Transvenous Lead Extraction in a European Low-Volume Center without On-Site Surgical Support
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Type
2.2. Study Participants
2.3. Definitions
2.4. TLE Indication
- Infectious indications for lead extraction
- Pocket infection
- Systemic infection and/or endocarditis
- Other indications for lead extraction
2.5. Statistical Analysis
2.6. TLE Procedure
3. Results
Reimplantation Strategy
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mond, H.G.; Proclemer, A. The 11th World Survey of Cardiac Pacing and Implantable Cardioverter-Defibrillators: Calendar Year 2009-A World Society of Arrhythmia’s Project. Pacing Clin. Electrophysiol. 2011, 34, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Borek, P.P.; Wilkoff, B.L. Pacemaker and ICD leads: Strategies for long-term management. J. Interv. Card. Electrophysiol. 2008, 23, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Kamireddy, S.; Nemec, J.; Voigt, A.; Saba, S. Predictors of Complications of Endovascular Chronic Lead Extractions from Pacemakers and Defibrillators: A Single-Operator Experience. J. Cardiovasc. Electrophysiol. 2009, 20, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Kirkfeldt, R.E.; Johansen, J.B.; Nohr, E.A.; Jørgensen, O.D.; Nielsen, J.C. Complications after cardiac implantable electronic device implantations: An analysis of a complete, nationwide cohort in Denmark. Eur. Heart J. 2013, 35, 1186–1194. [Google Scholar] [CrossRef]
- Eckstein, J.; Koller, M.T.; Zabel, M.; Kalusche, D.; Schaer, B.A.; Osswald, S.; Sticherling, C. Necessity for surgical revision of defibrillator leads implanted long-term: Causes and management. Circulation 2008, 117, 2727–2733. [Google Scholar] [CrossRef]
- Voigt, A.; Shalaby, A.; Saba, S. Continued Rise in Rates of Cardiovascular Implantable Electronic Device Infections in the United States: Temporal Trends and Causative Insights. Pacing Clin. Electrophysiol. 2010, 33, 414–419. [Google Scholar] [CrossRef]
- Cabell, C.H.; Heidenreich, P.; Chu, V.H.; Moore, C.M.; Stryjewski, M.; Corey, G.; Fowler, V.G. Increasing rates of cardiac device infections among medicare beneficiaries: 1990–1999. Am. Heart J. 2004, 147, 582–586. [Google Scholar] [CrossRef]
- Haqqani, H.M.; Mond, H.G. The Implantable Cardioverter-Defibrillator Lead: Principles, Progress, and Promises. Pacing Clin. Electrophysiol. 2009, 32, 1336–1353. [Google Scholar] [CrossRef]
- Kleemann, T.; Becker, T.; Doenges, K.; Vater, M.; Senges, J.; Schneider, S.; Saggau, W.; Weisse, U.; Seidl, K. Annual rate of transvenous defibrillation lead defects in implantable cardiovert-er-defibrillators over a period of >10 years. Circulation 2007, 115, 2474–2480. [Google Scholar] [CrossRef]
- Dorwarth, U.; Frey, B.; Dugas, M.; Matis, T.; Fiek, M.; Schmoeckel, M.; Remp, T.; Durchlaub, I.; Gerth, A.; Steinbeck, G.; et al. Transvenous defibrillation leads: High incidence of failure during long-term follow-up. J. Cardiovasc. Electrophysiol. 2003, 14, 38–43. [Google Scholar] [CrossRef]
- Ellenbogen, K.A.; Wood, M.A.; Shepard, R.K.; Clemo, H.F.; Vaughn, T.; Holloman, K.; Dow, M.; Leffler, J.; Abeyratne, A.; Verness, D. Detection and management of an implantable cardioverter defibrillator lead failure: Incidence and clinical implications. J. Am. Coll. Cardiol. 2003, 41, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Luria, D.; Glikson, M.; Brady, P.A.; Lexvold, N.Y.; Rasmussen, M.J.; Hodge, D.O.; Chugh, S.S.; Rea, R.F.; Hayes, D.L.; Hammill, S.C.; et al. Predictors and mode of detection of transvenous lead malfunction in implantable defib-rillators. Am. J. Cardiol. 2001, 87, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Pakarinen, S.; Oikarinen, L.; Toivonen, L. Short-term implantation-related complications of cardiac rhythm management device therapy: A retrospective single-centre 1-year survey. Europace 2010, 12, 103–108. [Google Scholar] [CrossRef]
- Vatankulu, M.A.; Goktekin, O.; Kaya, M.G.; Ayhan, S.; Kucukdurmaz, Z.; Sutton, R.; Henein, M. Effect of Long-Term Resynchronization Therapy on Left Ventricular Remodeling in Pacemaker Patients Upgraded to Biventricular Devices. Am. J. Cardiol. 2009, 103, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.O.; Shea, J.B.; Ellison, K.E. Upgrade of permanent pacemakers and single chamber implantable cardioverter defibril-lators to pectoral dual chamber implantable cardioverter defibrillators: Indications, surgical approach, and long-term clinical results. Pacing Clin. Electrophysiol. 2002, 25, 1715–1723. [Google Scholar] [CrossRef]
- Foley, P.W.; Muhyaldeen, S.A.; Chalil, S.; Smith, R.E.; Sanderson, J.E.; Leyva, F. Long-term effects of upgrading from right ventricular pacing to cardiac resynchronization therapy in patients with heart failure. Europace 2009, 11, 495–501. [Google Scholar] [CrossRef]
- Wilkoff, B.L.; Love, C.J.; Byrd, C.L.; Bongiorni, M.G.; Carrillo, R.G.; Crossley, G.H.; Epstein, L.M.; Friedman, R.A.; Kennergren, C.E.; Mitkowski, P.; et al. Transvenous Lead Extraction: Heart Rhythm Society Expert Consensus on Facilities, Training, Indications, and Patient Management: This document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009, 6, 1085–1104. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. Europace 2018, 20, 1217. [Google Scholar] [CrossRef]
- Sharma, S.; Ekeruo, I.A.; Nand, N.P.; Raman, A.S.; Zhang, X.; Reddy, S.K.; Hariharan, R. Safety and Efficacy of Transvenous Lead Extraction Utilizing the Evolution Mechanical Lead Extraction System. JACC Clin. Electrophysiol. 2018, 4, 212–220. [Google Scholar] [CrossRef]
- Pecha, S.; Ziegelhoeffer, T.; Yildirim, Y.; Choi, Y.-H.; Willems, S.; Reichenspurner, H.; Burger, H.; Hakmi, S. Safety and efficacy of transvenous lead extraction of very old leads. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 402–407. [Google Scholar] [CrossRef]
- Stefańczyk, P.; Nowosielecka, D.; Polewczyk, A.; Tułecki, Ł.; Tomków, K.; Jacheć, W.; Lewicka, E.; Tomaszewski, A.; Kutarski, A. Safety and Effectiveness of Transvenous Lead Extraction in Patients with Infected Cardiac Resynchronization Therapy Devices; Is It More Risky than Extraction of Other Systems? Int. J. Environ. Res. Public Health 2022, 19, 5803. [Google Scholar] [CrossRef] [PubMed]
- Segreti, L.; Santoro, M.G.; Di Cori, A.; Fiorentini, F.; Zucchelli, G.; Bernini, G.; De Lucia, R.; Viani, S.; Paperini, L.; Barletta, V.; et al. Safety and efficacy of transvenous mechanical lead extraction in patients with abandoned leads. Europace 2020, 22, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.-M.; Clancy, J.; Deharo, J.-C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, M.G.; Romano, S.L.; Kennergren, C.; Butter, C.; Deharo, J.C.; Kutarsky, A.; Rinaldi, C.A.; Maggioni, A.P.; Blomström-Lundqvist, C.; Auricchio, A. ELECTRa (European Lead Extraction ConTRolled) Registry—Shedding light on transvenous lead extraction real-world practice in Europe. Herzschrittmachertherapie Elektrophysiologie 2013, 24, 171–175. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Garisto, J.; Carrillo, R.G. Management of cardiac device-related infections: A review of protocol-driven care. Int. J. Cardiol. 2013, 166, 55–60. [Google Scholar] [CrossRef]
- Monkowski, D.; Axelrod, P.; Fekete, T.; Hollander, T.; Furukawa, S.; Samuel, R. Infections associated with ventricular assist devices: Epidemiology and effect on prognosis after transplantation. Transpl. Infect. Dis. 2007, 9, 114–120. [Google Scholar] [CrossRef]
- Toda, K.; Yonemoto, Y.; Fujita, T.; Shimahara, Y.; Sato, S.; Nakatani, T.; Kobayashi, J. Risk Analysis of Bloodstream Infection During Long-Term Left Ventricular Assist Device Support. Ann. Thorac. Surg. 2012, 94, 1387–1393. [Google Scholar] [CrossRef]
- Benaerts, P.; Ridler, B.; Vercaeren, P.; Thompson, J.; Campbell, W. Gentamicin beads in vascular surgery: Long-term results of implantation. Cardiovasc. Surg. 1999, 7, 447–450. [Google Scholar] [CrossRef]
- Deharo, J.-C.; Quatre, A.; Mancini, J.; Khairy, P.; Le Dolley, Y.; Casalta, J.-P.; Peyrouse, E.; Prévôt, S.; Thuny, F.; Collart, F.; et al. Long-term outcomes following infection of cardiac implantable electronic devices: A prospective matched cohort study. Heart 2012, 98, 724–731. [Google Scholar] [CrossRef]
- Franceschi, F.; Thuny, F.; Giorgi, R.; Sanaa, I.; Peyrouse, E.; Assouan, X.; Prévôt, S.; Bastard, E.; Habib, G.; Deharo, J.C. Incidence, risk factors, and outcome of traumatic tricuspid regurgitation after percuta-neous ventricular lead removal. J. Am. Coll. Cardiol. 2009, 53, 2168–2174. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Carabello, B.A.; Faxon, D.P.; Freed, M.D.; Lytle, B.W.; O’Gara, P.T.; O’Rourke, R.A.; Shah, P.M.; Bonow, R.O.; Carabello, B.A.; et al. ACC/AHA 2008 guideline update on valvular heart disease: Focused update on infective endocarditis: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Inter-ventions, and Society of Thoracic Surgeons. Circulation 2008, 118, 887–896. [Google Scholar] [PubMed]
- Wilkoff, B.L.; Byrd, C.L.; Love, C.J.; Hayes, D.L.; Sellers, T.; Schaerf, R.; Parsonnet, V.; Epstein, L.M.; A Sorrentino, R.; Reiser, C. Pacemaker lead extraction with the laser sheath: Results of the pacing lead extraction with the excimer sheath (PLEXES) trial. J. Am. Coll. Cardiol. 1999, 33, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.M.; Byrd, C.L.; Wilkoff, B.L.; Love, C.J.; Sellers, T.D.; Hayes, D.L.; Reiser, C. Initial experience with larger laser sheaths for the removal of transvenous pacemaker and implantable defibrillator leads. Circulation 1999, 100, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Kennergren, C.; Bucknall, C.A.; Butter, C.; Charles, R.; Fuhrer, J.; Grosfeld, M.; Tavernier, R.; Morgado, T.B.; Mortensen, P.; Paul, V.; et al. Laserassisted lead extraction: The European experience. Europace 2007, 9, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Wilkoff, B.L. Transvenous leads extraction with electrosurgical dissection sheaths. Initial experience. Pacing Clin. Electrophysiol. 2000, 23, 679–684. [Google Scholar]
- Neuzil, P.; Taborsky, M.; Rezek, Z.; Vopalka, R.; Sediva, L.; Niederle, P.; Reddy, V. Faculty Opinions recommendation of Pacemaker and ICD lead extraction with electrosurgical dissection sheaths and standard transvenous extraction systems: Results of a randomized trial. Europace 2007, 9, 98–104. [Google Scholar] [CrossRef]
- Mathur, G.; Stables, R.H.; Heaven, D.; Stack, Z.; Lovegrove, A.; Ingram, A.; Sutton, R. Cardiac pacemaker lead extraction using conventional techniques:a single centre experience. Int. J. Cardiol. 2003, 91, 215–219. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Soldati, E.; Zucchelli, G.; Di Cori, A.; Segreti, L.; De Lucia, R.; Solarino, G.; Balbarini, A.; Marzilli, M.; Mariani, M. Transvenous removal of pacing and implantable cardiac defibrillating leads using single sheath mechanical dilatation and multiple venous approaches: High success rate and safety in more than 2000 leads. Eur. Heart J. 2008, 29, 2886–2893. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. Faculty Opinions recommendation of 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Brunner, M.P.; Cronin, E.M.; Duarte, V.E.; Yu, C.; Tarakji, K.G.; Martin, D.O.; Callahan, T.; Cantillon, D.J.; Niebauer, M.J.; Saliba, W.I.; et al. Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm 2014, 11, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Kennergren, C.; Bjurman, C.; Wiklund, R.; Gäbel, J. A single-centre experience of over one thousand lead extractions. Europace 2009, 11, 612–617. [Google Scholar] [CrossRef]
- Breeman, K.T.N.; Beurskens, N.E.G.; Driessen, A.H.G.; Wilde, A.A.M.; Tjong, F.V.Y.; Knops, R.E. Timing and mid-term outcomes of using leadless pacemakers as replacement for infected cardiac implantable electronic devices. J. Interv. Card. Electrophysiol. 2022, 66, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Giacomin, E.; Falzone, P.V.; Dall’aglio, P.B.; Pittorru, R.; De Lazzari, M.; Vianello, R.; Bertaglia, E.; Tarzia, V.; Iliceto, S.; Gerosa, G.; et al. Subcutaneous implantable cardioverter defibrillator after transvenous lead extraction: Safety, efficacy and outcome. J. Interv. Card. Electrophysiol. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mitacchione, G.; Schiavone, M.; Gasperetti, A.; Arabia, G.; Breitenstein, A.; Cerini, M.; Palmisano, P.; Montemerlo, E.; Ziacchi, M.; Gulletta, S.; et al. Outcomes of leadless pacemaker implantation following transvenous lead extraction in high-volume referral centers: Real-world data from a large international registry. Heart Rhythm 2023, 20, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Deckx, S.; Marynissen, T.; Rega, F.; Ector, J.; Nuyens, D.; Heidbuchel, H.; Willems, R. Predictors of 30-day and 1-year mortality after transvenous lead extraction: A single-centre experience. Europace 2014, 16, 1218–1225. [Google Scholar] [CrossRef]
- Di Monaco, A.; Pelargonio, G.; Narducci, M.L.; Manzoli, L.; Boccia, S.; Flacco, M.E.; Capasso, L.; Barone, L.; Perna, F.; Bencardino, G.; et al. Safety of transvenous lead extraction according to centre volume: A systematic review and meta-analysis. Europace 2014, 16, 1496–1507. [Google Scholar] [CrossRef]
- Calvagna, G.M.; Evola, R.; Scardace, G.; Valsecchi, S. Single-operator experience with a mechanical approach for removal of pacing and implantable defibrillator leads. Europace 2009, 11, 1505–1509. [Google Scholar] [CrossRef]
- Sideris, S.; Kasiakogias, A.; Pirounaki, M.; Gatzoulis, K.; Sotiropoulos, I.; Dilaveris, P.; Traxanas, K.; Vouliotis, A.-I.; Voliotis, A.; Manakos, K.; et al. Transvenous extraction of cardiac rhythm device leads: A report of the experience from a single referral centre in Greece. Hell. J. Cardiol. 2015, 56, 55–60. [Google Scholar]
- Smith, H.J.; Fearnot, N.E.; Byrd, C.L.; Wilkoff, B.L.; Love, C.J.; Sellers, T.D.; Database, F.T.U.L.E. Five-Years Experience with Intravascular Lead Extraction. Pacing Clin. Electrophysiol. 1994, 17, 2016–2020. [Google Scholar] [CrossRef]
- Azevedo, A.I.; Primo, J.; Gonçalves, H.; Oliveira, M.; Adão, L.; Santos, E.; Ribeiro, J.; Fonseca, M.; Dias, A.V.; Vouga, L.; et al. Lead Extraction of Cardiac Rhythm Devices: A Report of a Single-Center Experience. Front. Cardiovasc. Med. 2017, 4, 18. [Google Scholar] [CrossRef]
- Kutarski, A.; Jacheć, W.; Polewczyk, A.; Nowosielecka, D.; Miszczak-Knecht, M.; Brzezinska, M.; Bieganowska, K. Transvenous Lead Extraction in Adult Patient with Leads Implanted in Childhood-Is That the Same Procedure as in Other Adult Patients? Int. J. Environ. Res. Public Health 2022, 19, 14594. [Google Scholar] [CrossRef] [PubMed]
- Deharo, J.C.; Bongiorni, M.G.; Rozkovec, A.; Bracke, F.; Defaye, P.; Fernandez-Lozano, I.; Golzio, P.G.; Hansky, B.; Kennergren, C.; Manolis, A.S.; et al. Pathways for training and accreditation for transvenous lead extraction: A European Heart Rhythm Association position paper. Europace 2012, 14, 124–134. [Google Scholar] [PubMed]
- Sood, N.; Martin, D.T.; Lampert, R.; Curtis, J.P.; Parzynski, C.; Clancy, J. Incidence and Predictors of Perioperative Complications with Transvenous Lead Extractions. Circ. Arrhythmia Electrophysiol. 2018, 11, e004768. [Google Scholar] [CrossRef] [PubMed]
- Kutarski, A.; Czajkowski, M.; Pietura, R.; Obszański, B.; Polewczyk, A.; Jacheć, W.; Polewczyk, M.; Młynarczyk, K.; Grabowski, M.; Opolski, G. Effectiveness, safety, and long-term outcomes of non-powered mechanical sheaths for transvenous lead extraction. Europace 2018, 20, 1324–1333. [Google Scholar] [CrossRef]
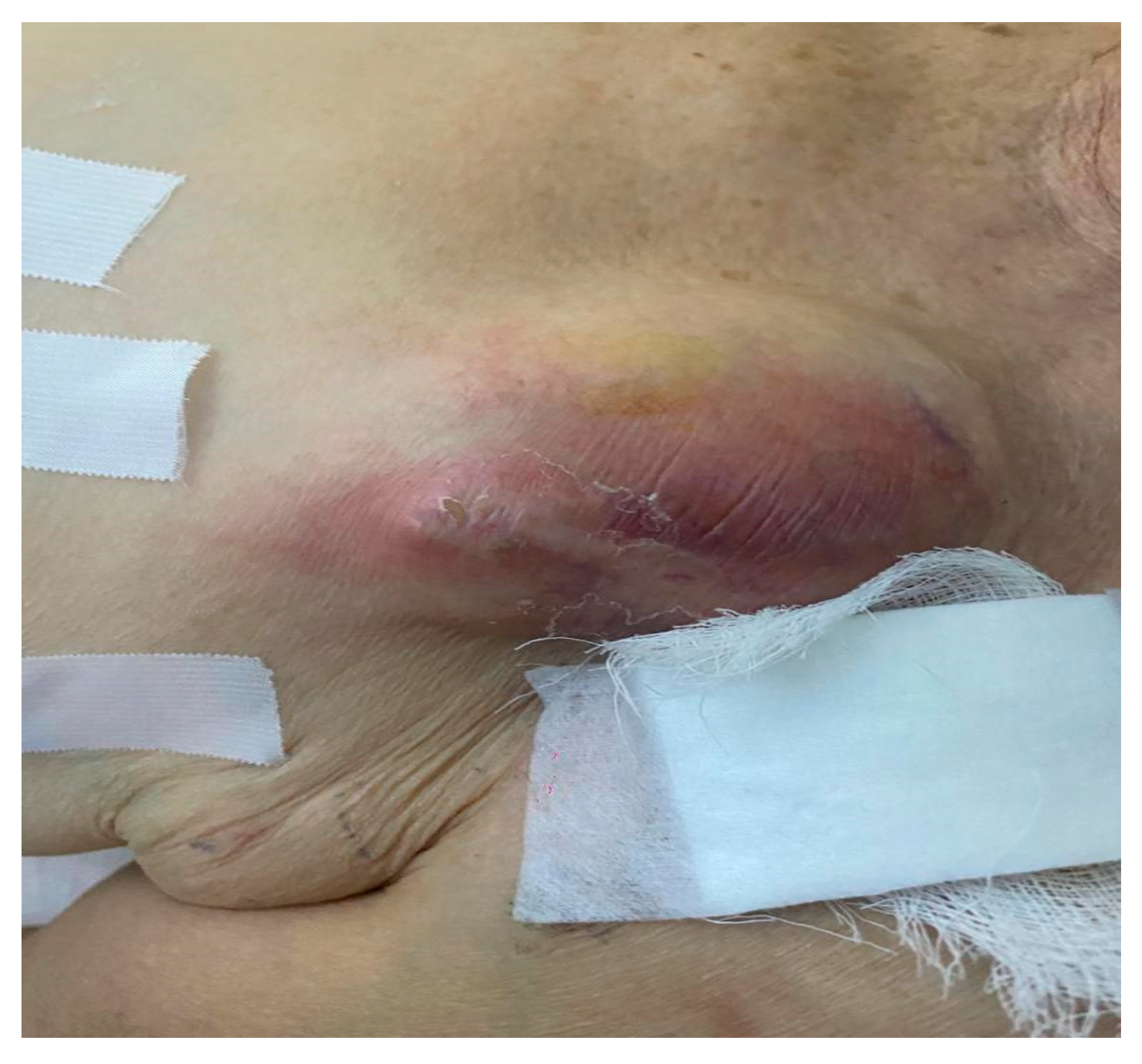

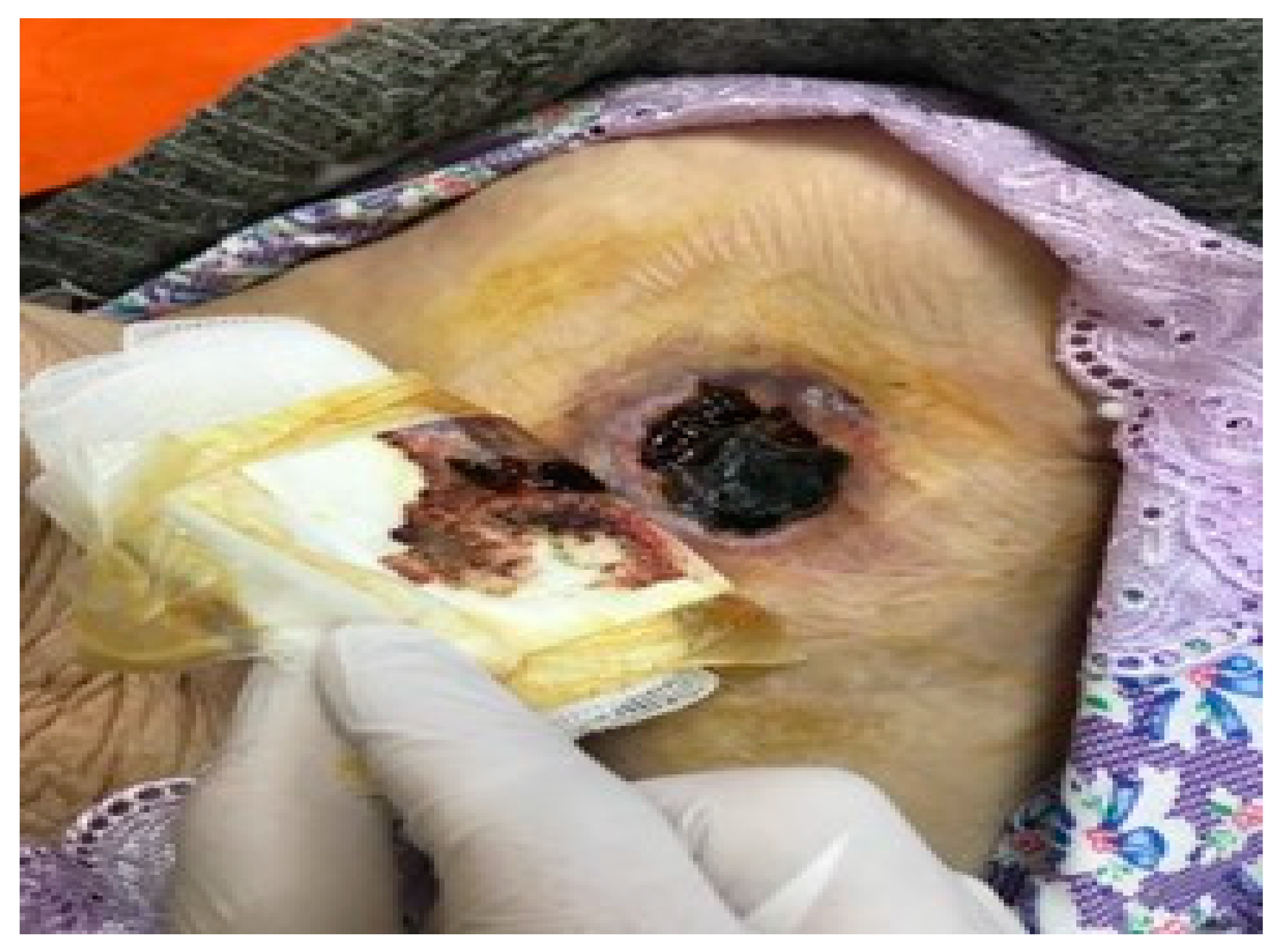
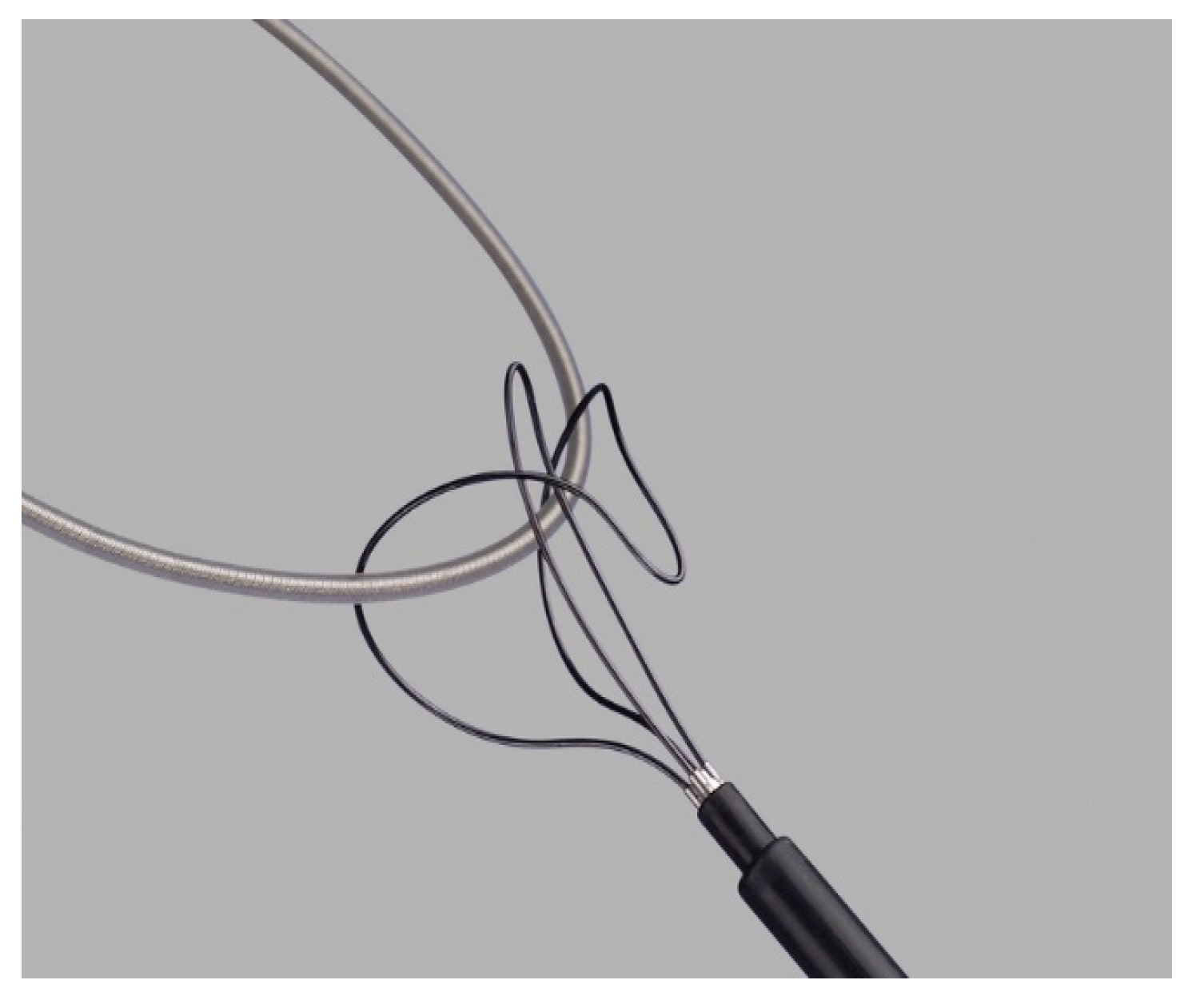

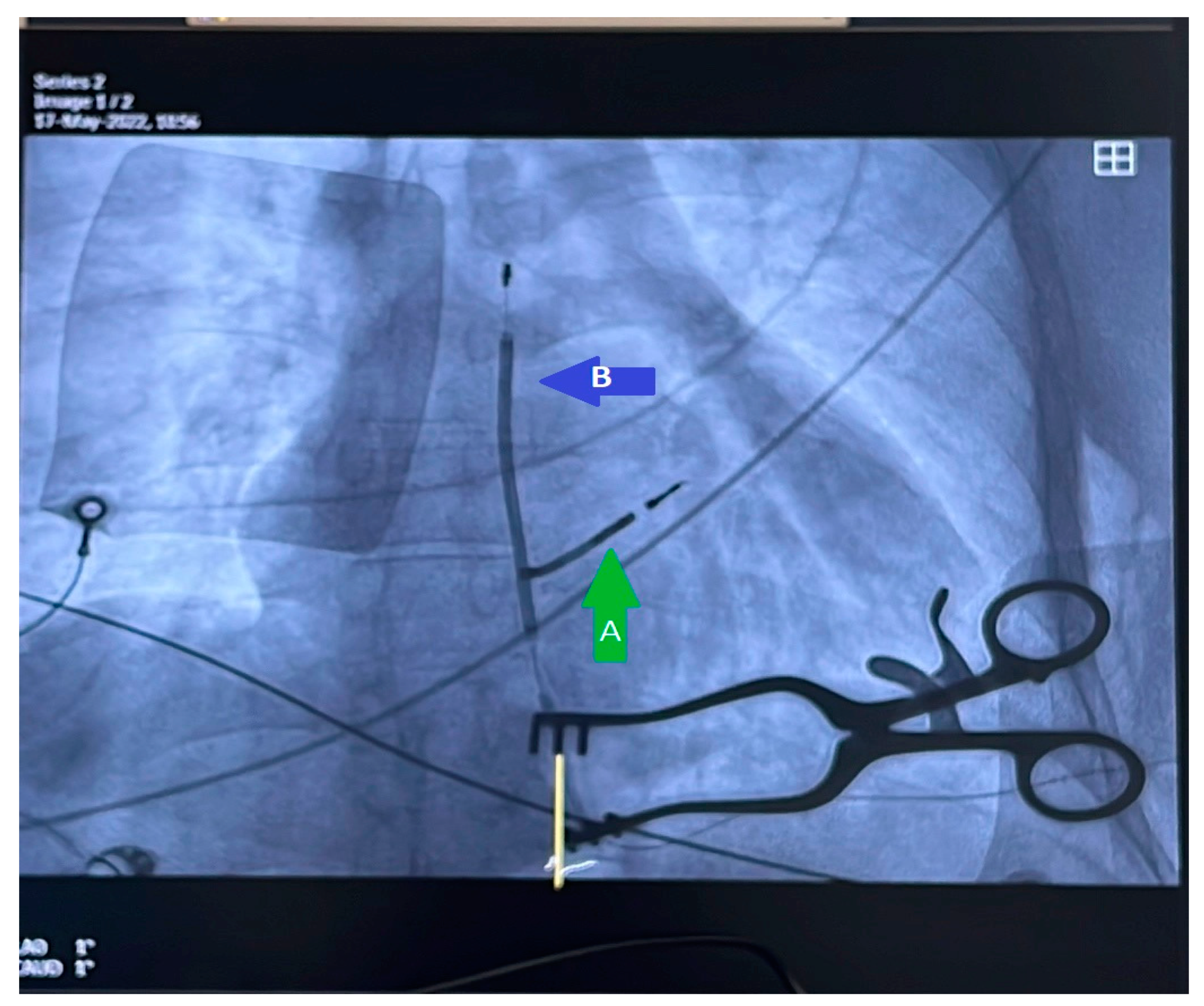
| Patient age, years, mean (standard deviation) | 66.16 (16.00) | |
| Time since first implant, years mean (standard deviation) | 6.92 (4.47) 0.477) | |
| Left ventricular EF, %, mean (standard deviation) | 43.8% (14.06) | |
| Creatinine, mg/dL, mean (standard deviation) | 1.00 (0.46) | |
| Frequency = n | Percent% | |
| Number of patients | 88 | 100 |
| Sex, male | 59 | 67.0% |
| Co-morbidities | ||
| HTN | 55 | 65.5% |
| Ischaemic Cardiomyopathy | 21 | 25% |
| Diabetes | 23 | 27.4% |
| Chronic kidney disease | 13 | 14.7% |
| Dyslipidemia | 10 | 11.9% |
| Atrial fibrillation | 36 | 42.9% |
| Anemia | 52 | 63.4% |
| Indications for removal | ||
| Infectious indication | 65 | 74% |
| Endocarditis | 28 | 31.8% |
| Pocket infection | 37 | 42% |
| Non-infectious indication | 23 | 26% |
| Venous occlusion | 9 | 10.2% |
| Abandoned/disfunctional lead | 14 | 15.9% |
| Device Type | Frequency = n | Percent% |
|---|---|---|
| VVI | 12 | 13.6 |
| DDD | 32 | 36.4 |
| CRT-P | 7 | 8.0 |
| SC-ICD | 15 | 17.0 |
| DC-ICD | 7 | 8.0 |
| CRT-D | 15 | 17 |
| Total | 88 | 100.0 |
| Lead Type | ||
|---|---|---|
| Lead age (mean, years) | 6.92 ± 4.47 (1–26) | |
| >5 (years, leads n) | 46 | 52.8% |
| >10 (years, leads n) | 14 | 15.8% |
| Leads extracted per procedure (=n) | ||
| Median | 2 (1–4) | |
| 1 | 31 | 38.2% |
| 2 | 34 | 42% |
| 3 | 14 | 17.3% |
| 4 | 2 | 2.5% |
| Type of extracted leads | Frequency = n | Percent% |
| RA/RV pacing | 102 | 68 |
| ICD S-C | 25 | 16.6 |
| ICD D-C | 7 | 4.6 |
| CS pace | 16 | 10.6 |
| Type of fixation | ||
| Active fixation | 139 | 92.6 |
| Passive fixation | 11 | 7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dardari, M.; Iorgulescu, C.; Bataila, V.; Deaconu, A.; Cinteza, E.; Vatasescu, R.; Padovani, P.; Vasile, C.M.; Dorobantu, M. Transvenous Lead Extraction in a European Low-Volume Center without On-Site Surgical Support. Reports 2023, 6, 41. https://doi.org/10.3390/reports6030041
Dardari M, Iorgulescu C, Bataila V, Deaconu A, Cinteza E, Vatasescu R, Padovani P, Vasile CM, Dorobantu M. Transvenous Lead Extraction in a European Low-Volume Center without On-Site Surgical Support. Reports. 2023; 6(3):41. https://doi.org/10.3390/reports6030041
Chicago/Turabian StyleDardari, Mohamed, Corneliu Iorgulescu, Vlad Bataila, Alexandru Deaconu, Eliza Cinteza, Radu Vatasescu, Paul Padovani, Corina Maria Vasile, and Maria Dorobantu. 2023. "Transvenous Lead Extraction in a European Low-Volume Center without On-Site Surgical Support" Reports 6, no. 3: 41. https://doi.org/10.3390/reports6030041
APA StyleDardari, M., Iorgulescu, C., Bataila, V., Deaconu, A., Cinteza, E., Vatasescu, R., Padovani, P., Vasile, C. M., & Dorobantu, M. (2023). Transvenous Lead Extraction in a European Low-Volume Center without On-Site Surgical Support. Reports, 6(3), 41. https://doi.org/10.3390/reports6030041









