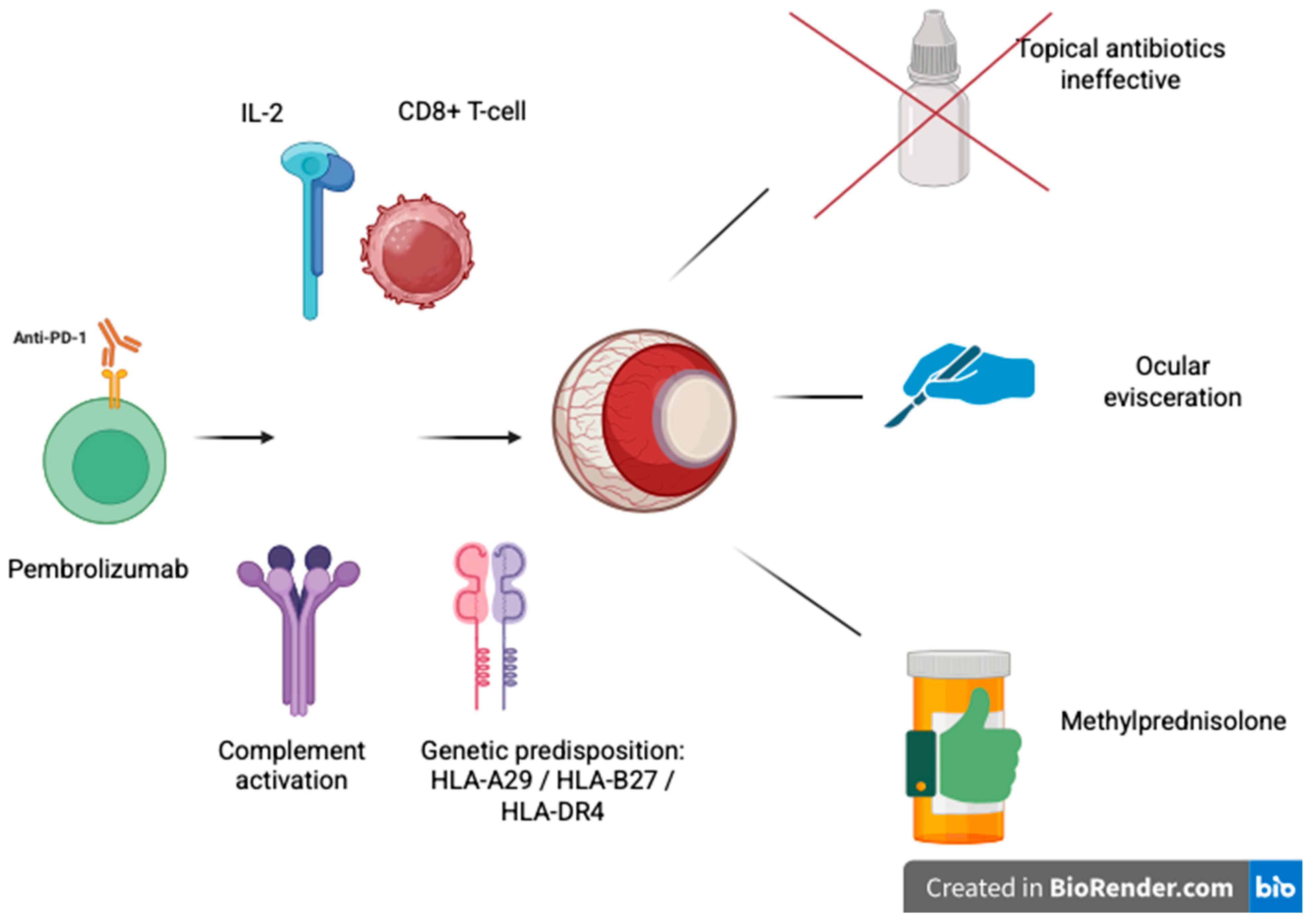
3. Discussion
3.1. Immune Checkpoint Inhibitors
3.2. Ocular Side Effects of Immune Checkpoint Inhibitors
3.3. Diagnosis
3.4. Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Oweira, H.; Petrausch, U.; Helbling, D.; Schmidt, J.; Mannhart, M.; Mehrabi, A.; Schöb, O.; Giryes, A. Immune-Related Ocular Toxicities in Solid Tumor Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review. Expert Rev. Anticancer Ther. 2017, 17, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Dalvin, L.A.; Shields, C.L.; Orloff, M.; Sato, T.; Shields, J.A. CHECKPOINT INHIBITOR IMMUNE THERAPY: Systemic Indications and Ophthalmic Side Effects. Retina Phila. 2018, 38, 1063–1078. [Google Scholar] [CrossRef]
- Iranzo, P.; Callejo, A.; Assaf, J.D.; Molina, G.; Lopez, D.E.; Garcia-Illescas, D.; Pardo, N.; Navarro, A.; Martinez-Marti, A.; Cedres, S.; et al. Overview of Checkpoint Inhibitors Mechanism of Action: Role of Immune-Related Adverse Events and Their Treatment on Progression of Underlying Cancer. Front. Med. 2022, 9, 875974. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef]
- Di Giacomo, A.M.; Lahn, M.; Eggermont, A.M.; Fox, B.; Ibrahim, R.; Sharma, P.; Allison, J.P.; Maio, M. The Future of Targeting Cytotoxic T-Lymphocyte-Associated Protein-4: Is There a Role? Eur. J. Cancer 2024, 198, 113501. [Google Scholar] [CrossRef]
- Bitton, K.; Michot, J.-M.; Barreau, E.; Lambotte, O.; Haigh, O.; Marabelle, A.; Voisin, A.-L.; Mateus, C.; Rémond, A.-L.; Couret, C.; et al. Prevalence and Clinical Patterns of Ocular Complications Associated With Anti-PD-1/PD-L1 Anticancer Immunotherapy. Am. J. Ophthalmol. 2019, 202, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Song, Y.; Tian, W. How to Select IgG Subclasses in Developing Anti-Tumor Therapeutic Antibodies. J. Hematol. Oncol. J. Hematol. Oncol. 2020, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Paul, S.; Echegaray, J. Update on Immune Checkpoint Inhibitor-Associated Uveitis. Curr. Ophthalmol. Rep. 2023, 11, 57–67. [Google Scholar] [CrossRef]
- Casselman, P.; Jacob, J.; Schauwvlieghe, P.-P. Relation between Ocular Paraneoplastic Syndromes and Immune Checkpoint Inhibitors (ICI): Review of Literature. J. Ophthalmic Inflamm. Infect. 2023, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-W.; Xu, Q.; Wang, Y.; Xia, R.-L.; Liu, J.-Y.; Ma, X.-L. Immune Checkpoint Inhibitor-Associated Ophthalmic Adverse Events: Current Understanding of Its Mechanisms, Diagnosis, and Management. Int. J. Ophthalmol. 2022, 15, 646–656. [Google Scholar] [CrossRef]
- Zhang, H.; Houadj, L.; Wu, K.Y.; Tran, S.D. Diagnosing and Managing Uveitis Associated with Immune Checkpoint Inhibitors: A Review. Diagnostics 2024, 14, 336. [Google Scholar] [CrossRef]
- Arai, T.; Harada, K.; Usui, Y.; Irisawa, R.; Tsuboi, R. Case of Acute Anterior Uveitis and Vogt–Koyanagi–Harada Syndrome-like Eruptions Induced by Nivolumab in a Melanoma Patient. J. Dermatol. 2017, 44, 975–976. [Google Scholar] [CrossRef]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary Expression of CTLA-4 Mediates Hypophysitis Secondary to Administration of CTLA-4 Blocking Antibody. Sci. Transl. Med. 2014, 6, ra45–ra230. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wan, Y.; Jiao, A.; Jiang, K.; Cui, G.; Tang, J.; Yu, S.; Hu, Z.; Zhao, S.; Yi, Z.; et al. Breaking Boundaries: Chronic Diseases and the Frontiers of Immune Microenvironments. Med. Res. 2025, 1, 62–102. [Google Scholar] [CrossRef]
- Khaghani, F.; Hemmati, M.; Ebrahimi, M.; Salmaninejad, A. Emerging Multi-Omic Approaches to the Molecular Diagnosis of Mitochondrial Disease and Available Strategies for Treatment and Prevention. Curr. Genom. 2024, 25, 358–379. [Google Scholar] [CrossRef]
- Abdollahi, E.; Johnston, T.P.; Ghaneifar, Z.; Vahedi, P.; Goleij, P.; Azhdari, S.; Moghaddam, A.S. Immunomodulatory Therapeutic Effects of Curcumin on M1/M2 Macrophage Polarization in Inflammatory Diseases. Curr. Mol. Pharmacol. 2023, 16, 2–14. [Google Scholar] [CrossRef]
- Manohar, S.M. At the Crossroads of TNF α Signaling and Cancer. Curr. Mol. Pharmacol. 2024, 17, e060923220758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.; Zhang, L. Emerging Trends and Hot Topics in the Application of Multi-Omics in Drug Discovery: A Bibliometric and Visualized Study. Curr. Pharm. Anal. 2024, 21, 20–32. [Google Scholar] [CrossRef]
- Comin-Anduix, B.; Escuin-Ordinas, H.; Ibarrondo, F.J. Tremelimumab: Research and Clinical Development. OncoTargets Ther. 2016, 23, 1767–1776. [Google Scholar] [CrossRef]
- Heng, J.S.; Kim, J.M.; Jones, D.K.; Stoessel, K.M.; Weiss, S.A.; Sznol, M.; Kluger, H.M.; Walter, S.D.; Silverstein, N.A.; Pointdujour-Lim, R. Autoimmune Retinopathy with Associated Anti-Retinal Antibodies as a Potential Immune-Related Adverse Event Associated with Immunotherapy in Patients with Advanced Cutaneous Melanoma: Case Series and Systematic Review. BMJ Open Ophthalmol. 2022, 7, e000889. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Eppley, S.; Baer, D.; Melles, R.B. Characteristics of Ocular Inflammatory Side Effects Associated with Immune Checkpoint Inhibitors in a Northern California Population. Ocul. Immunol. Inflamm. 2024, 32, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Adamus, G.; Champaigne, R.; Yang, S. Occurrence of Major Anti-Retinal Autoantibodies Associated with Paraneoplastic Autoimmune Retinopathy. Clin. Immunol. 2020, 210, 108317. [Google Scholar] [CrossRef]
- Papavasileiou, E.; Prasad, S.; Freitag, S.K.; Sobrin, L.; Lobo, A.-M. Ipilimumab-Induced Ocular and Orbital Inflammation—A Case Series and Review of the Literature. Ocul. Immunol. Inflamm. 2016, 24, 140–146. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Martens, A.; Schauwvlieghe, P.P.; Madoe, A.; Casteels, I.; Aspeslagh, S. Ocular Adverse Events Associated with Immune Checkpoint Inhibitors, a Scoping Review. J. Ophthalmic Inflamm. Infect. 2023, 13, 5. [Google Scholar] [CrossRef]
- Keltner, J.L.; Thirkill, C.E.; Yip, P.T. Clinical and Immunologic Characteristics of Melanoma-Associated Retinopathy Syndrome: Eleven New Cases and a Review of 51 Previously Published Cases. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2001, 21, 173–187. [Google Scholar] [CrossRef]
- Khaddour, K.; Khanna, S.; Ansstas, M.; Jakhar, I.; Dahiya, S.; Council, L.; Ansstas, G. Normalization of Electroretinogram and Symptom Resolution of Melanoma-Associated Retinopathy with Negative Autoantibodies after Treatment with Programmed Death-1 (PD-1) Inhibitors for Metastatic Melanoma. Cancer Immunol. Immunother. 2021, 70, 2497–2502. [Google Scholar] [CrossRef]
| Checkpoint Pathway | Target Molecule | Representative Drugs | Mechanism of Action | Common irAEs | References |
|---|---|---|---|---|---|
| CTLA-4 | CTLA-4 on T cells | Ipilimumab, Tremelimumab | Blocks CTLA-4/B7 interaction → enhances T-cell priming | Colitis, dermatitis, hypophysitis | [5,6,9] |
| PD-1 | PD-1 on T, B, and NK cells | Nivolumab, Pembrolizumab | Inhibits PD-1/PD-L1 binding → reverses T-cell exhaustion | Pneumonitis, thyroiditis, hepatitis, uveitis | [5,6,10] |
| PD-L1 | PD-L1 on tumor and immune cells | Atezolizumab, Durvalumab, Avelumab | Prevents PD-L1 from binding PD-1 → preserves T-cell activity | Like PD-1 inhibitors; generally milder | [5,6,8] |
| Ocular irAE | Clinical Features | Associated ICIs | Time of Onset | Prognosis | Management | References |
|---|---|---|---|---|---|---|
| Dry eye disease | Grittiness, foreign body sensation, blurry vision | All (PD-1, PD-L1, CTLA-4) | Weeks to months | Good | Artificial tears, topical cyclosporine | [12,25] |
| Uveitis (anterior/posterior) | Red eye, photophobia, eye pain, blurred vision | PD-1 (Nivolumab, Pembrolizumab), CTLA-4 | Median: 9 weeks | Variable | Topical/systemic steroids, +/− ICI suspension | [10,13,26] |
| VKH-like syndrome | Bilateral serous retinal detachments, headache, tinnitus | PD-1, CTLA-4 | Weeks to months | Moderate to poor | High-dose corticosteroids, ICI discontinuation | [14] |
| Corneal ulcer/perforation | Severe pain, photophobia, loss of vision | PD-1 (Pembrolizumab), rare with others | Variable | Often poor (may require evisceration) | Surgical repair, antibiotics, steroids | [12] |
| Melanoma-associated retinopathy (MAR) | Night blindness, shimmering, central scotomas, preserved a-wave on ERG | PD-1 in melanoma | 2 weeks to 2 years post-ICI | Poor (irreversible vision loss) | Immunosuppressants, ICI discontinuation | [22,23] |
| Cancer-associated retinopathy (CAR) | Painless vision loss, visual field constriction, photopsias | PD-1, rarely CTLA-4 | Variable | Poor | Limited response to therapy | [22,24] |
| AEPVM (vitelliform maculopathy) | Bilateral subretinal fluid, yellowish lesions, mild visual symptoms | PD-1, PD-L1, melanoma or carcinoma | Early to mid-course | Generally good | Often self-resolving; continue ICI | [11,27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldarelli, M.; Brisinda, D.; De Matteis, G.; De Vito, F.; Gambini, G.; Cianci, R.; Gambassi, G. Immune Checkpoint Inhibitor-Induced Ocular Toxicity: A Case of Pembrolizumab-Associated Corneal Ulceration and Evisceration. Reports 2025, 8, 154. https://doi.org/10.3390/reports8030154
Caldarelli M, Brisinda D, De Matteis G, De Vito F, Gambini G, Cianci R, Gambassi G. Immune Checkpoint Inhibitor-Induced Ocular Toxicity: A Case of Pembrolizumab-Associated Corneal Ulceration and Evisceration. Reports. 2025; 8(3):154. https://doi.org/10.3390/reports8030154
Chicago/Turabian StyleCaldarelli, Mario, Donatella Brisinda, Giuseppe De Matteis, Francesco De Vito, Gloria Gambini, Rossella Cianci, and Giovanni Gambassi. 2025. "Immune Checkpoint Inhibitor-Induced Ocular Toxicity: A Case of Pembrolizumab-Associated Corneal Ulceration and Evisceration" Reports 8, no. 3: 154. https://doi.org/10.3390/reports8030154
APA StyleCaldarelli, M., Brisinda, D., De Matteis, G., De Vito, F., Gambini, G., Cianci, R., & Gambassi, G. (2025). Immune Checkpoint Inhibitor-Induced Ocular Toxicity: A Case of Pembrolizumab-Associated Corneal Ulceration and Evisceration. Reports, 8(3), 154. https://doi.org/10.3390/reports8030154








