Abstract
Neuropeptides (NPs) are small molecular messengers synthesized in large dense core vesicles (LDCVs) and secreted to the extracellular space. In the central nervous system (CNS), NPs are secreted to the synaptic space, playing crucial roles in modulating neurons, astrocytes, microglia, oligodendrocytes, and other glial cells, through G-protein-coupled receptors, thereby influencing complex multicellular responses. During neuroinflammation, NPs regulate glial and neuronal reactions to inflammatory signals, promoting resolution and preventing chronic, non-resolving inflammation. For example, NPs inhibit apoptosis in neurons and oligodendrocytes while inducing anti-inflammatory effects in microglia and astrocytes, modulating cytokine secretion. Here, we present the notion that neuropeptides could participate in neuroinflammatory progression, altering glial responses, leading to excessive, non-resolutive inflammation when dysregulated. NP signaling—whether excessive or deficient—can disrupt specific cellular processes, leading to pathological inflammation, gliosis, and functional loss—hallmarks of neurodegenerative diseases. Despite their significance, the precise mechanisms underlying NP-mediated effects remain incompletely understood. This review synthesizes experimental and translational evidence highlighting the pivotal role of NPs in resolving neuroinflammation and explores how targeting NPs or their receptors could offer novel therapeutic strategies for neurodegenerative disorders. Further research is needed to elucidate the specific signaling pathways and receptor dynamics involved, which could pave the way for innovative treatments that address the root causes of these debilitating conditions.
1. Introduction
Neuropeptides (NPs) are small protein-like molecules that act as neuromodulators in central and peripheral nervous systems. They have received attention for their capability of modifying the biophysical properties of large groups of nerve cells, resulting in complex neuronal responses and circuit integration, and the corresponding coordination of multiple brain systems [1]. NPs act as relevant mediators of plasticity through their slow neuromodulatory effects, remodeling synaptic transmission and neuronal circuit plasticity [2]. However, this understanding implies that released neuropeptides diffusing in the parasynaptic space are able to modulate, in a multidirectional manner, the surrounding glial cells. The multicellular effects of NPs and their slow time-frame effects on circuit coherence reflect their significant role in physiological control of homeostasis, bridging physiology from multiple systems, which includes the glial responses to neuroinflammation [3].
NPs activate cell functions through stimulation of transmembrane G-protein-coupled receptors, widely distributed in nervous, endocrine, and immune cells. These receptors activate multiple pathways, which include the adenylate cyclase/cAMP pathway, phospholipase C (PLC)/calcium pathway, cAMP-response element-binding protein (CREB) pathway, and G-protein-independent pathways such as PI3K and MAPK cascades [4,5].
Among the several mechanisms involved in damage to nervous tissue, neuroinflammation represents one of the most finely tuned phenomena participating in homeostasis maintenance. Neuroinflammation, as a physiological process of the central nervous system (CNS), has the primary beneficial goal of eliminating the source of inflammation, allowing for tissue repair. However, neuroinflammation differs from other types of inflammation in that it triggers the activation of multiple neuronal and glial cells, localized in multiple but specific regions, leading to the production of pro-inflammatory cytokines and chemokines locally [6]. Importantly, if neuroprotective mechanisms fail in containing inflammation, the excessive stress to neurons can eventually lead to neuronal death and tissue scar formation (gliosis) [7]. Regulation of the neuroinflammatory response has captured the attention over the past few decades due to its profound impact and effects in the short (e.g., sickness behavior) and long terms (e.g., neurodegeneration) in such a way that it has become clear that the resolution of inflammation is crucial for functional behavioral, sensorimotor, and cognitive recovery [8,9]. Therefore, it is generally accepted that, if neuroinflammation becomes prolonged or inadequate (either deficient or excessive), it can lead to disease progression and tissue damage, progressing to a loss of function [10].
During neuroinflammatory responses, neurons release multiple signals, such as neurotransmitters and neuropeptides [7,11]. The latter, which are stored in large and dense core vesicles (LDCVs), are then secreted via exocytosis to the extracellular space. NPs then diffuse by volume transmission [12], extending their effects beyond the synaptic cleft, thus influencing glial cell functions locally. It is likely that this modality of transmission leads to integration, in a complex interplay, between neurons and glia [13]. This particular modality, based on induction of slow cellular responses, is mediated via second messengers, such as cyclic AMP, inositol triphosphate, and diacylglycerol [13,14], which enhance many cellular survival and trophic functions in both neurons and glial cells (for instance, increasing tolerance to cell death and cell resistance to reactive oxygen species (ROS)) [15], which results in being pivotal for the subsequent regulation of glial responses, promoting nervous tissue restoration.
Glial cells are provided multiple receptors, which play specific roles in neuro-immunomodulation (see Table 1 and Figure 1), and among the myriad of molecular signals that drive glial cells, neuropeptides have emerged as key players in this interplay as they can integrate multiple cell types in restricted periods of time, acting through membrane G-protein-coupled receptors [16]. Diffused neuropeptides, in turn, orchestrate cell communication between neurons (i.e., the main source of neuropeptides), glia (expressing prominent NP receptors [17]), and other local cell types (i.e., mast cells [18,19]), as well as modulating the transient peripherally invading monocytes and inflammatory lymphocytes (e.g., T CD4) that invade the brain in response to inflammatory signals [20,21].

Table 1.
Main neuropeptides discussed in this review, their receptors, and their expression in nervous tissue cells.
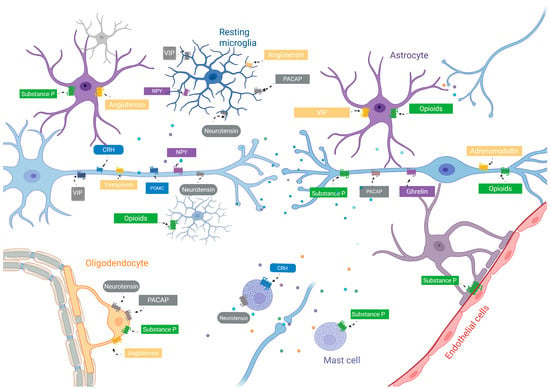
Figure 1.
Graphical scheme illustrating the proposed physiological model of neuropeptides in neuron–glia communication in a steady state. Note that NP receptors are widely distributed in glial cells, with cells exclusively expressing some receptors and sharing receptors with other glial cells, favoring multicellular simultaneous interactions. Neuropeptides are represented by different colored dots. Neuropeptides might be linked to steady-state maintenance of glial cellular responses and blood–brain barrier permeability. Abbreviations: CRH, corticotrophin-releasing hormone; VIP, vasoactive intestinal peptide; POMC, pro-opiomelanocortin; NPY, neuropeptide Y; PACAP, pituitary adenylate-cyclase-activating peptide.
Although research has focused mainly on the effects of NPs on neuronal modulation and co-transmission, their integrative role in glial cell biology has been largely overlooked. However, the relevance of neuropeptide–glial cell interactions has emerged and a plethora of accumulating emerging evidence of their role as orchestrators of neuroinflammation, synaptic regulation, and homeostasis has been discovered. Also, the interest in the potential therapeutic effects of NPs as multitarget modulatory molecules has increased recently, using neuropeptide mimetics, such as gastrin-releasing peptide (GLP-1) agonists [22], showing promising therapeutic effects.
2. Methodology
For this narrative review, MEDLINE/PubMed, Scopus, and Web of Science were consulted to identify experimental or clinical studies that examined the effects of neuropeptide function with a specific focus on glial pro- and anti-inflammatory response. The primary search terms included “Neuropeptide (each neuropeptide)”, which was crossed, using Boolean operators (AND, OR, IN) with “Astrocytes”, “Microglia”, “Oligodendrocytes”, “mast cells”, “myelin”, “Inflammation”, and to enhance the sensitivity and specificity for “Neurodegeneration”, “Alzheimer’s disease”, “Parkinson’s disease”, “Pain”, “stroke”, and “treatment”. Boolean operators were strategically employed to enhance both sensitivity and specificity. Selection of NPs was based on the number of published articles for a given neuropeptide, selecting the most frequently studied, and grouped by their anti- or pro-inflammatory effects, together with clustering by “families”, based on physiological functions (e.g., “adipokines”; see Supplementary Table S1) The exclusion criteria encompassed manuscripts not published in English and studies that did not provide detailed data on glial inflammatory processes. Articles were organized thematically based on families of neuropeptides analyzed, types of glial changes, clinical or experimental models, and therapeutic results. Schematic figures were created in https://BioRender.com, accessed on 1 July 2025.
3. Peptides with Anti-Inflammatory Cell-Resolving Actions
3.1. Vasoactive Intestinal Peptide (VIP) and Pituitary Adenylate Cyclase-Activated Poly-Peptide (PACAP)
The vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activated polypeptide (PACAP) are two amidated neuropeptides with pleiotropic neuromodulatory and immunomodulatory activities in the brain, acting as neuron-to-neuron transmitters, regulating the effect of neurotransmitters and influencing the inflammatory properties of glial cells. Both activate three types of G(q) receptors: PAC1, VPAC1, and VPAC2 [23]. It is widely accepted that VPAC1 and 2 exert immunomodulatory effects, besides their neuronal effects, whereas PAC1 activation has been related to neuroprotective and neurotrophic effects and cell death resistance [24].
In the context of neuroinflammation, PACAPs and VIPs play differential roles through these receptors. PAC1 predominantly mediates the release of growth/trophic factor and other neuroprotective actions, while VPAC1 and VPAC2 are implicated in mediating most of the immunomodulatory effects and prevent neuronal death by regulating microglial immune responses, inducing their proliferation and shift to the M2 (anti-inflammatory) phenotype, reducing the production and secretion of inflammatory cytokines, prostaglandins, iNOS, and NO, and increasing the secretion of anti-inflammatory cytokines (TNF-β, IL-10) [25].
Activation of VPAC1 mediates responses in microglial cells by increasing the intracellular amounts of cAMP, leading to the inhibition of NF-KB, the Janus kinase (JAK)/signal transducer and activator of the transcription 1 (STAT1) pathway, and inhibition of the MEKK1/MEK4/JNK pathway, controlling the gene expression and induction of CREB, a transcription factor involved with genes containing a cAMP-responsive element, which includes IL-2, IL-6, IL-10, and TNF-α [26,27]. PACAPs have been shown to inhibit the activation of the TLR4/MyD88/NF-κB signaling pathway, decreasing inflammatory cytokine levels and attenuating microglial polarization in response to hypoxic injury [28]. PACAPs and VIPs reduce cytokine secretion from M1-activated microglia, induce the shift to M2, and thus increase the survival and proliferation of oligodendrocytes and neurons, increasing phagocytosis of toxic myelin debris and further reducing the inflammatory microenvironment and favoring the remyelination processes [29]. Finally, the PACAP is a known stimulator of oligodendrocyte progenitor cell proliferation [30].
The deficiency of both PACAPs and VIPs has been reported in neurodegenerative inflammatory diseases, illustrating the effects of a system with low neuropeptide signaling [31]. For instance, in experimental models of multiple sclerosis, VIP administration increased phagocytosis by microglia, via protein kinase C (PKC) signaling, and PACAPs enhanced phagocytosis while inhibiting the release of pro-inflammatory cytokines [32]. This shift is paralleled by heightened phagocytic responses after PACAP or VIP treatment, suggesting that the peptides may trigger an anti-inflammatory phenotype in macrophages that, in turn, contributes to boosting cell-scavenging activities [25,29].
Another clinically relevant neurodegenerative disease where these two neuropeptides have shown promising prospects is Parkinson’s disease (PD), characterized by selective loss of dopamine (DA) neurons in the mesencephalic substantia nigra (SN) striatum pathway and the formation of Lewy bodies in the remaining neurons [33]. PACAP treatment attenuates apoptosis of DA neurons, improving behavioral and motor deficits, reducing severe acute hypokinesia [34]. These effects are linked to its anti-inflammatory activities and microglial regulation and activation of the PKA signaling pathway, enhancing the expression of BDNF and its signal transduction protein, p-CREB, inducing anti-autophagic properties [35]. In concordance, in a 6-OHDA-induced PD rat model, VIPs were found to reverse clinical mobility deficits, renovate the myelin sheet, and preserve neurons via the production of nerve growth factor by brain mast cells [36]. Administration of VIPs into the substantia nigra inhibited microglial activation by reducing local expression of TNF-α, IL-1β, iNOS, and NADPH oxidase, decreasing nigrostriatal nerve fiber loss and dopaminergic neuronal degeneration [37]. Therefore, VPAC1 seems to be the primary mediator of these protective actions in PD models.
Similarly, patients with neuropathologically confirmed Alzheimer dementia have reduced levels of PACAPs and VIPs in cerebrospinal fluid and brain expression in cortical structures [38]. The reduction in these peptides and their neuroprotective effects is related to the VIP suppression of the inflammatory response induced by beta-amyloid fibrils and by the neurotoxin MPTP on microglia [39]. Recent studies have shown that VIPs regulated microglial activation by fibrilized-Ab42, significantly promoted microglia phagocytosis of Ab1–42, and attenuated cerebral amyloidosis in a transgenic mouse model of Alzheimer’s disease [40]. These effects could also be explained by increased cell survival, as both PACAPs and VIPs reduce neuron and microglial cell death in local pro-inflammatory environments, promoting recovery [41].
Agonists of these neuropeptides have been developed since the past decade, showing promising pharmacological properties, even surpassing the effect of biologically derived peptide molecules [42]; however, to our knowledge, no clinical trials have been carried out reporting PACAP or VIP agonists as plausible therapeutics for these devastating conditions.
3.2. Neurotensin
Neurotensin is a 13-amino acid neuropeptide with potent vasoactive functions with neurotransmitter and neuromodulatory properties in the CNS, where it is involved in pain regulation, body temperature, and appetite, as well as learning and memory. It exerts its actions through three different receptors: neurotensin receptor (NTSR)1, NTSR2, and NTSR3, also known as sortilin (SORT1 and SORTla) [30]. NTSR1 and NTSR2 are class A GCPRs (Gq/11) while NTSR2 is a single-pass type I transmembrane receptor not coupled to G-proteins but capable of forming complexes with other neurotensin receptors [43] and modulating intracellular signaling and apoptosis [44].
NTSr1 is expressed mainly by neurons and is responsible for classic central effects of NT, such as modulation of nociception [45], thermoregulation (whose effect is mediated by neuronal and astrocyte receptors [46]), and dopaminergic transmission during motor learning and memory processes [47]. In the periphery, NT regulates digestive and cardiovascular function, and it has been linked to brain pathology as it is a central modulator of alcohol sensitivity [48] and psychosis [49]. NTSR2 is expressed throughout the CNS, particularly in the hippocampus, cerebral cortex, cerebellum, olfactory bulb, substantia nigra, and ventral tegmental area [50]. NTSR2 is widely expressed in astrocytes and other glial cells, where it regulates inflammation in response to excitotoxicity [51]. Contrastingly, NT secreted during inflammatory stress synergizes with corticotropin-releasing hormone (CRH) to activate mast cells [52], increasing vascular and blood–brain barrier permeability [53]. In microglia, NT induces the production and release of IL-1b and CXCL8 [54]. Through NTR3, in vitro, pro-inflammatory stimuli upregulate NTSR2 expression in astrocytes, and antagonism with SR142948A (NT antagonist) reduces glial reactivity, suggesting that NT is involved in modulating glial responses during neuroinflammation [51].
In several experimental models, NT has been shown to protect astrocytes against apoptosis, likely through NTSR2-dependent mechanisms [46]. However, in conditions involving excitotoxicity, NT can enhance NMDA receptor-mediated glutamate signaling, leading to intracellular calcium overload and neuronal injury [55]. This duality is evident in ischemic conditions, where NT has been shown to worsen tissue damage via mast cell degranulation [56,57] and affect blood–brain barrier permeability in vitro [53]. A reduction in neurotensin signaling has been found in Alzheimer’s disease (AD). Postmortem studies have shown a significant reduction in NTSR1 and NTSR2 mRNA levels in the temporal lobe, along with a notable loss of neurotensin binding sites in the hippocampus [58]. Given the role of NTSR2 in memory-related processes, its downregulation may contribute to the cognitive deficit characteristic of AD. However, neurotensin may also exert neuroprotective effects through CD36, a class B scavenger receptor involved in amyloid-beta (Aβ) uptake and microglial activation [59,60]. By binding to CD36, NT can inhibit Aβ-induced pro-inflammatory signaling in microglia. In contrast, the upregulation of NTSR3/sortilin in AD facilitates the formation of complexes with pro-neurotrophins and p75 NTR, promoting neuronal apoptosis [61]. These findings suggest that NT can play both protective and deleterious roles in AD, depending on the receptor and cellular context.
In contrast to Alzheimer’s disease, in Parkinson’s disease (PD), neurotensin levels are elevated in the substantia nigra and plasma, particularly in patients who are not treated with levodopa. Despite this elevation, receptor expression is reduced in dopaminergic regions such as the putamen. In animal models, dopaminergic injury induces upregulation of NT receptor mRNA in the substantia nigra and ventral tegmental area. Pharmacological administration of NT analogs, such as NT 8–13, NT2, and NT4, alleviates motor symptoms by enhancing striatal dopamine release. NT2 and NT4 also improve memory performance. However, NT may exacerbate glutamate excitotoxicity and calcium dysregulation, leading to potential risks in the long term. Similarly, in multiple sclerosis (MS), particularly relapsing–remitting MS (RRMS), NT contributes to immune regulation. Interferon-beta therapy upregulates NTSR1 expression in T cells, promoting anti-inflammatory cytokine production. NTSR1 is also found in active demyelinating lesions, suggesting its involvement in neuroimmune crosstalk [62].
NT is a good example on how neuropeptides can have a dual, bidirectional effect, and how this could contribute to pathophysiology. Although several studies have investigated its role, no insights into potential therapeutics have been addressed for specific neurodegenerative diseases yet.
3.3. Proopiomelanocortin
Proopiomelanocortin (POMC) is a precursor polypeptide of several important hormones and neuropeptides. Its main products are adrenocorticotropic hormone (ACTH), α-melanocyte-stimulating hormone (α-MSH), β-MSH, and γ-MSH, collectively known as melanocortins, and the endogenous opioid β-endorphin (β-END) [63]. Although melanocortins are better known for their involvement in the regulation of memory, food intake, energy expenditure, pigmentation, and gland secretion, among others [64], melanocortin receptors MC1R to MC5R are part of the family A GPCRs, and MC5R is the most often associated with the regulation of inflammation and immune responses [64]. Melanocortins are known to be responsible for regulating the differentiation of macrophages into their tissue specific variants, such as osteoclasts and microglial cells, but their regulatory functions on these differentiated cells are still being studied [65].
The most studied melanocortin, α-MSH, has shown several important functions as an immunoregulator: it inhibits the brain production of TNF-α [66] through the activation of MC1R present on astrocytes and microglia, most likely by a mechanism involving cAMP production. Microglial cells have been found to release α-MSH upon stimulation with LPS + IFN-γ, serving as a self-regulating autocrine anti-inflammatory loop [67]. α-MSH has been shown to suppress Toll-like receptor 4 responses to LPS in macrophages by reducing NO production and promoting IL-1 receptor-associated kinase (IRAK)-M binding to IRAK-1 [68]. α-MSH has been found to reduce the phagocytosis of apoptotic cells by macrophages reducing the oxidative state in inflammatory environments [69].
4. Neuropeptides with Pro-Inflammatory Effects
In the following section, NPs with pro-inflammatory effects are discussed. It is worth noting that some of these NPs act during inflammatory responses and that their sustained production could become pathological for tissue restoration (Figure 2); furthermore, NPs such as angiotensin can be cleaved by enzymes that modify their affinity for their receptors and therefore elicit dual pro- or anti-inflammatory effects, depending on the time and trigger nature.
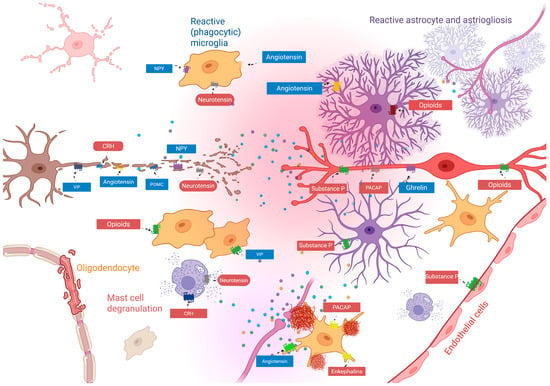
Figure 2.
Proposed pathological role of neuropeptides in neuron–glia communication in non-resolutive state. Increased and dysbalanced neuropeptide secretion and concentration (represented by different colored dots) induced by inflammatory response, dysregulate locally and time- and cell-dependent cellular responses, leading to uncontrolled redox state, inflammation, and accumulation of inflammatory debris, in turn leading to the consequent activation of M2 microglial responses, with pathological phagocytic effect, which is increased by massive degranulation of mast cells as well as increasing astrogliosis, leading to loss of circuit state. Note that some receptors or neuropeptide signals increase (reddish colors) or decrease (blueish colors) in response to inflammation. This disassembly of physiological network results in tissue damage and sustained inflammation, leading to changes in circuit connectivity, dysregulating cognitive, behavioral, and sensorimotor processing. Abbreviations: CRH, corticotrophin-releasing hormone; VIP, vasoactive intestinal peptide; POMC, pro-opiomelanocortin; NPY, neuropeptide Y; PACAP, pituitary adenylate-cyclase-activating peptide.
4.1. Substance P and Opioids
Substance P and opioids are two peptide families known by their role in pain triggering (SP) and pain modulation. In the central nervous system (CNS), substance P (SP) has been associated with various behavioral responses and with the regulation of neuronal survival and degeneration through its interaction with CNS-resident cells, including astrocytes and oligodendrocytes [70]. It is mainly secreted by neurons and has been involved in nociception, hypotension and muscular contraction, and inflammation [71]. These effects are primarily mediated by the neurokinin-1 receptor (NK1R), a class A G-protein-coupled receptor (GPCR) that signals predominantly through the Gq pathway [72]. It has been found to produce local inflammation of the hypothalamus and amygdala in neurodegenerative diseases [71].
Studies in rodents have demonstrated substance P production by macrophages, eosinophils, lymphocytes, and dendritic cells; also, it increases vascular permeability, facilitating the development of an inflammatory response [70,73]. In the brain, substance P induces the polarization of microglia and macrophages in inflammatory conditions [74]. These effects are notorious in Alzheimer’s disease, where SP upregulates ADAM9 via NK1R, promoting non-amyloidogenic amyloid precursor protein processing and reducing amyloid-β (Aβ) accumulation, and, through Aβ (25–35), it decreases hippocampal SP expression, exacerbating neuronal damage [75].
Similarly, substance P in Parkinson’s disease enhances microglial activation via the PKCδ–NOX2 axis, exacerbating dopaminergic neurodegeneration, and it reduces calcium influx, caspase activation, and oxidative stress, offering neuroprotection; therefore, dopaminergic replacement therapy may modulate SP expression, highlighting its complex role in PD [71]. Furthermore, SP-NK1R signaling drives glial activation and neuroinflammation induced by cytokine and reactive oxygen species release in astrocytes in demyelinating diseases such as multiple sclerosis; this increases endothelial permeability, facilitating leukocyte infiltration [76].
In contrast, in ischemic stroke models, acute SP administration promotes anti-inflammatory monocyte (M2 phenotype) recruitment, enhances CD163+ and CD206+ marker expression in microglia/macrophages, reduces neuronal apoptosis, preserves BBB integrity, and improves functional recovery. These findings position SP as an early modulator of post-ischemic inflammation [74].
Endogenous Opioids
Endogenous opioids—β-endorphin, enkephalins, dynorphin A, and nociceptin—share the N-terminal amino acid sequence Tyr-Gly-Gly-Phe [77]. Each preferentially activates a Gi/o-coupled opioid receptor: β-endorphin binds the μ receptor (MOR), enkephalins the δ receptor (DOR), dynorphin A the κ receptor (KOR), and nociceptin the NOP receptor [78,79]. These receptors, which belong to the class A GPCR family, are structurally similar and inhibit adenylate cyclase and multiple intracellular pathways upon activation [77].
In the central nervous system, neurons, microglia, and astrocytes can express these receptors, enabling them to participate in the regulation of neuroinflammation, driven by glial cells [80]. Acute activation of these receptors tends to polarize microglia toward an anti-inflammatory M2 phenotype by inhibiting TLR4 and NF-κB [81], also reducing the release of pro-inflammatory cytokines and limiting the progression of neuroinflammatory damage [82]. The MOR has a high affinity for β-endorphins and, to a lesser extent, for enkephalins [83]. It is widely expressed in CNS neurons, especially in the hypothalamus (arcuate nucleus), as well as in microglia and astrocytes [84]. MOR activation decreases the release of glutamate and substance P by inhibiting the AC–cAMP–PKA–CREB pathway and closing Ca2+ channels, exerting antinociceptive and indirectly anti-inflammatory effects [85]. It is also involved in the IL-10/β-endorphin axis that mediates neuroimmune analgesia [85,86].
In astrocytes, MOR stimulation has been associated with reduced GFAP and COX-2 levels and decreased astrogliosis, although its expression in these cells remains under debate [87]. Additionally, the MOR activates the ERK1/2 pathway, increasing BACE1 and Aβ production, which is associated with increased neurodegenerative risk in Alzheimer’s disease [88]. The DOR shows high affinity for enkephalins and is expressed in interneurons, microglia, and astrocytes [89]. In microglia, it also inhibits COX-2, TNF-α, and IL-1β via NF-κB and stimulates IL-10 through PI3K/Akt and SOCS3 [90]. Overexpression of the DOR curbs excessive glial activation and supports anti-inflammatory homeostasis, while bidirectionally modulating pain with IL10 [91]. Concomitantly, in Alzheimer’s models, dominant DOR signaling relative to the MOR is associated with lower amyloid burden and neuroprotection [88].
The KOR binds preferentially to dynorphin A. Its activation in neurons inhibits glutamate and substance P release via Gi/o-mediated cAMP inhibition, reducing excitability [92]. However, prolonged activation of the KOR recruits the β-arrestin 2 pathway, activating p38 and JNK, which can lead to dysphoria and negative plasticity [82]. In microglia, the KOR inhibits the TLR4–MyD88–IKK/NF-κB pathway, reducing TNF-α and IL-1β, promoting IL-10, and facilitating M2 polarization during antinociception, indicating that pain transmission is accompanied physiologically by KOR signaling in microglia to counter the effects of inflammation [93]. In the hippocampus, chronic KOR activity reduces BDNF and neurotrophin levels, induces synaptic loss, and alters reward circuits such as the amygdala and nucleus accumbens, contributing to anhedonia, anxiety, and depression [94]. The KOR thus exerts a dual effect: acutely anti-inflammatory, but disruptive in the long term to neural plasticity.
4.2. Corticotropin-Releasing Hormone
Corticotropin-releasing hormone (CRH) is synthesized by the hypothalamus and secreted to the hypothalamus–hypophyseal portal system, and to general circulation as a response to stress exposure, where it activates the hypothalamic–pituitary–adrenal axis (HPA), stimulating the release of adrenocorticotropic hormone in the pituitary gland, which stimulates the release and production of glucocorticoids from the adrenal cortex. CRH activates two G-protein (Gs)-coupled receptors, CRH-R1 and CRH-R2, which adds to increased intracellular concentrations of cyclic AMP (cAMP). These receptors are expressed in both hypothalamus and extra-hypothalamic regions, including the striatum, HP, cortex, and amygdala [95].
Opposite to the anti-inflammatory effects induced via the production of cortisol, CRH is considered to elicit pro-inflammatory effects directly [96]. CRH can act directly on mast cells, inducing MC maturation, producing neurogenic inflammation, and disrupting the blood–brain barrier in response to stress [97]. Also, histamine release is induced, and histamine has positive feedback, inducing the release of CRH by neurons. Mast-cell-derived IL1 and IL-6 also induce CRH release by hypothalamic neurons [98,99]. A recent study shows that CRH induces autophagy dysregulation and microglial activation following sleep restriction in rodents [100]. This process is relevant, as relevant studies have suggested that sleep restriction is a major risk factor for neurodegenerative diseases [101,102]; furthermore, dysregulated autophagy has been shown to play a role in inflammation and microglial activation [103].
CRH activates microglia by upregulating CD11b and mIL-1β l and nitric oxide (NO) release [104], which leads to increasing levels of reactive oxygen species (ROS) that, in response, induce interleukin-18, which is a critical molecule for integrating microglia and astrocyte-derived inflammatory responses in the brain [105,106]. These findings suggest that the CRH system may serve as a critical link between chronic stress and neuroinflammation in vivo. Furthermore, CRH clinical relevance has emerged as a potential cerebrospinal fluid biomarker for Lewy body disease and Parkinson’s disease, with studies showing reduced levels in patients with dementia, with Lewy bodies compared to those with other types of dementia [107]. On the other hand, it is well known that this neuropeptide, predominantly produced in the paraventricular nucleus of the hypothalamus, initiates activation of the hypothalamic–pituitary–adrenal axis, leading to cortisol secretion by the adrenal cortex, thus exerting anti-inflammatory effects.
4.3. Angiotensin
Angiotensin (Ang) and Ang-related peptide isoforms (e.g., Ang1–9) are a group of peptides with relevant roles in the control of blood pressure, as well as electrolyte balance through its crucial step in the renin–angiotensin–aldosterone system (RAAS) [108], which, in recent years, has received attention due to its role in inflammatory responses, particularly in some viral infections, such as SARS-CoV-2, in which the angiotensin-converting enzyme 2 (ACE2) serves as the viral entry to host cells, and its dysregulation has been implicated in COVID-19 pathophysiology, its progression, and clinical outcomes [103,104,105]. However, although angiotensin and Ang isoforms have been isolated in the brain, and the existence of a local brain renin–angiotensin system has been described and largely discussed [109], the evidence for angiotensin II or angiotensin-related peptides remains unclear. For instance, Ang is able to regulate hypothalamic neurons to maintain vascular resistance and blood pressure through acting in neurons of the paraventricular nucleus [104], and angiotensin II has been found to be colocalized with either GABA (gamma aminobutiric acid) or glutamate transporters in cortical and subcortical regions [110,111]; see [112] for an excellent review.
The brain renin–angiotensin system (bRAS) features several key receptors, including angiotensin type 1 (AT1R, with subtypes AT1A and AT1B), type 2 (AT2R), Mas (MasR), and type 4 (AT4R), each activated by specific angiotensin peptide isoforms and exhibiting distinct regional localizations that contribute to functions like blood pressure regulation, neuroprotection, and cognition.
Angiotensin receptors, integral to the brain renin–angiotensin system (bRAS), are expressed in glial cells, notably astrocytes and microglia, with distinct localization patterns that contribute to neuroinflammation, gliosis, and brain homeostasis. The angiotensin type 1 receptor (AT1R), primarily activated by angiotensin II (Ang II), is widely expressed in astrocytes across the cortex, hippocampus, and hypothalamus, where it drives reactive astrogliosis and pro-inflammatory responses, such as cytokine release (e.g., IL-1β, TNF-α) in conditions like hypertension or stroke [113]. The AT1R is also present in microglia, particularly in activated states in the cortex and brainstem, promoting microglial activation and oxidative stress, which exacerbate neuroinflammatory pathways [114]. The angiotensin type 2 receptor (AT2R), which binds Ang II and Ang III (with higher affinity for the latter), is less prevalent but detected in astrocytes in the cerebellum and hippocampus, potentially mediating anti-inflammatory or neuroprotective effects; its expression in microglia is context-dependent and less clear, often observed in injury models [115].
In contrast, the Mas receptor (MasR), activated by angiotensin-(1–7) [Ang-(1–7)] and alamandine, is expressed in astrocytes in the hippocampus, hypothalamus, and brainstem (e.g., nucleus tractus solitarii), supporting anti-inflammatory signaling and glial homeostasis, with limited evidence of low-level expression in resting microglia [116]. The angiotensin type 4 receptor (AT4R, or insulin-regulated aminopeptidase, IRAP), responsive to angiotensin IV (Ang IV), is primarily neuronal but identified in astrocytes in the cortex, hippocampus, and basal ganglia, where it may modulate glial support for synaptic plasticity and cognition; its presence in microglia or oligodendrocytes is understudied [117]. Notably, oligodendrocytes show minimal or no consistent expression of these receptors, with most bRAS studies focusing on astrocytes and microglia as primary glial sites. These glial localizations underscore the bRAS’s role in modulating neuroinflammatory and neuroprotective processes via glial–neuronal interactions.
5. Adipokines
Adipokines are a group of NPs that are known for their secretion by adipose tissue, but are also released as neuropeptides, playing multiple roles in food intake and energy expenditure. Given the relationship between adiposity, the amount of adipokines circulating throughout the cardiovascular system, and the inflammatory responses under the given circumstances, their role in neuroinflammation is a matter of discussion.
5.1. Ghrelin
Ghrelin, an endogenous 28-amino-acid peptide hormone, binds primarily to the GHSR1α and GHSR1β, both belonging to class A GCPR (Gq). These receptors signal predominantly through Gq/11 pathways, activating phospholipase C and inositol trisphosphate to regulate intracellular calcium, which mediates its neuroprotective, anti-inflammatory, and anti-apoptotic effects in the central nervous system [118,119].
Ghrelin is capable of crossing the blood–brain barrier, positioning both the ligand and its receptor to participate directly in CNS regulation [120]. While it is known mostly for its role in appetite control and metabolic homeostasis, it has been recognized as a neuromodulator with diverse actions in the CNS, including synaptic plasticity, neuroprotection, and immunomodulation [121]. Ghrelin and GHSR1α are widely expressed in regions critical for cognitive, affective, and motor functions, including the hippocampus, substantia nigra, ventral tegmental area, dorsal and medial raphe nuclei, sensorimotor cortex, and cingulate gyrus [122].
Beyond these physiological actions, ghrelin has demonstrated potent neuroprotective and anti-inflammatory properties in multiple experimental models. For instance, in a mouse model of spinal cord injury, ghrelin administration promoted functional recovery, mediated through the inhibition of microglial p38 MAPK activation and suppression of proNGF release [123]. It also prevented apoptosis in neurons and oligodendrocytes via a GHSR1α-dependent mechanism [124]. These have been confirmed in vitro using BV-2 microglia cells, where ghrelin blocked the LPS-induced activation of p38, MAPK, and JKN, reducing proNGF and ROS, indicating a direct neuroimmunomodulatory action of microglia [125].
Ghrelin has been found to be reduced in early stages of Parkinson’s disease [126], indicating that dysregulation in this hormonal system, together with susceptibility, could trigger neurodegeneration. Accordingly, the downregulation or loss of function of ghrelin receptors in dopaminergic neurons leads to motor dysfunction [127]. These effects are accompanied by reduced microglial activation and downregulation of TNF-α, IL-1β, matrix metalloproteinase-3 (MMP-3), and inducible nitric oxide synthase (iNOS) [128]. Notably, GHSR1α antagonists abolish these effects; however, the absence of GHSR1α in substantia nigra microglia suggests indirect modulation, possibly via neuronal MMP-3 regulation. In kainic-acid-induced hippocampal neurodegeneration models, ghrelin reduces neuronal death and microglial and astroglial activation by reducing the expression of pro-inflammatory cytokines, including TNF-α, IL-1β, and cyclooxygenase-2 (COX-2) [129].
Ghrelin modulates microglial responses to amyloid beta (Aβ) in AD models through CD36, a class B scavenger receptor expressed on microglia that plays a dual role by facilitating amyloid-beta (Aβ) uptake for clearance while also triggering pro-inflammatory cytokine production, such as IL-1β and IL-6, which exacerbates neuroinflammation [58]. In recent years, the acylated form of ghrelin has shown protective effects in Alzheimer’s and other neurodegenerative diseases [130], remarkably indicating that the post-translational modification of neuropeptides could be a milestone for their protective effects. In concordance, unacylated and synthetic growth hormone secretagogues bind CD36, inhibiting Aβ-induced inflammatory responses in a GHSR1α-independent manner, likely by suppressing NF-κB and MAPK signaling pathways. This contrasts with acylated ghrelin, which acts through GHSR1α and is ineffective in cells lacking this receptor, such as N9 microglia [131]. These findings highlight acylated ghrelin’s potential as a therapeutic agent to mitigate microglial-driven neuroinflammation in AD, offering a novel strategy to reduce neuronal damage while preserving Aβ clearance.
5.2. Leptin
Leptin, another adipocyte-derived hormone, exerts its effects primarily through receptors of the class I cytokine receptor family, particularly the short (LepRa) and long (LepRb) isoforms. These receptors are expressed in a wide variety of immune and neural cells, including hypothalamic and spinal microglia, perineural macrophages, and neurons in key brain regions such as the hippocampus. Through LepRb activation, leptin promotes the production of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) and pronociceptive mediators (COX-2, iNOS, MMP-9), contributing to immune cell chemotaxis, cytotoxicity, survival, and the modulation of autoimmune responses. This highlights leptin’s dual role in both immune regulation and neuroinflammatory processes.
Leptin is a key mediator of inflammatory responses in the CNS. It contributes to lipopolysaccharide (LPS)-induced fever and appetite suppression by inducing IL-1β in the brain, linking it to anorexia and cachexia during systemic inflammation [132]. Microglial cells have emerged as significant targets of leptin’s actions. Mouse primary hypothalamic microglia expresses both LepRa and LepRb, and their activation by leptin results in the release of IL-6 and TNF-α [133]. Leptin can acutely activate microglia, suggesting that these cells may directly mediate leptin’s pro-inflammatory effects under pathological conditions in the CNS.
Like other NPs that are referred here as “anti-inflammatory”, plasma levels of leptin have been found to be inversely related to the deposition of amyloid β deposition and tau deposition in the brain, suggesting that leptin exerts protective effects against Alzheimer’s disease.
6. Concluding Remarks
This review highlights neuropeptides (NPs) as critical regulators of glial cell responses in neuroinflammation. We have described that NP signaling in brain inflammatory responses is time-dependent, with NPs like VIPs and PACAPs promoting anti-inflammatory microglial phenotypes and enhancing neuroprotection in diseases such as multiple sclerosis and Parkinson’s disease, while others, like substance P and CRH, can exacerbate inflammation and tissue damage when dysregulated. These dual roles underscore NPs’ potential as therapeutic targets, offering opportunities to modulate glial function and mitigate neurodegeneration.
Future research should prioritize elucidating the specific signaling pathways mediating NP–glial interactions, particularly under pathological conditions. Developing NP mimetics or receptor antagonists for clinical use could unlock novel treatments, but this requires a deeper understanding of how NPs balance pro- and anti-inflammatory effects. By integrating experimental and translational insights, we can harness NP signaling to address the root causes of neurodegenerative disorders and improve patient outcomes.
This review illuminated the multifaceted role of NPs in the central nervous system, particularly their influence on glial cell responses during neuroinflammation and their relevance to neurodegenerative diseases. The evidence synthesized here reveals a dual nature of NPs: when appropriately regulated, peptides foster anti-inflammatory glial phenotypes and bolster neuroprotection, as observed in models of multiple sclerosis and Parkinson’s disease. Conversely, dysregulated NPs like substance P and corticotropin-releasing hormone (CRH) can amplify pro-inflammatory cascades, exacerbating tissue damage and contributing to disease progression. This duality underscores NPs as both key regulators of CNS homeostasis and potential drivers of pathology, positioning them as compelling targets for therapeutic innovation.
Despite its comprehensive scope, this review is not without limitations. The breadth of neuropeptide research is vast and rapidly evolving, and while we have aimed to capture critical insights, some relevant studies may have been excluded due to specific inclusion criteria or the sheer volume of emerging data. Much of the evidence presented relies on preclinical models—predominantly animal and in vitro studies—which may not fully mirror the complexity of human neurodegenerative conditions. Species differences, coupled with the intricate interplay of NP signaling in human pathology, introduce a translational gap that warrants cautious interpretation.
Additionally, the review may disproportionately focus on well-studied NPs, potentially overlooking insights from less-explored peptides. The molecular mechanisms underlying NP–glial interactions, especially under disease conditions, remain incompletely elucidated, complicating efforts to predict therapeutic outcomes. Finally, the scarcity of clinical data limits our ability to confidently extrapolate these findings to patient care, highlighting a critical need for human studies.
Potential and Limitations of Neuropeptide Mimetics for Neurodegenerative Disease Therapeutics
The use of neuropeptide mimetics as therapeutic agents is a standout implication of this review. These synthetic compounds, engineered to mimic or block NP actions, offer a precision approach to modulating glial cell activity and tempering neuroinflammation. For example, agonists targeting VIP receptor 1 (VPAC1) could promote microglial M2 polarization to mitigate Parkinson’s disease progression [34], while antagonists of the neurokinin-1 receptor (NK1R) might curb substance-P-driven inflammation in multiple sclerosis [71]; however, no pharmacological compounds have been tested in clinical trials to the best of our knowledge, remaining as an open gap [134]. Beyond their specificity, mimetics can be designed for enhanced stability—overcoming the rapid degradation of natural NPs—and optimized for blood–brain barrier penetration, a persistent challenge in CNS drug development. Their potential extends to combination therapies, where they could synergize existing treatments to enhance efficacy or reduce adverse effects, similar to what has been tested in other inflammatory conditions preclinically [135], offering a versatile toolset for personalized medicine.
The insights from this review lay a foundation for future exploration, but significant work remains to bridge the gaps identified. Advancing our understanding of NP–glial dynamics in human-derived models—such as induced pluripotent stem cell systems—will be crucial for closing the translational divide. Concurrently, preclinical and clinical studies must rigorously evaluate the safety and efficacy of NP mimetics, refining their design to overcome current limitations. This endeavor will demand interdisciplinary collaboration, uniting expertise from neuroscience, pharmacology, and clinical medicine to translate experimental promise into tangible therapies.
Ultimately, by addressing the shortcomings of this review and harnessing the full potential of neuropeptide mimetics, we can unlock innovative strategies to tackle the root causes of neurodegenerative disorders. Such advancements hold the promise of not only halting disease progression but also improving the quality of life for patients, marking a transformative step forward in neurological therapeutics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/neuroglia6030035/s1, Table S1: Criteria for NP selection based on MESH terms and Number of citations obtained.
Author Contributions
Conceptualization, M.A.Z.; methodology: M.A.Z., E.J.F.-S., V.U. and S.N.; validation, M.A.Z. and G.G.-V.; formal analysis, M.A.Z.; investigation, M.A.Z., E.J.F.-S., V.U., S.N., D.S. and G.G.-V.; resources, M.A.Z.; data curation, M.A.Z.; writing—original draft preparation, E.J.F.-S., V.U., S.N. and MZ.; writing—review and editing, M.A.Z. and G.G.-V.; visualization, S.N. and M.A.Z.; supervision, M.A.Z.; project administration, M.A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding but was associated to the project NEC-24-24 (M.A.Z.) from the Vice-rectorate of Research, La Salle University, Mexico.
Acknowledgments
During the preparation of this manuscript/study, the authors used AI generative intelligence (Grok 3), which was used for English editing; schematic figures were made using BioRender. The authors have reviewed and edited the output and take full responsibility for the content of this publication. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nusbaum, M.P.; Blitz, D.M. Neuropeptide Modulation of Microcircuits. Curr. Opin. Neurobiol. 2012, 22, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Eiden, L.E. Two Ancient Neuropeptides, PACAP and AVP, Modulate Motivated Behavior at Synapses in the Extrahypothalamic Brain: A Study in Contrast. Cell Tissue Res. 2019, 375, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R. Neuropeptide Signaling near and Far: How Localized and Timed Is the Action of Neuropeptides in Brain Circuits? Invert. Neurosci. 2009, 9, 57. [Google Scholar] [CrossRef]
- Langer, I.; Jeandriens, J.; Couvineau, A.; Sanmukh, S.; Latek, D. Signal Transduction by VIP and PACAP Receptors. Biomedicines 2022, 10, 406. [Google Scholar] [CrossRef]
- Hökfelt, T.; Bartfai, T.; Bloom, F. Neuropeptides: Opportunities for Drug Discovery. Lancet Neurol. 2003, 2, 463–472. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Kölliker-Frers, R.; Udovin, L.; Otero-Losada, M.; Kobiec, T.; Herrera, M.I.; Palacios, J.; Razzitte, G.; Capani, F. Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease. Mediat. Inflamm. 2021, 2021, 9999146. [Google Scholar] [CrossRef]
- Godbout, J.P.; Chen, J.; Abraham, J.; Richwine, A.F.; Berg, B.M.; Kelley, K.W.; Johnson, R.W. Exaggerated Neuroinflammation and Sickness Behavior in Aged Mice after Activation of the Peripheral Innate Immune System. FASEB J. 2005, 19, 1329–1331. [Google Scholar] [CrossRef]
- Biesmans, S.; Meert, T.F.; Bouwknecht, J.A.; Acton, P.D.; Davoodi, N.; De Haes, P.; Kuijlaars, J.; Langlois, X.; Matthews, L.J.; Ver Donck, L.; et al. Systemic Immune Activation Leads to Neuroinflammation and Sickness Behavior in Mice. Mediat. Inflamm. 2013, 2013, 271359. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of Inflammation, Neurogenic Inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Özçete, Ö.D.; Banerjee, A.; Kaeser, P.S. Mechanisms of Neuromodulatory Volume Transmission. Mol. Psychiatry 2024, 29, 3680–3693. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Agnati, L.F.; Bechter, K.; Jansson, A.; Tarakanov, A.O.; Fuxe, K. The Role of Transmitter Diffusion and Flow versus Extracellular Vesicles in Volume Transmission in the Brain Neural–Glial Networks. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140183. [Google Scholar] [CrossRef]
- Pandey, P.; Kaur, G.; Babu, K. Crosstalk between Neurons and Glia through G-Protein Coupled Receptors: Insights from Caenorhabditis Elegans. In Progress in Molecular Biology and Translational Science; Shukla, A.K., Ed.; G Protein-Coupled Receptors—Part A; Academic Press: Amsterdam, The Netherlands, 2022; Volume 193, pp. 119–144. [Google Scholar]
- Block, M.L.; Li, G.; Qin, L.; Wu, X.; Pei, Z.; Wang, T.; Wilson, B.; Yang, J.; Hong, J.S. Potent Regulation of Microglia-Derived Oxidative Stress and Dopaminergic Neuron Survival: Substance P vs. Dynorphin. FASEB J. 2006, 20, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hernandez, A.J.; Munguba, H.; Levitz, J. Emerging Modes of Regulation of Neuromodulatory G Protein-Coupled Receptors. Trends Neurosci. 2024, 47, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; Maura, G.; Agnati, L.F. Receptor–Receptor Interactions and Glial Cell Functions with a Special Focus on G Protein-Coupled Receptors. Int. J. Mol. Sci. 2021, 22, 8656. [Google Scholar] [CrossRef]
- Lauritano, D.; Mastrangelo, F.; D’Ovidio, C.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Frydas, I.; Kritas, S.K.; Trimarchi, M.; Carinci, F.; et al. Activation of Mast Cells by Neuropeptides: The Role of Pro-Inflammatory and Anti-Inflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 4811. [Google Scholar] [CrossRef] [PubMed]
- Kulka, M.; Sheen, C.H.; Tancowny, B.P.; Grammer, L.C.; Schleimer, R.P. Neuropeptides Activate Human Mast Cell Degranulation and Chemokine Production. Immunology 2008, 123, 398–410. [Google Scholar] [CrossRef]
- Kerage, D.; Sloan, E.K.; Mattarollo, S.R.; McCombe, P.A. Interaction of Neurotransmitters and Neurochemicals with Lymphocytes. J. Neuroimmunol. 2019, 332, 99–111. [Google Scholar] [CrossRef]
- Woods, T.A.; Du, M.; Carmody, A.; Peterson, K.E. Neuropeptide Y Negatively Influences Monocyte Recruitment to the Central Nervous System During Retrovirus Infection. J. Virol. 2015, 90, 2783–2793. [Google Scholar] [CrossRef]
- Zhang, X.; Du, P.; Bai, B.; Feng, P.; Lian, X.; Hölscher, C.; Wang, Y.; Xue, G. The GLP-1 Receptor Agonist Liraglutide Inhibits Necroptosis and Neuroinflammation in a Mouse Model of Parkinson’s Disease with Diabetes Co-Morbidity. Front. Neurosci. 2025, 19, 1596506. [Google Scholar] [CrossRef]
- Fahrenkrug, J.; Hannibal, J. Neurotransmitters Co-Existing with VIP or PACAP. Peptides 2004, 25, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J. VIP and PACAP: Neuropeptide Modulators of CNS Inflammation, Injury, and Repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Karunia, J.; Niaz, A.; Mandwie, M.; Thomas Broome, S.; Keay, K.A.; Waschek, J.A.; Al-Badri, G.; Castorina, A. PACAP and VIP Modulate LPS-Induced Microglial Activation and Trigger Distinct Phenotypic Changes in Murine BV2 Microglial Cells. Int. J. Mol. Sci. 2021, 22, 10947. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M. Inhibition of Interferon (IFN) γ-Induced Jak-STAT1 Activation in Microglia by Vasoactive Intestinal Peptide: Inhibitory Effect On CD40, IFN-Induced Protein-10, And Inducible Nitric-Oxide Synthase Expression. J. Biol. Chem. 2003, 278, 27620–27629. [Google Scholar] [CrossRef]
- Delgado, M.; Jonakait, G.M.; Ganea, D. Vasoactive Intestinal Peptide and Pituitary Adenylate Cyclase-Activating Polypeptide Inhibit Chemokine Production in Activated Microglia. Glia 2002, 39, 148–161. [Google Scholar] [CrossRef]
- Farnham, M.M.J.; Tallapragada, V.J.; O’Connor, E.T.; Nedoboy, P.E.; Dempsey, B.; Mohammed, S.; Fong, A.Y.; Lung, M.S.Y.; Derakhshan, F.; Wilson, R.J.A.; et al. PACAP-PAC1 Receptor Activation Is Necessary for the Sympathetic Response to Acute Intermittent Hypoxia. Front. Neurosci. 2019, 13, 881. [Google Scholar] [CrossRef]
- Witzel, R.; Block, A.; Pollmann, S.; Oetzel, L.; Fleck, F.; Bonaterra, G.A.; Kinscherf, R.; Schwarz, A. PACAP Regulates VPAC1 Expression, Inflammatory Processes and Lipid Homeostasis in M1- and M2-Macrophages. Front. Cardiovasc. Med. 2023, 10, 1264901. [Google Scholar] [CrossRef]
- Lelievre, V.; Ghiani, C.A.; Seksenyan, A.; Gressens, P.; de Vellis, J.; Waschek, J.A. Growth Factor-Dependent Actions of PACAP on Oligodendrocyte Progenitor Proliferation. Regul. Pept. 2006, 137, 58–66. [Google Scholar] [CrossRef]
- Al-Keilani, M.S.; Almomani, B.A.; Al-Sawalha, N.A.; Al Qawasmeh, M.; Jaradat, S.A. Significance of Serum VIP and PACAP in Multiple Sclerosis: An Exploratory Case–Control Study. Neurol. Sci. 2022, 43, 2621–2630. [Google Scholar] [CrossRef]
- De La Fuente, M.; Delgado, M.; del Rio, M.; Martinez, C.; Hernanz, A.; Gomariz, R.P. Stimulation by Vasoactive Intestinal Peptide (VIP) of Phagocytic Function in Rat Macrophages. Protein Kinase C Involvement. Regul. Pept. 1993, 48, 345–353. [Google Scholar] [CrossRef]
- Power, J.H.; Barnes, O.L.; Chegini, F. Lewy Bodies and the Mechanisms of Neuronal Cell Death in Parkinson’s Disease and Dementia with Lewy Bodies. Brain Pathol. 2017, 27, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Tamas, A.; Reglödi, D.; Tizabi, Y. PACAP Protects Against Salsolinol-Induced Toxicity in Dopaminergic SH-SY5Y Cells: Implication for Parkinson’s Disease. J. Mol. Neurosci. 2013, 50, 600–607. [Google Scholar] [CrossRef]
- Reglodi, D.; Maasz, G.; Pirger, Z.; Rivnyak, A.; Balogh, D.; Jungling, A.; Fulop, B.; Mark, L.; Tamas, A. Neurochemical Changes in Different Brain Regions Induced by PACAP—Relations to Neuroprotection. SpringerPlus 2015, 4, L56. [Google Scholar] [CrossRef]
- Korkmaz, O.T.; Tunçel, N.; Tunçel, M.; Öncü, E.M.; Şahintürk, V.; Çelik, M. Vasoactive Intestinal Peptide (VIP) Treatment of Parkinsonian Rats Increases Thalamic Gamma-Aminobutyric Acid (GABA) Levels and Alters the Release of Nerve Growth Factor (NGF) by Mast Cells. J. Mol. Neurosci. 2010, 41, 278–287. [Google Scholar] [CrossRef]
- Delgado, M.; Ganea, D. Neuroprotective Effect of Vasoactive Intestinal Peptide (VIP) in a Mouse Model of Parkinson’s Disease by Blocking Microglial Activation. FASEB J. 2003, 17, 1–18. [Google Scholar] [CrossRef]
- Han, P.; Caselli, R.J.; Baxter, L.; Serrano, G.; Yin, J.; Beach, T.G.; Reiman, E.M.; Shi, J. Association of Pituitary Adenylate Cyclase–Activating Polypeptide with Cognitive Decline in Mild Cognitive Impairment Due to Alzheimer Disease. JAMA Neurol. 2015, 72, 333–339. [Google Scholar] [CrossRef]
- Rat, D.; Schmitt, U.; Tippmann, F.; Dewachter, I.; Theunis, C.; Wieczerzak, E.; Postina, R.; van Leuven, F.; Fahrenholz, F.; Kojro, E. Neuropeptide Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Slows down Alzheimer’s Disease-like Pathology in Amyloid Precursor Protein-Transgenic Mice. FASEB J. 2011, 25, 3208–3218. [Google Scholar] [CrossRef]
- Song, M.; Xiong, J.X.; Wang, Y.Y.; Tang, J.; Zhang, B.; Bai, Y. VIP Enhances Phagocytosis of Fibrillar Beta-Amyloid by Microglia and Attenuates Amyloid Deposition in the Brain of APP/PS1 Mice. PLoS ONE 2012, 7, e29790. [Google Scholar] [CrossRef] [PubMed]
- Goksu, A.Y.; Kocanci, F.G.; Akinci, E.; Demir-Dora, D.; Erendor, F.; Sanlioglu, S.; Uysal, H. Microglia Cells Treated with Synthetic Vasoactive Intestinal Peptide or Transduced with LentiVIP Protect Neuronal Cells against Degeneration. Eur. J. Neurosci. 2024, 59, 1993–2015. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Álvarez, I.; Mantey, S.A.; Nakamura, T.; Nuche-Berenguer, B.; Moreno, P.; Moody, T.W.; Maderdrut, J.L.; Coy, D.H.; Jensen, R.T. A Structure-Function Study of PACAP Using Conformationally Restricted Analogs: Identification of PAC1 Receptor-Selective PACAP Agonists. Peptides 2015, 66, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Deluigi, M.; Klipp, A.; Klenk, C.; Merklinger, L.; Eberle, S.A.; Morstein, L.; Heine, P.; Mittl, P.R.E.; Ernst, P.; Kamenecka, T.M.; et al. Complexes of the Neurotensin Receptor 1 with Small-Molecule Ligands Reveal Structural Determinants of Full, Partial, and Inverse Agonism. Sci. Adv. 2021, 7, eabe5504. [Google Scholar] [CrossRef]
- Devader, C.; Béraud-Dufour, S.; Coppola, T.; Mazella, J. The Anti-Apoptotic Role of Neurotensin. Cells 2013, 2, 124–135. [Google Scholar] [CrossRef]
- Guo, F.; Du, Y.; Qu, F.H.; Lin, S.D.; Chen, Z.; Zhang, S.H. Dissecting the Neural Circuitry for Pain Modulation and Chronic Pain: Insights from Optogenetics. Neurosci. Bull. 2022, 38, 440–452. [Google Scholar] [CrossRef]
- Tabarean, I. Neurotensin Induces Hypothermia by Activating Both Neuronal Neurotensin Receptor 1 and Astrocytic Neurotensin Receptor 2 in the Median Preoptic Nucleus. Neuropharmacology 2020, 171, 108069. [Google Scholar] [CrossRef]
- Tschumi, C.W.; Blankenship, H.E.; Sharma, R.; Lynch, W.B.; Beckstead, M.J. Neurotensin Release from Dopamine Neurons Drives Long-Term Depression of Substantia Nigra Dopamine Signaling. J. Neurosci. 2022, 42, 6186–6194. [Google Scholar] [CrossRef]
- Ma, H.; Huang, Y.; Zhang, B.; Wang, Y.; Zhao, H.; Du, H.; Cong, Z.; Li, J.; Zhu, G. Association Between Neurotensin Receptor 1 Gene Polymorphisms and Alcohol Dependence in a Male Han Chinese Population. J. Mol. Neurosci. 2013, 51, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Servonnet, A.; Minogianis, E.A.; Bouchard, C.; Bédard, A.M.; Lévesque, D.; Rompré, P.P.; Samaha, A.N. Neurotensin in the Nucleus Accumbens Reverses Dopamine Supersensitivity Evoked by Antipsychotic Treatment. Neuropharmacology 2017, 123, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Sarret, P.; Perron, A.; Stroh, T.; Beaudet, A. Immunohistochemical distribution of NTS2 neurotensin receptors in the rat central nervous system. J. Comp. Neurol. 2003, 461, 520–538. [Google Scholar] [CrossRef]
- Kyriatzis, G.; Bernard, A.; Bôle, A.; Pflieger, G.; Chalas, P.; Masse, M.; Lécorché, P.; Jacquot, G.; Ferhat, L.; Khrestchatisky, M. Neurotensin Receptor 2 Is Induced in Astrocytes and Brain Endothelial Cells in Relation to Neuroinflammation Following Pilocarpine-Induced Seizures in Rats. Glia 2021, 69, 2618–2643. [Google Scholar] [CrossRef]
- Alysandratos, K.-D.; Asadi, S.; Angelidou, A.; Zhang, B.; Sismanopoulos, N.; Yang, H.; Critchfield, A.; Theoharides, T.C. Neurotensin and CRH Interactions Augment Human Mast Cell Activation. PLoS ONE 2012, 7, e48934. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.J.; Pervaiz, I.; Karamyan, V.T. Neurolysin Substrates Bradykinin, Neurotensin and Substance P Enhance Brain Microvascular Permeability in a Human in Vitro Model. J. Neuroendocrinol. 2021, 33, e12931. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Tsilioni, I.; Leeman, S.E.; Theoharides, T.C. Neurotensin Stimulates Sortilin and mTOR in Human Microglia Inhibitable by Methoxyluteolin, a Potential Therapeutic Target for Autism. Proc. Natl. Acad. Sci. USA 2016, 113, E7049–E7058. [Google Scholar] [CrossRef] [PubMed]
- Voyer, D.; Lévesque, D.; Rompré, P.-P. Repeated Ventral Midbrain Neurotensin Injections Sensitize to Amphetamine-Induced Locomotion and ERK Activation: A Role for NMDA Receptors. Neuropharmacology 2017, 112, 150–163. [Google Scholar] [CrossRef]
- Coll, R.C.; Vargas, P.M.; Mariani, M.L.; Penissi, A.B. Natural α,β-Unsaturated Lactones Inhibit Neuropeptide-Induced Mast Cell Activation in an in Vitro Model of Neurogenic Inflammation. Inflamm. Res. 2020, 69, 1039–1051. [Google Scholar] [CrossRef]
- Miller, L.A.; Cochrane, D.E.; Feldberg, R.S.; Carraway, R.E. Inhibition of Neurotensin-Stimulated Mast Cell Secretion and Carboxypeptidase A Activity by the Peptide Inhibitor of Carboxypeptidase A and Neurotensin-Receptor Antagonist SR 48692. Int. Arch. Allergy Immunol. 1998, 116, 147–153. [Google Scholar] [CrossRef]
- Gahete, M.D.; Rubio, A.; Córdoba-Chacón, J.; Gracia-Navarro, F.; Kineman, R.D.; Avila, J.; Luque, R.M.; Castaño, J.P. Expression of the Ghrelin and Neurotensin Systems Is Altered in the Temporal Lobe of Alzheimer’s Disease Patients. J. Alzheimers Dis. 2010, 22, 819–828. [Google Scholar] [CrossRef]
- Martin, S.; Dicou, E.; Vincent, J.-P.; Mazella, J. Neurotensin and the neurotensin receptor-3 in microglial cells. J. Neurosci. Res. 2005, 81, 322–326. [Google Scholar] [CrossRef]
- Grajchen, E.; Wouters, E.; van de Haterd, B.; Haidar, M.; Hardonnière, K.; Dierckx, T.; Van Broeckhoven, J.; Erens, C.; Hendrix, S.; Kerdine-Römer, S.; et al. CD36-Mediated Uptake of Myelin Debris by Macrophages and Microglia Reduces Neuroinflammation. J. Neuroinflamm. 2020, 17, 224. [Google Scholar] [CrossRef]
- Martin, S.; Vincent, J.P.; Mazella, J. Involvement of the Neurotensin Receptor-3 in the Neurotensin-Induced Migration of Human Microglia. J. Neurosci. 2003, 23, 1198–1205. [Google Scholar] [CrossRef]
- Soltys, J.; Knight, J.; Scharf, E.; Pitt, D.; Mao-Draayer, Y. IFN-β Alters Neurotrophic Factor Expression in T Cells Isolated from Multiple Sclerosis Patients—Implication of Novel Neurotensin/NTSR1 Pathway in Neuroprotection. Am. J. Transl. Res. 2014, 6, 312–319. [Google Scholar] [PubMed]
- Carniglia, L.; Ramírez, D.; Durand, D.; Saba, J.; Turati, J.; Caruso, C.; Scimonelli, T.N.; Lasaga, M. Neuropeptides and Microglial Activation in Inflammation, Pain, and Neurodegenerative Diseases. Mediat. Inflamm. 2017, 2017, 5048616. [Google Scholar] [CrossRef]
- Ji, L.-Q.; Hong, Y.; Tao, Y.-X. Melanocortin-5 Receptor: Pharmacology and Its Regulation of Energy Metabolism. Int. J. Mol. Sci. 2022, 23, 8727. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.B.; Montero-Melendez, T.; Greco, K.V.; Perretti, M. Melanocortin Receptors as Novel Effectors of Macrophage Responses in Inflammation. Front. Immunol. 2011, 2, 41. [Google Scholar] [CrossRef][Green Version]
- Rajora, N.; Boccoli, G.; Burns, D.; Sharma, S.; Catania, A.P.; Lipton, J.M. Alpha-MSH Modulates Local and Circulating Tumor Necrosis Factor-Alpha in Experimental Brain Inflammation. J. Neurosci. 1997, 17, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Star, R.A.; Rajora, N.; Huang, J.; Stock, R.C.; Catania, A.; Lipton, J.M. Evidence of Autocrine Modulation of Macrophage Nitric Oxide Synthase by Alpha-Melanocyte-Stimulating Hormone. Proc. Natl. Acad. Sci. USA 1995, 92, 8016–8020. [Google Scholar] [CrossRef]
- Taylor, A.W. The Immunomodulating Neuropeptide Alpha-Melanocyte-Stimulating Hormone (Alpha-MSH) Suppresses LPS-Stimulated TLR4 with IRAK-M in Macrophages. J. Neuroimmunol. 2005, 162, 43–50. [Google Scholar] [CrossRef]
- Taylor, A.W. Alpha-Melanocyte Stimulating Hormone (α-MSH) Is a Post-Caspase Suppressor of Apoptosis in RAW 264.7 Macrophages. PLoS ONE 2013, 8, e74488. [Google Scholar] [CrossRef][Green Version]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The Role of Substance P in Inflammatory Disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef]
- Wang, Q.; Oyarzabal, E.; Wilson, B.; Qian, L.; Hong, J.-S. Substance P Enhances Microglial Density in the Substantia Nigra through Neurokinin-1 Receptor/NADPH Oxidase-Mediated Chemotaxis in Mice. Clin. Sci. 2015, 129, 757–767. [Google Scholar] [CrossRef]
- Chowdari Gurram, P.; Satarker, S.; Nampoothiri, M. Recent Advances in the Molecular Signaling Pathways of Substance P in Alzheimer’s Disease: Link to Neuroinflammation Associated with Toll-like Receptors. Biochem. Biophys. Res. Commun. 2024, 733, 150597. [Google Scholar] [CrossRef]
- Pascual, D.W.; Bost, K.L. Substance P Production by P388D1 Macrophages: A Possible Autocrine Function for This Neuropeptide. Immunology 1990, 71, 52–56. [Google Scholar]
- Ahn, W.; Chi, G.; Kim, S.; Son, Y.; Zhang, M. Substance P Reduces Infarct Size and Mortality After Ischemic Stroke, Possibly Through the M2 Polarization of Microglia/Macrophages and Neuroprotection in the Ischemic Rat Brain. Cell Mol. Neurobiol. 2023, 43, 2035–2052. [Google Scholar] [CrossRef]
- Nag, S.; Yee, B.K.; Tang, F. Reduction in Somatostatin and Substance P Levels and Choline Acetyltransferase Activity in the Cortex and Hippocampus of the Rat after Chronic Intracerebroventricular Infusion of β-Amyloid (1-40). Brain Res. Bull. 1999, 50, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Vilisaar, J.; Kawabe, K.; Braitch, M.; Aram, J.; Furtun, Y.; Fahey, A.J.; Chopra, M.; Tanasescu, R.; Tighe, P.J.; Gran, B.; et al. Reciprocal Regulation of Substance P and IL-12/IL-23 and the Associated Cytokines, IFNγ/IL-17: A Perspective on the Relevance of This Interaction to Multiple Sclerosis. J. Neuroimmune Pharmacol. 2015, 10, 457–467. [Google Scholar] [CrossRef]
- Coutens, B.; Ingram, S.L. Key Differences in Regulation of Opioid Receptors Localized to Presynaptic Terminals Compared to Somas: Relevance for Novel Therapeutics. Neuropharmacology 2023, 226, 109408. [Google Scholar] [CrossRef]
- Zhao, J.; Elgeti, M.; O’Brien, E.S.; Sár, C.P.; Daibani, A.E.; Heng, J.; Sun, X.; Che, T.; Hubbell, W.L.; Kobilka, B.K.; et al. Conformational Dynamics of the μ-Opioid Receptor Determine Ligand Intrinsic Efficacy. BioRxiv 2023, 629, 474–480. [Google Scholar]
- Takayama, N.; Ueda, H. Morphine-Induced Overexpression of Prepro-Nociceptin/Orphanin FQ in Cultured Astrocytes. Peptides 2005, 26, 2513–2517. [Google Scholar] [CrossRef]
- Corkrum, M.; Rothwell, P.E.; Thomas, M.J.; Kofuji, P.; Araque, A. Opioid-Mediated Astrocyte–Neuron Signaling in the Nucleus Accumbens. Cells 2019, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Gessi, S.; Borea, P.A.; Bencivenni, S.; Fazzi, D.; Varani, K.; Merighi, S. The Activation of μ-Opioid Receptor Potentiates LPS-Induced NF-kB Promoting an Inflammatory Phenotype in Microglia. FEBS Lett. 2016, 590, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wu, C.-Y.; Burton, F.H.; Loh, H.H.; Wei, L.-N. β-Arrestin Protects Neurons by Mediating Endogenous Opioid Arrest of Inflammatory Microglia. Cell Death Differ. 2014, 21, 397–406. [Google Scholar] [CrossRef]
- Birdsong, W.T.; Arttamangkul, S.; Clark, M.J.; Cheng, K.; Rice, K.C.; Traynor, J.R.; Williams, J.T. Increased Agonist Affinity at the μ-Opioid Receptor Induced by Prolonged Agonist Exposure. J. Neurosci. 2013, 33, 4118–4127. [Google Scholar] [CrossRef]
- Chao, C.C.; Hu, S.; Shark, K.B.; Sheng, W.S.; Gekker, G.; Peterson, P.K. Activation of Mu Opioid Receptors Inhibits Microglial Cell Chemotaxis1. J. Pharmacol. Exp. Ther. 1997, 281, 998–1004. [Google Scholar] [CrossRef]
- Ma, L.; Peng, S.; Wei, J.; Zhao, M.; Ahmad, K.A.; Chen, J.; Wang, Y.-X. Spinal Microglial β-Endorphin Signaling Mediates IL-10 and Exenatide-Induced Inhibition of Synaptic Plasticity in Neuropathic Pain. CNS Neurosci. Ther. 2021, 27, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Leiguarda, C.; Potilinski, C.; Rubione, J.; Tate, P.; Villar, M.J.; Montaner, A.; Bisagno, V.; Constandil, L.; Brumovsky, P.R. IMT504 Provides Analgesia by Modulating Cell Infiltrate and Inflammatory Milieu in a Chronic Pain Model. J. Neuroimmune Pharmacol. 2021, 16, 651–666. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhang, H.; Tan, Z.; Zheng, Y.; Ping, J.; Zhang, J.; Luo, J.; Li, L.; Lu, L.; et al. Elevated Circulating Levels of GFAP Associated with Reduced Volumes in Hippocampal Subregions Linked to Mild Cognitive Impairment among Community-Dwelling Elderly Individuals. Front. Aging Neurosci. 2024, 16, 1461556. [Google Scholar] [CrossRef]
- Xu, Y.; Zhi, F.; Balboni, G.; Yang, Y.; Xia, Y. Opposite Roles of δ- and μ-Opioid Receptors in BACE1 Regulation and Alzheimer’s Injury. Front. Cell. Neurosci. 2020, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Nix, M.; Abdul, Y.; Singh, S.; Husain, S. Delta-Opioid Receptors Attenuate TNF-α–Induced MMP-2 Secretion from Human ONH Astrocytes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6605–6611. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.S. Exploring the Role of Opioid Signaling in Modulation of Microglial Function. Ph.D. Thesis, Charles University, Prague, Czech Republic, 2023. [Google Scholar]
- Xu, Y.; Chen, R.; Zhi, F.; Sheng, S.; Khiati, L.; Yang, Y.; Peng, Y.; Xia, Y. δ-Opioid Receptor, Microglia and Neuroinflammation. Aging Dis. 2023, 14, 778–793. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Xia, H.; Hu, M.; Chen, C.; Fu, J.; Shi, G.; Guo, Q.; Zhou, Y.; Wang, W.; Shi, J.; et al. Isotalatizidine, a C19-Diterpenoid Alkaloid, Attenuates Chronic Neuropathic Pain through Stimulating ERK/CREB Signaling Pathway-Mediated Microglial Dynorphin A Expression. J. Neuroinflamm. 2020, 17, 13. [Google Scholar] [CrossRef]
- Belo, T.C.A.; Santos, G.X.; da Silva, B.E.G.; Rocha, B.L.G.; Abdala, D.W.; Freire, L.A.M.; Rocha, F.S.; Galdino, G. IL-10/β-Endorphin-Mediated Neuroimmune Modulation on Microglia during Antinociception. Brain Sci. 2023, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Cuitavi, J.; Torres-Pérez, J.V.; Lorente, J.D.; Campos-Jurado, Y.; Andrés-Herrera, P.; Polache, A.; Agustín-Pavón, C.; Hipólito, L. Crosstalk between Mu-Opioid Receptors and Neuroinflammation: Consequences for Drug Addiction and Pain. Neurosci. Biobehav. Rev. 2023, 145, 105011. [Google Scholar] [CrossRef]
- Sukhareva, E.V. The Role of the Corticotropin-Releasing Hormone and Its Receptors in the Regulation of Stress Response. Vavilovskii Zhurnal Genet. Sel. 2021, 25, 216–223. [Google Scholar] [CrossRef]
- Mastorakos, G.; Karoutsou, E.I.; Mizamtsidi, M. Corticotropin Releasing Hormone and the Immune/Inflammatory response. Eur. J. Endocrinol. 2006, 155, S77–S84. [Google Scholar] [CrossRef]
- Esposito, P.; Chandler, N.; Kandere, K.; Basu, S.; Jacobson, S.; Connolly, R.; Tutor, D.; Theoharides, T.C. Corticotropin-Releasing Hormone and Brain Mast Cells Regulate Blood-Brain-Barrier Permeability Induced by Acute Stress. J. Pharmacol. Exp. Ther. 2002, 303, 1061–1066. [Google Scholar] [CrossRef]
- Cao, J.; Papadopoulou, N.; Kempuraj, D.; Boucher, W.S.; Sugimoto, K.; Cetrulo, C.L.; Theoharides, T.C. Human Mast Cells Express Corticotropin-Releasing Hormone (CRH) Receptors and CRH Leads to Selective Secretion of Vascular Endothelial Growth Factor. J. Immunol. 2005, 174, 7665–7675. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, T.; Li, Y.; Li, T.; Ding, Z.; Liu, L. Paraventricular Mast Cell-Derived Histamine Activates CRH Neurons to Mediate Adult Visceral Hypersensitivity Induced by Neonatal Maternal Separation. Brain Sci. 2023, 13, 1595. [Google Scholar] [CrossRef]
- Guo, L.; Reed, K.M.; Carter, A.; Cheng, Y.; Roodsari, S.K.; Martinez Pineda, D.; Wellman, L.L.; Sanford, L.D.; Guo, M.-L. Sleep-Disturbance-Induced Microglial Activation Involves CRH-Mediated Galectin 3 and Autophagy Dysregulation. Cells 2022, 12, 160. [Google Scholar] [CrossRef]
- Anghel, L.; Ciubară, A.; Nechita, A.; Nechita, L.; Manole, C.; Baroiu, L.; Ciubară, A.B.; Mușat, C.L. Sleep Disorders Associated with Neurodegenerative Diseases. Diagnostics 2023, 13, 2898. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.K. Neurodegenerative Disorders and Sleep. Sleep Med. Clin. 2018, 13, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wang, F.; Qi, D.; Liu, W.; Gu, C.; Mao, C.-J.; Yang, Y.-P.; Zhao, Z.; Hu, L.-F.; Liu, C.-F. A Critical Role of Autophagy in Regulating Microglia Polarization in Neurodegeneration. Front. Aging Neurosci. 2018, 10, 378. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Cho, D.-H. Enhancement of Nitric Oxide Production by Corticotropin-Releasing Hormone (CRH) in Murine Microglial Cells, BV2. Immune Netw. 2004, 4, 60–64. [Google Scholar] [CrossRef]
- Yang, Y.; Hahm, E.; Kim, Y.; Kang, J.; Lee, W.; Han, I.; Myung, P.; Kang, H.; Park, H.; Cho, D. Regulation of IL-18 Expression by CRH in Mouse Microglial Cells. Immunol. Lett. 2005, 98, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Lin, Y.; Lu, Z.; Xiao, Z. Microglia-Astrocyte Cross Talk through IL-18/IL-18R Signaling Modulates Migraine-like Behavior in Experimental Models of Migraine. Neuroscience 2020, 451, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Gomes, B.; Kumar, A.; Ashton, N.J.; Hall, S.; Stomrud, E.; Smith, R.; Zetterberg, H.; Blennow, K.; Mattsson-Carlgren, N.; Hansson, O. Corticotropin-Releasing Hormone as a Candidate Biomarker for Parkinsonian Disorders. Brain Commun. 2024, 6, fcae414. [Google Scholar] [CrossRef] [PubMed]
- Fountain, J.H.; Kaur, J.; Lappin, S.L. Physiology, Renin Angiotensin System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Abiodun, O.A.; Ola, M.S. Role of Brain Renin Angiotensin System in Neurodegeneration: An Update. Saudi J. Biol. Sci. 2020, 27, 905–912. [Google Scholar] [CrossRef]
- Deliu, E.; Brailoiu, G.C.; Eguchi, S.; Hoffman, N.E.; Rabinowitz, J.E.; Tilley, D.G.; Madesh, M.; Koch, W.J.; Brailoiu, E. Direct Evidence of Intracrine Angiotensin II Signaling in Neurons. Am. J. Physiol.-Cell Physiol. 2014, 306, C736–C744. [Google Scholar] [CrossRef]
- Erdmann, B.; Fuxe, K.; Ganten, D. Subcellular Localization of Angiotensin II Immunoreactivity in the Rat Cerebellar Cortex. Hypertension 1996, 28, 818–824. [Google Scholar] [CrossRef]
- Pan, H.-L. Brain Angiotensin II and Synaptic Transmission. Neuroscientist 2004, 10, 422–431. [Google Scholar] [CrossRef]
- Saavedra, J.M. Angiotensin II AT1 Receptor Blockers as Treatments for Inflammatory Brain Disorders. Clin. Sci. 2012, 123, 567–590. [Google Scholar] [CrossRef]
- Biancardi, V.C.; Stranahan, A.M.; Krause, E.G.; de Kloet, A.D.; Stern, J.E. Cross Talk between AT1 Receptors and Toll-like Receptor 4 in Microglia Contributes to Angiotensin II-Derived ROS Production in the Hypothalamic Paraventricular Nucleus. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H404–H415. [Google Scholar] [CrossRef]
- Sun, H.; Wu, H.; Yu, X.; Zhang, G.; Zhang, R.; Zhan, S.; Wang, H.; Bu, N.; Ma, X.; Li, Y. Angiotensin II and Its Receptor in Activated Microglia Enhanced Neuronal Loss and Cognitive Impairment Following Pilocarpine-Induced Status Epilepticus. Mol. Cell Neurosci. 2015, 65, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.C.; Kimbark, K.D.; Vernail, V.L.; Silberman, Y.; Arnold, A.C. Angiotensin-(1-7) Protective Effects in Neurocognitive Disorders: Molecular Mechanisms to Therapeutic Implications. Front. Physiol. 2025, 16, 1565270. [Google Scholar] [CrossRef]
- Wright, J.W.; Harding, J.W. Brain Angiotensin Receptor Subtypes AT1, AT2, and AT4 and Their Functions. Regul. Pept. 1995, 59, 269–295. [Google Scholar] [CrossRef]
- Camiña, J.P. Cell Biology of the Ghrelin Receptor. J. Neuroendocrinol. 2006, 18, 65–76. [Google Scholar] [CrossRef]
- de Candia, P.; Matarese, G. Leptin and Ghrelin: Sewing Metabolism onto Neurodegeneration. Neuropharmacology 2018, 136, 307–316. [Google Scholar] [CrossRef]
- Rhea, E.M.; Salameh, T.S.; Gray, S.; Niu, J.; Banks, W.A.; Tong, J. Ghrelin Transport across the Blood–Brain Barrier Can Occur Independently of the Growth Hormone Secretagogue Receptor. Mol. Metab. 2018, 18, 88–96. [Google Scholar] [CrossRef]
- Cavalier, M.; Crouzin, N.; Ben Sedrine, A.; de Jesus Ferreira, M.C.; Guiramand, J.; Cohen-Solal, C.; Fehrentz, J.-A.; Martinez, J.; Barbanel, G.; Vignes, M. Involvement of PKA and ERK Pathways in Ghrelin-Induced Long-Lasting Potentiation of Excitatory Synaptic Transmission in the CA1 Area of Rat Hippocampus. Eur. J. Neurosci. 2015, 42, 2568–2576. [Google Scholar] [CrossRef]
- Jones, R. Ghrelin on the Brain. Nat. Rev. Neurosci. 2003, 4, 246. [Google Scholar] [CrossRef]
- Wu, C.-R.; Yang, Q.-Y.; Chen, Q.-W.; Li, C.-Q.; He, W.-Y.; Zhao, Y.-P.; Wang, L. Ghrelin Attenuate Cerebral Microvascular Leakage by Regulating Inflammation and Apoptosis Potentially via a P38 MAPK-JNK Dependent Pathway. Biochem. Biophys. Res. Commun. 2021, 552, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yune, T.Y. Ghrelin Inhibits Oligodendrocyte Cell Death by Attenuating Microglial Activation. Endocrinol. Metab. 2024, 29, 371–378. [Google Scholar] [CrossRef]
- Russo, C.; Valle, M.S.; Russo, A.; Malaguarnera, L. The Interplay between Ghrelin and Microglia in Neuroinflammation: Implications for Obesity and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 13432. [Google Scholar] [CrossRef]
- Song, N.; Wang, W.; Jia, F.; Du, X.; Xie, A.; He, Q.; Shen, X.; Zhang, J.; Rogers, J.T.; Xie, J.; et al. Assessments of Plasma Ghrelin Levels in the Early Stages of Parkinson’s Disease. Mov. Disord. 2017, 32, 1487–1491. [Google Scholar] [CrossRef]
- Suda, Y.; Kuzumaki, N.; Sone, T.; Narita, M.; Tanaka, K.; Hamada, Y.; Iwasawa, C.; Shibasaki, M.; Maekawa, A.; Matsuo, M.; et al. Down-Regulation of Ghrelin Receptors on Dopaminergic Neurons in the Substantia Nigra Contributes to Parkinson’s Disease-like Motor Dysfunction. Mol. Brain 2018, 11, 6. [Google Scholar] [CrossRef]
- Moon, M.; Kim, H.G.; Hwang, L.; Seo, J.-H.; Kim, S.; Hwang, S.; Kim, S.; Lee, D.; Chung, H.; Oh, M.S.; et al. Neuroprotective Effect of Ghrelin in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Mouse Model of Parkinson’s Disease by Blocking Microglial Activation. Neurotox. Res. 2009, 15, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lim, E.; Kim, Y.; Li, E.; Park, S. Ghrelin Attenuates Kainic Acid-Induced Neuronal Cell Death in the Mouse Hippocampus. J. Endocrinol. 2010, 205, 263–270. Available online: https://joe.bioscientifica.com/view/journals/joe/205/3/263.xml?ijkey=028a1396e4cea94fb91c36d67d2e16486332415d&keytype2=tf_ipsecsha&legid=joe%3B205%2F3%2F263&related-urls=yes (accessed on 1 August 2025). [CrossRef]
- Reich, N.; Hölscher, C. Acylated Ghrelin as a Multi-Targeted Therapy for Alzheimer’s and Parkinson’s Disease. Front. Neurosci. 2020, 14, 614828. Available online: https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2020.614828/full (accessed on 1 August 2025).
- Thomas, A.S.; Sassi, M.; Angelini, R.; Morgan, A.H.; Davies, J.S. Acylation, a Conductor of Ghrelin Function in Brain Health and Disease. Front. Physiol. 2022, 13, 831641. [Google Scholar] [CrossRef]
- Engineer, D.R.; Garcia, J.M. Leptin in Anorexia and Cachexia Syndrome. Int. J. Pept. 2012, 2012, 287457. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, V.; Inoue, W.; Kan, B.; Luheshi, G.N. Leptin Modulates Cell Morphology and Cytokine Release in Microglia. Brain Behav. Immun. 2010, 24, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Cherait, A.; Xifró, X.; Reglodi, D.; Vaudry, D. More Than Three Decades After Discovery of the Neuroprotective Effect of PACAP, What Is Still Preventing Its Clinical Use? J. Mol. Neurosci. 2025, 75, 80. [Google Scholar] [CrossRef] [PubMed]
- Zetter, M.; Barrios-Payán, J.; Mata-Espinosa, D.; Marquina-Castillo, B.; Quintanar-Stephano, A.; Hernández-Pando, R. Involvement of Vasopressin in the Pathogenesis of Pulmonary Tuberculosis: A New Therapeutic Target? Front. Endocrinol. 2019, 10, 351. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).