Current Understanding Regarding the Glioma Microenvironment and Impact of the Immune System
Abstract
1. Introduction
2. TME of HGG and Involved Cells/Signaling Pathways
2.1. Glioma Stem Cells
2.2. Identification of the TME
2.3. Perivascular TME
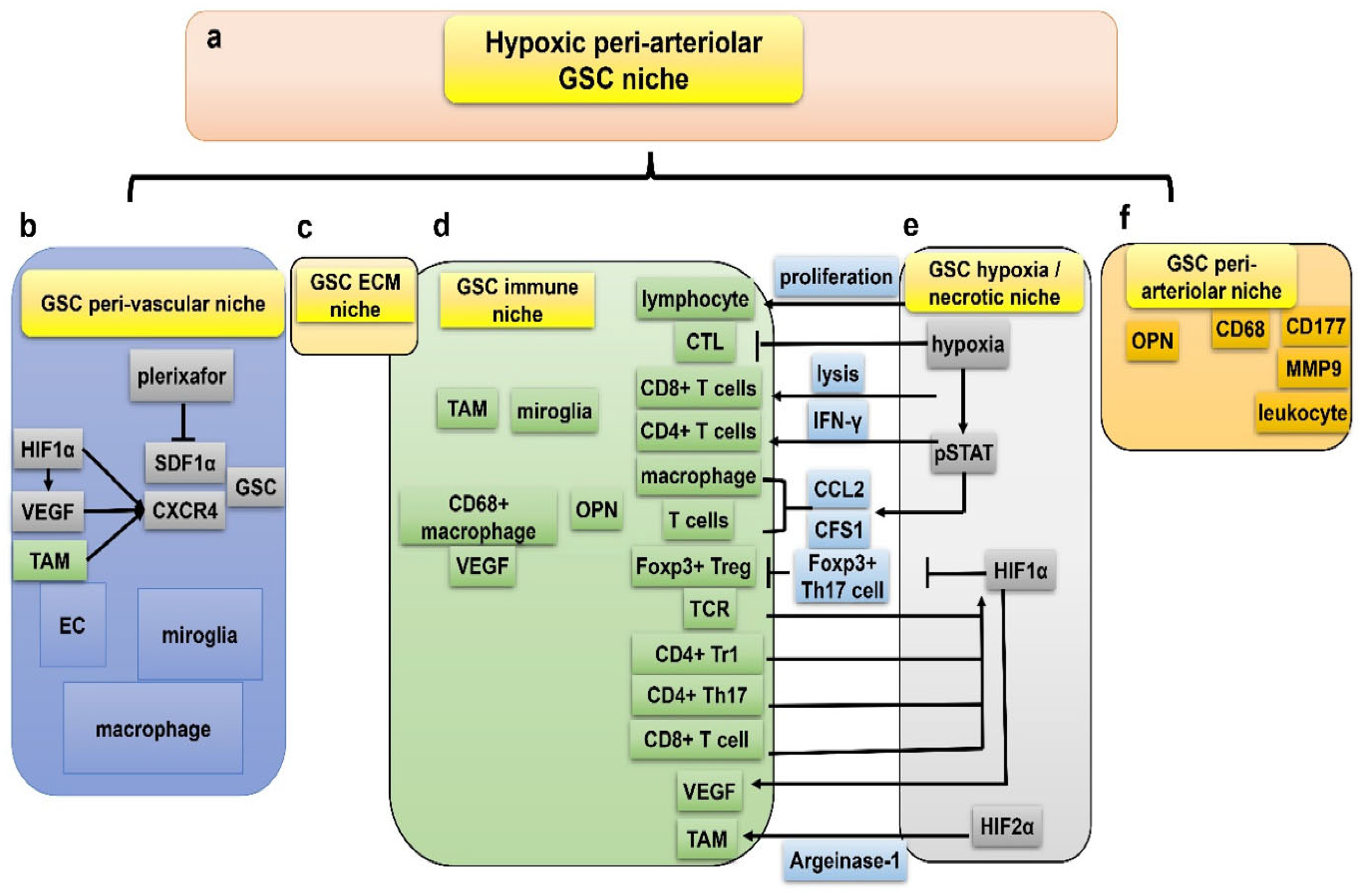
2.4. Hypoxic TME
2.5. Invasive TME
3. Interaction Between the Glioma Tumor Cells and the Immune System
3.1. Role of Blood-Brain-Barrier; Changes in HGG
3.2. Tregs and Their Involvement in HGG
3.3. NK Cells and Their Involvement in HGG
3.4. Neutrophils
3.5. Tumor-Associated Macrophages and Their Involvement in HGG
4. Interactions Between Glioma Tumor Cells and the Normal Brain Environment
4.1. Alpha-amino-3-hydroxy-5-methyl-4-isoxazole Propionic Acid Receptors (AMPARs)
4.2. Extracellular Vesicles (EVs)
4.3. Scherer Secondary Structures
4.4. Tumor Microtubes (TMs)
4.5. Neuron-Glioma Cross-Talk and Neuroligin-3 (NLGN3)
4.6. Astrocytes
4.7. Potential Target: Interactions Between Glioma Tumor Cells and Normal Brain Cells
5. Targeting the Immune System to Treat HGG
5.1. Innate Immune Treatment
5.2. Checkpoint Inhibitors
5.3. Chimeric Antigen Receptor (CAR) T Cells
5.4. Tumor Vaccines
5.5. Oncolytic Viral Therapies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obrador, E.; Moreno-Murciano, P.; Oriol-Caballo, M.; Lopez-Blanch, R.; Pineda, B.; Gutierrez-Arroyo, J.L.; Loras, A.; Gonzalez-Bonet, L.G.; Martinez-Cadenas, C.; Estrela, J.M.; et al. Glioblastoma Therapy: Past, Present and Future. Int. J. Mol. Sci. 2024, 25, 2529. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Barthel, L.; Hadamitzky, M.; Dammann, P.; Schedlowski, M.; Sure, U.; Thakur, B.K.; Hetze, S. Glioma: Molecular signature and crossroads with tumor microenvironment. Cancer Metastasis Rev. 2022, 41, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, M.A.; Phillips, J.J. Role of the Microenvironment in Glioma Pathogenesis. Annu. Rev. Pathol. 2024, 19, 181–201. [Google Scholar] [CrossRef]
- Thomas, B.C.; Staudt, D.E.; Douglas, A.M.; Monje, M.; Vitanza, N.A.; Dun, M.D. CAR T cell therapies for diffuse midline glioma. Trends Cancer 2023, 9, 791–804. [Google Scholar] [CrossRef]
- Yasinjan, F.; Xing, Y.; Geng, H.; Guo, R.; Yang, L.; Liu, Z.; Wang, H. Immunotherapy: A promising approach for glioma treatment. Front. Immunol. 2023, 14, 1255611. [Google Scholar] [CrossRef]
- Shi, T.; Zhu, J.; Zhang, X.; Mao, X. The Role of Hypoxia and Cancer Stem Cells in Development of Glioblastoma. Cancers 2023, 15, 2613. [Google Scholar] [CrossRef]
- Sattiraju, A.; Kang, S.; Giotti, B.; Chen, Z.; Marallano, V.J.; Brusco, C.; Ramakrishnan, A.; Shen, L.; Tsankov, A.M.; Hambardzumyan, D.; et al. Hypoxic niches attract and sequester tumor-associated macrophages and cytotoxic T cells and reprogram them for immunosuppression. Immunity 2023, 56, 1825–1843.e1826. [Google Scholar] [CrossRef]
- Barzegar Behrooz, A.; Syahir, A.; Ahmad, S. CD133: Beyond a cancer stem cell biomarker. J. Drug Target. 2019, 27, 257–269. [Google Scholar] [CrossRef]
- Suva, M.L.; Tirosh, I. The Glioma Stem Cell Model in the Era of Single-Cell Genomics. Cancer Cell 2020, 37, 630–636. [Google Scholar] [CrossRef]
- Gillespie, S.; Monje, M. An active role for neurons in glioma progression: Making sense of Scherer’s structures. Neuro-Oncology 2018, 20, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.L.; Mumert, M.L.; Gillespie, D.L.; Kinney, A.Y.; Schabel, M.C.; Salzman, K.L. Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro-Oncology 2014, 16, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Underhill, H.R.; Karsy, M.; Davidson, C.J.; Hellwig, S.; Stevenson, S.; Goold, E.A.; Vincenti, S.; Sellers, D.L.; Dean, C.; Harrison, B.E.; et al. Subclonal Cancer Driver Mutations Are Prevalent in the Unresected Peritumoral Edema of Adult Diffuse Gliomas. Cancer Res. 2024, 84, 1149–1164. [Google Scholar] [CrossRef]
- LeBlanc, V.G.; Trinh, D.L.; Aslanpour, S.; Hughes, M.; Livingstone, D.; Jin, D.; Ahn, B.Y.; Blough, M.D.; Cairncross, J.G.; Chan, J.A.; et al. Single-cell landscapes of primary glioblastomas and matched explants and cell lines show variable retention of inter- and intratumor heterogeneity. Cancer Cell 2022, 40, 379–392.e379. [Google Scholar] [CrossRef]
- Liu, J.; Gao, L.; Zhan, N.; Xu, P.; Yang, J.; Yuan, F.; Xu, Y.; Cai, Q.; Geng, R.; Chen, Q. Hypoxia induced ferritin light chain (FTL) promoted epithelia mesenchymal transition and chemoresistance of glioma. J. Exp. Clin. Cancer Res. 2020, 39, 137. [Google Scholar] [CrossRef]
- Mathur, R.; Wang, Q.; Schupp, P.G.; Nikolic, A.; Hilz, S.; Hong, C.; Grishanina, N.R.; Kwok, D.; Stevers, N.O.; Jin, Q.; et al. Glioblastoma evolution and heterogeneity from a 3D whole-tumor perspective. Cell 2024, 187, 446–463.e416. [Google Scholar] [CrossRef]
- Kwiatkowska, A.; Symons, M. Signaling Determinants of Glioma Cell Invasion. Adv. Exp. Med. Biol. 2020, 1202, 129–149. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; Sathornsumetee, S.; Hao, Y.; Li, Z.; Hjelmeland, A.B.; Shi, Q.; McLendon, R.E.; Bigner, D.D.; Rich, J.N. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006, 66, 7843–7848. [Google Scholar] [CrossRef]
- Ahir, B.K.; Engelhard, H.H.; Lakka, S.S. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol. Neurobiol. 2020, 57, 2461–2478. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Aderetti, D.A.; Hira, V.V.V.; Molenaar, R.J.; van Noorden, C.J.F. The hypoxic peri-arteriolar glioma stem cell niche, an integrated concept of five types of niches in human glioblastoma. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Zadeh, G. Metabolic reprogramming in glioblastoma: The influence of cancer metabolism on epigenetics and unanswered questions. Neuro-Oncology 2016, 18, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Abbadi, S.; Rampazzo, E.; Persano, L.; Della Puppa, A.; Frasson, C.; Sarto, E.; Scienza, R.; D’Avella, D.; Basso, G. Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem Cells 2010, 28, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Soni, N.; Pinero, G.; Giotti, B.; Eddins, D.J.; Lindblad, K.E.; Ross, J.L.; Puigdelloses Vallcorba, M.; Joshi, T.; Angione, A.; et al. Monocyte depletion enhances neutrophil influx and proneural to mesenchymal transition in glioblastoma. Nat. Commun. 2023, 14, 1839. [Google Scholar] [CrossRef]
- Feng, Q.; Qian, C.; Fan, S. A Hypoxia-Related Long Non-Coding RNAs Signature Associated with Prognosis in Lower-Grade Glioma. Front. Oncol. 2021, 11, 771512. [Google Scholar] [CrossRef]
- Karsy, M.; Gillespie, D.L.; Horn, K.P.; Burrell, L.D.; Yap, J.T.; Jensen, R.L. Correlation of Glioma Proliferation and Hypoxia by Luciferase, Magnetic Resonance, and Positron Emission Tomography Imaging. Methods Mol. Biol. 2018, 1742, 301–320. [Google Scholar] [CrossRef]
- Karsy, M.; Guan, J.; Jensen, R.; Huang, L.E.; Colman, H. The Impact of Hypoxia and Mesenchymal Transition on Glioblastoma Pathogenesis and Cancer Stem Cells Regulation. World Neurosurg. 2016, 88, 222–236. [Google Scholar] [CrossRef]
- Xiong, Z.; Liu, H.; He, C.; Li, X. Hypoxia Contributes to Poor Prognosis in Primary IDH-wt GBM by Inducing Tumor Cells MES-Like Transformation Trend and Inhibiting Immune Cells Activity. Front. Oncol. 2021, 11, 782043. [Google Scholar] [CrossRef]
- Infanger, D.W.; Cho, Y.; Lopez, B.S.; Mohanan, S.; Liu, S.C.; Gursel, D.; Boockvar, J.A.; Fischbach, C. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res. 2013, 73, 7079–7089. [Google Scholar] [CrossRef]
- Latour, M.; Her, N.G.; Kesari, S.; Nurmemmedov, E. WNT Signaling as a Therapeutic Target for Glioblastoma. Int. J. Mol. Sci. 2021, 22, 8428. [Google Scholar] [CrossRef]
- Huang, W.; Ding, X.; Ye, H.; Wang, J.; Shao, J.; Huang, T. Hypoxia enhances the migration and invasion of human glioblastoma U87 cells through PI3K/Akt/mTOR/HIF-1alpha pathway. Neuroreport 2018, 29, 1578–1585. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Robel, S.; Watkins, S.; Sontheimer, H. A neurocentric perspective on glioma invasion. Nat. Rev. Neurosci. 2014, 15, 455–465. [Google Scholar] [CrossRef]
- Zhu, T.S.; Costello, M.A.; Talsma, C.E.; Flack, C.G.; Crowley, J.G.; Hamm, L.L.; He, X.; Hervey-Jumper, S.L.; Heth, J.A.; Muraszko, K.M.; et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011, 71, 6061–6072. [Google Scholar] [CrossRef]
- Mentlein, R.; Hattermann, K.; Held-Feindt, J. Lost in disruption: Role of proteases in glioma invasion and progression. Biochim. Biophys. Acta 2012, 1825, 178–185. [Google Scholar] [CrossRef]
- Reinhard, J.; Brosicke, N.; Theocharidis, U.; Faissner, A. The extracellular matrix niche microenvironment of neural and cancer stem cells in the brain. Int. J. Biochem. Cell Biol. 2016, 81 Pt A, 174–183. [Google Scholar] [CrossRef]
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017, 18, 123–131. [Google Scholar] [CrossRef]
- Sampson, J.H.; Gunn, M.D.; Fecci, P.E.; Ashley, D.M. Brain immunology and immunotherapy in brain tumours. Nat. Rev. Cancer 2020, 20, 12–25. [Google Scholar] [CrossRef]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef]
- Louveau, A.; Herz, J.; Alme, M.N.; Salvador, A.F.; Dong, M.Q.; Viar, K.E.; Herod, S.G.; Knopp, J.; Setliff, J.C.; Lupi, A.L.; et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018, 21, 1380–1391. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Owens, T.; Bechmann, I.; Engelhardt, B. Perivascular spaces and the two steps to neuroinflammation. J. Neuropathol. Exp. Neurol. 2008, 67, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Schlager, C.; Korner, H.; Krueger, M.; Vidoli, S.; Haberl, M.; Mielke, D.; Brylla, E.; Issekutz, T.; Cabanas, C.; Nelson, P.J.; et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 2016, 530, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.Y.; Dutoit, V.; Tran Thang, N.N.; Walker, P.R. T-cell immunotherapy for malignant glioma: Toward a combined approach. Curr. Opin. Oncol. 2010, 22, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Linares, C.A.; Varghese, A.; Ghose, A.; Shinde, S.D.; Adeleke, S.; Sanchez, E.; Sheriff, M.; Chargari, C.; Rassy, E.; Boussios, S. Hallmarks of the Tumour Microenvironment of Gliomas and Its Interaction with Emerging Immunotherapy Modalities. Int. J. Mol. Sci. 2023, 24, 13215. [Google Scholar] [CrossRef]
- Platten, M.; Ochs, K.; Lemke, D.; Opitz, C.; Wick, W. Microenvironmental clues for glioma immunotherapy. Curr. Neurol. Neurosci. Rep. 2014, 14, 440. [Google Scholar] [CrossRef]
- Tran Thang, N.N.; Derouazi, M.; Philippin, G.; Arcidiaco, S.; Di Berardino-Besson, W.; Masson, F.; Hoepner, S.; Riccadonna, C.; Burkhardt, K.; Guha, A.; et al. Immune infiltration of spontaneous mouse astrocytomas is dominated by immunosuppressive cells from early stages of tumor development. Cancer Res. 2010, 70, 4829–4839. [Google Scholar] [CrossRef]
- El Andaloussi, A.; Lesniak, M.S. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-Oncology 2006, 8, 234–243. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2018, 33, 152. [Google Scholar] [CrossRef]
- von Boehmer, H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 2005, 6, 338–344. [Google Scholar] [CrossRef]
- Grossman, W.J.; Verbsky, J.W.; Barchet, W.; Colonna, M.; Atkinson, J.P.; Ley, T.J. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 2004, 21, 589–601. [Google Scholar] [CrossRef]
- Jonuleit, H.; Schmitt, E.; Stassen, M.; Tuettenberg, A.; Knop, J.; Enk, A.H. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 2001, 193, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kitani, A.; Strober, W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 2001, 194, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.; Roettinger, B.; Kyewski, B. Sampling of complementing self-antigen pools by thymic stromal cells maximizes the scope of central T cell tolerance. Eur. J. Immunol. 2001, 31, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Kreiter, S.; Vormehr, M.; van de Roemer, N.; Diken, M.; Lower, M.; Diekmann, J.; Boegel, S.; Schrors, B.; Vascotto, F.; Castle, J.C.; et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015, 520, 692–696. [Google Scholar] [CrossRef]
- El Andaloussi, A.; Lesniak, M.S. CD4+CD25+FoxP3+ T-cell infiltration and heme oxygenase-1 expression correlate with tumor grade in human gliomas. J. Neuro-Oncol. 2007, 83, 145–152. [Google Scholar] [CrossRef]
- Fecci, P.E.; Mitchell, D.A.; Whitesides, J.F.; Xie, W.; Friedman, A.H.; Archer, G.E.; Herndon, J.E., 2nd; Bigner, D.D.; Dranoff, G.; Sampson, J.H. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006, 66, 3294–3302. [Google Scholar] [CrossRef]
- Hussain, S.F.; Yang, D.; Suki, D.; Aldape, K.; Grimm, E.; Heimberger, A.B. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-Oncology 2006, 8, 261–279. [Google Scholar] [CrossRef]
- Maes, W.; Rosas, G.G.; Verbinnen, B.; Boon, L.; De Vleeschouwer, S.; Ceuppens, J.L.; Van Gool, S.W. DC vaccination with anti-CD25 treatment leads to long-term immunity against experimental glioma. Neuro-Oncology 2009, 11, 529–542. [Google Scholar] [CrossRef]
- Fecci, P.E.; Ochiai, H.; Mitchell, D.A.; Grossi, P.M.; Sweeney, A.E.; Archer, G.E.; Cummings, T.; Allison, J.P.; Bigner, D.D.; Sampson, J.H. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin. Cancer Res. 2007, 13, 2158–2167. [Google Scholar] [CrossRef]
- Kong, L.Y.; Wei, J.; Sharma, A.K.; Barr, J.; Abou-Ghazal, M.K.; Fokt, I.; Weinberg, J.; Rao, G.; Grimm, E.; Priebe, W.; et al. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol. Immunother. 2009, 58, 1023–1032. [Google Scholar] [CrossRef][Green Version]
- Fecci, P.E.; Sweeney, A.E.; Grossi, P.M.; Nair, S.K.; Learn, C.A.; Mitchell, D.A.; Cui, X.; Cummings, T.J.; Bigner, D.D.; Gilboa, E.; et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin. Cancer Res. 2006, 12 Pt 1, 4294–4305. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.T.; Sun, W.; Hussain, S.F.; DeAngulo, G.; Prabhu, S.S.; Heimberger, A.B. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol. Immunother. 2008, 57, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Golan, I.; Rodriguez de la Fuente, L.; Costoya, J.A. NK Cell-Based Glioblastoma Immunotherapy. Cancers 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Baker, G.J.; Chockley, P.; Zamler, D.; Castro, M.G.; Lowenstein, P.R. Natural killer cells require monocytic Gr-1+/CD11b+ myeloid cells to eradicate orthotopically engrafted glioma cells. Oncoimmunology 2016, 5, e1163461. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Menard, C.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E.; et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef]
- Yan, D.; Li, W.; Liu, Q.; Yang, K. Advances in Immune Microenvironment and Immunotherapy of Isocitrate Dehydrogenase Mutated Glioma. Front. Immunol. 2022, 13, 914618. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Engler, J.R.; Robinson, A.E.; Smirnov, I.; Hodgson, J.G.; Berger, M.S.; Gupta, N.; James, C.D.; Molinaro, A.; Phillips, J.J. Increased microglia/macrophage gene expression in a subset of adult and pediatric astrocytomas. PLoS ONE 2012, 7, e43339. [Google Scholar] [CrossRef]
- Lin, C.; Wang, N.; Xu, C. Glioma-associated microglia/macrophages (GAMs) in glioblastoma: Immune function in the tumor microenvironment and implications for immunotherapy. Front. Immunol. 2023, 14, 1123853. [Google Scholar] [CrossRef]
- Orlikowsky, T.; Dannecker, G.E.; Wang, Z.; Horowitz, H.; Niethammer, D.; Hoffmann, M.K. Activation or destruction of T cells via macrophages. Pathobiology 1999, 67, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Gieryng, A.; Pszczolkowska, D.; Walentynowicz, K.A.; Rajan, W.D.; Kaminska, B. Immune microenvironment of gliomas. Lab. Investig. 2017, 97, 498–518. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.B.; Wu, W.; Zhang, L.; Wang, Z.; Dai, Z.; Feng, S.; Cao, H.; Cheng, Q.; Liu, Z. The molecular feature of macrophages in tumor immune microenvironment of glioma patients. Comput. Struct. Biotechnol. J. 2021, 19, 4603–4618. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Kiesel, B.; Widhalm, G.; Rajky, O.; Ricken, G.; Wohrer, A.; Dieckmann, K.; Filipits, M.; Brandstetter, A.; Weller, M.; et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-Oncology 2015, 17, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Crane, C.A.; Kaur, R.; Safaee, M.; Rutkowski, M.J.; Parsa, A.T. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res. 2013, 19, 3165–3175. [Google Scholar] [CrossRef]
- Anido, J.; Saez-Borderias, A.; Gonzalez-Junca, A.; Rodon, L.; Folch, G.; Carmona, M.A.; Prieto-Sanchez, R.M.; Barba, I.; Martinez-Saez, E.; Prudkin, L.; et al. TGF-beta Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell 2010, 18, 655–668. [Google Scholar] [CrossRef]
- Huang, S.P.; Wu, M.S.; Shun, C.T.; Wang, H.P.; Lin, M.T.; Kuo, M.L.; Lin, J.T. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J. Biomed. Sci. 2004, 11, 517–527. [Google Scholar] [CrossRef]
- Jansen, T.; Tyler, B.; Mankowski, J.L.; Recinos, V.R.; Pradilla, G.; Legnani, F.; Laterra, J.; Olivi, A. FasL gene knock-down therapy enhances the antiglioma immune response. Neuro-Oncology 2010, 12, 482–489. [Google Scholar] [CrossRef][Green Version]
- Pan, Y.; Monje, M. Neuron-Glial Interactions in Health and Brain Cancer. Adv. Biol. 2022, 6, e2200122. [Google Scholar] [CrossRef]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Korber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Z.; Mashimo, T.; Shen, B.; Nyagilo, J.; Wang, H.; Wang, Y.; Liu, Z.; Mulgaonkar, A.; Hu, X.L.; et al. Gliomas Interact with Non-glioma Brain Cells via Extracellular Vesicles. Cell Rep. 2020, 30, 2489–2500.e2485. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wissmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 2022, 185, 2899–2917.e2831. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, H.S.; Johung, T.B.; Caretti, V.; Noll, A.; Tang, Y.; Nagaraja, S.; Gibson, E.M.; Mount, C.W.; Polepalli, J.; Mitra, S.S.; et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 2015, 161, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.N.; Li, X.B.; Zhang, M.; Han, C.; Fan, X.Y.; Huang, S.H. NLGN3 Upregulates Expression of ADAM10 to Promote the Cleavage of NLGN3 via Activating the LYN Pathway in Human Gliomas. Front. Cell Dev. Biol. 2021, 9, 662763. [Google Scholar] [CrossRef]
- Kamran, N.; Alghamri, M.S.; Nunez, F.J.; Shah, D.; Asad, A.S.; Candolfi, M.; Altshuler, D.; Lowenstein, P.R.; Castro, M.G. Current state and future prospects of immunotherapy for glioma. Immunotherapy 2018, 10, 317–339. [Google Scholar] [CrossRef]
- Perng, P.; Lim, M. Immunosuppressive Mechanisms of Malignant Gliomas: Parallels at Non-CNS Sites. Front. Oncol. 2015, 5, 153. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Hata, A. Targeting the TGFbeta signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef]
- Fontana, A.; Bodmer, S.; Frei, K.; Malipiero, U.; Siepl, C. Expression of TGF-beta 2 in human glioblastoma: A role in resistance to immune rejection? Ciba Found. Symp. 1991, 157, 232–238; discussion 238–241. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, W.; Zhao, D.; Han, Y.; Liu, B.; Zhao, H.; Wang, H.; Zhang, Q.; Xu, G. Correlation between TSP-1, TGF-beta and PPAR-gamma expression levels and glioma microvascular density. Oncol. Lett. 2014, 7, 95–100. [Google Scholar] [CrossRef]
- Platten, M.; Wick, W.; Weller, M. Malignant glioma biology: Role for TGF-beta in growth, motility, angiogenesis, and immune escape. Microsc. Res. Tech. 2001, 52, 401–410. [Google Scholar] [CrossRef]
- Vega, E.A.; Graner, M.W.; Sampson, J.H. Combating immunosuppression in glioma. Future Oncol. 2008, 4, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.E.; Ellison, M.D.; Young, H.F. Immunotherapy for malignant glioma using human recombinant interleukin-2 and activated autologous lymphocytes. A review of pre-clinical and clinical investigations. J. Neuro-Oncol. 1990, 8, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, N.K.; Gilbert, M.R. Immunotherapeutic Approaches for Glioblastoma Treatment. Biomedicines 2022, 10, 427. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Kurz, S.C.; Cabrera, L.P.; Hastie, D.; Huang, R.; Unadkat, P.; Rinne, M.; Nayak, L.; Lee, E.Q.; Reardon, D.A.; Wen, P.Y. PD-1 inhibition has only limited clinical benefit in patients with recurrent high-grade glioma. Neurology 2018, 91, e1355–e1359. [Google Scholar] [CrossRef]
- Lim, M.; Weller, M.; Idbaih, A.; Steinbach, J.; Finocchiaro, G.; Raval, R.R.; Ansstas, G.; Baehring, J.; Taylor, J.W.; Honnorat, J.; et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro-Oncology 2022, 24, 1935–1949. [Google Scholar] [CrossRef]
- Belcaid, Z.; Phallen, J.A.; Zeng, J.; See, A.P.; Mathios, D.; Gottschalk, C.; Nicholas, S.; Kellett, M.; Ruzevick, J.; Jackson, C.; et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS ONE 2014, 9, e101764. [Google Scholar] [CrossRef]
- Wainwright, D.A.; Chang, A.L.; Dey, M.; Balyasnikova, I.V.; Kim, C.K.; Tobias, A.; Cheng, Y.; Kim, J.W.; Qiao, J.; Zhang, L.; et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 2014, 20, 5290–5301. [Google Scholar] [CrossRef]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C.; et al. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin. Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef]
- Brown, C.E.; Starr, R.; Aguilar, B.; Shami, A.F.; Martinez, C.; D’Apuzzo, M.; Barish, M.E.; Forman, S.J.; Jensen, M.C. Stem-like tumor-initiating cells isolated from IL13Ralpha2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin. Cancer Res. 2012, 18, 2199–2209. [Google Scholar] [CrossRef]
- Ahmed, N.; Salsman, V.S.; Kew, Y.; Shaffer, D.; Powell, S.; Zhang, Y.J.; Grossman, R.G.; Heslop, H.E.; Gottschalk, S. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin. Cancer Res. 2010, 16, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Gale, N.W.; Guo, H.; Qian, J.; Petty, A.; Kaspar, J.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.; Hambardzumyan, D.; et al. EphA2 promotes infiltrative invasion of glioma stem cells in vivo through cross-talk with Akt and regulates stem cell properties. Oncogene 2015, 34, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Johnson, L.A.; Davis, J.L.; Zheng, Z.; Woolard, K.D.; Reap, E.A.; Feldman, S.A.; Chinnasamy, N.; Kuan, C.T.; Song, H.; et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum. Gene Ther. 2012, 23, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef]
- Heimberger, A.B.; Crotty, L.E.; Archer, G.E.; McLendon, R.E.; Friedman, A.; Dranoff, G.; Bigner, D.D.; Sampson, J.H. Bone marrow-derived dendritic cells pulsed with tumor homogenate induce immunity against syngeneic intracerebral glioma. J. Neuroimmunol. 2000, 103, 16–25. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Schumacher, T.; Bunse, L.; Wick, W.; Platten, M. Mutant IDH1: An immunotherapeutic target in tumors. Oncoimmunology 2014, 3, e974392. [Google Scholar] [CrossRef]
- Wang, Q.T.; Nie, Y.; Sun, S.N.; Lin, T.; Han, R.J.; Jiang, J.; Li, Z.; Li, J.Q.; Xiao, Y.P.; Fan, Y.Y.; et al. Tumor-associated antigen-based personalized dendritic cell vaccine in solid tumor patients. Cancer Immunol. Immunother. 2020, 69, 1375–1387. [Google Scholar] [CrossRef]
- Markert, J.M.; Medlock, M.D.; Rabkin, S.D.; Gillespie, G.Y.; Todo, T.; Hunter, W.D.; Palmer, C.A.; Feigenbaum, F.; Tornatore, C.; Tufaro, F.; et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000, 7, 867–874. [Google Scholar] [CrossRef]
- Rampling, R.; Cruickshank, G.; Papanastassiou, V.; Nicoll, J.; Hadley, D.; Brennan, D.; Petty, R.; MacLean, A.; Harland, J.; McKie, E.; et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000, 7, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Cassady, K.A.; Parker, J.N. Herpesvirus vectors for therapy of brain tumors. Open Virol. J. 2010, 4, 103–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cloughesy, T.F.; Petrecca, K.; Walbert, T.; Butowski, N.; Salacz, M.; Perry, J.; Damek, D.; Bota, D.; Bettegowda, C.; Zhu, J.J.; et al. Effect of Vocimagene Amiretrorepvec in Combination with Flucytosine vs Standard of Care on Survival Following Tumor Resection in Patients with Recurrent High-Grade Glioma: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Gromeier, M.; Herndon, J.E., 2nd; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Nassiri, F.; Wang, J.; Peruzzi, P.; Zadeh, G. Viral and other therapies for recurrent glioblastoma: Is a 24-month durable response unusual? Neuro-Oncology 2019, 21, 14–25. [Google Scholar] [CrossRef]



| Molecular Factor | Interaction |
|---|---|
| EGFR | Promotes glioma cell migration and reduces inflammatory response; induces macrophage infiltration; support neo-angiogenesis; increased in a hypoxic environment |
| EGFRvIII | Supports glioma cell survival, invasion, and stemness; inflammatory triggering properties; increased sensitivity to temozolomide; macrophage infiltration; support neo-angiogenesis |
| IDH | Promotes tumor-infiltrating lymphocytes, with less antitumor T-cell response; higher expression of PD-L1 |
| IDH1mut | Favorable response to chemotherapy and radiation; reduced IFN-γ and CD8; less antitumor T-cell response |
| ATRX | Mutation: stabilization of the glioma cell; deletion: promotes expression of (type I) interferon |
| KIAA1549-BRAF fusion | BRAF activation promotes pro-cancerogenic senescence via a p16 (INK4a) pathway, pro-cancerogenic TME via the CCL2/CCR2 axis; microglia recruitment |
| NF1 | NF1 incompetence: decreased cancer cell homogeneity; enhanced NF1 expression: diminished microglia activity; NF1 deactivation: increased macrophage activation |
| PTEN | PTEN mutation: immunosuppressive TME, PDL-1 enhancement; PTEN absences/deficiency: immune resistance, increased T-cell apoptosis, promoting cross-linking of proteins; supports VEGF |
| MGMT | Hypermethylation: better therapy response, promoted by hypoxic TME; reduced in presence of decreased Wnt-signaling; methylations seem to influences immune response |
| p53 | Dysfunction: cell invasion and migration of glioma cells and supports inflammatory processes; loss: pro-cancerogenic activities of SASP, resulting in immunosuppressive TME; activation: immune invigoration |
| CDK4/6 | Dysfunction: promotes phosphorylation of RB1, resulting in glioma cells’ division; lack of CDK4; prevents glioma cell development |
| RB1 | Mutation: increased glioma cell proliferation rate |
| HIF | Upregulation of VEGF and IL-8; support stem cell presence; reduction of IFN-y and TNF |
| Approach | Phase | Completed/Ongoing | Sample Size | PFS (m) | OS (m) | Year Published | References |
|---|---|---|---|---|---|---|---|
| Adaptive T cells | |||||||
| CAR-T cells (IL13Rα2) | I | Completed | 3 | NR | 11 | 2015 | NCT00730613 |
| Assessment of the feasibility and safety of cellular immunotherapy utilizing ex vivo expanded autologous CD8-positive T-cell clones genetically modified to express the IL-13 zetakine chimeric immunoreceptor and the Hy/TK selection/suicide fusion protein in patients with recurrent or refractory, high-grade malignant glioma. | |||||||
| T cells (HER2-CAR-CMV) | I | Completed | 16 | 3.5 | 24.5 | 2017 | NCT01109095 |
| Evaluation of the safety of escalating doses of autologous CMV-specific cytotoxic T-lymphocytes (CTL) genetically modified to express chimeric antigen receptors targeting the HER2 molecule in patients with HER2-positive glioblastoma multiforme, who have recurrent or progressive disease after front line therapy. | |||||||
| T cells (CMV specific) | I | Completed | 19 | 8.2 | 13.3 | 2014 | ACTRN12609000338268 |
| Assessment of the safety and tolerability of autologous CMV-specific T-cell therapy for recurrent GBM. | |||||||
| Immuncell-LC-T cells | III | Completed | 180 | 8.1 | 22.5 | 2017 | NCT00807027 |
| Assessment of the superiority of INNOCELL Corp. “Immuncell-LC” in aspects of therapeutic efficacy and safety when administered with temozolomide to glioblastoma patients when compared with the control group who did not receive administration of the drug. | |||||||
| Checkpoint inhibitors | |||||||
| Ipilimumab (BMS-734016) | II | Completed | 72 | NR | 7/4 | 2012 | NCT00623766 |
| Assessment of the response of melanoma with brain metastases to ipilimumab treatment while maintaining acceptable tolerability. | |||||||
| Nivolumab, anti-PD-1 antibody | III | Completed | 369 | 1.5 | 9.8 | 2020 | NCT02017717 |
| Comparison of the efficacy and safety of nivolumab administered alone versus bevacizumab in patients diagnosed with recurrent; evaluation of the safety and tolerability of nivolumab administered alone or in combination with ipilimumab in patients with different lines of GBM therapy (CheckMate143). | |||||||
| Nivolumab, anti-PD-1 antibody | III | Completed (last update posted: 3 March 2023) | NR | NR | NR | NR | NCT02617589 |
| Evaluation of patients with glioblastoma that is MGMT unmethylated (the MGMT gene is not altered by a chemical change). Comparison with patients receiving standard therapy with temozolomide in addition to radiation therapy (CheckMate498). | |||||||
| Nivolumab, anti-PD-1 antibody | III | Completed | 716 | 10.6 | 28.9 | 2022 | NCT02667587 |
| Evaluation of patients with glioblastoma that is MGMT methylated (the MGMT gene is altered by a chemical change). Patients will receive temozolomide plus radiation therapy. They will be compared to patients receiving nivolumab in addition to temozolomide plus radiation therapy (CheckMate548). | |||||||
| Vaccines | |||||||
| IMA950-vac | I | Completed | 45 | NR | 15.3 | 2016 | NCT01222221 |
| The aim of the study was to elucidate the side effects of vaccine therapy when administered together with temozolomide and radiation therapy in treating patients with newly diagnosed glioblastoma multiforme. | |||||||
| DCs vaccine | II | Completed | 26 | 12.7 | 23.4 | 2017 | NCT01006044 |
| Investigation of efficacy and safety of autologous dendritic cell vaccination in glioblastoma multiforme patients after complete surgical resection with a fluorescence microscope. | |||||||
| CDX-110 (rindopepimut) | III | Completed | 745 | 8 | 20.1 | 2017 | NCT01480479 |
| Investigation whether an adding of the experimental vaccine rindopepimut (also known as CDX-110) to the commonly used chemotherapy drug temozolomide can help improve the life expectancy of patients with newly diagnosed, resected EGFRvIII positive glioblastoma. CDX-110 was admixed with granulocyte macrophage-colony stimulating factor. | |||||||
| CDX-110 (rindopepimut) | II | Completed | 85 | 5.5 | 21.8 | 2015 | NCT00458601 |
| Evaluation of CDX-110 vaccination when provided with standard care treatment in glioblastoma (maintenance temozolomide therapy). Study treatment was given until disease progression. Follow-up for long-term survival information. Efficacy was measured by the progression-free survival status at 5.5 months from the date of the first dose. CDX-110 was admixed with Granulocyte macrophage-colony stimulating factor. | |||||||
| Dendritic cell (DC)-based vaccine (targeting cancer stem cells) | I | Completed | 20 | 23.1 | 25.5 | 2013 | NCT00846456 |
| Evaluation of immunological response, time to disease progression and survival time (time frame: five years) in patients with glioblastoma. | |||||||
| GP96 heat shock protein-peptide complex | I/II | Completed | 41 | 4.5 | 9.5 | 2014 | NCT00293423 |
| Investigation of the side effects and best dose of gp96 heat shock protein–peptide complex vaccine to see how well it worked in treating patients with recurrent or progressive high-grade glioma over time. | |||||||
| Survivin peptide mimic SurVaxM (SVN53-67/M57-KLH) | I | Completed | 9 | 17.6 | 86.6 | 2016 | NCT01250470 |
| Study of the side effects of vaccine therapy when given together with sargramostim in treating patients with malignant glioma. | |||||||
| Cytomegalovirus pp65-targeted vaccination | I/II | Completed | 11 | 25.3 | 41.1 | 2015; 2017 | NCT00639639 |
| Study of how well vaccine therapy worked in treating patients with newly diagnosed glioblastoma multiforme recovering from lymphopenia caused by temozolomide. | |||||||
| GVAX vaccine | I | Completed | 11 | NR | 8.8 | 2016 | NCT00694330 |
| The aim was to test the safety of vaccination of cells called GM-K562 cells mixed with the participant’s own irradiated tumor cells in glioblastoma. GM-K562 is a granulocyte-macrophage colony stimulating factor producing cell line. | |||||||
| DCVax®-L | III | Completed | 331 | NR | 34.7/19.8 | 2018; 2023 | NCT00045968; NCT02146066 |
| Investigation of the efficacy of an investigational therapy called DCVax(R)-L in patients with newly diagnosed GBM for whom surgery was indicated (NCT00045968). Open-label expanded access to study for patients for whom the vaccine was manufactured during the Northwest Biotherapeutics’ 020,221 DCVax-L for GBM screening process; however, they subsequently failed to meet specific enrollment criteria (NCT02146066). | |||||||
| NOA-16 | I | Completed | 39 | NR | NR | 2021 | NCT02454634 |
| Evaluation of safety and tolerability of and immune response to the IDH1 peptide vaccine in patients with IDH1R132H-mutated, WHO grade III–IV gliomas. | |||||||
| DNX-2401 (formerly known as delta-24-RGD-4C) | I/I | Completed | 37 | NR | 9.5 | 2018 | NCT00805376 |
| The aim was to find the highest tolerable dose of DNX-2401 that can be injected directly into brain tumors and into the surrounding brain tissue where tumor cells can multiply. A second goal was to study how the new drug DNX-2401 affects brain tumor cells and the body in general. | |||||||
| Personalized neoantigen cancer-vaccine-wRT | I/Ib | Recruiting (last update posted: 28 May 2024) | NR | NR | NR | NR | NCT02287428 |
| Study of a new type of vaccine as a possible treatment for patients with glioblastoma. It tested the safety of an investigational intervention and tried to define the appropriate dose of the intervention to use for further studies. | |||||||
| PVSRIPO | I/II | Completed | 61 | NR | 12.5 | 2018 | NCT01491893 |
| The aim iwas to determine the maximally tolerated dose (MTD) and the recommended phase 2 dose (RP2D) of PVSRIPO when delivered intracerebrally by convection-enhanced delivery (CED) | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demir, E.; Montgomery, D.; Saloum, A.; Yaghi, N.; Karsy, M. Current Understanding Regarding the Glioma Microenvironment and Impact of the Immune System. Neuroglia 2025, 6, 13. https://doi.org/10.3390/neuroglia6010013
Demir E, Montgomery D, Saloum A, Yaghi N, Karsy M. Current Understanding Regarding the Glioma Microenvironment and Impact of the Immune System. Neuroglia. 2025; 6(1):13. https://doi.org/10.3390/neuroglia6010013
Chicago/Turabian StyleDemir, Enes, Deondra Montgomery, Ammar Saloum, Nasser Yaghi, and Michael Karsy. 2025. "Current Understanding Regarding the Glioma Microenvironment and Impact of the Immune System" Neuroglia 6, no. 1: 13. https://doi.org/10.3390/neuroglia6010013
APA StyleDemir, E., Montgomery, D., Saloum, A., Yaghi, N., & Karsy, M. (2025). Current Understanding Regarding the Glioma Microenvironment and Impact of the Immune System. Neuroglia, 6(1), 13. https://doi.org/10.3390/neuroglia6010013






