The Role of Pharmacogenomics in Optimizing Ketamine Therapy for Post-Amputation Pain
Abstract
1. Introduction and Clinical Significance
2. Methods
3. Mechanism of Action of Ketamine
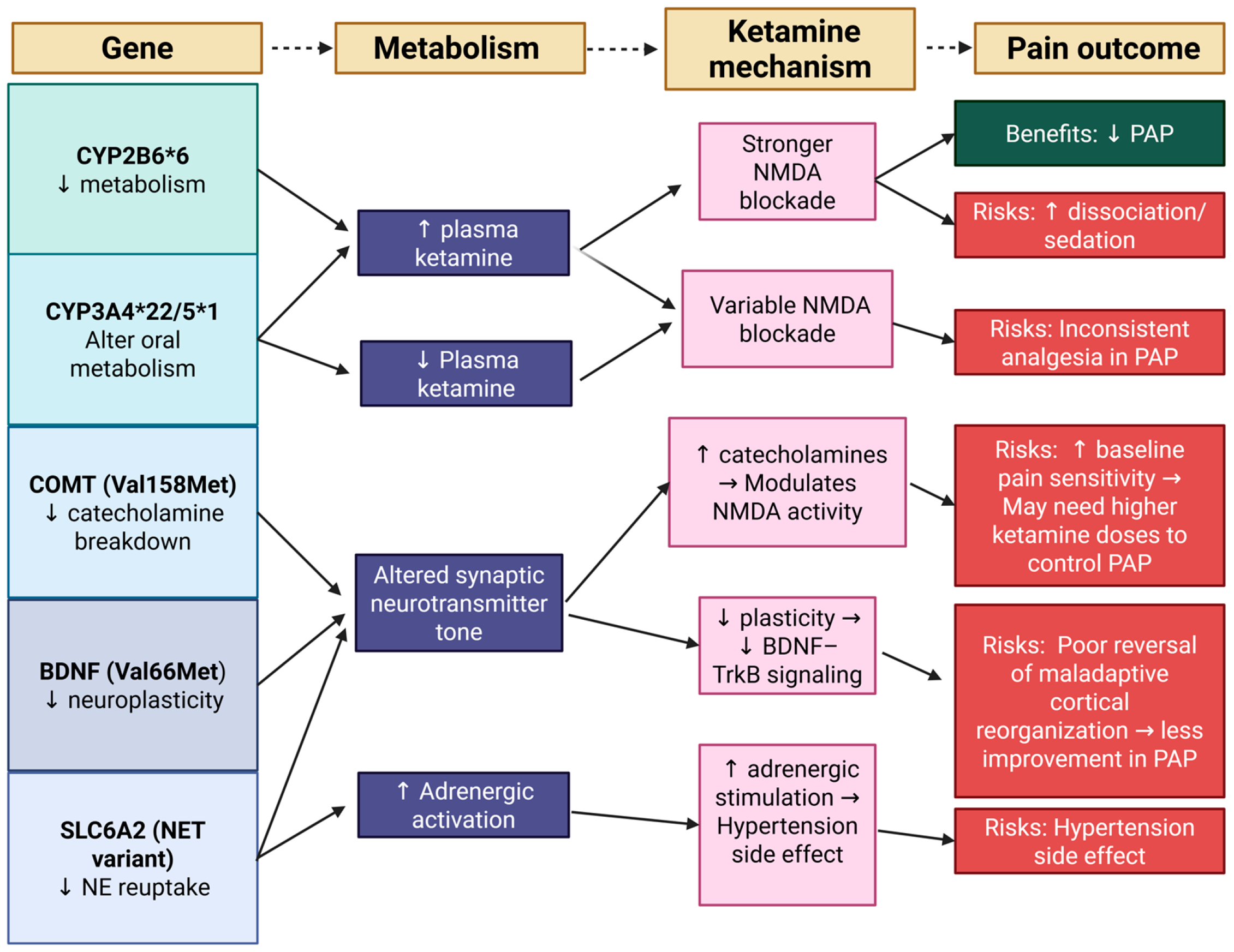
4. Pharmacogenomics of Ketamine: Genetic Determinants of Response
4.1. CYP2B6 Alleles
4.2. Brain-Derived Neurotrophic Factor (BDNF)
4.3. CYP3A4/5
4.4. Catechol-O-Methyltransferase (COMT)
4.5. SLC6A2 (Norepinephrine Transporter, NET)
5. Comparison with Other Treatment Modalities
6. Impact of Pharmacogenomics on Ketamine’s Safety Profile in Post-Amputation Pain Management
7. Clinical Implications for Personalized Ketamine Therapy
8. Limitations of Current Research
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKA | Above-Knee Amputation |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid |
| BDNF | Brain-Derived Neurotrophic Factor |
| BKA | Below-Knee Amputation |
| CBT | Cognitive Behavioral Therapy |
| CNS | Central Nervous System |
| COMT | Catechol-O-Methyltransferase |
| CYP | Cytochrome P450 |
| DNA | Deoxyribonucleic Acid |
| FDA | Food and Drug Administration |
| GABA | Gamma-Aminobutyric Acid |
| GABAa | Ionotropic GABA Receptor |
| GRIN2B | Glutamate Ionotropic Receptor NMDA Type Subunit 2B |
| H2O2 | Hydrogen Peroxide |
| IV | Intravenous |
| KCNS1 | Potassium Voltage-Gated Channel Subfamily S Member 1 |
| Met | Methionine Allele (in Polymorphisms) |
| mTOR | Mechanistic Target of Rapamycin |
| NE | Norepinephrine |
| NET | Norepinephrine Transporter |
| NMDA | N-Methyl-D-Aspartate |
| PAP | Post-Amputation Pain |
| PK/PD | Pharmacokinetics/Pharmacodynamics |
| PLP | Phantom Limb Pain |
| POD | Postoperative Day |
| PPI | Present Pain Intensity |
| RCT | Randomized Controlled Trial |
| ROS | Reactive Oxygen Species |
| RLP | Residual Limb Pain |
| SCS | Spinal Cord Stimulation |
| SLC6A2 | Solute Carrier Family 6 Member 2 |
| SNRI | Serotonin-Norepinephrine Reuptake Inhibitor |
| SSRI | Selective Serotonin Reuptake Inhibitor |
| TCAs | Tricyclic Antidepressants |
| TENS | Transcutaneous Electrical Nerve Stimulation |
| TrkB | Tropomyosin Receptor Kinase B |
| Val | Valine Allele (in Polymorphisms) |
References
- Ephraim, P.L.; Wegener, S.T.; MacKenzie, E.J.; Dillingham, T.R.; Pezzin, L.E. Phantom Pain, Residual Limb Pain, and Back Pain in Amputees: Results of a National Survey. Arch. Phys. Med. Rehabil. 2005, 86, 1910–1919. [Google Scholar] [CrossRef]
- Cohen, S.P.; Hsu, E. Postamputation Pain: Epidemiology, Mechanisms, and Treatment. J. Pain Res. 2013, 6, 121–136. [Google Scholar] [CrossRef]
- Nikolajsen, L.; Hansen, C.L.; Nielsen, J.; Keller, J.; Arendt-Nielsen, L.; Jensen, T.S. The Effect of Ketamine on Phantom Pain: A Central Neuropathic Disorder Maintained by Peripheral Input. Pain 1996, 67, 69–77. [Google Scholar] [CrossRef]
- Wilson, J.A.; Nimmo, A.F.; Fleetwood-Walker, S.M.; Colvin, L.A. A Randomised Double Blind Trial of the Effect of Pre-Emptive Epidural Ketamine on Persistent Pain after Lower Limb Amputation. Pain 2008, 135, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.; Armstrong-Brown, A.; Burstal, R. Perioperative Intravenous Ketamine Infusion for the Prevention of Persistent Post-Amputation Pain: A Randomized, Controlled Trial. Anaesth. Intensive Care 2004, 32, 330–338. [Google Scholar] [CrossRef]
- Eichenberger, U.; Neff, F.; Sveticic, G.; Björgo, S.; Petersen-Felix, S.; Arendt-Nielsen, L.; Curatolo, M. Chronic Phantom Limb Pain: The Effects of Calcitonin, Ketamine, and Their Combination on Pain and Sensory Thresholds. Anesth. Analg. 2008, 106, 1265–1273. [Google Scholar] [CrossRef]
- Polomano, R.C.; Buckenmaier, C.C.; Kwon, K.H.; Hanlon, A.L.; Rupprecht, C.; Goldberg, C.; Gallagher, R.M. Effects of Low-Dose IV Ketamine on Peripheral and Central Pain from Major Limb Injuries Sustained in Combat. Pain Med. 2013, 14, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Buvanendran, A.; Kroin, J.S.; Rajagopal, A.; Robison, S.J.; Moric, M.; Tuman, K.J. Oral Ketamine for Acute Pain Management After Amputation Surgery. Pain Med. 2018, 19, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Vlisides, P.E. Ketamine: 50 Years of Modulating the Mind. Front. Hum. Neurosci. 2016, 10, 612. [Google Scholar] [CrossRef]
- Pai, A.; Heining, M. Ketamine. Contin. Educ. Anaesth. Crit. Care Pain 2007, 7, 59–63. [Google Scholar] [CrossRef]
- Andoh, J.; Milde, C.; Diers, M.; Bekrater-Bodmann, R.; Trojan, J.; Fuchs, X.; Becker, S.; Desch, S.; Flor, H. Assessment of Cortical Reorganization and Preserved Function in Phantom Limb Pain: A Methodological Perspective. Sci. Rep. 2020, 10, 11504. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Grossberg, G.T. Phantom Limb Pain: Mechanisms and Treatment Approaches. Pain Res. Treat. 2011, 2011, 864605. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Lambert, D.G. Ketamine: Its Mechanism(s) of Action and Unusual Clinical Uses. Br. J. Anaesth. 1996, 77, 441–444. [Google Scholar] [CrossRef]
- Israel, J.E.; St Pierre, S.; Ellis, E.; Hanukaai, J.S.; Noor, N.; Varrassi, G.; Wells, M.; Kaye, A.D. Ketamine for the Treatment of Chronic Pain: A Comprehensive Review. Health Psychol. Res. 2021, 9, 25535. [Google Scholar] [CrossRef]
- Lullau, A.P.M.; Haga, E.M.W.; Ronold, E.H.; Dwyer, G.E. Antidepressant Mechanisms of Ketamine: A Review of Actions with Relevance to Treatment-Resistance and Neuroprogression. Front. Neurosci. 2023, 17, 1223145. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak-Bębenista, M.; Sokołowska, P.; Wiktorowska-Owczarek, A.; Kowalczyk, E.; Sienkiewicz, M. Ketamine—A New Antidepressant Drug with Anti-Inflammatory Properties. J. Pharmacol. Exp. Ther. 2024, 388, 134–144. [Google Scholar] [CrossRef]
- Erlenwein, J.; Diers, M.; Ernst, J.; Schulz, F.; Petzke, F. Clinical Updates on Phantom Limb Pain. Pain Rep. 2021, 6, e888. [Google Scholar] [CrossRef]
- Hashimoto, K.; Zhao, M.; Zhu, T.; Wang, X.; Yang, J. Ketamine and Its Two Enantiomers in Anesthesiology and Psychiatry: A Historical Review and Future Directions. J. Anesth. Transl. Med. 2024, 3, 65–75. [Google Scholar] [CrossRef]
- Yang, C.; Shirayama, Y.; Zhang, J.; Ren, Q.; Yao, W.; Ma, M.; Dong, C.; Hashimoto, K. R-Ketamine: A Rapid-Onset and Sustained Antidepressant without Psychotomimetic Side Effects. Transl. Psychiatry 2015, 5, e632. [Google Scholar] [CrossRef]
- Jóźwiak-Bębenista, M.; Wiktorowska-Owczarek, A.; Siatkowska, M.; Komorowski, P.; Włodarczyk, A.; Kowalczyk, E.; Sokołowska, P. Modulation of ER Stress and Inflammation by S-Ketamine, R-Ketamine, and Their Metabolites in Human Microglial Cells: Insights into Novel Targets for Depression Therapy. Cells 2025, 14, 831. [Google Scholar] [CrossRef]
- Cook-Sather, S.D.; Adamson, P.C.; Li, J.; Hakonarson, H. CYP2B6*6 or Not CYP2B6*6—That Remains a Question for Precision Medicine and Ketamine! Anesthesiology 2016, 125, 1085–1087. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Jackson, K.A.; Slon, B.; Hardy, J.R.; Franco, M.; William, L.; Poon, P.; Coller, J.K.; Hutchinson, M.R.; Currow, D.C.; et al. CYP2B6 * 6 Allele and Age Substantially Reduce Steady-state Ketamine Clearance in Chronic Pain Patients: Impact on Adverse Effects. Br. J. Clin. Pharmacol. 2015, 80, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Langmia, I.M.; Just, K.S.; Yamoune, S.; Müller, J.P.; Stingl, J.C. Pharmacogenetic and Drug Interaction Aspects on Ketamine Safety in Its Use as Antidepressant - Implications for Precision Dosing in a Global Perspective. Br. J. Clin. Pharmacol. 2022, 88, 5149–5165. [Google Scholar] [CrossRef]
- Langmia, I.M.; Just, K.S.; Yamoune, S.; Brockmöller, J.; Masimirembwa, C.; Stingl, J.C. CYP2B6 Functional Variability in Drug Metabolism and Exposure Across Populations—Implication for Drug Safety, Dosing, and Individualized Therapy. Front. Genet. 2021, 12, 692234. [Google Scholar] [CrossRef]
- Meshkat, S.; Rodrigues, N.B.; Di Vincenzo, J.D.; Ceban, F.; Jaberi, S.; McIntyre, R.S.; Lui, L.M.W.; Rosenblat, J.D. Pharmacogenomics of Ketamine: A Systematic Review. J. Psychiatr. Res. 2022, 145, 27–34. [Google Scholar] [CrossRef]
- Yamoune, S.; Müller, J.P.; Langmia, I.M.; Scholl, C.; Stingl, J.C. Uncoupling of Cytochrome P450 2B6 and Stimulation of Reactive Oxygen Species Production in Pharmacogenomic Alleles Affected by Interethnic Variability. Biochim. Biophy.s Acta Gen. Subj. 2024, 1868, 130595. [Google Scholar] [CrossRef] [PubMed]
- Yamoune, S.; Wintz, K.; Niederau, C.; Craveiro, R.B.; Wolf, M.; Stingl, J. Role of Cytochrome P450 2C8 Genetic Polymorphism and Epoxygenase Uncoupling in Periodontal Remodelling Affecting Orthodontic Treatment. Basic Clin. Pharmacol. Toxicol. 2022, 130, 132–140. [Google Scholar] [CrossRef]
- Mangó, K.; Kiss, Á.F.; Fekete, F.; Erdős, R.; Monostory, K. CYP2B6 Allelic Variants and Non-Genetic Factors Influence CYP2B6 Enzyme Function. Sci. Rep. 2022, 12, 2984. [Google Scholar] [CrossRef]
- Rodrigues, N.B.; Chen-Li, D.; Di Vincenzo, J.D.; Juneja, A.; Pinder, B.D.; McIntyre, R.S.; Rosenblat, J.D. Brain-Derived Neurotrophic Factor Val66Met and CYP2B6 Polymorphisms as Predictors for Ketamine Effectiveness in Patients with Treatment-Resistant Depression. J. Psychopharmacol. 2024, 38, 375–381. [Google Scholar] [CrossRef]
- Casarotto, P.; Umemori, J.; Castrén, E. BDNF Receptor TrkB as the Mediator of the Antidepressant Drug Action. Front. Mol. Neurosci. 2022, 15, 1032224. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, X.; Xu, G.; Zhang, Q.; Qian, P.; Liu, S.; Zhu, J.; Shen, R. Association between COMT Polymorphism Val158Met and Opioid Consumption in Patients with Postoperative Pain: A Meta-Analysis. Neurosignals 2018, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Fragoso, R.; Pereira, D.; Medeiros, R. Pain Polymorphisms and Opioids: An Evidence Based Review. Mol. Med. Rep. 2018, 19, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Searle, R.; Hopkins, P.M. Pharmacogenomic Variability and Anaesthesia. Br. J. Anaesth. 2009, 103, 14–25. [Google Scholar] [CrossRef]
- Liebe, T.; Li, S.; Lord, A.; Colic, L.; Krause, A.L.; Batra, A.; Kretzschmar, M.A.; Sweeney-Reed, C.M.; Behnisch, G.; Schott, B.H.; et al. Factors Influencing the Cardiovascular Response to Subanesthetic Ketamine: A Randomized, Placebo-Controlled Trial. Int. J. Neuropsychopharmacol. 2017, 20, 909–918. [Google Scholar] [CrossRef]
- Beanes, G.; Caliman-Fontes, A.T.; Souza-Marques, B.; Silva, H.D.S.; Leal, G.C.; Carneiro, B.A.; Guerreiro-Costa, L.N.F.; Figueiredo, A.V.; Figueiredo, C.A.V.; Lacerda, A.L.T.; et al. Effects of GRIN2B, GRIA1, and BDNF Polymorphisms on the Therapeutic Action of Ketamine and Esketamine in Treatment-Resistant Depression Patients: Secondary Analysis From a Randomized Clinical Trial. Clin. Neuropharmacol. 2022, 45, 151–156. [Google Scholar] [CrossRef]
- Kaye, A.D.; Garcia, A.J.; Hall, O.M.; Jeha, G.M.; Cramer, K.D.; Granier, A.L.; Kallurkar, A.; Cornett, E.M.; Urman, R.D. Update on the Pharmacogenomics of Pain Management. Pharmgenom. Pers. Med. 2019, 12, 125–143. [Google Scholar] [CrossRef]
- Gao, M.; Rejaei, D.; Liu, H. Ketamine Use in Current Clinical Practice. Acta Pharmacol. Sin. 2016, 37, 865–872. [Google Scholar] [CrossRef]
- Hall, N.; Eldabe, S. Phantom Limb Pain: A Review of Pharmacological Management. Br. J. Pain 2018, 12, 202–207. [Google Scholar] [CrossRef]
- Ahuja, V.; Thapa, D.; Ghai, B. Strategies for Prevention of Lower Limb Post-Amputation Pain: A Clinical Narrative Review. J. Anaesthesiol. Clin. Pharmacol. 2018, 34, 439. [Google Scholar] [CrossRef]
- Duncan, W.C.; Zarate, C.A. Ketamine, Sleep, and Depression: Current Status and New Questions. Curr. Psychiatry Rep. 2013, 15, 394. [Google Scholar] [CrossRef] [PubMed]
- Alviar, M.J.M.; Hale, T.; Lim-Dungca, M. Pharmacologic Interventions for Treating Phantom Limb Pain. Cochrane Database Syst. Rev. 2016, 2016, CD006380. [Google Scholar] [CrossRef] [PubMed]
- Von Plato, H.; Kontinen, V.; Hamunen, K. Efficacy and Safety of Epidural, Continuous Perineural Infusion and Adjuvant Analgesics for Acute Postoperative Pain after Major Limb Amputation—A Systematic Review. Scand. J. Pain 2018, 18, 3–17. [Google Scholar] [CrossRef]
- Ayling, O.G.S.; Montbriand, J.; Jiang, J.; Ladak, S.; Love, L.; Eisenberg, N.; Katz, J.; Clarke, H.; Roche-Nagle, G. Continuous Regional Anaesthesia Provides Effective Pain Management and Reduces Opioid Requirement Following Major Lower Limb Amputation. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.B.; Marouani, N.; Weinbroum, A.A. Dextromethorphan Mitigates Phantom Pain in Cancer Amputees. Ann. Surg. Oncol. 2003, 10, 268–274. [Google Scholar] [CrossRef]
- Johnson, M.I.; Paley, C.A.; Jones, G.; Mulvey, M.R.; Wittkopf, P.G. Efficacy and Safety of Transcutaneous Electrical Nerve Stimulation (TENS) for Acute and Chronic Pain in Adults: A Systematic Review and Meta-Analysis of 381 Studies (the Meta-TENS Study). BMJ Open 2022, 12, e051073. [Google Scholar] [CrossRef]
- Kalsi, S.S.; Wood, D.M.; Dargan, P.I. The Epidemiology and Patterns of Acute and Chronic Toxicity Associated with Recreational Ketamine Use. Emerg. Health Threats J. 2011, 4, 7107. [Google Scholar] [CrossRef]
- Bankoglu, E.E.; Stopper, H. Obesity-Related Genomic Instability and Altered Xenobiotic Metabolism: Possible Consequences for Cancer Risk and Chemotherapy. Expert Rev. Mol. Med. 2022, 24, e28. [Google Scholar] [CrossRef]
- Olujimi Odutola, P.; Gupta, R. IV Ketamine Infusion Therapy for Chronic Pain: A Systematic Review and Meta-analysis. Med. Adv. 2023, 1, 394–407. [Google Scholar] [CrossRef]

| Author(s) | Route of Administration | Timing | Dose | Duration | |
|---|---|---|---|---|---|
| Nikolajsen et al. [3] | IV | Postoperative (PLP present) | Bolus 0.1 mg/kg over 5 min, then infusion 7 mcg/kg/min for up to 45 min | Up to 45 min | n = 11; uncontrolled; short relief only. |
| Wilson et al. [4] | Epidural | Perioperative | Bolus 0.5 mg/kg, infusion 3.3 mg/kg/L at 15 mL/h, post-op 10–20 mL/h | 48–72 h | RCT; small sample; significant pain reduction. |
| Hayes et al. [5] | IV | Perioperative | Bolus 0.5 mg/kg, infusion 0.15 mg/kg/h | 72 h | RCT; underpowered; no significant benefit. |
| Eichenberger et al. [6] | IV | Postoperative (chronic PLP) | Not fully specified | 48 h | Moderate sample; partial blinding; combo with calcitonin significant. |
| Polomano et al. [7] | IV | Acute trauma recovery (combat-related) | 120 mcg/kg/h for most; two patients received 60 or 100 mcg/kg/h | 3 days | n = 19; non-randomized; benefit in subset only. |
| Buvanendran et al. [8] | Oral | Pre- and postoperative | 1 mg/kg 1h pre-op, repeated 8h later; 1 mg/kg TID POD1, 0.5 mg/kg TID POD2 | 2 days | Case series (n = 3); low quality; reported benefit. |
| Gene (Variant) | Effect on Ketamine PK/PD | Clinical Impact | References |
|---|---|---|---|
| CYP2B6 (*6 allele) | ↓ Enzyme activity → slower N-demethylation of ketamine. Higher ketamine/norketamine ratio (accumulation). | ↑ Ketamine levels: prolonged effect. More dissociative side effects were observed in *6 carriers. Consider dose reduction. | [22] |
| BDNF (Val66Met) | Val66Met polymorphism → ↓ activity-dependent BDNF secretion → impaired neuroplasticity. No direct effect on ketamine metabolism, but may modulate response via synaptic plasticity. | May reduce response to ketamine in CNS conditions (e.g., depression, potentially chronic pain). In pain, impaired neuroplasticity may contribute to persistent central sensitization and reduced ketamine efficacy, especially in PLP. Interaction with CYP2B6 and COMT variants may affect tolerability and dosing. | [25,29,30,31] |
| CYP3A4/5 (*22 or *1) | CYP3A4: major contributor, especially orally. 22 allele = ↓CYP3A4; CYP3A5*1 = additional metabolism. | Oral ketamine: high first-pass metabolism (CYP3A4) polymorphism or inhibitors cause significant PK changes. IV: CYP3A4 vs. CYP2B6 balance affects clearance. Monitor for drug interactions. | [22] |
| COMT (Val158Met) | Met allele → ~75% ↓COMT activity → ↑ dopamine/NE levels in CNS. It does not metabolize ketamine but alters pain modulation. | Low COMT activity is associated with ↑ pain sensitivity and variable opioid requirements. It may influence ketamine analgesic needs (e.g., high-pain-sensitivity patients might require higher or prolonged dosing; still under study). | [31,32] |
| SLC6A2 (NET rs28386840) | T allele → ↓ NET expression → impaired NE reuptake. No direct effect on ketamine metabolism. | Exaggerated cardiovascular response: T carriers have faster, higher blood pressure rise on ketamine. Higher risk of acute hypertension necessitates close monitoring or dose caution. | [34] |
| KCNS1 (Ile/Val SNP) | Polymorphism in Kv9.1 potassium channel; no effect on ketamine PK. | Val variant linked to a higher incidence of PLP. Identifies patients with severe pain phenotype who may particularly benefit from NMDA blockade (theoretical use in preemptive ketamine analgesia). | [36] |
| Treatment | Mechanism | Duration | Effectiveness in PAP | Side Effects | Limitations | Primary or Adjunct Use | References |
|---|---|---|---|---|---|---|---|
| Ketamine | NMDA receptor antagonist, reduces central sensitization and neuroinflammation | Short-term to long-term effects possible | High efficacy in refractory cases, perioperative administration reduces long-term pain | Neuropsychiatric effects (hallucinations, dissociation), hypertension, nausea | Neuropsychiatric side effects, unclear long-term safety, lack of standardized protocols | Adjunct, potentially primary in refractory cases | [2,13,37] |
| Opioids | Binds to opioid receptors, inhibits pain transmission | Short-term (hours) | Effective for acute pain but limited for chronic PAP | Addiction risk, tolerance, opioid-induced hyperalgesia, respiratory depression | High addiction potential, limited chronic pain efficacy, cognitive effects | Primary for acute pain, adjunct for chronic | [2,38,39] |
| Antidepressants (TCAs, SNRIs) | Enhances serotonin and norepinephrine inhibition of pain pathways | Long-term if effective | Limited evidence, may help with associated depression | Sedation, dry mouth, dizziness, cardiac arrhythmias | Delayed onset, high side effect burden, weak evidence for PAP | Adjunct | [38,40,41] |
| Anticonvulsants (Gabapentin, Pregabalin) | Modulates calcium channels to reduce excitatory neurotransmission | Long-term | Mixed results, may help in neuropathic components | Dizziness, peripheral edema, renal impairment, drowsiness | Need for titration, mixed results for PAP | Adjunct | [2,38,39] |
| Local Anesthetics | Blocks sodium channels to prevent pain signal transmission | Short-term (hours) | Useful for acute pain but requires repeat administration | Motor weakness, cardiotoxicity with bupivacaine | Short duration, limited efficacy in neuropathic pain | Adjunct | [41,42,43] |
| NMDA Antagonists (Memantine, Dextromethorphan) | NMDA receptor antagonists but weaker effects than ketamine | Short-term to none | Weak or inconsistent efficacy | Dizziness, nausea, agitation, headache | Weak NMDA antagonism, inconsistent results | Adjunct | [2,41,44] |
| Calcitonin | Modulates neurogenic inflammation and neuronal firing | Short-term | Some benefit, particularly in combination with ketamine | Flushing, hypocalcemia, dizziness, nausea | Limited data, side effects reduce tolerability | Adjunct | [2,38,41] |
| Non-Pharmacological Therapies | Includes mirror therapy, CBT, spinal cord stimulation, TENS | Varies | Effective for some, especially mirror therapy and spinal cord stimulation | None for psychological therapies, device-related risks for TENS, SCS | Variable effectiveness, requires adherence and training | Adjunct | [2,39,45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tappe, A.; Burzynski, E.; Patel, J.; Cheyne, I.; Mikaszewska-Sokolewicz, M. The Role of Pharmacogenomics in Optimizing Ketamine Therapy for Post-Amputation Pain. Reports 2025, 8, 156. https://doi.org/10.3390/reports8030156
Tappe A, Burzynski E, Patel J, Cheyne I, Mikaszewska-Sokolewicz M. The Role of Pharmacogenomics in Optimizing Ketamine Therapy for Post-Amputation Pain. Reports. 2025; 8(3):156. https://doi.org/10.3390/reports8030156
Chicago/Turabian StyleTappe, Alix, Emily Burzynski, Jhanvi Patel, Ithamar Cheyne, and Małgorzata Mikaszewska-Sokolewicz. 2025. "The Role of Pharmacogenomics in Optimizing Ketamine Therapy for Post-Amputation Pain" Reports 8, no. 3: 156. https://doi.org/10.3390/reports8030156
APA StyleTappe, A., Burzynski, E., Patel, J., Cheyne, I., & Mikaszewska-Sokolewicz, M. (2025). The Role of Pharmacogenomics in Optimizing Ketamine Therapy for Post-Amputation Pain. Reports, 8(3), 156. https://doi.org/10.3390/reports8030156






