- Review
The Role of Oligodendrocytes in Alzheimer’s Disease Pathogenesis and Therapy
- Shihui Guo,
- Xinyi Yu and
- Hongsheng Zhang
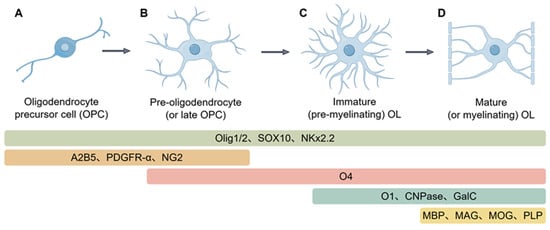
Oligodendrocytes (OLs) constitute the main glial population in the central nervous system and are indispensable for the stability and performance of neural networks. Although best known for generating and maintaining myelin to speed impulse conduction, their influence extends further. By modulating myelin in response to activity, supplying metabolic substrates, and engaging in neuroimmune communication, OLs help preserve the structural integrity and plasticity of neuronal circuits. Growing evidence now positions defective OLs as central players in Alzheimer’s disease (AD). Experimental work suggests that OL injury can act as an early trigger, fostering amyloid-β (Aβ) deposition and Tau hyperphosphorylation. Conversely, toxic Aβ aggregates and pathological Tau proteins damage OLs, causing myelin breakdown and progressive neurodegeneration that fuels a self-perpetuating cycle. Here, we synthesize current knowledge of OL physiology and its multifaceted contributions to AD pathogenesis, with particular attention to the bidirectional interplay between OL dysfunction and the disease’s core features—Aβ and tau. On this basis, we outline prospective therapeutic avenues to protect or restore oligodendrocyte function in AD.
11 December 2025