Pro- and Anti-Inflammatory Neuropeptides and Glia: The Balance Between Neuroprotection and Neuroinflammation
Abstract
1. Introduction
2. Methodology
3. Peptides with Anti-Inflammatory Cell-Resolving Actions
3.1. Vasoactive Intestinal Peptide (VIP) and Pituitary Adenylate Cyclase-Activated Poly-Peptide (PACAP)
3.2. Neurotensin
3.3. Proopiomelanocortin
4. Neuropeptides with Pro-Inflammatory Effects
4.1. Substance P and Opioids
Endogenous Opioids
4.2. Corticotropin-Releasing Hormone
4.3. Angiotensin
5. Adipokines
5.1. Ghrelin
5.2. Leptin
6. Concluding Remarks
Potential and Limitations of Neuropeptide Mimetics for Neurodegenerative Disease Therapeutics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nusbaum, M.P.; Blitz, D.M. Neuropeptide Modulation of Microcircuits. Curr. Opin. Neurobiol. 2012, 22, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Eiden, L.E. Two Ancient Neuropeptides, PACAP and AVP, Modulate Motivated Behavior at Synapses in the Extrahypothalamic Brain: A Study in Contrast. Cell Tissue Res. 2019, 375, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R. Neuropeptide Signaling near and Far: How Localized and Timed Is the Action of Neuropeptides in Brain Circuits? Invert. Neurosci. 2009, 9, 57. [Google Scholar] [CrossRef]
- Langer, I.; Jeandriens, J.; Couvineau, A.; Sanmukh, S.; Latek, D. Signal Transduction by VIP and PACAP Receptors. Biomedicines 2022, 10, 406. [Google Scholar] [CrossRef]
- Hökfelt, T.; Bartfai, T.; Bloom, F. Neuropeptides: Opportunities for Drug Discovery. Lancet Neurol. 2003, 2, 463–472. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Kölliker-Frers, R.; Udovin, L.; Otero-Losada, M.; Kobiec, T.; Herrera, M.I.; Palacios, J.; Razzitte, G.; Capani, F. Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease. Mediat. Inflamm. 2021, 2021, 9999146. [Google Scholar] [CrossRef]
- Godbout, J.P.; Chen, J.; Abraham, J.; Richwine, A.F.; Berg, B.M.; Kelley, K.W.; Johnson, R.W. Exaggerated Neuroinflammation and Sickness Behavior in Aged Mice after Activation of the Peripheral Innate Immune System. FASEB J. 2005, 19, 1329–1331. [Google Scholar] [CrossRef]
- Biesmans, S.; Meert, T.F.; Bouwknecht, J.A.; Acton, P.D.; Davoodi, N.; De Haes, P.; Kuijlaars, J.; Langlois, X.; Matthews, L.J.; Ver Donck, L.; et al. Systemic Immune Activation Leads to Neuroinflammation and Sickness Behavior in Mice. Mediat. Inflamm. 2013, 2013, 271359. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of Inflammation, Neurogenic Inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Özçete, Ö.D.; Banerjee, A.; Kaeser, P.S. Mechanisms of Neuromodulatory Volume Transmission. Mol. Psychiatry 2024, 29, 3680–3693. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Agnati, L.F.; Bechter, K.; Jansson, A.; Tarakanov, A.O.; Fuxe, K. The Role of Transmitter Diffusion and Flow versus Extracellular Vesicles in Volume Transmission in the Brain Neural–Glial Networks. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140183. [Google Scholar] [CrossRef]
- Pandey, P.; Kaur, G.; Babu, K. Crosstalk between Neurons and Glia through G-Protein Coupled Receptors: Insights from Caenorhabditis Elegans. In Progress in Molecular Biology and Translational Science; Shukla, A.K., Ed.; G Protein-Coupled Receptors—Part A; Academic Press: Amsterdam, The Netherlands, 2022; Volume 193, pp. 119–144. [Google Scholar]
- Block, M.L.; Li, G.; Qin, L.; Wu, X.; Pei, Z.; Wang, T.; Wilson, B.; Yang, J.; Hong, J.S. Potent Regulation of Microglia-Derived Oxidative Stress and Dopaminergic Neuron Survival: Substance P vs. Dynorphin. FASEB J. 2006, 20, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hernandez, A.J.; Munguba, H.; Levitz, J. Emerging Modes of Regulation of Neuromodulatory G Protein-Coupled Receptors. Trends Neurosci. 2024, 47, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; Maura, G.; Agnati, L.F. Receptor–Receptor Interactions and Glial Cell Functions with a Special Focus on G Protein-Coupled Receptors. Int. J. Mol. Sci. 2021, 22, 8656. [Google Scholar] [CrossRef]
- Lauritano, D.; Mastrangelo, F.; D’Ovidio, C.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Frydas, I.; Kritas, S.K.; Trimarchi, M.; Carinci, F.; et al. Activation of Mast Cells by Neuropeptides: The Role of Pro-Inflammatory and Anti-Inflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 4811. [Google Scholar] [CrossRef] [PubMed]
- Kulka, M.; Sheen, C.H.; Tancowny, B.P.; Grammer, L.C.; Schleimer, R.P. Neuropeptides Activate Human Mast Cell Degranulation and Chemokine Production. Immunology 2008, 123, 398–410. [Google Scholar] [CrossRef]
- Kerage, D.; Sloan, E.K.; Mattarollo, S.R.; McCombe, P.A. Interaction of Neurotransmitters and Neurochemicals with Lymphocytes. J. Neuroimmunol. 2019, 332, 99–111. [Google Scholar] [CrossRef]
- Woods, T.A.; Du, M.; Carmody, A.; Peterson, K.E. Neuropeptide Y Negatively Influences Monocyte Recruitment to the Central Nervous System During Retrovirus Infection. J. Virol. 2015, 90, 2783–2793. [Google Scholar] [CrossRef]
- Zhang, X.; Du, P.; Bai, B.; Feng, P.; Lian, X.; Hölscher, C.; Wang, Y.; Xue, G. The GLP-1 Receptor Agonist Liraglutide Inhibits Necroptosis and Neuroinflammation in a Mouse Model of Parkinson’s Disease with Diabetes Co-Morbidity. Front. Neurosci. 2025, 19, 1596506. [Google Scholar] [CrossRef]
- Fahrenkrug, J.; Hannibal, J. Neurotransmitters Co-Existing with VIP or PACAP. Peptides 2004, 25, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J. VIP and PACAP: Neuropeptide Modulators of CNS Inflammation, Injury, and Repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Karunia, J.; Niaz, A.; Mandwie, M.; Thomas Broome, S.; Keay, K.A.; Waschek, J.A.; Al-Badri, G.; Castorina, A. PACAP and VIP Modulate LPS-Induced Microglial Activation and Trigger Distinct Phenotypic Changes in Murine BV2 Microglial Cells. Int. J. Mol. Sci. 2021, 22, 10947. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M. Inhibition of Interferon (IFN) γ-Induced Jak-STAT1 Activation in Microglia by Vasoactive Intestinal Peptide: Inhibitory Effect On CD40, IFN-Induced Protein-10, And Inducible Nitric-Oxide Synthase Expression. J. Biol. Chem. 2003, 278, 27620–27629. [Google Scholar] [CrossRef]
- Delgado, M.; Jonakait, G.M.; Ganea, D. Vasoactive Intestinal Peptide and Pituitary Adenylate Cyclase-Activating Polypeptide Inhibit Chemokine Production in Activated Microglia. Glia 2002, 39, 148–161. [Google Scholar] [CrossRef]
- Farnham, M.M.J.; Tallapragada, V.J.; O’Connor, E.T.; Nedoboy, P.E.; Dempsey, B.; Mohammed, S.; Fong, A.Y.; Lung, M.S.Y.; Derakhshan, F.; Wilson, R.J.A.; et al. PACAP-PAC1 Receptor Activation Is Necessary for the Sympathetic Response to Acute Intermittent Hypoxia. Front. Neurosci. 2019, 13, 881. [Google Scholar] [CrossRef]
- Witzel, R.; Block, A.; Pollmann, S.; Oetzel, L.; Fleck, F.; Bonaterra, G.A.; Kinscherf, R.; Schwarz, A. PACAP Regulates VPAC1 Expression, Inflammatory Processes and Lipid Homeostasis in M1- and M2-Macrophages. Front. Cardiovasc. Med. 2023, 10, 1264901. [Google Scholar] [CrossRef]
- Lelievre, V.; Ghiani, C.A.; Seksenyan, A.; Gressens, P.; de Vellis, J.; Waschek, J.A. Growth Factor-Dependent Actions of PACAP on Oligodendrocyte Progenitor Proliferation. Regul. Pept. 2006, 137, 58–66. [Google Scholar] [CrossRef]
- Al-Keilani, M.S.; Almomani, B.A.; Al-Sawalha, N.A.; Al Qawasmeh, M.; Jaradat, S.A. Significance of Serum VIP and PACAP in Multiple Sclerosis: An Exploratory Case–Control Study. Neurol. Sci. 2022, 43, 2621–2630. [Google Scholar] [CrossRef]
- De La Fuente, M.; Delgado, M.; del Rio, M.; Martinez, C.; Hernanz, A.; Gomariz, R.P. Stimulation by Vasoactive Intestinal Peptide (VIP) of Phagocytic Function in Rat Macrophages. Protein Kinase C Involvement. Regul. Pept. 1993, 48, 345–353. [Google Scholar] [CrossRef]
- Power, J.H.; Barnes, O.L.; Chegini, F. Lewy Bodies and the Mechanisms of Neuronal Cell Death in Parkinson’s Disease and Dementia with Lewy Bodies. Brain Pathol. 2017, 27, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Tamas, A.; Reglödi, D.; Tizabi, Y. PACAP Protects Against Salsolinol-Induced Toxicity in Dopaminergic SH-SY5Y Cells: Implication for Parkinson’s Disease. J. Mol. Neurosci. 2013, 50, 600–607. [Google Scholar] [CrossRef]
- Reglodi, D.; Maasz, G.; Pirger, Z.; Rivnyak, A.; Balogh, D.; Jungling, A.; Fulop, B.; Mark, L.; Tamas, A. Neurochemical Changes in Different Brain Regions Induced by PACAP—Relations to Neuroprotection. SpringerPlus 2015, 4, L56. [Google Scholar] [CrossRef]
- Korkmaz, O.T.; Tunçel, N.; Tunçel, M.; Öncü, E.M.; Şahintürk, V.; Çelik, M. Vasoactive Intestinal Peptide (VIP) Treatment of Parkinsonian Rats Increases Thalamic Gamma-Aminobutyric Acid (GABA) Levels and Alters the Release of Nerve Growth Factor (NGF) by Mast Cells. J. Mol. Neurosci. 2010, 41, 278–287. [Google Scholar] [CrossRef]
- Delgado, M.; Ganea, D. Neuroprotective Effect of Vasoactive Intestinal Peptide (VIP) in a Mouse Model of Parkinson’s Disease by Blocking Microglial Activation. FASEB J. 2003, 17, 1–18. [Google Scholar] [CrossRef]
- Han, P.; Caselli, R.J.; Baxter, L.; Serrano, G.; Yin, J.; Beach, T.G.; Reiman, E.M.; Shi, J. Association of Pituitary Adenylate Cyclase–Activating Polypeptide with Cognitive Decline in Mild Cognitive Impairment Due to Alzheimer Disease. JAMA Neurol. 2015, 72, 333–339. [Google Scholar] [CrossRef]
- Rat, D.; Schmitt, U.; Tippmann, F.; Dewachter, I.; Theunis, C.; Wieczerzak, E.; Postina, R.; van Leuven, F.; Fahrenholz, F.; Kojro, E. Neuropeptide Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Slows down Alzheimer’s Disease-like Pathology in Amyloid Precursor Protein-Transgenic Mice. FASEB J. 2011, 25, 3208–3218. [Google Scholar] [CrossRef]
- Song, M.; Xiong, J.X.; Wang, Y.Y.; Tang, J.; Zhang, B.; Bai, Y. VIP Enhances Phagocytosis of Fibrillar Beta-Amyloid by Microglia and Attenuates Amyloid Deposition in the Brain of APP/PS1 Mice. PLoS ONE 2012, 7, e29790. [Google Scholar] [CrossRef] [PubMed]
- Goksu, A.Y.; Kocanci, F.G.; Akinci, E.; Demir-Dora, D.; Erendor, F.; Sanlioglu, S.; Uysal, H. Microglia Cells Treated with Synthetic Vasoactive Intestinal Peptide or Transduced with LentiVIP Protect Neuronal Cells against Degeneration. Eur. J. Neurosci. 2024, 59, 1993–2015. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Álvarez, I.; Mantey, S.A.; Nakamura, T.; Nuche-Berenguer, B.; Moreno, P.; Moody, T.W.; Maderdrut, J.L.; Coy, D.H.; Jensen, R.T. A Structure-Function Study of PACAP Using Conformationally Restricted Analogs: Identification of PAC1 Receptor-Selective PACAP Agonists. Peptides 2015, 66, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Deluigi, M.; Klipp, A.; Klenk, C.; Merklinger, L.; Eberle, S.A.; Morstein, L.; Heine, P.; Mittl, P.R.E.; Ernst, P.; Kamenecka, T.M.; et al. Complexes of the Neurotensin Receptor 1 with Small-Molecule Ligands Reveal Structural Determinants of Full, Partial, and Inverse Agonism. Sci. Adv. 2021, 7, eabe5504. [Google Scholar] [CrossRef]
- Devader, C.; Béraud-Dufour, S.; Coppola, T.; Mazella, J. The Anti-Apoptotic Role of Neurotensin. Cells 2013, 2, 124–135. [Google Scholar] [CrossRef]
- Guo, F.; Du, Y.; Qu, F.H.; Lin, S.D.; Chen, Z.; Zhang, S.H. Dissecting the Neural Circuitry for Pain Modulation and Chronic Pain: Insights from Optogenetics. Neurosci. Bull. 2022, 38, 440–452. [Google Scholar] [CrossRef]
- Tabarean, I. Neurotensin Induces Hypothermia by Activating Both Neuronal Neurotensin Receptor 1 and Astrocytic Neurotensin Receptor 2 in the Median Preoptic Nucleus. Neuropharmacology 2020, 171, 108069. [Google Scholar] [CrossRef]
- Tschumi, C.W.; Blankenship, H.E.; Sharma, R.; Lynch, W.B.; Beckstead, M.J. Neurotensin Release from Dopamine Neurons Drives Long-Term Depression of Substantia Nigra Dopamine Signaling. J. Neurosci. 2022, 42, 6186–6194. [Google Scholar] [CrossRef]
- Ma, H.; Huang, Y.; Zhang, B.; Wang, Y.; Zhao, H.; Du, H.; Cong, Z.; Li, J.; Zhu, G. Association Between Neurotensin Receptor 1 Gene Polymorphisms and Alcohol Dependence in a Male Han Chinese Population. J. Mol. Neurosci. 2013, 51, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Servonnet, A.; Minogianis, E.A.; Bouchard, C.; Bédard, A.M.; Lévesque, D.; Rompré, P.P.; Samaha, A.N. Neurotensin in the Nucleus Accumbens Reverses Dopamine Supersensitivity Evoked by Antipsychotic Treatment. Neuropharmacology 2017, 123, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Sarret, P.; Perron, A.; Stroh, T.; Beaudet, A. Immunohistochemical distribution of NTS2 neurotensin receptors in the rat central nervous system. J. Comp. Neurol. 2003, 461, 520–538. [Google Scholar] [CrossRef]
- Kyriatzis, G.; Bernard, A.; Bôle, A.; Pflieger, G.; Chalas, P.; Masse, M.; Lécorché, P.; Jacquot, G.; Ferhat, L.; Khrestchatisky, M. Neurotensin Receptor 2 Is Induced in Astrocytes and Brain Endothelial Cells in Relation to Neuroinflammation Following Pilocarpine-Induced Seizures in Rats. Glia 2021, 69, 2618–2643. [Google Scholar] [CrossRef]
- Alysandratos, K.-D.; Asadi, S.; Angelidou, A.; Zhang, B.; Sismanopoulos, N.; Yang, H.; Critchfield, A.; Theoharides, T.C. Neurotensin and CRH Interactions Augment Human Mast Cell Activation. PLoS ONE 2012, 7, e48934. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.J.; Pervaiz, I.; Karamyan, V.T. Neurolysin Substrates Bradykinin, Neurotensin and Substance P Enhance Brain Microvascular Permeability in a Human in Vitro Model. J. Neuroendocrinol. 2021, 33, e12931. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Tsilioni, I.; Leeman, S.E.; Theoharides, T.C. Neurotensin Stimulates Sortilin and mTOR in Human Microglia Inhibitable by Methoxyluteolin, a Potential Therapeutic Target for Autism. Proc. Natl. Acad. Sci. USA 2016, 113, E7049–E7058. [Google Scholar] [CrossRef] [PubMed]
- Voyer, D.; Lévesque, D.; Rompré, P.-P. Repeated Ventral Midbrain Neurotensin Injections Sensitize to Amphetamine-Induced Locomotion and ERK Activation: A Role for NMDA Receptors. Neuropharmacology 2017, 112, 150–163. [Google Scholar] [CrossRef]
- Coll, R.C.; Vargas, P.M.; Mariani, M.L.; Penissi, A.B. Natural α,β-Unsaturated Lactones Inhibit Neuropeptide-Induced Mast Cell Activation in an in Vitro Model of Neurogenic Inflammation. Inflamm. Res. 2020, 69, 1039–1051. [Google Scholar] [CrossRef]
- Miller, L.A.; Cochrane, D.E.; Feldberg, R.S.; Carraway, R.E. Inhibition of Neurotensin-Stimulated Mast Cell Secretion and Carboxypeptidase A Activity by the Peptide Inhibitor of Carboxypeptidase A and Neurotensin-Receptor Antagonist SR 48692. Int. Arch. Allergy Immunol. 1998, 116, 147–153. [Google Scholar] [CrossRef]
- Gahete, M.D.; Rubio, A.; Córdoba-Chacón, J.; Gracia-Navarro, F.; Kineman, R.D.; Avila, J.; Luque, R.M.; Castaño, J.P. Expression of the Ghrelin and Neurotensin Systems Is Altered in the Temporal Lobe of Alzheimer’s Disease Patients. J. Alzheimers Dis. 2010, 22, 819–828. [Google Scholar] [CrossRef]
- Martin, S.; Dicou, E.; Vincent, J.-P.; Mazella, J. Neurotensin and the neurotensin receptor-3 in microglial cells. J. Neurosci. Res. 2005, 81, 322–326. [Google Scholar] [CrossRef]
- Grajchen, E.; Wouters, E.; van de Haterd, B.; Haidar, M.; Hardonnière, K.; Dierckx, T.; Van Broeckhoven, J.; Erens, C.; Hendrix, S.; Kerdine-Römer, S.; et al. CD36-Mediated Uptake of Myelin Debris by Macrophages and Microglia Reduces Neuroinflammation. J. Neuroinflamm. 2020, 17, 224. [Google Scholar] [CrossRef]
- Martin, S.; Vincent, J.P.; Mazella, J. Involvement of the Neurotensin Receptor-3 in the Neurotensin-Induced Migration of Human Microglia. J. Neurosci. 2003, 23, 1198–1205. [Google Scholar] [CrossRef]
- Soltys, J.; Knight, J.; Scharf, E.; Pitt, D.; Mao-Draayer, Y. IFN-β Alters Neurotrophic Factor Expression in T Cells Isolated from Multiple Sclerosis Patients—Implication of Novel Neurotensin/NTSR1 Pathway in Neuroprotection. Am. J. Transl. Res. 2014, 6, 312–319. [Google Scholar] [PubMed]
- Carniglia, L.; Ramírez, D.; Durand, D.; Saba, J.; Turati, J.; Caruso, C.; Scimonelli, T.N.; Lasaga, M. Neuropeptides and Microglial Activation in Inflammation, Pain, and Neurodegenerative Diseases. Mediat. Inflamm. 2017, 2017, 5048616. [Google Scholar] [CrossRef]
- Ji, L.-Q.; Hong, Y.; Tao, Y.-X. Melanocortin-5 Receptor: Pharmacology and Its Regulation of Energy Metabolism. Int. J. Mol. Sci. 2022, 23, 8727. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.B.; Montero-Melendez, T.; Greco, K.V.; Perretti, M. Melanocortin Receptors as Novel Effectors of Macrophage Responses in Inflammation. Front. Immunol. 2011, 2, 41. [Google Scholar] [CrossRef][Green Version]
- Rajora, N.; Boccoli, G.; Burns, D.; Sharma, S.; Catania, A.P.; Lipton, J.M. Alpha-MSH Modulates Local and Circulating Tumor Necrosis Factor-Alpha in Experimental Brain Inflammation. J. Neurosci. 1997, 17, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Star, R.A.; Rajora, N.; Huang, J.; Stock, R.C.; Catania, A.; Lipton, J.M. Evidence of Autocrine Modulation of Macrophage Nitric Oxide Synthase by Alpha-Melanocyte-Stimulating Hormone. Proc. Natl. Acad. Sci. USA 1995, 92, 8016–8020. [Google Scholar] [CrossRef]
- Taylor, A.W. The Immunomodulating Neuropeptide Alpha-Melanocyte-Stimulating Hormone (Alpha-MSH) Suppresses LPS-Stimulated TLR4 with IRAK-M in Macrophages. J. Neuroimmunol. 2005, 162, 43–50. [Google Scholar] [CrossRef]
- Taylor, A.W. Alpha-Melanocyte Stimulating Hormone (α-MSH) Is a Post-Caspase Suppressor of Apoptosis in RAW 264.7 Macrophages. PLoS ONE 2013, 8, e74488. [Google Scholar] [CrossRef][Green Version]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The Role of Substance P in Inflammatory Disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef]
- Wang, Q.; Oyarzabal, E.; Wilson, B.; Qian, L.; Hong, J.-S. Substance P Enhances Microglial Density in the Substantia Nigra through Neurokinin-1 Receptor/NADPH Oxidase-Mediated Chemotaxis in Mice. Clin. Sci. 2015, 129, 757–767. [Google Scholar] [CrossRef]
- Chowdari Gurram, P.; Satarker, S.; Nampoothiri, M. Recent Advances in the Molecular Signaling Pathways of Substance P in Alzheimer’s Disease: Link to Neuroinflammation Associated with Toll-like Receptors. Biochem. Biophys. Res. Commun. 2024, 733, 150597. [Google Scholar] [CrossRef]
- Pascual, D.W.; Bost, K.L. Substance P Production by P388D1 Macrophages: A Possible Autocrine Function for This Neuropeptide. Immunology 1990, 71, 52–56. [Google Scholar]
- Ahn, W.; Chi, G.; Kim, S.; Son, Y.; Zhang, M. Substance P Reduces Infarct Size and Mortality After Ischemic Stroke, Possibly Through the M2 Polarization of Microglia/Macrophages and Neuroprotection in the Ischemic Rat Brain. Cell Mol. Neurobiol. 2023, 43, 2035–2052. [Google Scholar] [CrossRef]
- Nag, S.; Yee, B.K.; Tang, F. Reduction in Somatostatin and Substance P Levels and Choline Acetyltransferase Activity in the Cortex and Hippocampus of the Rat after Chronic Intracerebroventricular Infusion of β-Amyloid (1-40). Brain Res. Bull. 1999, 50, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Vilisaar, J.; Kawabe, K.; Braitch, M.; Aram, J.; Furtun, Y.; Fahey, A.J.; Chopra, M.; Tanasescu, R.; Tighe, P.J.; Gran, B.; et al. Reciprocal Regulation of Substance P and IL-12/IL-23 and the Associated Cytokines, IFNγ/IL-17: A Perspective on the Relevance of This Interaction to Multiple Sclerosis. J. Neuroimmune Pharmacol. 2015, 10, 457–467. [Google Scholar] [CrossRef]
- Coutens, B.; Ingram, S.L. Key Differences in Regulation of Opioid Receptors Localized to Presynaptic Terminals Compared to Somas: Relevance for Novel Therapeutics. Neuropharmacology 2023, 226, 109408. [Google Scholar] [CrossRef]
- Zhao, J.; Elgeti, M.; O’Brien, E.S.; Sár, C.P.; Daibani, A.E.; Heng, J.; Sun, X.; Che, T.; Hubbell, W.L.; Kobilka, B.K.; et al. Conformational Dynamics of the μ-Opioid Receptor Determine Ligand Intrinsic Efficacy. BioRxiv 2023, 629, 474–480. [Google Scholar]
- Takayama, N.; Ueda, H. Morphine-Induced Overexpression of Prepro-Nociceptin/Orphanin FQ in Cultured Astrocytes. Peptides 2005, 26, 2513–2517. [Google Scholar] [CrossRef]
- Corkrum, M.; Rothwell, P.E.; Thomas, M.J.; Kofuji, P.; Araque, A. Opioid-Mediated Astrocyte–Neuron Signaling in the Nucleus Accumbens. Cells 2019, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Gessi, S.; Borea, P.A.; Bencivenni, S.; Fazzi, D.; Varani, K.; Merighi, S. The Activation of μ-Opioid Receptor Potentiates LPS-Induced NF-kB Promoting an Inflammatory Phenotype in Microglia. FEBS Lett. 2016, 590, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wu, C.-Y.; Burton, F.H.; Loh, H.H.; Wei, L.-N. β-Arrestin Protects Neurons by Mediating Endogenous Opioid Arrest of Inflammatory Microglia. Cell Death Differ. 2014, 21, 397–406. [Google Scholar] [CrossRef]
- Birdsong, W.T.; Arttamangkul, S.; Clark, M.J.; Cheng, K.; Rice, K.C.; Traynor, J.R.; Williams, J.T. Increased Agonist Affinity at the μ-Opioid Receptor Induced by Prolonged Agonist Exposure. J. Neurosci. 2013, 33, 4118–4127. [Google Scholar] [CrossRef]
- Chao, C.C.; Hu, S.; Shark, K.B.; Sheng, W.S.; Gekker, G.; Peterson, P.K. Activation of Mu Opioid Receptors Inhibits Microglial Cell Chemotaxis1. J. Pharmacol. Exp. Ther. 1997, 281, 998–1004. [Google Scholar] [CrossRef]
- Ma, L.; Peng, S.; Wei, J.; Zhao, M.; Ahmad, K.A.; Chen, J.; Wang, Y.-X. Spinal Microglial β-Endorphin Signaling Mediates IL-10 and Exenatide-Induced Inhibition of Synaptic Plasticity in Neuropathic Pain. CNS Neurosci. Ther. 2021, 27, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Leiguarda, C.; Potilinski, C.; Rubione, J.; Tate, P.; Villar, M.J.; Montaner, A.; Bisagno, V.; Constandil, L.; Brumovsky, P.R. IMT504 Provides Analgesia by Modulating Cell Infiltrate and Inflammatory Milieu in a Chronic Pain Model. J. Neuroimmune Pharmacol. 2021, 16, 651–666. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhang, H.; Tan, Z.; Zheng, Y.; Ping, J.; Zhang, J.; Luo, J.; Li, L.; Lu, L.; et al. Elevated Circulating Levels of GFAP Associated with Reduced Volumes in Hippocampal Subregions Linked to Mild Cognitive Impairment among Community-Dwelling Elderly Individuals. Front. Aging Neurosci. 2024, 16, 1461556. [Google Scholar] [CrossRef]
- Xu, Y.; Zhi, F.; Balboni, G.; Yang, Y.; Xia, Y. Opposite Roles of δ- and μ-Opioid Receptors in BACE1 Regulation and Alzheimer’s Injury. Front. Cell. Neurosci. 2020, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Nix, M.; Abdul, Y.; Singh, S.; Husain, S. Delta-Opioid Receptors Attenuate TNF-α–Induced MMP-2 Secretion from Human ONH Astrocytes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6605–6611. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.S. Exploring the Role of Opioid Signaling in Modulation of Microglial Function. Ph.D. Thesis, Charles University, Prague, Czech Republic, 2023. [Google Scholar]
- Xu, Y.; Chen, R.; Zhi, F.; Sheng, S.; Khiati, L.; Yang, Y.; Peng, Y.; Xia, Y. δ-Opioid Receptor, Microglia and Neuroinflammation. Aging Dis. 2023, 14, 778–793. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Xia, H.; Hu, M.; Chen, C.; Fu, J.; Shi, G.; Guo, Q.; Zhou, Y.; Wang, W.; Shi, J.; et al. Isotalatizidine, a C19-Diterpenoid Alkaloid, Attenuates Chronic Neuropathic Pain through Stimulating ERK/CREB Signaling Pathway-Mediated Microglial Dynorphin A Expression. J. Neuroinflamm. 2020, 17, 13. [Google Scholar] [CrossRef]
- Belo, T.C.A.; Santos, G.X.; da Silva, B.E.G.; Rocha, B.L.G.; Abdala, D.W.; Freire, L.A.M.; Rocha, F.S.; Galdino, G. IL-10/β-Endorphin-Mediated Neuroimmune Modulation on Microglia during Antinociception. Brain Sci. 2023, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Cuitavi, J.; Torres-Pérez, J.V.; Lorente, J.D.; Campos-Jurado, Y.; Andrés-Herrera, P.; Polache, A.; Agustín-Pavón, C.; Hipólito, L. Crosstalk between Mu-Opioid Receptors and Neuroinflammation: Consequences for Drug Addiction and Pain. Neurosci. Biobehav. Rev. 2023, 145, 105011. [Google Scholar] [CrossRef]
- Sukhareva, E.V. The Role of the Corticotropin-Releasing Hormone and Its Receptors in the Regulation of Stress Response. Vavilovskii Zhurnal Genet. Sel. 2021, 25, 216–223. [Google Scholar] [CrossRef]
- Mastorakos, G.; Karoutsou, E.I.; Mizamtsidi, M. Corticotropin Releasing Hormone and the Immune/Inflammatory response. Eur. J. Endocrinol. 2006, 155, S77–S84. [Google Scholar] [CrossRef]
- Esposito, P.; Chandler, N.; Kandere, K.; Basu, S.; Jacobson, S.; Connolly, R.; Tutor, D.; Theoharides, T.C. Corticotropin-Releasing Hormone and Brain Mast Cells Regulate Blood-Brain-Barrier Permeability Induced by Acute Stress. J. Pharmacol. Exp. Ther. 2002, 303, 1061–1066. [Google Scholar] [CrossRef]
- Cao, J.; Papadopoulou, N.; Kempuraj, D.; Boucher, W.S.; Sugimoto, K.; Cetrulo, C.L.; Theoharides, T.C. Human Mast Cells Express Corticotropin-Releasing Hormone (CRH) Receptors and CRH Leads to Selective Secretion of Vascular Endothelial Growth Factor. J. Immunol. 2005, 174, 7665–7675. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, T.; Li, Y.; Li, T.; Ding, Z.; Liu, L. Paraventricular Mast Cell-Derived Histamine Activates CRH Neurons to Mediate Adult Visceral Hypersensitivity Induced by Neonatal Maternal Separation. Brain Sci. 2023, 13, 1595. [Google Scholar] [CrossRef]
- Guo, L.; Reed, K.M.; Carter, A.; Cheng, Y.; Roodsari, S.K.; Martinez Pineda, D.; Wellman, L.L.; Sanford, L.D.; Guo, M.-L. Sleep-Disturbance-Induced Microglial Activation Involves CRH-Mediated Galectin 3 and Autophagy Dysregulation. Cells 2022, 12, 160. [Google Scholar] [CrossRef]
- Anghel, L.; Ciubară, A.; Nechita, A.; Nechita, L.; Manole, C.; Baroiu, L.; Ciubară, A.B.; Mușat, C.L. Sleep Disorders Associated with Neurodegenerative Diseases. Diagnostics 2023, 13, 2898. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.K. Neurodegenerative Disorders and Sleep. Sleep Med. Clin. 2018, 13, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wang, F.; Qi, D.; Liu, W.; Gu, C.; Mao, C.-J.; Yang, Y.-P.; Zhao, Z.; Hu, L.-F.; Liu, C.-F. A Critical Role of Autophagy in Regulating Microglia Polarization in Neurodegeneration. Front. Aging Neurosci. 2018, 10, 378. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Cho, D.-H. Enhancement of Nitric Oxide Production by Corticotropin-Releasing Hormone (CRH) in Murine Microglial Cells, BV2. Immune Netw. 2004, 4, 60–64. [Google Scholar] [CrossRef]
- Yang, Y.; Hahm, E.; Kim, Y.; Kang, J.; Lee, W.; Han, I.; Myung, P.; Kang, H.; Park, H.; Cho, D. Regulation of IL-18 Expression by CRH in Mouse Microglial Cells. Immunol. Lett. 2005, 98, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Lin, Y.; Lu, Z.; Xiao, Z. Microglia-Astrocyte Cross Talk through IL-18/IL-18R Signaling Modulates Migraine-like Behavior in Experimental Models of Migraine. Neuroscience 2020, 451, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Gomes, B.; Kumar, A.; Ashton, N.J.; Hall, S.; Stomrud, E.; Smith, R.; Zetterberg, H.; Blennow, K.; Mattsson-Carlgren, N.; Hansson, O. Corticotropin-Releasing Hormone as a Candidate Biomarker for Parkinsonian Disorders. Brain Commun. 2024, 6, fcae414. [Google Scholar] [CrossRef] [PubMed]
- Fountain, J.H.; Kaur, J.; Lappin, S.L. Physiology, Renin Angiotensin System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Abiodun, O.A.; Ola, M.S. Role of Brain Renin Angiotensin System in Neurodegeneration: An Update. Saudi J. Biol. Sci. 2020, 27, 905–912. [Google Scholar] [CrossRef]
- Deliu, E.; Brailoiu, G.C.; Eguchi, S.; Hoffman, N.E.; Rabinowitz, J.E.; Tilley, D.G.; Madesh, M.; Koch, W.J.; Brailoiu, E. Direct Evidence of Intracrine Angiotensin II Signaling in Neurons. Am. J. Physiol.-Cell Physiol. 2014, 306, C736–C744. [Google Scholar] [CrossRef]
- Erdmann, B.; Fuxe, K.; Ganten, D. Subcellular Localization of Angiotensin II Immunoreactivity in the Rat Cerebellar Cortex. Hypertension 1996, 28, 818–824. [Google Scholar] [CrossRef]
- Pan, H.-L. Brain Angiotensin II and Synaptic Transmission. Neuroscientist 2004, 10, 422–431. [Google Scholar] [CrossRef]
- Saavedra, J.M. Angiotensin II AT1 Receptor Blockers as Treatments for Inflammatory Brain Disorders. Clin. Sci. 2012, 123, 567–590. [Google Scholar] [CrossRef]
- Biancardi, V.C.; Stranahan, A.M.; Krause, E.G.; de Kloet, A.D.; Stern, J.E. Cross Talk between AT1 Receptors and Toll-like Receptor 4 in Microglia Contributes to Angiotensin II-Derived ROS Production in the Hypothalamic Paraventricular Nucleus. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H404–H415. [Google Scholar] [CrossRef]
- Sun, H.; Wu, H.; Yu, X.; Zhang, G.; Zhang, R.; Zhan, S.; Wang, H.; Bu, N.; Ma, X.; Li, Y. Angiotensin II and Its Receptor in Activated Microglia Enhanced Neuronal Loss and Cognitive Impairment Following Pilocarpine-Induced Status Epilepticus. Mol. Cell Neurosci. 2015, 65, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.C.; Kimbark, K.D.; Vernail, V.L.; Silberman, Y.; Arnold, A.C. Angiotensin-(1-7) Protective Effects in Neurocognitive Disorders: Molecular Mechanisms to Therapeutic Implications. Front. Physiol. 2025, 16, 1565270. [Google Scholar] [CrossRef]
- Wright, J.W.; Harding, J.W. Brain Angiotensin Receptor Subtypes AT1, AT2, and AT4 and Their Functions. Regul. Pept. 1995, 59, 269–295. [Google Scholar] [CrossRef]
- Camiña, J.P. Cell Biology of the Ghrelin Receptor. J. Neuroendocrinol. 2006, 18, 65–76. [Google Scholar] [CrossRef]
- de Candia, P.; Matarese, G. Leptin and Ghrelin: Sewing Metabolism onto Neurodegeneration. Neuropharmacology 2018, 136, 307–316. [Google Scholar] [CrossRef]
- Rhea, E.M.; Salameh, T.S.; Gray, S.; Niu, J.; Banks, W.A.; Tong, J. Ghrelin Transport across the Blood–Brain Barrier Can Occur Independently of the Growth Hormone Secretagogue Receptor. Mol. Metab. 2018, 18, 88–96. [Google Scholar] [CrossRef]
- Cavalier, M.; Crouzin, N.; Ben Sedrine, A.; de Jesus Ferreira, M.C.; Guiramand, J.; Cohen-Solal, C.; Fehrentz, J.-A.; Martinez, J.; Barbanel, G.; Vignes, M. Involvement of PKA and ERK Pathways in Ghrelin-Induced Long-Lasting Potentiation of Excitatory Synaptic Transmission in the CA1 Area of Rat Hippocampus. Eur. J. Neurosci. 2015, 42, 2568–2576. [Google Scholar] [CrossRef]
- Jones, R. Ghrelin on the Brain. Nat. Rev. Neurosci. 2003, 4, 246. [Google Scholar] [CrossRef]
- Wu, C.-R.; Yang, Q.-Y.; Chen, Q.-W.; Li, C.-Q.; He, W.-Y.; Zhao, Y.-P.; Wang, L. Ghrelin Attenuate Cerebral Microvascular Leakage by Regulating Inflammation and Apoptosis Potentially via a P38 MAPK-JNK Dependent Pathway. Biochem. Biophys. Res. Commun. 2021, 552, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yune, T.Y. Ghrelin Inhibits Oligodendrocyte Cell Death by Attenuating Microglial Activation. Endocrinol. Metab. 2024, 29, 371–378. [Google Scholar] [CrossRef]
- Russo, C.; Valle, M.S.; Russo, A.; Malaguarnera, L. The Interplay between Ghrelin and Microglia in Neuroinflammation: Implications for Obesity and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 13432. [Google Scholar] [CrossRef]
- Song, N.; Wang, W.; Jia, F.; Du, X.; Xie, A.; He, Q.; Shen, X.; Zhang, J.; Rogers, J.T.; Xie, J.; et al. Assessments of Plasma Ghrelin Levels in the Early Stages of Parkinson’s Disease. Mov. Disord. 2017, 32, 1487–1491. [Google Scholar] [CrossRef]
- Suda, Y.; Kuzumaki, N.; Sone, T.; Narita, M.; Tanaka, K.; Hamada, Y.; Iwasawa, C.; Shibasaki, M.; Maekawa, A.; Matsuo, M.; et al. Down-Regulation of Ghrelin Receptors on Dopaminergic Neurons in the Substantia Nigra Contributes to Parkinson’s Disease-like Motor Dysfunction. Mol. Brain 2018, 11, 6. [Google Scholar] [CrossRef]
- Moon, M.; Kim, H.G.; Hwang, L.; Seo, J.-H.; Kim, S.; Hwang, S.; Kim, S.; Lee, D.; Chung, H.; Oh, M.S.; et al. Neuroprotective Effect of Ghrelin in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Mouse Model of Parkinson’s Disease by Blocking Microglial Activation. Neurotox. Res. 2009, 15, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lim, E.; Kim, Y.; Li, E.; Park, S. Ghrelin Attenuates Kainic Acid-Induced Neuronal Cell Death in the Mouse Hippocampus. J. Endocrinol. 2010, 205, 263–270. Available online: https://joe.bioscientifica.com/view/journals/joe/205/3/263.xml?ijkey=028a1396e4cea94fb91c36d67d2e16486332415d&keytype2=tf_ipsecsha&legid=joe%3B205%2F3%2F263&related-urls=yes (accessed on 1 August 2025). [CrossRef]
- Reich, N.; Hölscher, C. Acylated Ghrelin as a Multi-Targeted Therapy for Alzheimer’s and Parkinson’s Disease. Front. Neurosci. 2020, 14, 614828. Available online: https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2020.614828/full (accessed on 1 August 2025).
- Thomas, A.S.; Sassi, M.; Angelini, R.; Morgan, A.H.; Davies, J.S. Acylation, a Conductor of Ghrelin Function in Brain Health and Disease. Front. Physiol. 2022, 13, 831641. [Google Scholar] [CrossRef]
- Engineer, D.R.; Garcia, J.M. Leptin in Anorexia and Cachexia Syndrome. Int. J. Pept. 2012, 2012, 287457. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, V.; Inoue, W.; Kan, B.; Luheshi, G.N. Leptin Modulates Cell Morphology and Cytokine Release in Microglia. Brain Behav. Immun. 2010, 24, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Cherait, A.; Xifró, X.; Reglodi, D.; Vaudry, D. More Than Three Decades After Discovery of the Neuroprotective Effect of PACAP, What Is Still Preventing Its Clinical Use? J. Mol. Neurosci. 2025, 75, 80. [Google Scholar] [CrossRef] [PubMed]
- Zetter, M.; Barrios-Payán, J.; Mata-Espinosa, D.; Marquina-Castillo, B.; Quintanar-Stephano, A.; Hernández-Pando, R. Involvement of Vasopressin in the Pathogenesis of Pulmonary Tuberculosis: A New Therapeutic Target? Front. Endocrinol. 2019, 10, 351. [Google Scholar] [CrossRef] [PubMed]
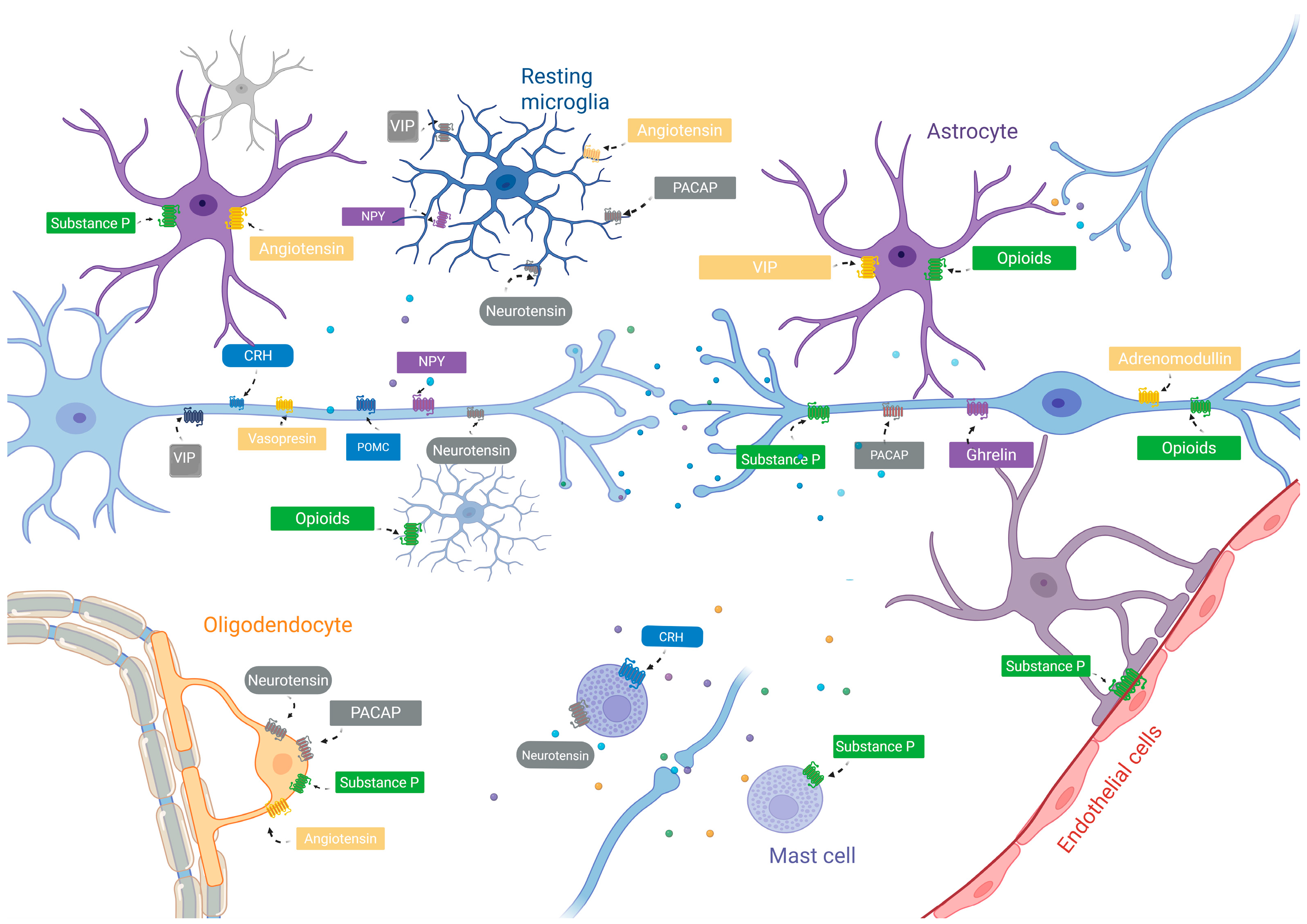
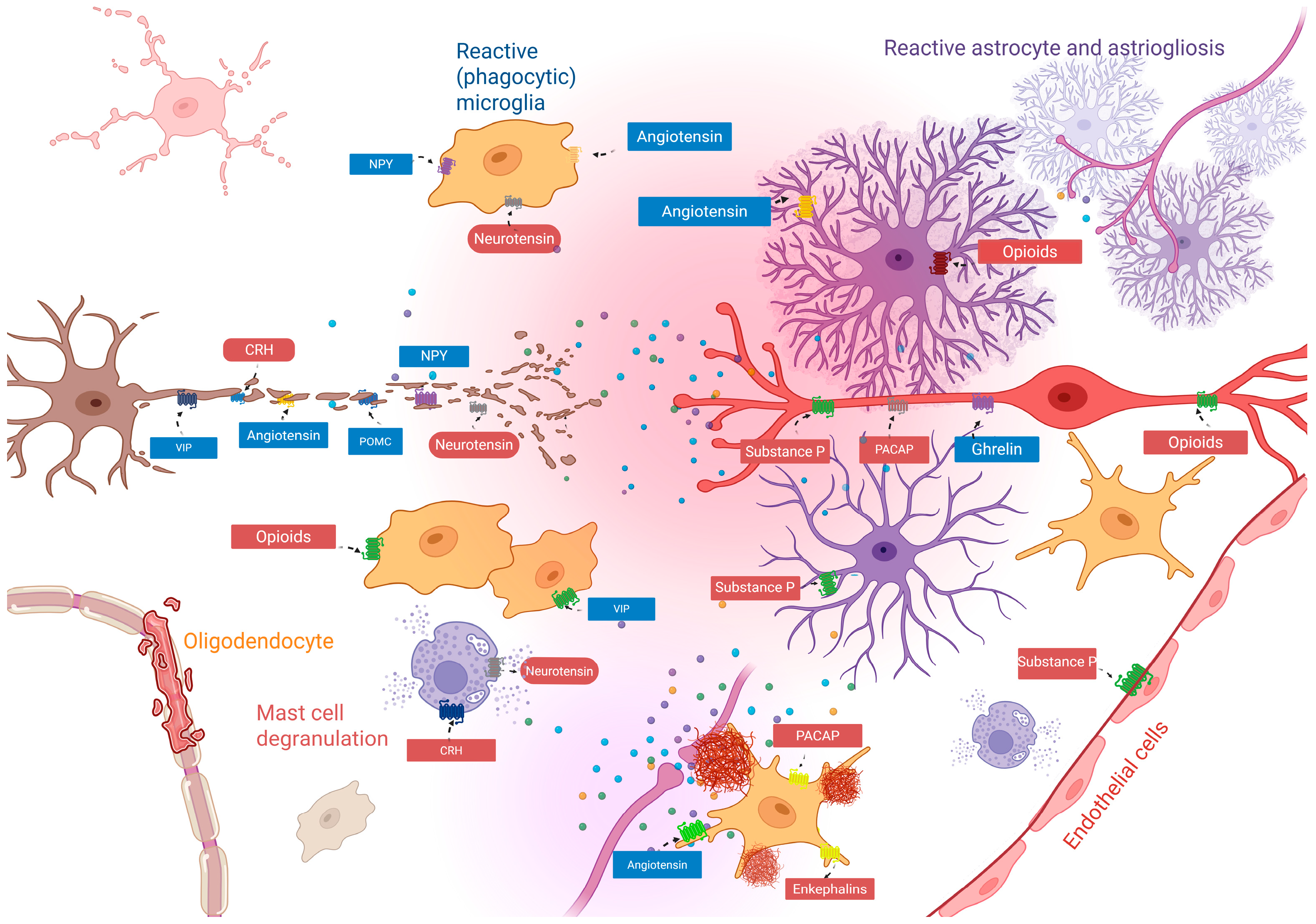
| Neuropeptide | Receptor | Receptor Type | Target Cell |
|---|---|---|---|
| VIP | VPAC1, VPAC2 | Class B GPCR (Gs) | Microglia, neurons |
| PACAP | PAC1, VPAC1, VPAC2 | PAC1: Class B GPCR (Gs/Gq); VPAC1/2: Class B GPCR (Gs) | Microglia, neurons, oligodendrocytes |
| Neurotensin | NTS1, NTS2, SORT1, SORT1a | NTS1/2: Class A GPCR (Gq); SORT1: Trafficking/sorting receptor | Microglia, neurons, mast cells |
| Pain-related neuropeptides | |||
| Substance P | NK1r | Class A GPCR (Gq) | Mast cells, endothelial cells, neurons, astrocytes, oligodendrocytes |
| Endogenous opioids | β-END: μ > δ | Class A GPCR (Gi/o) | Central neurons (hypothalamus, arcuate nucleus), microglia, astroglia) |
| Enkephalin: δ > μ | Class A GPCR (Gi/o) | Interneurons, astrocytes, microglia | |
| Dynorphin A: κ (KOR) | Class A GPCR (Gi/o) | Neurons, astrocytes, microglia | |
| Neuroendocrine and hormonal pro-inflammatory neuropeptides | |||
| CRH | CRH1r, CRH2r (alpha, beta) | Class B GPCR (Gs) | Mast cells, neurons, astrocytes, microglia |
| POMC | ACTH: MC2R | Class A GPCR (Gs) | Neurons (hypothalamus) |
| α-MSH: MC1R, MC3R, MC4R | Class A GPCR (Gs) | Neurons | |
| Adipokines and related neuropeptides | |||
| NPY | Y1R, Y2R, Y4R, Y5R, and Y6R | GPCRs Gi/0 proteins and inhibition of cAMP production | Neurons, microglia, astrocytes |
| Ghrelin | GHSR1α and GHSR1β | Class A GPCR (Gq/11) | Neurons, pituitary cells |
| Leptin | LepR, LepRa, LepRb | Class I cytokine receptor family | Hypothalamic neurons |
| Angiotensin | AT1R, AT2R, MasR, AT4R | Class A GPCR (Gq, Gi/o) | Neurons, glia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Futran-Sheinberg, E.J.; Urbina, V.; Nava, S.; Sanchez, D.; Guzmán-Valdivia, G.; Zetter, M.A. Pro- and Anti-Inflammatory Neuropeptides and Glia: The Balance Between Neuroprotection and Neuroinflammation. Neuroglia 2025, 6, 35. https://doi.org/10.3390/neuroglia6030035
Futran-Sheinberg EJ, Urbina V, Nava S, Sanchez D, Guzmán-Valdivia G, Zetter MA. Pro- and Anti-Inflammatory Neuropeptides and Glia: The Balance Between Neuroprotection and Neuroinflammation. Neuroglia. 2025; 6(3):35. https://doi.org/10.3390/neuroglia6030035
Chicago/Turabian StyleFutran-Sheinberg, Eli J., Victoria Urbina, Sofia Nava, Daniel Sanchez, Gilberto Guzmán-Valdivia, and Mario A. Zetter. 2025. "Pro- and Anti-Inflammatory Neuropeptides and Glia: The Balance Between Neuroprotection and Neuroinflammation" Neuroglia 6, no. 3: 35. https://doi.org/10.3390/neuroglia6030035
APA StyleFutran-Sheinberg, E. J., Urbina, V., Nava, S., Sanchez, D., Guzmán-Valdivia, G., & Zetter, M. A. (2025). Pro- and Anti-Inflammatory Neuropeptides and Glia: The Balance Between Neuroprotection and Neuroinflammation. Neuroglia, 6(3), 35. https://doi.org/10.3390/neuroglia6030035






