Insights into Parkinson’s Disease Pathology Focusing on Glial Response and Apoptosis in a Classic Rat Model of Dopaminergic Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Experimental Procedures
2.3. Behavioral Tests
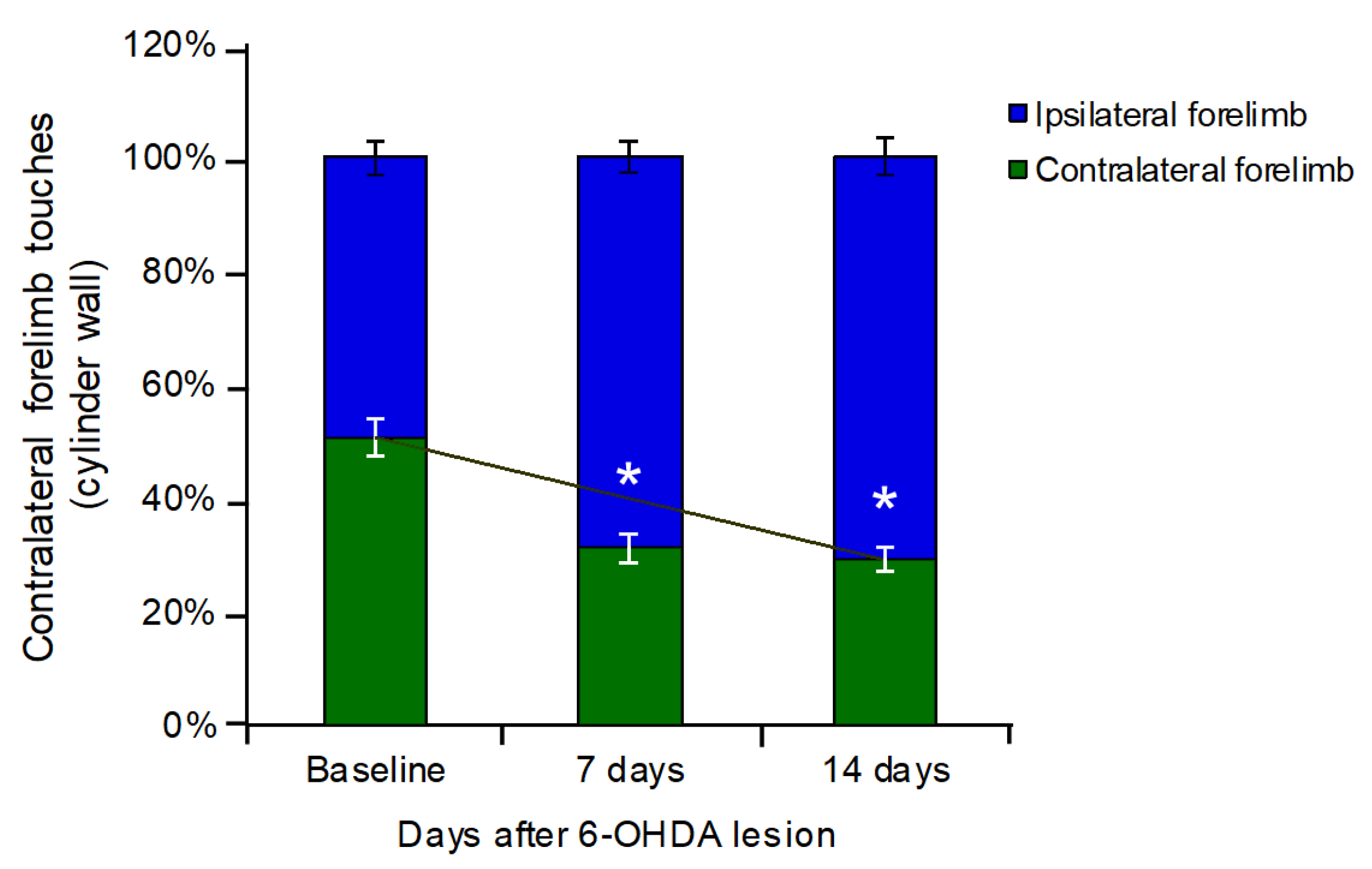
2.3.1. Cylinder Test
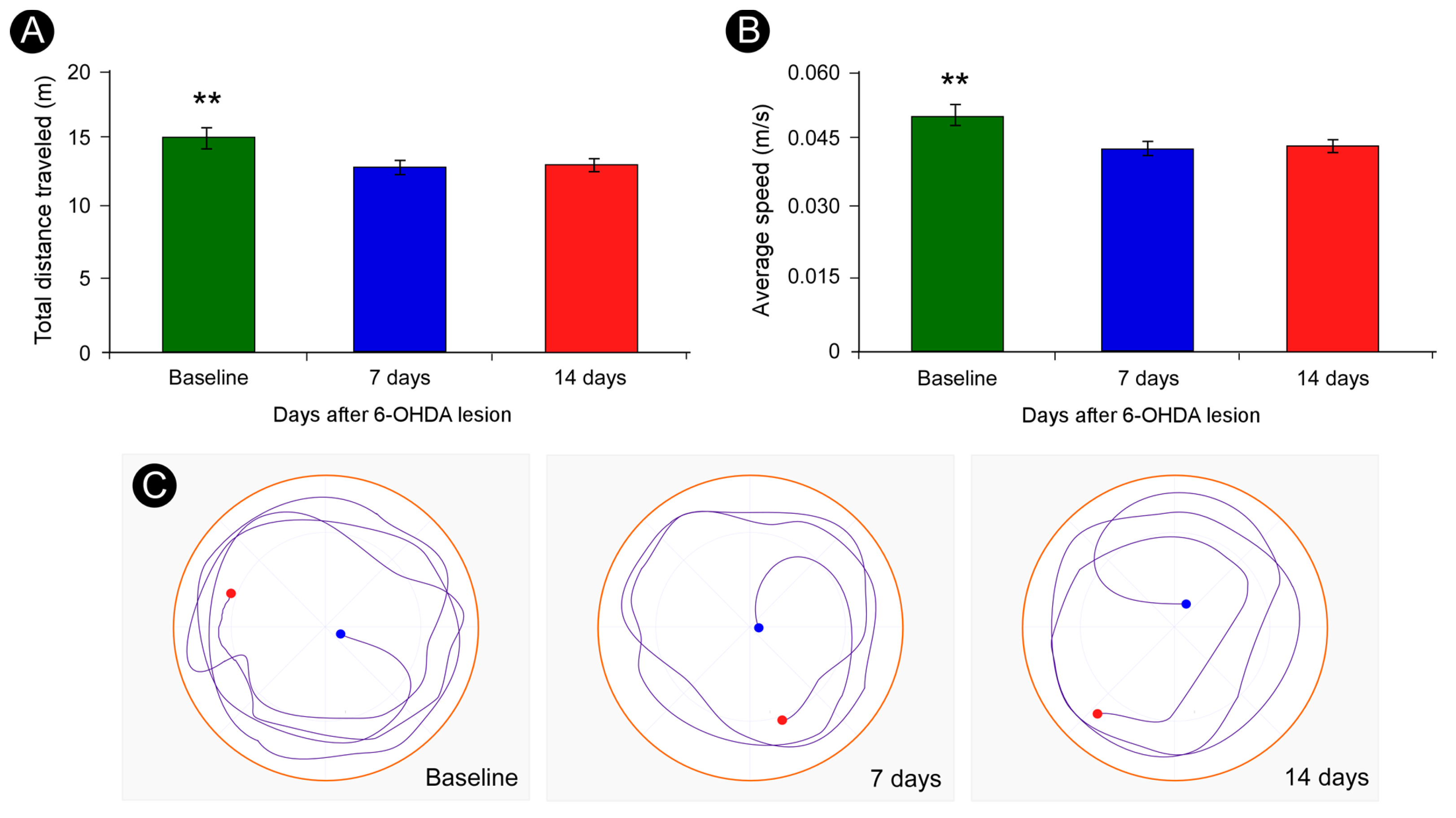
2.3.2. Open Field Test
2.4. Perfusion and Immunohistochemical Procedures
2.5. Qualitative and Quantitative Analysis
2.6. Statistical Analysis
3. Results
3.1. Cylinder Test
3.2. Open Field Test
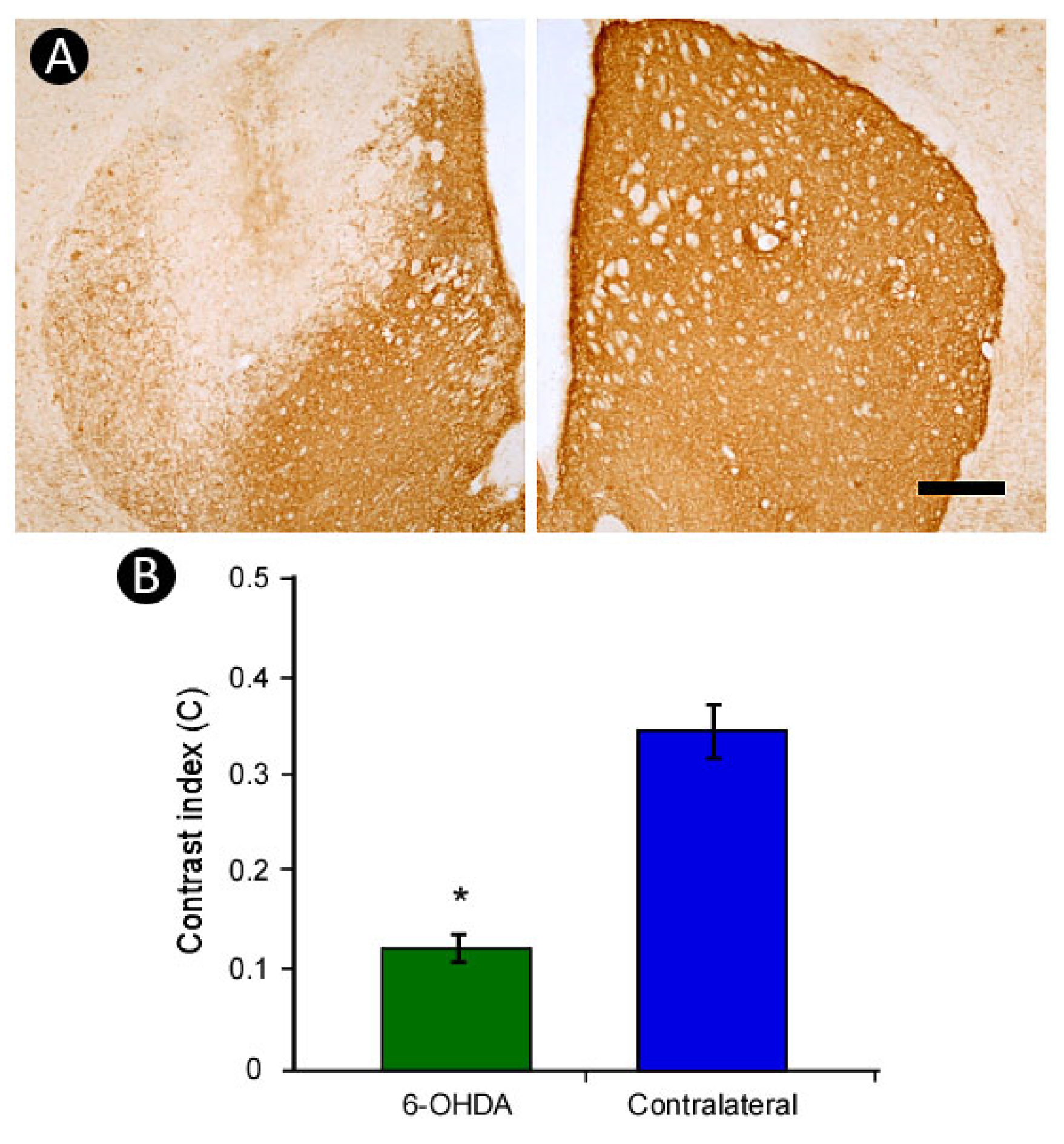
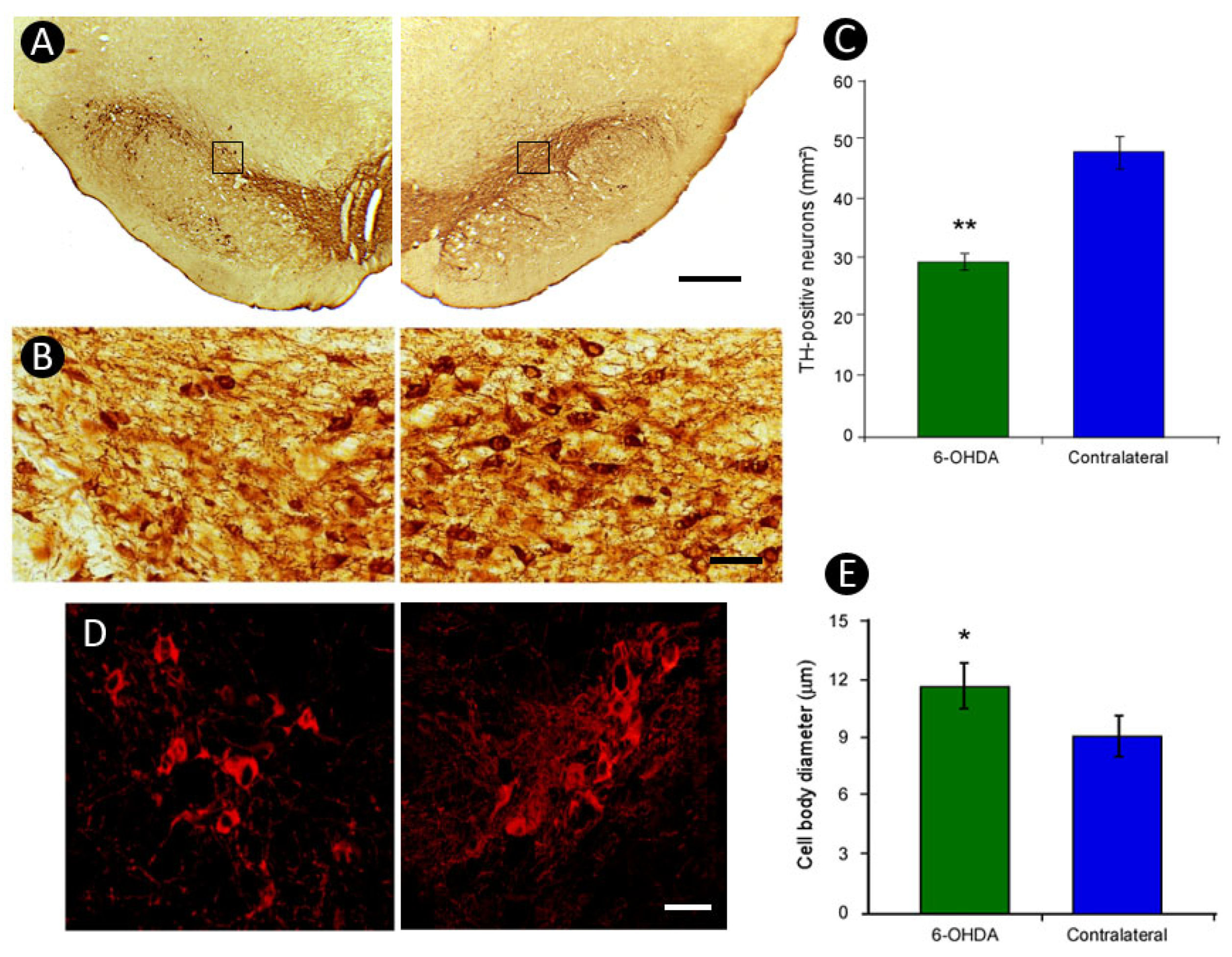
3.3. Pattern of TH-Immunoreactivity
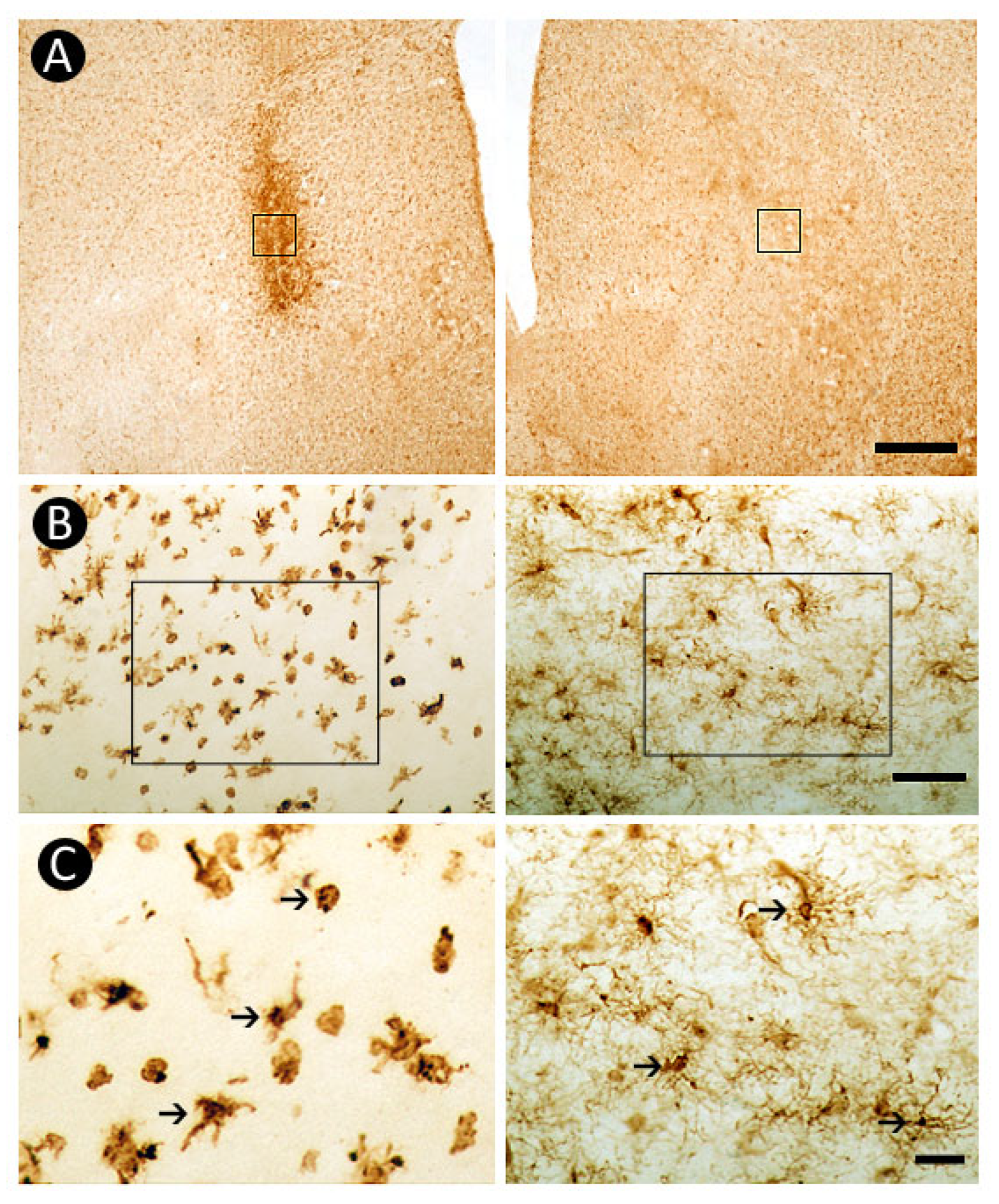
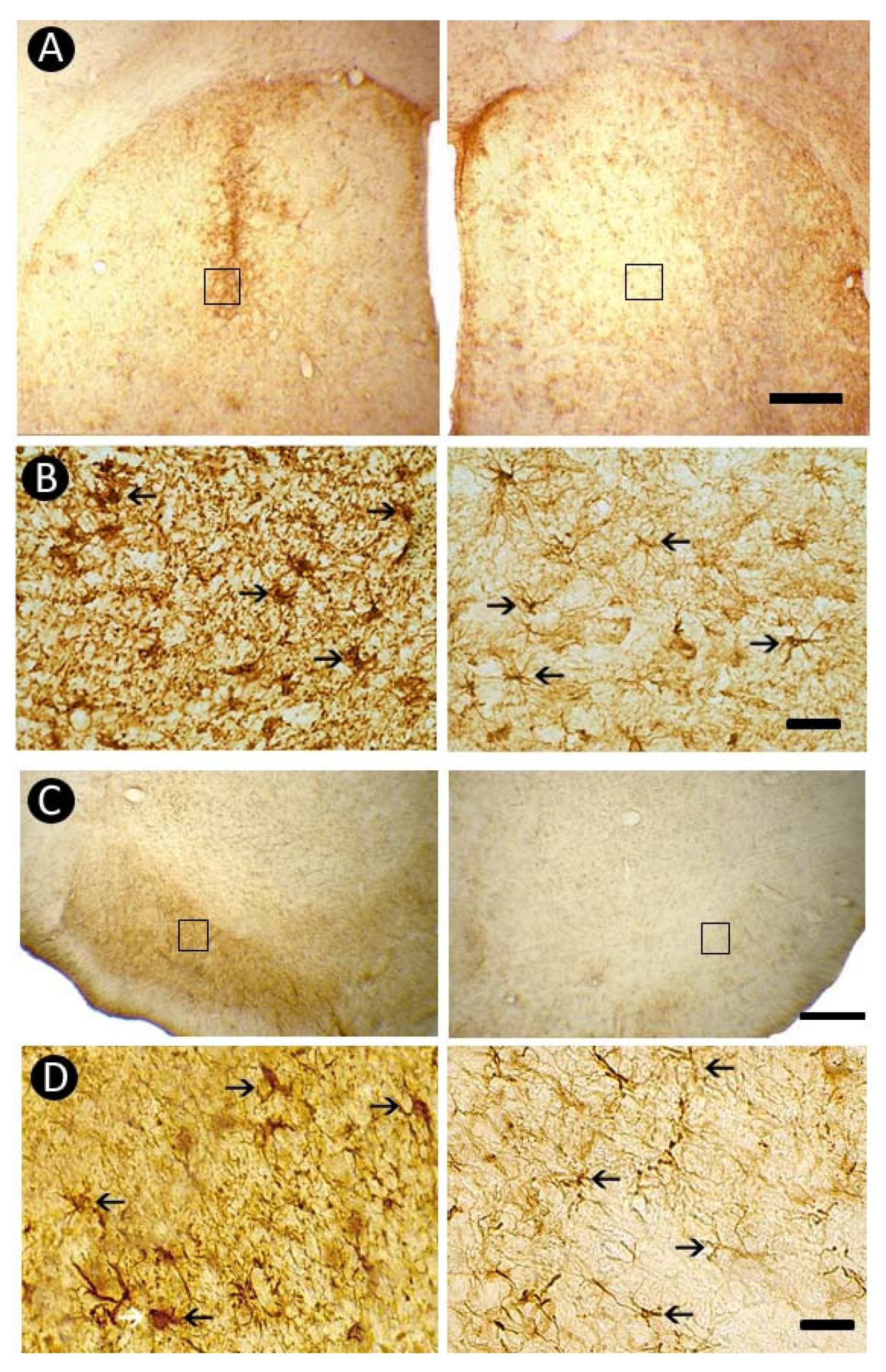
3.4. Pattern of Inflammatory Response
3.5. Pattern of Astrocytic Response
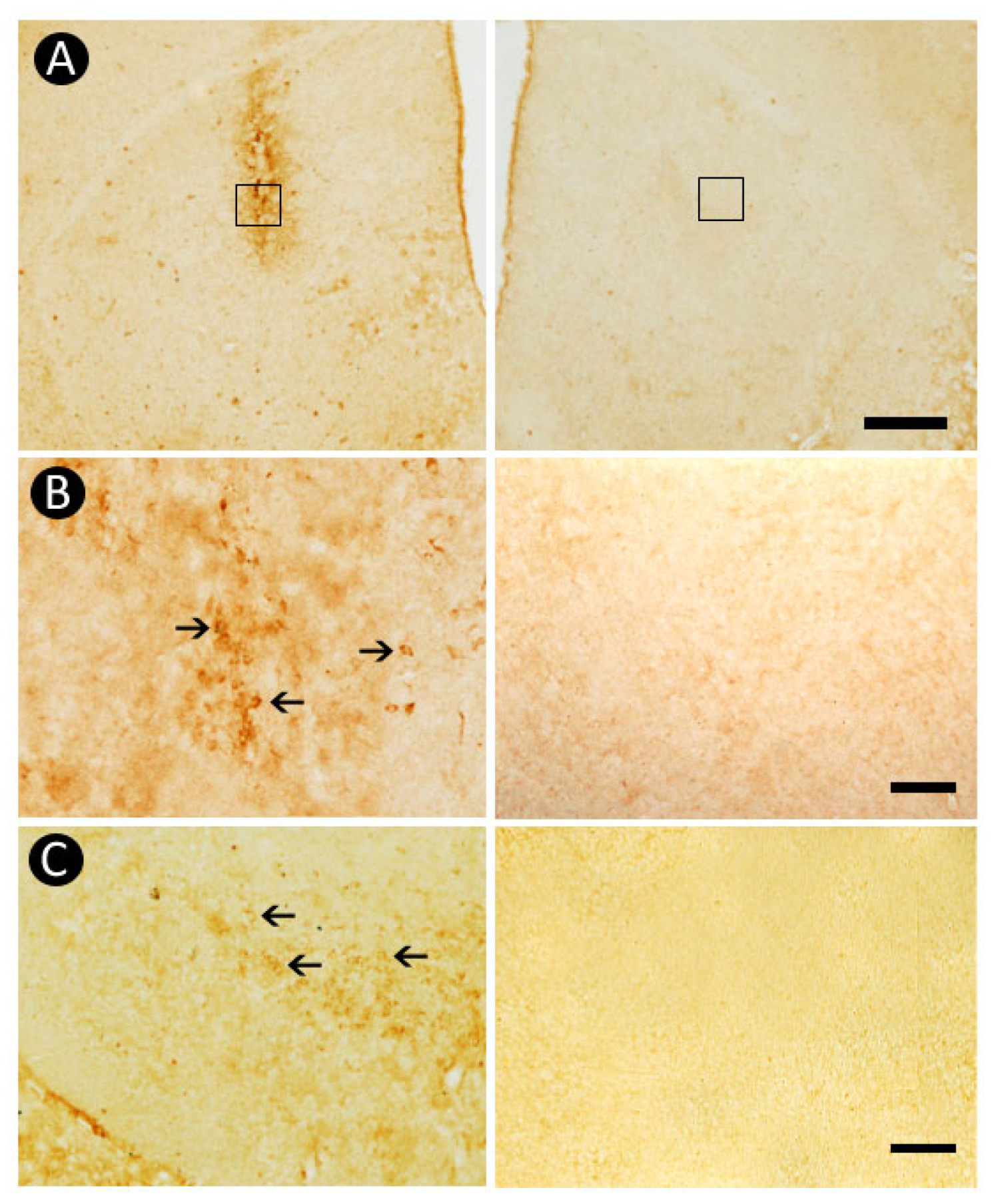
3.6. Pattern of Caspase-3-Immunolabeling
4. Discussion
Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| DRN | Dorsal raphe nucleus |
| GFAP | Glial fibrillary acid protein |
| Iba-1 | Ionized calcium-binding adapter molecule 1 |
| IL-1β | Interleukin-1 beta |
| iNOS | Inducible nitric oxide synthase |
| MFB | Medial forebrain bundle |
| OD | Optical density |
| PD | Parkinson’s disease |
| PLD | Post-lesion days |
| ROS | Reactive oxygen species |
| SN | Substantia nigra |
| SNpc | Substantia nigra pars compacta |
| SNpl | Substantia nigra pars lateralis |
| SNpr | Substantia nigra pars reticulata |
| TH | Tyrosine hydroxylase |
| TNF-α | Tumor necrosis factor alpha |
| VTA | Ventral tegmental area |
References
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D.L. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.; Halliday, G.M.; Brundin, P.; Volkman, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Chaudhuri, K.R.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar] [CrossRef]
- Hussein, A.; Guevara, C.A.; Del Valle, P.; Gupta, S.; Benson, D.L.; Huntley, G.W. Non-motor symptoms of Parkinson’s disease: The neurobiology of early psychiatric and cognitive dysfunction. Neuroscientist 2023, 29, 97–116. [Google Scholar] [CrossRef]
- Wang, T.; Shi, C.; Luo, H.; Zheng, H.; Fan, L.; Tang, M.; Su, Y.; Yang, J.; Mao, C.; Xu, Y. Neuroinflammation in Parkinson’s Disease: Triggers, Mechanisms, and Immunotherapies. Neuroscientist 2022, 28, 364–381. [Google Scholar] [CrossRef]
- Tansey, M.G.; McCoy, M.K.; Frank-Cannon, T.C. Neuroinflammatory mechanisms in Parkinson’s disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007, 208, 1–25. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Thiele, S.L.; Warre, R.; Nash, J.E. Development of a unilaterally-lesioned 6-OHDA mouse model of Parkinson’s disease. J. Vis. Exp. 2012, 60, 3234. [Google Scholar] [CrossRef]
- Jagmag, S.A.; Tripathi, N.; Shukla, S.D.; Maiti, S.; Khurana, S. Evaluation of models of Parkinson’s Disease. Front. Neurosci. 2016, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, U. 6-hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 1968, 5, 107–110. [Google Scholar] [CrossRef]
- Simola, N.; Morelli, M.; Carta, A.R. The 6-Hydroxydopamine model of Parkinson’s disease. Neurotox. Res. 2007, 11, 151–167. [Google Scholar] [CrossRef]
- Vivekanantham, S.; Shah, S.; Dewji, R.; Dewji, A.; Khatri, C.; Ologunde, R. Neuroinflammation in Parkinson’s disease: Role in neurodegeneration and tissue repair. Int. J. Neurosci. 2015, 125, 717–725. [Google Scholar] [CrossRef]
- Bayo-Olugbami, A.; Nafiu, A.B.; Amin, A.; Ogundele, O.M.; Lee, C.C.; Owoyele, B.V. Vitamin D attenuated 6-OHDA-induced behavioural deficits, dopamine dysmetabolism, oxidative stress, and neuro-inflammation in mice. Nutr. Neurosci. 2022, 25, 823–834. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef]
- Barata-Antunes, S.; Teixeira, F.G.; Mendes-Pinheiro, B.; Domingues, A.V.; Vilaça-Faria, H.; Marote, A.; Silva, D.; Sousa, R.A.; Salgado, A.J. Impact of aging on the 6-OHDA-induced rat model of Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 3459. [Google Scholar] [CrossRef]
- Su, R.J.; Zhen, J.L.; Wang, W.; Zhang, J.L.; Zheng, Y.; Wang, X.M. Time-course behavioral features are correlated with Parkinson’s disease-associated pathology in a 6-hydroxydopamine hemiparkinsonian rat model. Mol. Med. Rep. 2018, 17, 3356–3363. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Nikkhah, G.; Bentlage, C.; Björklund, A. Forelimb akinesia in the rat Parkinson model: Differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J. Neurosci. 1995, 5, 3863–3875. [Google Scholar] [CrossRef] [PubMed]
- Iancu, R.; Mohapel, P.; Brundin, P.; Paul, G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav. Brain Res. 2005, 162, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, I.; Nsibi, A.; Saoud, H.; Saidi, N.E.; Mani, S. Unilateral 6-hydroxydopamine-lesioned rat as relevant model to study the pain related to Parkinson’s disease. Neurol. Neurobiol. 2020, 3, 1–5. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Li, J.; Sun, Y.; Chen, J. Transcriptome sequencing in a 6-hydroxydopamine rat model of Parkinson’s disease. Genes Genet. Syst. 2019, 94, 61–69. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Tian, Y.; Li, H.; Ren, Z.; Liang, S.; Zhang, G.; Zhao, C.; Li, X.; Wang, T.; et al. Moxibustion Exerts a Neuroprotective Effect through Antiferroptosis in Parkinson’s Disease. Evid. Based Complement. Alternat Med. 2019, 2019, 2735492. [Google Scholar] [CrossRef]
- Schallert, T.; Fleming, S.M.; Leasure, J.L.; Tillerson, J.L.; Bland, S.T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 2000, 39, 777–787. [Google Scholar] [CrossRef]
- Boix, J.; Padel, T.; Paul, G. A partial lesion model of Parkinson’s disease in mice--characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behav. Brain Res. 2015, 284, 196–206. [Google Scholar] [CrossRef]
- Carvalho, M.M.; Campos, F.L.; Coimbra, B.; Pego, J.M.; Rodrigues, C.; Lima, R.; Rodrigues, A.J.; Sousa, N.; Salgado, A.J. Behavioral characterization of the 6-hydroxidopamine model of Parkinson’s disease and pharmacological rescuing of non-motor deficits. Mol. Neurodegener. 2013, 8, 14. [Google Scholar] [CrossRef]
- Freire, M.A.M.; Guimaraes, J.S.; Santos, J.R.; Simplicio, H.; Gomes-Leal, W. Morphometric analysis of NADPH diaphorase reactive neurons in a rat model of focal excitotoxic striatal injury. Neuropathology 2016, 36, 527–534. [Google Scholar] [CrossRef]
- Abercrombie, M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946, 94, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, X.; Zhang, L.; Zhang, Y.; Qi, X.; Wang, S.; Qin, C. Longitudinal assessment of motor function following the unilateral intrastriatal 6-hydroxydopamine lesion model in mice. Front. Behav. Neurosci. 2022, 16, 982218. [Google Scholar] [CrossRef] [PubMed]
- Glajch, K.E.; Fleming, S.M.; Surmeier, D.J.; Osten, P. Sensorimotor assessment of the unilateral 6-hydroxydopamine mouse model of Parkinson’s disease. Behav. Brain Res. 2012, 230, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.B.; Halje, P.; Simplício, H.; Richter, U.; Freire, M.A.M.; Petersson, P.; Nicolelis, M. Spinal cord stimulation alleviates motor deficits in a primate model of Parkinson Disease. Neuron 2014, 84, 716–722. [Google Scholar] [CrossRef]
- Stott, S.R.W.; Barker, R.A. Time course of dopamine neuron loss and glial response in the 6-OHDA striatal mouse model of Parkinson’s disease. Eur. J. Neurosci. 2014, 39, 1042–1056. [Google Scholar] [CrossRef]
- Bustelli, I.B.; Oliveira, L.M.; Netto, N.F.C.; Stilhano, R.S.; Caetano, A.L. Behavioral effects of 6-hydroxydopamine-induced damage to nigro-striatal pathway and locus coeruleus as a rodent model of Parkinson’s disease. Behav. Brain Res. 2024, 462, 114873. [Google Scholar] [CrossRef]
- Bagga, V.; Dunnett, S.B.; Fricker, R.A. The 6-OHDA mouse model of Parkinson’s disease—Terminal striatal lesions provide a superior measure of neuronal loss and replacement than median forebrain bundle lesions. Behav. Brain Res. 2015, 288, 107–117. [Google Scholar] [CrossRef]
- Porras, A.R.B.; Ramirez, L.B.; Turner, L.F. Striatal vs. Nigral 6-OHDA lesion models: A closer look at Parkinson’s disease pathology. Braz. J. Anim. Environ. Res. 2025, 8, e80427. [Google Scholar] [CrossRef]
- Grealish, S.; Mattsson, B.; Draxler, P.; Björklund, A. Characterisation of behavioural and neurodegenerative changes induced by intrastatial 6-OHDA lesion in mice. Eur. J. Neurosci. 2010, 31, 2266–2278. [Google Scholar] [CrossRef]
- Francardo, V.; Recchia, A.; Popovic, N.; Andersson, D.; Nissbrandt, H.; Cenci, M.A. Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson’s disease. Neurobiol. Dis. 2011, 42, 327–340. [Google Scholar] [CrossRef]
- Branchi, I.; D’Andrea, I.; Armida, M.; Cassano, T.; Pezzola, A.; Potenza, R.L.; Morgese, M.G.; Popoli, P.; Alleva, E. Nonmotor symptoms in Parkinson’s disease: Investigating early-phase onset of behavioral dysfunction in the 6-hydroxydopamine-lesioned rat model. J. Neurosci. Res. 2010, 88, 2051–2062. [Google Scholar] [CrossRef]
- Collier, T.J.; Kanaan, N.M.; Kordower, J.H. Ageing as a primary risk factor for Parkinson’s disease: Evidence from studies of non-human primates. Nat. Rev. Neurosci. 2007, 8, 499–504. [Google Scholar] [CrossRef]
- Norrara, B.; Arrais, A.C.; Costa, I.M.; Santos, J.R.; Engelberth, R.C.G.J.; Cavalcante, J.S.; Guzen, F.; Cavalcanti, J.R.; Freire, M.A.M. Pattern of tyrosine hydroxylase expression during aging of mesolimbic pathway of the rat. J. Chem. Neuroanat. 2018, 92, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.S.L.; Valente-Amaral, A.; Monteiro, R.F.M.; Meira, C.L.S.; de Meira, N.S.; da Silva, M.N.; Pinheiro, J.J.V.; Bastos, G.N.T.; Felicio, J.S.; Yamada, E.S. Aqueous extract of Swietenia macrophylla leaf exerts an anti-inflammatory effect in a murine model of Parkinson’s disease induced by 6-OHDA. Front. Neurosci. 2024, 18, 1351718. [Google Scholar] [CrossRef] [PubMed]
- Koprich, J.B.; Reske-Nielsen, C.; Mithal, P.; Isacson, O. Neuroinflammation mediated by IL-1β increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J. Neuroinflamm. 2008, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Villar-Cheda, B.; Valenzuela, R.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L. Aging-related changes in the nigral angiotensin system enhances proinflammatory and pro-oxidative markers and 6-OHDA-induced dopaminergic degeneration. Neurobiol. Aging 2012, 33, 204.e1–204.e11. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat. Disord. 2004, 10 (Suppl. S1), S3–S7. [Google Scholar] [CrossRef]
- Peterson, L.J.; Flood, P.M. Oxidative stress and microglial cells in Parkinson’s disease. Mediators Inflamm. 2012, 2012, 401264. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.A.M.; Rocha, G.S.; Bittencourt, L.O.; Falcao, D.; Lima, R.R.; Cavalcanti, J.R. Cellular and molecular pathophysiology of traumatic brain injury: What have we learned so far? Biology 2023, 12, 1139. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef]
- Cieri, M.B.; Ramos, A.J. Astrocytes, reactive astrogliosis, and glial scar formation in traumatic brain injury. Neural Regen. Res. 2025, 20, 973–989. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Federoff, H.J. Targeting microglial activation states as a therapeutic avenue in Parkinson’s disease. Front. Aging Neurosci. 2017, 9, 176. [Google Scholar] [CrossRef]
- Wong, Y.C.; Luk, K.; Purtell, K.; Nanni, S.B.; Stoessi, A.J.; Trudeau, L.E.; Yue, Z.; Krainc, D.; Oertel, W.; Obeso, J.A.; et al. Neuronal vulnerability in Parkinson disease: Should the focus be on axons and synaptic terminals? Mov. Disord. 2019, 34, 1406–1422. [Google Scholar] [CrossRef]
- Rocha, G.S.; Freire, M.A.M.; Falcao, D.; Outeiro, T.F.; Lima, R.R.; Santos, J.R. Neurodegeneration in Parkinson’s disease: Are we looking at the right spot? Mol. Brain 2025, 18, 68. [Google Scholar] [CrossRef]
- Graeber, M.B.; Streit, W.J. Microglia: Biology and pathology. Acta Neuropathol. 2020, 119, 89–105. [Google Scholar] [CrossRef]
- Le, T.M.; Takarada-Iemata, M.; Ta, H.M.; Roboon, J.; Ishii, H.; Tamatani, T.; Hattori, T.; Hori, O. Ndrg2 deficiency ameliorates neurodegeneration in experimental autoimmune encephalomyelitis. J. Neurochem. 2018, 145, 139–153. [Google Scholar] [CrossRef]
- Guo, M.; Lu, B.; Gan, J.; Wang, S.; Jiang, X.; Li, H. Apoptosis detection: A purpose-dependent approach selection. Cell Cycle 2021, 20, 1033–1040. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, X.; Xu, J.; Wen, Y.; Li, A.; Lu, M.; Zhou, J. Astrocytic JWA deletion exacerbates dopaminergic neurodegeneration by decreasing glutamate transporters in mice. Cell Death Dis. 2018, 9, 352. [Google Scholar] [CrossRef]
- Li, M.; Chen, M.; Li, H.; Gao, D.; Zhao, L.; Zhu, M. Glial cells improve Parkinson’s disease by modulating neuronal function and regulating neuronal ferroptosis. Front. Cell Dev. Biol. 2025, 12, 1510897. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, Y.; Kim, J.S.; Lee, H.; Ju, I.G.; Yoo, N.Y.; La, S.; Jeong, D.H.; Na, C.; Park, H.-J.; et al. Stimulation of microneedles alleviates pathology of Parkinson’s disease in mice by regulating the CD4+/CD8+ cells from the periphery to the brain. Front. Immunol. 2024, 15, 1454102. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.A.M.; Santos, J.R. Parkinson’s disease: General features, effects of levodopa treatment and future directions. Front. Neuroanat. 2010, 4, 146. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.J.; Clarke, P.B.S. Susceptibility of ascending dopamine projections to 6-hydroxydopamine in rats: Effect of hypothermia. Neuroscience 2002, 115, 1281–1294. [Google Scholar] [CrossRef]
- Tanguay, W.; Ducrot, C.; Giguère, N.; Bourque, M.J.; Trudeau, L.E. Neonatal 6-OHDA lesion of the SNc induces striatal compensatory sprouting from surviving SNc dopaminergic neurons without VTA contribution. Eur. J. Neurosci. 2021, 54, 6618–6632. [Google Scholar] [CrossRef]
- Paß, T.; Ricke, K.M.; Hofmann, P.H.; Chowdhury, R.S.; Nie, Y.; Chinnery, P.; Endepols, H.; Neumaier, B.; Carvalho, A.; Rigoux, L.; et al. Preserved striatal innervation maintains motor function despite severe loss of nigral dopaminergic neurons. Brain 2024, 147, 3189–3203. [Google Scholar] [CrossRef]
- Salmani, B.Y.; Lahti, L.; Gillberg, L.; Jacobsen, J.K.; Mantas, I.; Svenningsson, P.; Perlmann, T. Transcriptomic atlas of midbrain dopamine neurons uncovers differential vulnerability in a Parkinsonism lesion model. Elife 2024, 12, RP89482. [Google Scholar] [CrossRef]
| Primary Antibodies | Secondary Antibodies | Labeling Purpose |
|---|---|---|
| TH (1:5000— Merck Millipore, Burlington, MA, USA) | Goat anti-rabbit (1:1000—Vector Labs, Burlingame, CA, USA)/(1:700—Alexa Fluor 555—Invitrogen, Grand Island, NY, USA) | Dopaminergic neurons |
| GFAP (1:1000— Dako, Glostrup, Denmark) | Goat anti-rabbit (1:1000—Vector Labs, Burlingame, CA, USA) | Astrocytes |
| Iba1 (1:1000— Wako, Osaka, Japan) | Goat anti-rabbit (1:1000—Vector Labs, Burlingame, CA, USA) | Microglial cells |
| Caspase-3 (1:250— Promega, Madison, WI, USA) | Goat anti-rabbit (1:1000—Vector Labs, Burlingame, CA, USA) | Apoptotic cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire, M.A.M.; Rocha, G.S.; Lemos, N.A.M.; Lima, R.R.; Bittar, S.; Jenkins, L.B.; Falcao, D.; Steinbusch, H.W.M.; Santos, J.R. Insights into Parkinson’s Disease Pathology Focusing on Glial Response and Apoptosis in a Classic Rat Model of Dopaminergic Degeneration. Neuroglia 2025, 6, 36. https://doi.org/10.3390/neuroglia6030036
Freire MAM, Rocha GS, Lemos NAM, Lima RR, Bittar S, Jenkins LB, Falcao D, Steinbusch HWM, Santos JR. Insights into Parkinson’s Disease Pathology Focusing on Glial Response and Apoptosis in a Classic Rat Model of Dopaminergic Degeneration. Neuroglia. 2025; 6(3):36. https://doi.org/10.3390/neuroglia6030036
Chicago/Turabian StyleFreire, Marco Aurelio M., Gabriel S. Rocha, Nelson Alessandretti M. Lemos, Rafael R. Lima, Stanley Bittar, Lissandra B. Jenkins, Daniel Falcao, Harry W. M. Steinbusch, and Jose Ronaldo Santos. 2025. "Insights into Parkinson’s Disease Pathology Focusing on Glial Response and Apoptosis in a Classic Rat Model of Dopaminergic Degeneration" Neuroglia 6, no. 3: 36. https://doi.org/10.3390/neuroglia6030036
APA StyleFreire, M. A. M., Rocha, G. S., Lemos, N. A. M., Lima, R. R., Bittar, S., Jenkins, L. B., Falcao, D., Steinbusch, H. W. M., & Santos, J. R. (2025). Insights into Parkinson’s Disease Pathology Focusing on Glial Response and Apoptosis in a Classic Rat Model of Dopaminergic Degeneration. Neuroglia, 6(3), 36. https://doi.org/10.3390/neuroglia6030036








