Abstract
The central nervous system (CNS) relies on complex and dynamic interactions between neurons and glial cells. Among glial cells, astrocytes regulate the chemical environment surrounding neurons and supply essential nutrients for brain metabolism whereas microglia, the resident macrophages of the CNS, play critical roles in homeostasis, defense, and responses to injury. Both microglia and astrocytes contribute to the regulation of excitotoxicity and inflammation mediated by the metabolism of tryptophan (Trp) via the kynurenine pathway. Trp metabolism generates several bioactive metabolites, including quinolinic acid (QUIN) and kynurenic acid (KYNA), which have opposing effects. QUIN, produced by activated microglia, acts as an agonist for NMDA receptors; excessive stimulation of these receptors can lead to excitotoxicity and neuronal death. Conversely, KYNA, primarily produced by astrocytes via kynurenine 2,3-aminotransferases (KAT), acts as an NMDA receptor antagonist, conferring neuroprotection by mitigating excitotoxicity. Dysregulation of the Trp metabolism is implicated in many neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis and amyotrophic lateral sclerosis, as well as in various neuropsychiatric disorders. This review examines the cellular and molecular mechanisms underlying Trp metabolism in glial cells, highlighting the unique contributions of each glial phenotype, the implications for CNS pathologies, and the potential biomarkers and therapeutic targets for restoring homeostasis and preventing disease progression.
1. Introduction
The central nervous system (CNS) is a highly complex structure composed of various cell types dynamically interacting to maintain homeostasis, neuronal functions, and immune response. Glial cells play a crucial role not only in providing structural support but also in regulating metabolic, immunological, and neuroprotective processes [1]. Among the metabolic pathways associated with these cells, Trp metabolism via the kynurenine pathway (KP) has emerged as a critical link for understanding the interaction between inflammation, neurodegeneration, and neuroprotection. This review explores the mechanisms associated with Trp metabolism in the CNS, focusing on the implication of glial cells in the neuroinflammatory response and the pathogenesis of neurodegenerative diseases (NDD), such as Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS). Key metabolic and inflammatory markers of NDD progression are also discussed to enhance our understanding of the molecular mechanisms underlying these diseases and to provide a foundation for the development of new biomarkers and effective therapies.
2. Communication Between Microglia and Astrocytes During Neuroinflammation and Activation of the KP
In the CNS, microglia, oligodendrocytes, and astrocytes are the glial cells responsible for providing support and protection to neurons [1,2,3]. Oligodendrocytes are responsible for the production and maintenance of myelin, which is essential for the rapid transmission of action potentials, as well as for axonal stabilization and nutrition. In response to injury or damage, the inflammatory response is triggered by the activation of microglia and astrocytes and the infiltration of immune system cells, playing key roles as immune effectors within the CNS. Astrocytes are responsible for regulating the chemical environment around neurons and supplying essential nutrients for brain metabolism [4].
In the human cerebral cortex, the number of astrocytes is comparable to the number of neurons, but their size is larger compared to other species [5]. Pelvig et al. (2008) estimated that astrocytes account for approximately 20% of all glial cells in the human cortex, where they provide structural support to neurons and other cells, contributing to the three-dimensional architecture of the CNS [5,6,7] (Figure 1). The diverse functions of astrocytes are related to the protection and maintenance of the brain’s chemical environment, regulation of ionic balance, pH, and neurotransmitter levels in the extracellular environment, all of which contribute to the proper transmission of intercellular signals. Astrocytes also play a key role in neuronal development by releasing neurotrophins such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) [8], as well as in the regulation of neuronal metabolism by storing glucose in glycogen granules, which is then converted into lactate and pyruvate to meet the high ATP demands of neurons [9]. Additionally, astrocytes are also crucial for the formation of the blood–brain barrier, synapse plasticity, and tissue recovery after injury [9].

Figure 1.
Cellular components of SNC. Astrocytes (purple) surround blood vessels in order to select metabolites that can pass through the blood–brain barrier. Astrocytes also support neuron (yellow) metabolism and function. Oligodendrocytes (pink) create a myelin sheath that wraps around axons and helps in the transmission of signaling. Microglia (red) is the immune resident cell in the CNS which controls not only immune signaling but also synapse growth and pruning in physiological conditions.
Astrocytes’ morphology varies according to the region in which they are found, and they are classically divided into the following: (i) protoplasmic astrocytes of the gray matter, with a spongy morphology, where the processes branch profusely to contact and enclose synapses and blood vessels [9]; (ii) fibrous astrocytes of the white matter, which are characterized by long, non-uniform fibers [10]; (iii) radial astrocytes, which are more abundant during development and transpose into cortical astrocytes. Astrocytes are identified by the expression of glial fibrillary acidic protein (GFAP), although levels of this protein vary in different brain regions, as well as other proteins, such as interleukin (IL) 6 and STAT3 [6].
In response to CNS injury, astrocytes undergo phenotypic changes, generally associated with increased GFAP expression and morphological hypertrophy, termed astrogliosis, together with altered metabolism and expression of cytokines, proteases, and transcription factors [11]. These pathological responses are termed astrocyte reactivity, with phenotype modification accompanied by gradual changes in molecular mechanisms that can promote the survival or the programmed death of surrounding neurons [12]. The factors inducing astrocyte reactivity are diverse and highly context-dependent, including tumor necrosis factor alpha (TNF-α), a potent inflammatory inducer of astrogliosis [13], chemoattraction of peripheral immune cells, and subsequent activation of microglia, promoting an inflammatory response [14]. Reactive astrocytes participate in the immune response, activating defense mechanisms, including those against pathogens, through the release of immunomodulatory substances [15,16]. Their production of antioxidant enzymes and neurotrophic factors helps protect and recover neurons under conditions of oxidative stress or injury [17]. It is important to highlight that astrocyte activation occurs throughout life in a healthy organism and is part of the plasticity and homeostasis of the CNS.
Microglia play a crucial role in the defense and maintenance of brain homeostasis by monitoring the neuronal environment for signs of injury, inflammation, or infection, which lead to microglial activation. Astrocytes play a key role in modulating microglial activation by regulating the inflammatory response, producing several cytokines such as IL-1, IL-3, IL-5, IL-6, and IL-8, and secreting TNF-α, transforming growth factor beta (TGF-β), Interferon gamma (INF-γ), granulocyte–macrophage colony-stimulating factor (GM-CSF), colony-stimulating factor (M-CSF), and the mediators prostaglandin E2 (PGE2), monocyte chemoattractant protein-1 (MCP-1) and INF-γ-inducible protein-10 (IP-10). These molecules signal and influence the neuroinflammatory response either by recruiting other cells or acting in a chemotactic manner [16]. A primary function of activated microglia is phagocytosis, engulfing and eliminating pathogens, abnormal proteins, dead cells, or cellular debris [18]. Microglia also process and present antigens to T lymphocytes, in addition to regulating the inflammatory response by recruiting adaptive immune cells and releasing cytokines, chemokines, and other inflammatory mediators [19]. Microglial activation is a graded response, and an exacerbated microglial response to injury can contribute to chronic neurodegenerative and inflammatory processes [20]. Under these conditions, there is an increased release of pro-inflammatory cytokines, such as IL-1β, TNF- α and IL-6. These cytokines are primarily secreted by activated immune cells and, when present in excess and for prolonged periods, they lead to uncontrolled activation, causing damage to neurons and other cells of the CNS. Furthermore, microglia produce chemokines from the CXC family or α-family (IL-8, IL-10) and the CC family or β-family (MIP-1α, MIP-1β, MCP-1, RANTES [21]), which contribute to the recruitment of T cells, macrophages, and dendritic cells during neuroinflammation [14,18]. Additionally, these chemokines can also stimulate microglial migration to injured or other inflammatory sites [22]. The imbalance in the production and proportion of pro-inflammatory and anti-inflammatory cytokines, as well as the dysfunction in the regulation of chemokines in the CNS, plays an important role in chronic neuroinflammation and the development of neurological diseases, such as MS, AD and PD. Therefore, understanding these complex interactions is fundamental for advancing the development of therapies that aim to restore the balance of cytokines and chemokines in neuroinflammation, thus promoting immunological homeostasis in the CNS [23].
3. Trp Catabolism Through the KP During Neuroinflammatory Conditions
Trp is an essential amino acid obtained by dietary intake or synthesis in the microbiota in humans. During evolution, Trp metabolism became part of cellular and organismal communication strategies that align food availability with physiology and behavior. Besides being a constituent in protein synthesis, it coordinates organismal responses to environmental and dietary signals. Ninety percent of Trp is bound to serum albumin, while the remaining portion is freely available to be metabolized by the liver, kidney, and brain, where it crosses the blood–brain barrier through a system of neutral amino acid transporters and generates several metabolites with distinct biological activities in the immune response and neurotransmission [24] (Figure 2). Trp is primarily degraded by three distinct metabolic pathways. About 2% of it is directed to the serotonin pathway, and approximately 3% is metabolized, generating catabolites such as tryptamine, skatole, and indole acetate, which act as ligands for aryl hydrocarbon receptors (AhRs) [25]. However, most of this amino acid is catabolized through the KP [26,27], a route generating neuroactive intermediate metabolites with crucial physiological roles in both the CNS and the immune system [28].
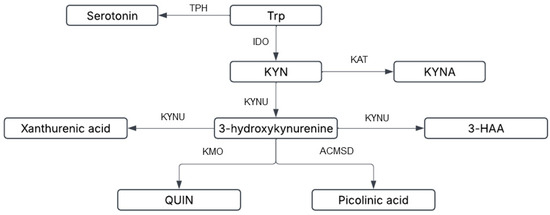
Figure 2.
Tryptophan can be metabolized into serotonin and melatonin through sequential multi-step reactions or alternatively metabolized via the kynurenine pathway (KP). Kynurenine (KYN) is the rate-limiting KP product of initial tryptophan metabolism by the enzymes indoleamine-2,3-dioxygenase (IDO-1) and tryptophan dioxygenase (orange box). Kynurenine is then converted via kynurenine aminotransferases (KAT I/II/III) into kynurenic acid (KYNA), a neuroprotective molecule that antagonizes glutamate receptor-induced neurotoxicity. 3-Hydroxykynurenine (3-HK) is produced through further metabolism of kynurenine, with accumulating evidence suggesting its neurotoxic potential. The conversion of quinolinic acid (QUIN) into the essential cofactor NAD+ is catalyzed by quinolinate phosphoribosyltransferase (QPRT).
Trp is a substrate for tryptophan 2,3-dioxygenase (TDO E.C. 1. 13.11.11) and indoleamine 2,3-dioxygenase (IDO1 E.C.1. 13.11.52). TDO has substantial activity localized in the liver and is activated by dietary proteins and corticosteroids [29], but it is not induced by pro-inflammatory stimuli such as IFN-γ [30]. The basal serum level of Trp is primarily controlled by TDO [31]. In turn, IDO1, a heme protein, is expressed in cells such as macrophages, astrocytes, and microglia, and may exist in the IDO1 or IDO2 isoforms, depending on the tissue [31,32]. IDO1 initially converts Trp to formyl kynurenine and subsequently to L-kynurenine (KYN), allowing the generation of many biologically active metabolites (Figure 3). Four isozymes, kynurenine 2,3-aminotransferases (KAT I, II, III, and IV), are involved in the synthesis of kynurenic acid (KYNA) from KYN in the human and rodent CNS. Macrophages and microglia express all enzymes of the kynurenine monooxygenase (KMO, E.C.1.14.13.9) branch of KP, allowing their primary contributions to local QUIN production in response to inflammatory stimuli within the CNS [33]. These isoforms share similarities in their structure and function, being involved in the conversion of Trp into other metabolites, with broad immunomodulatory functions [30].
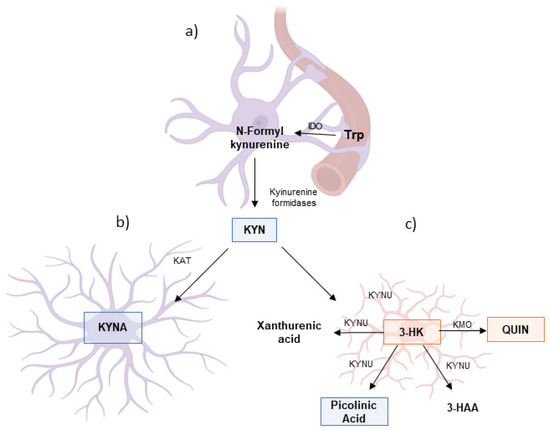
Figure 3.
Metabolism of the kynurenine pathway in the glial cells. As shown above, (a) tryptophan (Trp) is converted into kynurenine (KYN) via the enzymes indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO). In astrocytes (b), KYN is preferentially metabolized by kynurenine aminotransferases (KAT) into kynurenic acid (KYNA), a neuroprotective metabolite with anti-excitotoxic and anti-inflammatory properties. In contrast, microglia (c) favor the metabolism of KYN via kynurenine monooxygenase (KMO) and kynureninase (KYNU), leading to the production of 3-hydroxykynurenine (3-HK), a pro-oxidant compound, and quinolinic acid (QUIN), a neurotoxic NMDA receptor agonist associated with neurodegenerative processes. Although frequently associated with neuroinflammatory function, KP in microglia also can produce neuroprotective metabolites such as picolinic acid and 3-hydroxyanthranilic acid (3-HAA). Furthermore, astrocytes and neurons can uptake QUIN and produce the neuroprotective metabolite nicotinamide adenine dinucleotide (NAD). This differential metabolism of KYN in astrocytes and microglia, as well as its concentration, is crucial in determining the balance between neuroprotection and neurotoxicity in the central nervous system. Adapted from Guillemin et al. (2012) [29].
The human and rodent genomes encode the four KAT isozymes, which demonstrate the ability to use KYN as an amino group donor in the first half-reaction: KAT I (also known as glutamine transaminase K or cysteine conjugate beta-lyase), KAT II (α-aminoadipate aminotransferase), KAT III (glutamine transaminase L), and KAT IV (mitochondrial aspartate aminotransferase) [34]. A comparative analysis between species reveals a high degree of conservation of the primary sequence of these isozymes, with KAT IV being the isoform that is most phylogenetically conserved [35]. On the other hand, within the same species, KAT II and KAT IV are the most divergent isozymes, probably due to their specific characteristics and the different roles played by the N-terminal regions of the proteins [36]. Changes in KAT II activity are considered important for the pathogenesis of neurological diseases, given the neuroprotective role of KYNA [28]. Studies have shown that genetic alterations for KAT II present perinatal reductions in brain KYNA levels, accompanied by behavioral and pathological abnormalities [36]. However, these alterations are progressively repaired as the individual reaches adulthood. These observations suggest that another KAT isoform can compensate for the absence of KAT II, and, in mice, normalization of KYNA levels has been observed [37].
Pro-inflammatory stimuli, such as IFN-γ, TNF-α, IFN-β or LPS, recruit macrophages from the periphery into the brain and the enzyme KMO is activated, leading to increased production of 3-hydroxy kynurenine (3-HK) and exacerbating the KP’s oxidative profile. KMO is a mitochondrial flavoprotein expressed in several peripheral tissues [38,39,40], including the liver, kidney, and phagocytes, such as macrophages and monocytes [34]. This enzyme uses O2 as a co-substrate and NADPH as a cofactor [40]. Being a flavin adenine dinucleotide-dependent enzyme, its activity might be expected to be decreased in riboflavin (vitamin B2) deficiency [26,41]. In the CNS, KMO is expressed predominantly in microglia [41]. The continuity of the oxidative branch of KP leads to the production of 3-hydroxyanthranilic acid (3-HAA), xanthurenic acid (XA) and anthranilic acid (AA) [38], which, through the action of kynureninase (KYNU), contributes to the production of QUIN [26,42]. Independently, KMO regulates the conversion of Kyn into neuroactive and neurotoxic KP metabolites, including quinolinic acid (QUIN). QUIN can be converted into NAD+—a key coenzyme in energy metabolism—by certain cell types, but the physiological significance of this de novo NAD+ production via the KP is unclear, as NAD+ is mainly produced by salvage pathways [41]. The enzyme quinolinate phosphoribosyl transferase (QPRTase), responsible for the synthesis of NAD from QUIN, has been detected in rat brain in astrocytes, tanycytes, ependymal cells, and some neurons. The expression of this enzyme in the human brain is partially understood, with immunoreactivity observed in glial cells and neurons [43,44,45]. Guillemin et al.’s (2005) findings suggest high expression of QPRTase in human fetal astrocytes and neurons, indicating that both can catabolize QUIN [43]. However, the exact mechanism by which QUIN is absorbed by these cell types remains unclear [44,45]. QUIN is an NMDA receptor agonist, which can mediate neuronal death by excitotoxicity when excessively stimulated [37,38].
In cultured rat and mouse cortical cells, the metabolite 3-HK is an intermediate that induces the selective death of neuronal cells in specific brain regions, exhibiting pro-apoptotic characteristics that may be relevant to the pathology of neurodegenerative disorders [45,46]. Moreover, in physiological conditions, QUIN participates in the production of Nicotinamide Adenine Dinucleotide (NAD), but an excess of QUIN in astrocytes and neurons induces apoptosis through activation of the NMDA receptor [47]. This condition also increases astrocyte expression of GFAP, along with cell proliferation [37,48,49].
It is known that KYN is actively transported across the blood–brain barrier by neutral amino acid transporters. QUIN and KYNA do not have access to brain tissue where they are generated by the direct degradation of endogenous Trp [50]. In pathological conditions, activated astrocytes can be stimulated to produce KYN in large quantities [51]. This metabolite is captured by microglia, increasing QUIN synthesis, since only this phenotype exhibits gene expression for the KMO enzyme [37]. Activation of the KMO branch that induces elevated levels of 3-HK and QUIN in the CNS is observed in most inflammatory neurological diseases [28].
Although the kynurenine and serotonin pathways are almost entirely responsible for Trp catabolism, a small portion is directed to the tryptamine pathway. In this pathway, Trp is decarboxylated by the enzyme amino acid decarboxylase (AADC) to produce tryptamine, which is then demethylated to S-adenosyl methionine (SAM). Through a nucleophilic attack in a reaction catalyzed by indolethylamine-N-methyltransferase (INMT), N-methyltryptamine and dimethyltryptamine (DMT) are formed, metabolites that are ligands for aryl hydrocarbon receptors (AhRs) [51]. High levels of KYN also interact with AhRs, stimulating transcription factors found in most human cells related to cell proliferation, by a functional alteration of cell signaling, involving GCN2 and mTOR kinases [52]. Several molecules, such as the adenosine/purinergic pathway, CTLA-4 and PD-L1, collaborate with kynurenine and its metabolites to induce the immune response [52]. This is achieved through different mechanisms, including Trp exhaustion, TH17 cell induction, dendritic cell and macrophage trans-differentiation, CD4 T cell differentiation into Treg cells, and IL-2 inhibition, which prevents CD4 T cell survival [52]. These mechanisms are attributed to astrocytes in the brain immune microenvironment [53].
Trp catabolism may act in immune defense mechanisms against pathogen invasion by producing reactive oxygen species (ROS), including superoxide anion, during the respiratory burst induced by phagocytes. By degrading Trp, IDO1 also contributes to controlling the proliferation of pathogens [54,55,56]. IDO1 acts as a link between the innate and adaptive immune systems, as well as communication between the nervous system and the peripheral immune system. In a study using glial cells infected with Neospora caninum, Argolo et al. (2021) observed genotypic modulation of the inflammatory profile through KP activity [56]. The parasite uses the KP as a cellular escape and evasion mechanism and promotes tissue preservation through the production of KYNA [56].
The extreme scarcity of Trp (<1 µM) leads to the accumulation of uncharged tRNAs, which activate the general control nonderepressible kinase 2 (GCN2) pathway, resulting in dysfunction of T cells and antigen-presenting cells (APCs) [56]. However, even under conditions of forced dioxygenase expression, the Trp levels in the local microenvironment do not decrease enough to activate GCN2 [57]. Studies have shown that there are differences in QUIN concentrations in neurodegenerative diseases among individuals of different ages, and that these differences may be associated with the levels of intermediate metabolites such as 3-HK and XA, reinforcing the neurotoxic role of 3-HK and QUIN [58,59]. Other studies indicate differences in QUIN concentrations between men and women [60,61]. This may suggest that the KP influences the hormonal response [62,63]. It is well established that KYN concentrations below the picomolar level are sufficient to bind to AhRs [64]. In turn, the activities of these enzymes result in the accumulation of KP metabolites, mainly KYN. Kyn can be converted into AA by kynureninase (KYNU) and kynurenic acid (KYNA) by kynurenine aminotransferases (KATI–KATIII), with the latter step being important for controlling the production of neuroprotective KYNA [65]. Particularly in the brain, KYN can be transaminated into KYNA by mitochondrial aspartate aminotransferase (GOT2) [43,66].
It has been shown that kynurenic acid (KYNA) is present in the CNS of various animal species, including humans, with extracellular concentrations or levels in the cerebrospinal fluid (CSF) ranging from 15 to 150 nM, which accumulate with age [67,68]. However, it should be noted that extracellular concentrations of KYNA in mammalian brains are in the low nM range, whereas its affinity for the glycine site of the NMDA receptor complex is approximately 10–20 µM [67]. The levels of KYNA in the brain can be increased by administering direct or indirect precursors, transport inhibitors, or inhibitors of KMO, the most abundant enzyme involved in kynurenine metabolism [68,69]. An elegant study reported that inhibiting kynurenine 3-monooxygenase in peripheral organs, in turn increasing kynurenine levels in the blood and KYNA content in the brain, significantly reduced neurodegeneration in various transgenic models of Huntington’s and AD [70]. In recent decades, crucial information about the action of KYNA has been revealed. KYNA not only acts as an endogenous antagonist of ionotropic excitatory amino acid receptors for glutamate, such as the N-methyl-D-aspartate (NMDAR), alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA), and kainate receptors [5,40] and the nicotinic acetylcholine receptor subtype α-7 (α-7nAChR) [37], but also acts as an agonist of the G-protein-coupled receptor GPR-35 [43,44], with anticonvulsant and neuroprotective activities [41,71]. Regarding the importance of KYNA and XA involvement with the GPR-35 receptor in the gut microbiota, specific positive GPR-35 signals have been detected in the gastrointestinal tract [26,42,72], which should be further investigated.
4. KP and Neurodegenerative Diseases
The KP contributes to understanding neurodegenerative disorders and neuropsychiatric diseases, such as PD, AD, MS, Huntington’s disease (HD), and ALS, as well as mood and personality disorders, depression, and schizophrenia, among other nervous system disorders [28,73]. As previously discussed, inflammatory cytokines such as TNF-α, IL-1β and IL-6 are inducers of the IDO1 enzyme, stimulating the metabolism of Trp to synthetize different kynurenines that are responsible for the modulatory response in nervous tissue [74,75,76].
The levels of this amino acid, its metabolites, and enzymes have been studied in the brain, liver, and kidney of female Wistar rats, showing that Trp concentration and IDO1 activity decrease with age in all these tissues [77]. Another study showed that IDO1 activity increases in the brain but decreases in the liver and kidneys of Wistar rats with age [78]. The inflammatory processes observed in aging act as a driving force to activate the KP, with excess production of QUIN, which may increase the likelihood of neurodegenerative diseases. It was also observed that reduced IDO1 activity suppresses the toxicity of α-synuclein (αSyn) [79]. Several studies have shown an association between serum or CSF levels of KP catabolites and neuropsychiatric pathologies [43,65]. The correlation between neurodegeneration and neuropreservation mediated by the KP involves interactions between microglia and astrocytes. Depending on the stimulus, pathological responses and the emergence of diseases can occur, which are aggravated by the loss of neural network function and failure in synaptic communication [80]. This mechanism can be understood in the various neuroinflammatory manifestations that lead to neurodegenerative and neuropsychiatric diseases.
4.1. Trp Catabolism and KP in AD
AD is the most common form of dementia and is defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques resulting from the accumulation of beta-amyloid and neurofibrillary tangles [81]. The most affected areas of the brain are the medial temporal lobe and neocortical structures [82]. The disease is characterized by progressive cognitive impairment, including memory loss, disorientation, language impairment, and executive dysfunction. The “amyloid hypothesis” as the cause of AD has been strongly questioned in recent years. According to this hypothesis, deposits of Aβ oligomers originating from misfolded amyloid precursor protein (APP) and higher-order aggregates such as fibrils favor the formation of so-called amyloid plaques [83]. APP, a transmembrane glycoprotein, is cleaved by amyloid secretase, an enzyme with several variants at the α, β, and γ cleavage sites [84]. The cleavage of APP by γ-secretase produces sAPPβ peptides and amyloid β (Aβ) monomers. Amyloid β aggregates, particularly small oligomers, are considered neurotoxic and neuroinflammatory [85]. Although they are not the root cause of AD, they have a pronounced cytotoxic effect and may contribute to disease progression [85,86].
In AD-associated dementia, behavioral changes are usually the first symptoms to appear, while memory and perceptual spatial skills are not initially affected. In the early stages, the patient begins to develop motor symptoms [87]. Currently, there are no available biomarkers for the diagnosis of dementia or neurodegenerative diseases, limiting this work to behavioral and cognitive characteristics only [88,89]. A molecularly precise diagnosis is currently only possible by studying the patient’s tissue in a post-mortem process [88].
Studies have shown that Trp and its metabolites modulate Aβ biochemistry in a potentially beneficial manner by interacting with various enzymes or by interacting directly with Aβ itself [28]. 3-Hydroxyanthranilic acid, generated by the KP, is an endogenous inhibitor of Aβ aggregation [90]. Among other enzymes implicated in the biochemistry of Aβ oligomers and influenced by Trp, a crucial role is played by neprilysin (NEP), a metalloproteinase that regulates the brain clearance of Aβ peptides. A decrease in the elimination of these oligomers significantly contributes to the pathogenesis of AD [26,42]. A study demonstrated that two Trp metabolites, 5-hydroxy indoleacetic acid (5-HIAA) and KYNA, stimulate the activity and expression of NEP and prevent Aβ peptide-induced neurotoxicity, possibly by interacting with the AhR [42].
Finally, Trp metabolism correlates with cellular physiology and contributes to the understanding of the interaction between amyloid biochemistry and mechanisms associated with AD. This knowledge allows for the exploration of effective therapeutic targets and the analysis of biomarkers aimed at the prevention and diagnosis of AD, helping to reduce the impacts and burden of neurodegeneration associated with the disease.
4.2. Trp Catabolism and KP in PD
PD is a neurodegenerative disease that ranks second only to AD as the most common neurodegenerative condition. With increasing life expectancy and fewer competing causes of death, its prevalence is expected to increase to 12 to 17 million people by 2040 [88,89,91]. The cause of PD is multifactorial, and although there is consensus among experts that it is an age-related disease, questions remain about the extent to which external factors (such as pollutants) contribute to its development [92].
The pathogenesis of PD involves the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), resulting in a dopamine deficit in the basal ganglia, which leads to the motor symptoms of classic parkinsonism [93], including bradykinesia, ataxia, muscle rigidity, postural instability, and resting tremor. Approximately 15% of PD patients have the familial form of the disease, and 5–10% have a monogenic Mendelian form, with 23 loci and 19 causative genes already identified [94].
In PD, Trp metabolism is modulated, showing an increase in IDO1 activity, elevated KMO expression, and, consequently, increased QUIN synthesis [73]. Elevated QUIN results in strong sensitization of NMDA receptors in dopaminergic neurons, leading to neuronal death due to excitotoxicity [74]. Additionally, there is an accumulation of NAD+, which induces oxidative imbalance within mitochondria, a set of reactions well known in this disease [73].
Dhivyahave et al. (2020) demonstrated in experimental models the involvement of KP in modulating phenomena associated with PD. For example, they infused QUIN into the brains of rats, resulting in a reduction in KYN and KYNA levels [93,94,95]. Moreover, increased levels of KYNA in the brain of monkeys may protect nigrostriatal dopaminergic neurons from damage caused by QUIN-induced excitotoxicity [73]. PD patients have shown lower Trp concentrations, a higher KYN/TRP ratio, and elevated levels of KYN, AA, and KYNA compared to controls [75]. Other studies have shown reduced KYNA levels in the cortical regions, caudate putamen, SNpc, and cerebellum of PD patients [96]. The molar ratio between Trp and KYN, as well as KYN and KYNA, remains unchanged in PD patients treated with or without L-DOPA [44]. Although significant progress has been made in correlating the KP with PD, further in-depth studies are still needed to better understand the molecular and biochemical mechanisms underlying the etiopathogenesis of PD.
4.3. Trp Catabolism in ALS
ALS is a progressive disease that affects motor neurons, resulting in signs of upper motor neuron involvement, such as spasticity and hyperreflexia, and lower motor neuron involvement, such as muscle weakness, atrophy, and weight loss [97,98]. According to the El Escorial criteria, the diagnosis of ALS requires evidence of progressive deficits in both upper and lower motor neurons in at least one limb or body region. Alternatively, deficits of the lower motor neuron can be confirmed through clinical examination (in one region) and/or by electromyography in two body regions, defined as bulbar, cervical, thoracic, and lumbosacral [99]. Overall, the levels of metabolites from the KP were significantly lower in ALS patients compared to healthy individuals in the frontal cortex, substantia nigra, hippocampus, and neostriatum regions. The levels of AA and the ratios of kynurenine to Trp showed consistent reductions across all brain regions investigated in ALS [100].
Several mutations are associated with the onset and progression of ALS. Interestingly, among these are the SOD1 gene, which encodes copper–zinc superoxide dismutase (SOD1), the TARDBP gene, which encodes Tar DNA binding protein 43 (TDP-43), and the human chromosome 9 open reading frame 72 hexanucleotide repeat (C9orf72) [101]. Both the loss of function and toxic gain of function of these proteins are related to the development of inflammatory and oxidative cascades that culminate in motor neuron death [102]. A recent study conducted by Fifita et al. (2023) identified variations in two genes related to KYNA metabolism (KYAT1/CCBL1 and GOT2) and two related to QUIN metabolism (KYNU and HAAO) that may be involved in the sporadic form of ALS [100].
Inflammation and oxidative stress play crucial roles in the degeneration of motor neurons and the neuromuscular junction in ALS [43]. Reactive astrocytes are found around primary and secondary motor neurons throughout the lateral corticospinal tract, along with invasive microglia and T cells, all of which contribute to motor degeneration [102]. Astrogliosis in ALS is characterized by increased GFAP and S100β, in addition to markers such as COX-2, iNOS, and IDO1 [103,104]. Astrocytes are the main producers of KYNA in the nervous system through the enzyme KAT, and KYNA levels are altered depending on the onset and severity of ALS [105]. In patients with bulbar onset, KYNA levels are higher than in patients with limb onset. Serum KYNA concentrations are lower in patients with more severe clinical symptoms [106,107].
Although there is evidence that ALS modulates the Trp pathway, the mechanisms involved in this pathology and its correlation with the KP require further investigation for a better understanding.
4.4. Trp Catabolism and KP in MS
MS is a chronic inflammatory disease of the CNS that causes demyelination and disseminated or plaque-like lesions in the white matter and spinal cord. Its etiology is multifactorial and still not fully understood, primarily affecting young adults and being more prevalent in females [3]. The disease manifests in different clinical forms, including primary or secondary progressive, relapsing–remitting types, all of which significantly impair the quality of life of patients, often in their productive years [105]. It is believed that, like other neurodegenerative diseases, the pathological mechanism underlying MS is not singular, but rather a complex set of cellular and biochemical alterations that ultimately trigger degeneration [106,107].
Microglia are intrinsic immune cells of the CNS involved in the processes of demyelination and remyelination. Specifically, the infiltration of immune cells into the CNS results in the loss of myelin and oligodendrocyte [16]. Myelin loss has devastating effects on CNS function and ultimately leads to neuronal degeneration, both of which are hallmarks of MS and other related neuropathologies [107]. Therapies currently used for MS include immunomodulatory agents that aim to reduce inflammation and myelin damage, and immunosuppressants that target the autoimmune reaction [108]. However, such treatments have limited efficacy and can cause collateral damage, failing to ensure significant improvement or stabilization of remyelination [108]. Recently, it has been shown that the modulation of mitochondrial activity can mitigate neuronal damage associated with MS [109]. Indeed, mitochondria are also associated with immune activation in macrophages and microglia [110,111,112].
The experimental autoimmune encephalomyelitis (EAE) model has been widely used to investigate the underlying mechanisms of MS [113]. Evidence suggests that the activation of the enzyme IDO and the KP plays a critical role in inflammation-related diseases. Studies have shown that IDO inhibition can reduce the clinical signs of EAE, as well as cytokine production and neurotoxic metabolites such as quinolinic acid. Similarly, progressive MS, characterized by metabolic dysfunctions in neurons and glial cells, presents therapeutic challenges due to the complexity of the mechanisms involved, including mitochondrial dysfunction, oxidative stress, and incomplete myelin repair. Integrating therapeutic strategies to modulate the KP, improve cellular metabolism, and promote remyelination may offer promising new approaches for the treatment of both relapsing and progressive forms of MS [45,114].
Clinical trials based on the KP have explored the levels of catabolites or the expression/activation of their enzymes [115]. For example, Aeinehband et al. (2016) found an increase in QUIN in the cerebrospinal fluid of patients with MS, attributed to the activation of the KMO enzyme [115]. These same authors reported that it is possible to monitor the course of the disease based on the increased QUIN/KYN ratio in patients with MS relapses, while patients in primary MS crises presented increased levels of all metabolites. Additionally, Mancuso et al. (2015) reported that significant levels of KYN in the serum of patients with MS result from the activation of the IDO enzyme [116]. Due to these findings, KP enzymes have been explored in the diagnosis and prediction of progression of MS [46,71,117] and also as therapeutic targets in MS and other neurological diseases [118,119,120,121].
5. Biomarkers and Pharmacological Targets in KP for Neurodegenerative Diseases
Neuroinflammation is considered one of the main features of several neurodegenerative diseases, as it can exacerbate the pathological formation and accumulation of toxic proteins [122,123]. One pathway through which neuroinflammation can worsen neurodegeneration involves the (super)activation of the KP, the primary catabolic pathway of Trp.
Neurodegenerative and neuroinflammatory diseases involve the death of neurons, leading to a gradual loss of synaptic integrity, which may remain imperceptible to the individual during the clinical stage. The KP metabolites can be detected in serum, plasma, or CSF and may serve as effective biomarkers and pharmacological targets, facilitating early diagnosis and clinical monitoring in a range of pathologies.
To propose new methods for early diagnosis, Lovelace (2016) correlated concentrations of KP metabolites in biological materials including blood, CSF and urine in models involving cardiovascular diseases. The study analyzed CSF samples from patients with bacterial meningitis to evaluate the expression of Trp catabolism enzymes through polymerase chain reaction (PCR) gene sequences [46]. Other studies have also evaluated serum levels of Trp, KYN, KYNA, AA, 3-HK, and XA using liquid chromatography–tandem mass spectrometry (LC-MS/MS) to correlate these metabolites as biomarkers with clinical and pathophysiological findings in neuroinflammatory diseases [124]. Additionally, other authors report increased plasma levels of KYNA in diseases such as schizophrenia, drawing correlations with mood disorders and psychiatric comorbidities [125]. Other studies have linked associations between serum 3-HK and BDNF levels in individuals exposed to stress and elderly individuals with a predisposition to dementia, showing that in situations of stress and cognitive deficit, there is an increase in 3-HK concentrations and a decrease in BDNF concentrations [126]. Furthermore, these studies observed that after 10 weeks of physical and cognitive training, BDNF levels increased and 3-HK decreased, respectively. Fujigaki et al. (2017) compared the concentrations of KP metabolites in various biological fluids in neuropsychiatric disorders [78], highlighting their potential role in diagnostic and therapeutic application. The detection of these metabolites can provide valuable clues for the investigation, diagnosis, and clinical monitoring of pathologies, enabling more precise diagnoses and effective therapies [79].
The association of Trp metabolites with various diseases has led to significant efforts to therapeutically modulate the KP, particularly through the inhibition of the key enzymes involved, such as IDO1, TDO, and KMO (Figure 1). In the case of CNS disorders, there is a growing interest in correcting the altered balance of KP metabolites, targeting specific KP enzymes to achieve a neuroprotective effect, as well as the role of Trp and its metabolites in mediating interactions between the gut microbiome and the brain (the “gut–brain axis”) [127]. In oncology, IDO1 inhibitors have been extensively investigated for cancer immunotherapy in recent years, with multiple compounds in clinical trials, typically in combination with other drugs, such as immune checkpoint inhibitors [128]. Since the discovery of its immunosuppressive effects [57], a growing body of evidence supports the fundamental role of IDO1 in immune regulation [129]. The activation of TDO, which catalyzes the same reaction as IDO1, similarly affects the immune response by inhibiting T cell proliferation, limiting tumor immune infiltration, and restraining antitumor immune responses [130]. Although the IDO2 enzyme, related to IDO1, may support IDO1-mediated immune tolerance, the physiological functions of IDO2 and its roles in disease conditions involving KP activity are not yet fully understood [131]. The immunoregulatory properties of Trp metabolism are primarily a result of KP metabolites rather than Trp depletion [131]. To date, there are no Food and Drug Administration (FDA)- or European Medicines Agency (EMA)-approved treatments for frontotemporal dementia [89], with available treatments primarily acting on the inhibition of monoamines. For ALS, only two treatments are currently approved: riluzole, a glutamate antagonist, and edaravone, an antioxidant [97,99,132].
6. Conclusions
This review explores the importance of Trp metabolism and its derivatives produced via the KP in glial cells (mostly astrocytes and microglia) in their roles in maintaining homeostasis and their immunoinflammatory responses. Trp metabolism and the production of KP metabolites play a key role in the regulation of the immune system in the brain during pathological conditions, such as NDDs. Table 1 summarizes the progress over time in studying the Trp pathway in relation to the CNS and diseases, by measuring KP metabolite ratios and/or enzymatic activity and using glial phenotypes, indicating potential upregulation and/or downregulation of KP enzymes and metabolites, while also evidencing that the advances in studies in neurodegeneration were mostly focused on AD. Trp metabolism emerges as a key to understanding neuroinflammatory processes, helping to identify new prognostic biomarkers and potentially lead to therapeutic translation. The importance of metabolites such as KYN, KYNA and QUIN on excitotoxic and neuroprotective pathways illustrates the delicate balance between processes that can promote neuronal protection or degeneration. The involvement of multiple factors including inflammatory cytokines, oxidative stress, and enzymes regulating the KP in astrocytes and microglia highlight the extreme complexity of these interactions in the CNS. Understanding Trp metabolism and the roles played by KP metabolites in NDDs will accelerate our comprehension of the progression of these pathologies, potentially predicting morbidity and mortality while facilitating the development of more efficient therapeutic strategies. Furthermore, the analysis of bioactive compounds capable of modulating microglial inflammation and astrocyte response to protect neurons highlights their possible effects on the KP and their therapeutic potential for NDDs. The insights gained from this dynamic may not only improve the understanding of the molecular basis of neurological diseases but also provide valuable tools for early diagnosis, prognosis and the development of more effective treatments. Future studies should aim to further elucidate these pathways and explore the practical application of these findings through clinical trials.

Table 1.
Contribution on understanding the role of Trp metabolism in health disorders.
Author Contributions
Conceptualization, S.L.C. and M.d.F.D.C.; literature search, writing the original manuscript draft, and illustration preparation, D.S.A. and L.M.G.d.O.; writing—editing, reviewing, and finalizing the manuscript, G.J.G., G.E.B., A.M.B., S.L.C. and M.d.F.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Bahia State Research Support Foundation (FAPESB) (PhD fellowship for D.S.A. Process BOL0284/2020) and by the National Council for Scientific and Technological Development (CNPq) (Research Productivity Fellowship to S.L.C. Processes No. 312388/2021-7, EU-Process No. 407833/2023-4 and National Institute for Translational Neuroscience—INNT Brazil).
Acknowledgments
We would like to thank the Postgraduate Program in Immunology, Federal University of Bahia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stassart, R.M.; Möbius, W.; Nave, K.A.; Edgar, J.M. The Axon-Myelin Unit in Development and Degenerative Disease. Front. Neurosci. 2018, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, S.; Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell. Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef]
- Harkany, T.; Hökfelt, T. Neuroglia: Function and Pathology by Alexei Verkhratsky and Arthur M. Butt. Acta Physiol. 2023, 239, e14033. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The glia/neuron ratio: How it varies uniformly across brain structures and species and what that Means for brain physiology and evolution. Glia 2014, 62, 1377–1391. [Google Scholar] [CrossRef]
- Zhang, Y.; Barres, B.A. Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010, 20, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Bonvento, G. Targeted Activation of Astrocytes: A Potential Neuroprotective Strategy. Mol. Neurobiol. 2008, 38, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Butt, A.; Li, B.; Illes, P.; Zorec, R.; Semyanov, A.; Tang, Y.; Sofroniew, M.V. Astrocytes in human central nervous system diseases: A frontier for new therapies. Signal Transduct. Target. Ther. 2023, 8, 396. [Google Scholar] [CrossRef]
- Bayraktar, O.A.; Fuentealba, L.C.; Alvarez-Buylla, A.; Rowitch, D.H. Astrocyte Development and Heterogeneity. Cold Spring Harb. Perspect. Biol. 2015, 7, a020362. [Google Scholar] [CrossRef]
- Yoon, H.; Walters, G.; Paulsen, A.R.; Scarisbrick, I.A. Astrocyte Heterogeneity across the brain and spinal cord occurs developmentally, in adulthood and in response to demyelination. PLoS ONE 2017, 12, e0180697. [Google Scholar] [CrossRef]
- Yang, R.; Yang, B.; Liu, W.; Tan, C.; Chen, H.; Wang, X. Emerging role of non-coding RNAs in neuroinflammation mediated by microglia and astrocytes. J. Neuroinflammation 2023, 20, 173. [Google Scholar] [CrossRef]
- Abd-El-Basset, E.M.; Rao, M.S.; Alshawaf, S.M.; Ashkanani, H.K.; Kabli, A.H. Tumor Necrosis Factor (TNF) induces astrogliosis, microgliosis and promotes survival of cortical neurons. AIMS Neurosci. 2021, 8, 558–584. [Google Scholar] [CrossRef]
- Szelényi, J. Cytokines and the central nervous system. Brain Res. Bull. 2001, 54, 329–338. [Google Scholar] [CrossRef]
- Williamson, M.R.; Deneen, B. Astrocytes remember inflammation. Immunity 2024, 57, 938–940. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Pal, P.; Gupta, S.K.; Potdar, M.B.; Belgamwar, A.V. Microglial-mediated immune mechanisms in autoimmune uveitis: Elucidating pathogenic pathways and targeted therapeutics. J. Neuroimmunol. 2024, 395, 578433. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Xie, S.; Li, W.; Zhang, H.; Huang, L.; Qian, Z.; Zhao, C.; Zhang, L. Hidden role of microglia during neurodegenerative disorders and neurocritical Care: A mitochondrial perspective. Int. Immunopharmacol. 2024, 142, 113024. [Google Scholar] [CrossRef]
- Mondal, H.; Mondal, S. Microglia and Neuroinflammation. In Physiology and Function of Glial Cells in Health and Disease; IGI Global Scientific Publishing: Hershey, PA, USA, 2024; pp. 100–119. [Google Scholar] [CrossRef]
- Durán Laforet, V.; Schafer, D.P. Microglia: Activity-dependent regulators of neural circuits. Ann. N. Y. Acad. Sci. 2024, 1533, 38–50. [Google Scholar] [CrossRef]
- Kumari, S.; Dhapola, R.; Sharma, P.; Nagar, P.; Medhi, B.; HariKrishnaReddy, D. The impact of cytokines in neuroinflammation-mediated stroke. Cytokine Growth Factor Rev. 2024, 78, 105–119. [Google Scholar] [CrossRef]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Astrocyte–neuron metabolic relationships: For better and for worse. Trends Neurosci. 2011, 34, 76–87. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science (1979) 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Iwaniak, P.; Owe-Larsson, M.; Urbańska, E.M. Microbiota, Tryptophan, and Aryl Hydrocarbon Receptors as the Target Triad in Parkinson’s Disease—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 2915. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.B.; Guillemin, G. The Plasma [Kynurenine]/[Tryptophan] Ratio and Indoleamine 2,3-Dioxygenase: Time for Appraisal. Int. J. Tryptophan Res. 2019, 12, 1178646919868978. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Nadar, R.; Kim, S.; Liu, K.; Govindarajulu, M.; Cook, P.; Alexander, C.S.W.; Dhanasekaran, M.; Moore, T. The Influence of Kynurenine Metabolites on Neurodegenerative Pathologies. Int. J. Mol. Sci. 2024, 25, 853. [Google Scholar] [CrossRef]
- Fiore, A.; Murray, P.J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef]
- Lashgari, N.A.; Roudsari, N.M.; Shayan, M.; Niazi Shahraki, F.; Hosseini, Y.; Momtaz, S.; Abdolghaffari, A.H. IDO/Kynurenine: Novel insight for treatment of inflammatory diseases. Cytokine 2023, 166, 156206. [Google Scholar] [CrossRef]
- Guillemin, G.J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012, 279, 1356–1365. [Google Scholar] [CrossRef]
- Batabyal, D.; Yeh, S.R. Human Tryptophan Dioxygenase: A Comparison to Indoleamine 2,3-Dioxygenase. J. Am. Chem. Soc. 2007, 129, 15690–15701. [Google Scholar] [CrossRef]
- Heyes, M.P.; Saito, K.; Markey, S.P. Human macrophages convert l-tryptophan into the neurotoxin quinolinic acid. Biochem. J. 1992, 283, 633–635. [Google Scholar] [CrossRef]
- Rossi, F.; Miggiano, R.; Ferraris, D.M.; Rizzi, M. The Synthesis of Kynurenic Acid in Mammals: An Updated Kynurenine Aminotransferase Structural KATalogue. Front. Mol. Biosci. 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Li, Z.; Zhang, L.; Tagle, D.A.; Cai, T. Characterization of kynurenine aminotransferase III, a novel member of a phylogenetically conserved KAT family. Gene 2006, 365, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Robinson, H.; Cai, T.; Tagle, D.A.; Li, J. Biochemical and Structural Properties of Mouse Kynurenine Aminotransferase III. Mol. Cell. Biol. 2009, 29, 784–793. [Google Scholar] [CrossRef]
- Yu, P.; Di Prospero, N.A.; Sapko, M.T.; Cai, T.; Chen, A.; Melendez-Ferro, M.; Du, F.; Whetsell, W.O.; Guidetti, P.; Schwarcz, R.; et al. Biochemical and Phenotypic Abnormalities in Kynurenine Aminotransferase II-Deficient Mice. Mol. Cell. Biol. 2004, 24, 6919–6930. [Google Scholar] [CrossRef]
- Liang, J.; Cheng, Z.Y.; Shan, F.; Cao, Y.; Xia, Q.R. Serum indoleamine 2, 3-dioxygenase and tryptophan-2, 3-dioxygenase: Potential biomarkers for the diagnosis of major depressive disorder. Psychopharmacology 2024, 241, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Kerr, S.J.; Smythe, G.A.; Smith, D.G.; Kapoor, V.; Armati, P.J.; Croitoru, J.; Brew, B.J. Kynurenine pathway metabolism in human astrocytes: A paradox for neuronal protection. J. Neurochem. 2001, 78, 842–853. [Google Scholar] [CrossRef]
- Sahm, F.; Oezen, I.; Opitz, C.A.; Radlwimmer, B.; von Deimling, A.; Ahrendt, T.; Adams, S.; Bode, H.B.; Guillemin, G.J.; Wick, W.; et al. The Endogenous Tryptophan Metabolite and NAD+ Precursor Quinolinic Acid Confers Resistance of Gliomas to Oxidative Stress. Cancer Res. 2013, 73, 3225–3234. [Google Scholar] [CrossRef]
- Müller, F. Flavin-dependent hydroxylases. Biochem. Soc. Trans. 1985, 13, 443–447. [Google Scholar] [CrossRef]
- Pabarcus, M.K.; Casida, J.E. Cloning, expression, and catalytic triad of recombinant arylformamidase. Protein Expr. Purif. 2005, 44, 39–44. [Google Scholar] [CrossRef]
- Kim, J.; Porciuncula, F.; Yang, H.D.; Wendel, N.; Baker, T.; Chin, A.; Ellis, T.D.; Walsh, C.J. Soft robotic apparel to avert freezing of gait in parkinson’s disease. Nat. Med. 2024, 30, 177–185. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Meininger, V.; Brew, B.J. Implications for the Kynurenine Pathway and Quinolinic Acid in Amyotrophic Lateral Sclerosis. Neurodegener. Dis. 2005, 2, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Okuno, E.; Whetsell, W.O.; Köhler, C.; Schwarcz, R. Immunohistochemical localization of quinolinic acid phosphoribosyltransferase in the human neostriatum. Neuroscience 1991, 42, 397–406. [Google Scholar] [CrossRef]
- Wejksza, K.; Rzeski, W.; Okuno, E.; Kandefer-Szerszen, M.; Albrecht, J.; Turski, W.A. Demonstration of Kynurenine Aminotransferases I and II and Characterization of Kynurenic Acid Synthesis in Oligodendrocyte Cell Line (OLN-93). Neurochem. Res. 2005, 30, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Schardien, K.; Wigdahl, B.; Nonnemacher, M.R. roles of neuropathology-associated reactive astrocytes: A systematic review. Acta Neuropathol. Commun. 2023, 11, 42. [Google Scholar] [CrossRef]
- Okuda, S.; Nishiyama, N.; Saito, H.; Katsuki, H. 3-Hydroxykynurenine, an Endogenous Oxidative Stress Generator, Causes Neuronal Cell Death with Apoptotic Features and Region Selectivity. J. Neurochem. 1998, 70, 299–307. [Google Scholar] [CrossRef]
- Colín-González, A.L.; Maldonado, P.D.; Santamaría, A. 3-Hydroxykynurenine: An intriguing molecule exerting dual actions in the Central Nervous System. Neurotoxicology 2013, 34, 189–204. [Google Scholar] [CrossRef]
- Kadi, L.; Selvaraju, R.; de Lys, P.; Proudfoot, A.E.I.; Wells, T.N.C.; Boschert, U. Differential Effects of chemokines on oligodendrocyte precursor proliferation and myelin formation in vitro. J. Neuroimmunol. 2006, 174, 133–146. [Google Scholar] [CrossRef]
- Jia, L.; Tian, P.; Ding, C. Immunoregulatory effects of indoleamine 2,3-dioxygenase in transplantation. Transpl. Immunol. 2009, 21, 18–22. [Google Scholar] [CrossRef]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2–KYN–AhR Pathway for Cancer Immunotherapy—Challenges and Opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Anu, R.I.; Shiu, K.K.; Khan, K.H. The immunomodulatory role of IDO1-Kynurenine-NAD+ pathway in switching cold tumor microenvironment in PDAC. Front. Oncol. 2023, 13, 1142838. [Google Scholar] [CrossRef]
- Thomas, S.R.; Stocker, R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 1999, 4, 199–220. [Google Scholar] [CrossRef]
- Argolo, D.S.; Borges, J.M.P.; Freitas, L.D.S.; Pina, G.A.; Grangeiro, M.S.; da Silva, V.D.A.; Pinheiro, A.M.; Conceição, R.S.; Branco, A.; Guillemin, G.; et al. Activation of the Kynurenine Pathway and Production of Inflammatory Cytokines by Astrocytes and Microglia Infected with Neospora caninum. Int. J. Tryptophan Res. 2022, 15, 117864692110699. [Google Scholar] [CrossRef]
- Del’Arco, A.E.; Argolo, D.S.; Guillemin, G.; Costa, M.D.F.; Costa, S.L.; Pinheiro, A.M. Neurological Infection, Kynurenine Pathway, and Parasitic Infection by Neospora caninum. Front. Immunol. 2022, 12, 714248. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Conway, S.J.; Marshall, B.; Brown, C.; Mellor, A.L. Prevention of Allogeneic Fetal Rejection by Tryptophan Catabolism. Science (1979) 1998, 281, 1191–1193. [Google Scholar] [CrossRef]
- Sonner, J.K.; Deumelandt, K.; Ott, M.; Thomé, C.M.; Rauschenbach, K.J.; Schulz, S.; Munteanu, B.; Mohapatra, S.; Adam, I.; Hofer, A.-C.; et al. The stress kinase GCN2 does not mediate suppression of antitumor T cell responses by tryptophan catabolism in experimental melanomas. Oncoimmunology 2016, 5, e1240858. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-associated depression: From Serotonin to kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Aaldijk, E.; Vermeiren, Y. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: A narrative review. Ageing Res. Rev. 2022, 75, 101556. [Google Scholar] [CrossRef]
- Meier, T.B.; Drevets, W.C.; Teague, T.K.; Wurfel, B.E.; Mueller, S.C.; Bodurka, J.; Dantzer, R.; Savitz, J. Kynurenic acid is reduced in females and oral contraceptive users: Implications for depression. Brain Behav. Immun. 2018, 67, 59–64. [Google Scholar] [CrossRef]
- Widner, B.; Werner, E.R.; Schennach, H.; Wachter, H.; Fuchs, D. Simultaneous Measurement of Serum Tryptophan and Kynurenine by HPLC. Clin. Chem. 1997, 43, 2424–2426. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Dalkner, N.; Riedrich, K.; Fuchs, D.; Gostner, J.M.; Reininghaus, B. Sex-Specific Changes in Tryptophan Breakdown Over a 6 Week Treatment Period. Front. Psychiatry 2019, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Seok, S.H.; Ma, Z.X.; Feltenberger, J.B.; Chen, H.; Chen, H.; Scarlett, C.; Lin, Z.; Satyshur, K.A.; Cortopassi, M.; Jefcoate, C.R.; et al. Trace derivatives of kynurenine potently activate the aryl hydrocarbon receptor (AHR). J. Biol. Chem. 2018, 293, 1994–2005. [Google Scholar] [CrossRef]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Smythe, G.; Takikawa, O.; Brew, B.J. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 2005, 49, 15–23. [Google Scholar] [CrossRef]
- Moroni, F.; Russi, P.; Carlá, V.; Lombardi, G. Kynurenic acid is present in the rat brain and its content increases during development and aging processes. Neurosci. Lett. 1988, 94, 145–150. [Google Scholar] [CrossRef]
- Turski, W.A.; Nakamura, M.; Todd, W.P.; Carpenter, B.K.; Whetsell, W.O.; Schwarcz, R. Identification and quantification of kynurenic acid in human brain tissue. Brain Res. 1988, 454, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Bacciottini, L.; Pellegrini-Giampietro, D.E.; Bongianni, F.; de Luca, G.; Politi, V.; Moroni, F. Biochemical and behavioural studies on indole-pyruvic acid: A keto-analogue of tryptophan. Pharmacol. Res. Commun. 1987, 19, 803–817. [Google Scholar] [CrossRef]
- Zwilling, D.; Huang, S.Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Guidetti, P.; Wu, H.Q.; Lee, J.; Truong, J.; Andrews-Zwilling, Y.; Hsieh, E.W.; et al. Kynurenine 3-Monooxygenase Inhibition in Blood Ameliorates Neurodegeneration. Cell 2011, 145, 863–874. [Google Scholar] [CrossRef]
- Lim, C.K.; Bilgin, A.; Lovejoy, D.B.; Tan, V.; Bustamante, S.; Taylor, B.V.; Bessede, A.; Brew, B.J.; Guillemin, G.J. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci. Rep. 2017, 7, 41473. [Google Scholar] [CrossRef]
- Stone, T.W.; Stoy, N.; Darlington, L.G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci. 2013, 34, 136–143. [Google Scholar] [CrossRef]
- Bosch, J.; Roca, T.; Armengol, M.; Fernández-Forner, D. Synthesis of 5-(sulfamoylmethyl)indoles. Tetrahedron 2001, 57, 1041–1048. [Google Scholar] [CrossRef]
- Hetherington-Rauth, M.; Johnson, E.; Migliavacca, E.; Parimi, N.; Langsetmo, L.; Hepple, R.T.; Grzywinski, Y.; Corthesy, J.; Ryan, T.E.; Ferrucci, L.; et al. Nutrient Metabolites Associated with Low D3Cr Muscle Mass, Strength, and Physical Performance in Older Men. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad217. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Dieterich, W.; Natarajan, A.; Schwappacher, R.; Reljic, D.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Influence of Amino Acids and Exercise on Muscle Protein Turnover, Particularly in Cancer Cachexia. Cancers 2024, 16, 1921. [Google Scholar] [CrossRef] [PubMed]
- Grishanova, A.Y.; Perepechaeva, M.L. Kynurenic Acid/AhR Signaling at the Junction of Inflammation and Cardiovascular Diseases. Int. J. Mol. Sci. 2024, 25, 6933. [Google Scholar] [CrossRef] [PubMed]
- Del Tredici, K.; Braak, H. A not entirely benign procedure: Progression of parkinson’s disease. Acta Neuropathol. 2008, 115, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic targets for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Fujigaki, H.; Mouri, A.; Yamamoto, Y.; Nabeshima, T.; Saito, K. Linking phencyclidine intoxication to the tryptophan-kynurenine pathway: Therapeutic implications for schizophrenia. Neurochem. Int. 2019, 125, 94–105. [Google Scholar] [CrossRef]
- De-Paula, V.J.; Radanovic, M.; Diniz, B.S.; Forlenza, O.V. Alzheimer’s disease. Sub-Cell. Biochem. 2012, 65, 329–352. [Google Scholar] [CrossRef]
- Penke, B.; Bogár, F.; Fülöp, L. β-Amyloid and the Pathomechanisms of Alzheimer’s Disease: A Comprehensive View. Molecules 2017, 22, 1692. [Google Scholar] [CrossRef]
- Tomita, S.; Kirino, Y.; Suzuki, T. Cleavage of Alzheimer’s Amyloid Precursor Protein (APP) by Secretases Occurs after O-Glycosylation of APP in the Protein Secretory Pathway. J. Biol. Chem. 1998, 273, 6277–6284. [Google Scholar] [CrossRef]
- Deng, J.; Liu, B.; Tao, Q.; Luo, Y.; Zhu, Y.; Huang, X.; Yue, F. The Co-Oligomers of Aβ42 and Human Islet Amyloid Polypeptide Exacerbate Neurotoxicity and Alzheimer-Like Pathology at Cellular Level. Neuroscience 2024, 547, 37–55. [Google Scholar] [CrossRef]
- Meng, X.; Song, Q.; Liu, Z.; Liu, X.; Wang, Y.; Liu, J. Neurotoxic β-amyloid oligomers cause mitochondrial dysfunction—The trigger for PANoptosis in neurons. Front. Aging Neurosci. 2024, 16, 1400544. [Google Scholar] [CrossRef] [PubMed]
- Sorgdrager, F.J.H.; Vermeiren, Y.; Van Faassen, M.; van der Ley, C.; Nollen, E.A.A.; Kema, I.P.; De Deyn, P.P. Age- and disease-specific changes of the kynurenine pathway in parkinson’s and alzheimer’s disease. J. Neurochem. 2019, 151, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Perdew, G.H. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes 2020, 12, 1859812. [Google Scholar] [CrossRef]
- Verma, A.; Waiker, D.K.; Singh, N.; Roy, A.; Singh, N.; Saraf, P.; Bhardwaj, B.; Krishnamurthy, S.; Trigun, S.K.; Shrivastava, S.K. Design, Synthesis, and Biological Investigation of Quinazoline Derivatives as Multitargeting Therapeutics in Alzheimer’s Disease Therapy. ACS Chem. Neurosci. 2024, 15, 745–771. [Google Scholar] [CrossRef]
- Rascovsky, K.; Grossman, M. Clinical diagnostic criteria and classification controversies in frontotemporal lobar degeneration. Int. Rev. Psychiatry 2013, 25, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- The Lancet. What next in parkinson’s disease? Lancet 2024, 403, 219. [Google Scholar] [CrossRef]
- Goldman, J.G.; Postuma, R. Premotor and nonmotor features of Parkinson’s disease. Curr. Opin. Neurol. 2014, 27, 434–441. [Google Scholar] [CrossRef]
- Deng, H.; Wang, P.; Jankovic, J. The genetics of parkinson disease. Ageing Res. Rev. 2018, 42, 72–85. [Google Scholar] [CrossRef]
- Venkatesan, D.; Iyer, M.; Narayanasamy, A.; Siva, K.; Vellingiri, B. Kynurenine pathway in Parkinson’s disease—An update. eNeurologicalSci 2020, 21, 100270. [Google Scholar] [CrossRef]
- Jacobs, K.R.; Lim, C.K.; Blennow, K.; Zetterberg, H.; Chatterjee, P.; Martins, R.N.; Brew, B.J.; Guillemin, G.J.; Lovejoy, D.B. Correlation between plasma and CSF concentrations of kynurenine pathway metabolites in Alzheimer’s disease and relationship to amyloid-β and Tau. Neurobiol. Aging 2019, 80, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Neary, D.; Snowden, J.S.; Gustafson, L.; Passant, U.; Stuss, D.; Black, S.; Freedman, M.; Kertesz, A.; Robert, P.H.; Albert, M.; et al. Frontotemporal lobar degeneration. Neurology 1998, 51, 1546–1554. [Google Scholar] [CrossRef]
- Corcia, P.; Beltran, S.; Bakkouche, S.E.; Couratier, P. Therapeutic news in ALS. Rev. Neurol. 2021, 177, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Ludolph, A.C.; Riepe, M.; Ullrich, K. Excitotoxicity, Energy Metabolism, and Neurodegeneration. Meldrum Garthwaite Excit. Neurodegener. 1993, 16, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Salameh, J.; Brown, R.; Berry, J. Amyotrophic Lateral Sclerosis: Review. Semin. Neurol. 2015, 35, 469–476. [Google Scholar] [CrossRef]
- Ludolph, A.; Drory, V.; Hardiman, O.; Nakano, I.; Ravits, J.; Robberecht, W.; Shefner, J.; The WFN Research Group On ALS/MND. A revision of the El Escorial criteria—2015. Amyotroph. Lateral Scler. Frontotemporal Degener. 2015, 16, 291–292. [Google Scholar] [CrossRef]
- Heylen, A.; Vermeiren, Y.; Kema, I.P.; van Faassen, M.; van der Ley, C.; Van Dam, D.; De Deyn, P.P. Brain Kynurenine Pathway Metabolite Levels May Reflect Extent of Neuroinflammation in ALS, FTD and Early Onset AD. Pharmaceuticals 2023, 16, 615. [Google Scholar] [CrossRef]
- Fifita, J.A.; Chan Moi Fat, S.; McCann, E.P.; Williams, K.L.; Twine, N.A.; Bauer, D.C.; Rowe, D.B.; Pamphlett, R.; Kiernan, M.C.; Tan, V.X.; et al. Genetic Analysis of Tryptophan Metabolism Genes in Sporadic Amyotrophic Lateral Sclerosis. Front. Immunol. 2021, 12, 701550. [Google Scholar] [CrossRef]
- Pollari, E.; Goldsteins, G.; Bart, G.; Koistinaho, J.; Giniatullin, R. The role of oxidative stress in degeneration of the neuromuscular junction in amyotrophic lateral sclerosis. Front. Cell Neurosci. 2014, 8, 131. [Google Scholar] [CrossRef]
- Iłżecka, J.; Kocki, T.; Stelmasiak, Z.; Turski, W.A. Endogenous protectant kynurenic acid in amyotrophic lateral sclerosis. Acta Neurol. Scand. 2003, 107, 412–418. [Google Scholar] [CrossRef]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links Behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef] [PubMed]
- Ysrraelit, M.C.; Fiol, M.P.; Gaitán, M.I.; Correale, J. Quality of Life Assessment in Multiple Sclerosis: Different Perception between Patients and Neurologists. Front. Neurol. 2018, 8, 729. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Chen, W.W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis, and treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef]
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef] [PubMed]
- Gruchot, J.; Weyers, V.; Göttle, P.; Förster, M.; Hartung, H.P.; Küry, P.; Kremer, D. The Molecular Basis for Remyelination Failure in Multiple Sclerosis. Cells 2019, 8, 825. [Google Scholar] [CrossRef]
- Rosenkranz, S.C.; Shaposhnykov, A.A.; Träger, S.; Engler, J.B.; Witte, M.E.; Roth, V.; Vieira, V.; Paauw, N.; Bauer, S.; Schwencke-Westphal, C.; et al. Enhancing mitochondrial activity in neurons protects against neurodegeneration in a mouse model of multiple sclerosis. Elife 2021, 10, e61798. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, M.; Zhu, X.; Gu, X.; Zhang, T.; Xia, C.; Yang, L.; Xu, Y.; Zhou, M. The role of microglia immunometabolism in neurodegeneration: Focus on molecular determinants and metabolic intermediates of metabolic reprogramming. Biomed. Pharmacother. 2022, 153, 113412. [Google Scholar] [CrossRef]
- Castro-Portuguez, R.; Sutphin, G.L. Kynurenine Pathway, NAD+ Synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp. Gerontol. 2020, 132, 110841. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, Z.; Chen, X.; Liu, M.; Li, J.; Liu, N. Electron transport chain inhibitors induce microglia activation through enhancing mitochondrial reactive oxygen species production. Exp. Cell Res. 2016, 340, 315–326. [Google Scholar] [CrossRef]
- Zarzecki, M.S.; Cattelan Souza, L.; Giacomeli, R.; Silva, M.R.P.; Prigol, M.; Boeira, S.P.; Jesse, C.R. Involvement of Indoleamine-2,3-Dioxygenase and Kynurenine Pathway in Experimental Autoimmune Encephalomyelitis in Mice. Neurochem. Res. 2020, 45, 2959–2977. [Google Scholar] [CrossRef]
- Heidker, R.; Emerson, M.; LeVine, S. Metabolic pathways as possible therapeutic targets for progressive multiple sclerosis. Neural Regen. Res. 2017, 12, 1262. [Google Scholar] [PubMed]
- Fathi, M.; Vakili, K.; Yaghoobpoor, S.; Tavasol, A.; Jazi, K.; Mohamadkhani, A.; Klegeris, A.; McElhinney, A.; Mafi, Z.; Hajiesmaeili, M.; et al. Dynamic changes in kynurenine pathway metabolites in multiple sclerosis: A systematic review. Front. Immunol. 2022, 13, 1013784. [Google Scholar] [CrossRef]
- Aeinehband, S.; Brenner, P.; Ståhl, S.; Bhat, M.; Fidock, M.D.; Khademi, M.; Olsson, T.; Engberg, G.; Jokinen, J.; Erhardt, S.; et al. Cerebrospinal fluid kynurenines in multiple sclerosis; relation to disease course and neurocognitive symptoms. Brain Behav. Immun. 2016, 51, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Hernis, A.; Agostini, S.; Rovaris, M.; Caputo, D.; Fuchs, D.; Clerici, M. Indoleamine 2,3-Dioxygenase (IDO) Expression and Activity in Relapsing-Remitting Multiple Sclerosis. PLoS ONE 2015, 10, e0130715. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.K.; Fernández-Gomez, F.J.; Braidy, N.; Estrada, C.; Costa, C.; Costa, S.; Bessede, A.; Fernandez-Villalba, E.; Zinger, A.; Herrero, M.T.; et al. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog. Neurobiol. 2017, 155, 76–95. [Google Scholar] [CrossRef]
- Isık, S.M.T.; Onmaz, D.E.; Ekmekci, A.H.; Ozturk, S.; Unlu, A.; Abusoglu, S. Relationship of tryptophan metabolites with the type and severity of multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 77, 104898. [Google Scholar] [CrossRef]
- Polyák, H.; Galla, Z.; Nánási, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabó, Á.; Klivényi, P.; Tanaka, M.; et al. The Tryptophan-Kynurenine Metabolic System Is Suppressed in Cuprizone-Induced Model of Demyelination Simulating Progressive Multiple Sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef]
- Gaetani, L.; Boscaro, F.; Pieraccini, G.; Calabresi, P.; Romani, L.; Di Filippo, M.; Zelante, T. Host and Microbial Tryptophan Metabolic Profiling in Multiple Sclerosis. Front. Immunol. 2020, 11, 157. [Google Scholar] [CrossRef]
- Maddison, D.C.; Giorgini, F. The Kynurenine pathway and neurodegenerative disease. Semin. Cell Dev. Biol. 2015, 40, 134–141. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [PubMed]
- Skorobogatov, K.; Autier, V.; Foiselle, M.; Richard, J.R.; Boukouaci, W.; Wu, C.L.; Raynal, S.; Carbonne, C.; Laukens, K.; Meysman, P.; et al. kynurenine pathway abnormalities are state-specific but not diagnosis-specific in schizophrenia and bipolar disorder. Brain Behav. Immun. Health 2023, 27, 100584. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.K.; Miller, B.J. Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr. Bull. 2018, 44, 75–83. [Google Scholar] [CrossRef]
- Salinas, J.; Beiser, A.; Himali, J.J.; Satizabal, C.L.; Aparicio, H.J.; Weinstein, G.; Mateen, F.J.; Berkman, L.F.; Rosand, J.; Seshadri, S. Associations between social relationship measures, serum brain-derived neurotrophic factor, and risk of stroke and dementia. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Prendergast, G.C.; Malachowski, W.J.; Mondal, A.; Scherle, P.; Muller, A.J. Indoleamine 2,3-Dioxygenase and Its Therapeutic Inhibition in Cancer. In Advances in Cancer Research; Elsevier: Oxford, UK, 2018; pp. 175–203. [Google Scholar]
- Mellor, A.L.; Lemos, H.; Huang, L. Indoleamine 2,3-Dioxygenase and Tolerance: Where Are We Now? Front. Immunol. 2017, 8, 1360. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Badawy, A.A.B.; Namboodiri, A.M.A.; Moffett, J.R. The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin. Sci. 2016, 130, 1327–1333. [Google Scholar] [CrossRef]
- Miller, R.; Mitchell, J.; Lyon, M.; Moore, D. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Foster, A.C.; Vezzani, A.; French, E.D.; Schwarcz, R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci. Lett. 1984, 48, 273–278. [Google Scholar] [CrossRef]
- Hilmas, C.; Pereira, E.F.R.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The Brain Metabolite Kynurenic Acid Inhibits α7 Nicotinic Receptor Activity and Increases Non-α7 Nicotinic Receptor Expression: Physiopathological Implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Croitoru-Lamoury, J.; Dormont, D.; Armati, P.J.; Brew, B.J. quinolinic acid upregulates chemokine production and chemokine receptor expression in astrocytes. Glia 2003, 41, 371–381. [Google Scholar] [CrossRef]
- Ting, K.K.; Brew, B.J.; Guillemin, G.J. Effect of quinolinic acid on human astrocytes morphology and functions: Implications in Alzheimer’s disease. J. Neuroinflammation 2009, 6, 36. [Google Scholar] [CrossRef]
- Kaur, G.; Han, S.J.; Yang, I.; Crane, C. Microglia and Central Nervous System Immunity. Neurosurg. Clin. N. Am. 2010, 21, 43–51. [Google Scholar] [CrossRef]
- Müller, N.; Myint, A.M.; Schwarz, M.J. The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders—Relation to drug treatment. Dialogues Clin. Neurosci. 2009, 11, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Giorgini, F.; Huang, S.Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Thomas, M.A.R.; Tararina, M.; Wu, H.-Q.; Schwarcz, R.; Muchowski, P.J. Targeted Deletion of Kynurenine 3-Monooxygenase in Mice. J. Biol. Chem. 2013, 288, 36554–36566. [Google Scholar] [CrossRef] [PubMed]
- Giil, L.M.; Midttun, Ø.; Refsum, H.; Ulvik, A.; Advani, R.; Smith, A.D.; Ueland, P.M. Kynurenine Pathway Metabolites in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 60, 495–504. [Google Scholar] [CrossRef]
- Bratek-Gerej, E.; Ziembowicz, A.; Godlewski, J.; Salinska, E. The Mechanism of the Neuroprotective Effect of Kynurenic Acid in the Experimental Model of Neonatal Hypoxia–Ischemia: The Link to Oxidative Stress. Antioxidants 2021, 10, 1775. [Google Scholar] [CrossRef]
- Whiley, L.; Chappell, K.E.; D’Hondt, E.; Lewis, M.R.; Jiménez, B.; Snowden, S.G.; Soininen, H.; Kłoszewska, I.; Mecocci, P.; AddNeuroMed Consortium; et al. Metabolic phenotyping reveals a reduction in the bioavailability of serotonin and kynurenine pathway metabolites in both the urine and serum of individuals living with Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 20. [Google Scholar] [CrossRef]
- Knapskog, A.B.; Edwin, T.H.; Ueland, P.M.; Ulvik, A.; Fang, E.F.; Eldholm, R.S.; Halaas, N.B.; Giil, L.M.; Saltvedt, I.; Watne, L.O.; et al. Sex-specific associations of kynurenic acid with neopterin in Alzheimer’s disease. Alzheimers Res. Ther. 2024, 16, 167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).