A Circular Economy Perspective: Recycling Wastes through the CO2 Capture Process in Gypsum Products. Fire Resistance, Mechanical Properties, and Life Cycle Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Procedure for CO2 Capture
2.2. Physicochemical Characterization of FGD Gypsum before and after CO2 Capture
2.3. Fabrication Process of the Fire Resistance Materials
2.4. Methods for the Evaluation of Physical, Mechanical, and Fire Resistance Properties of the Gypsum-Based Products
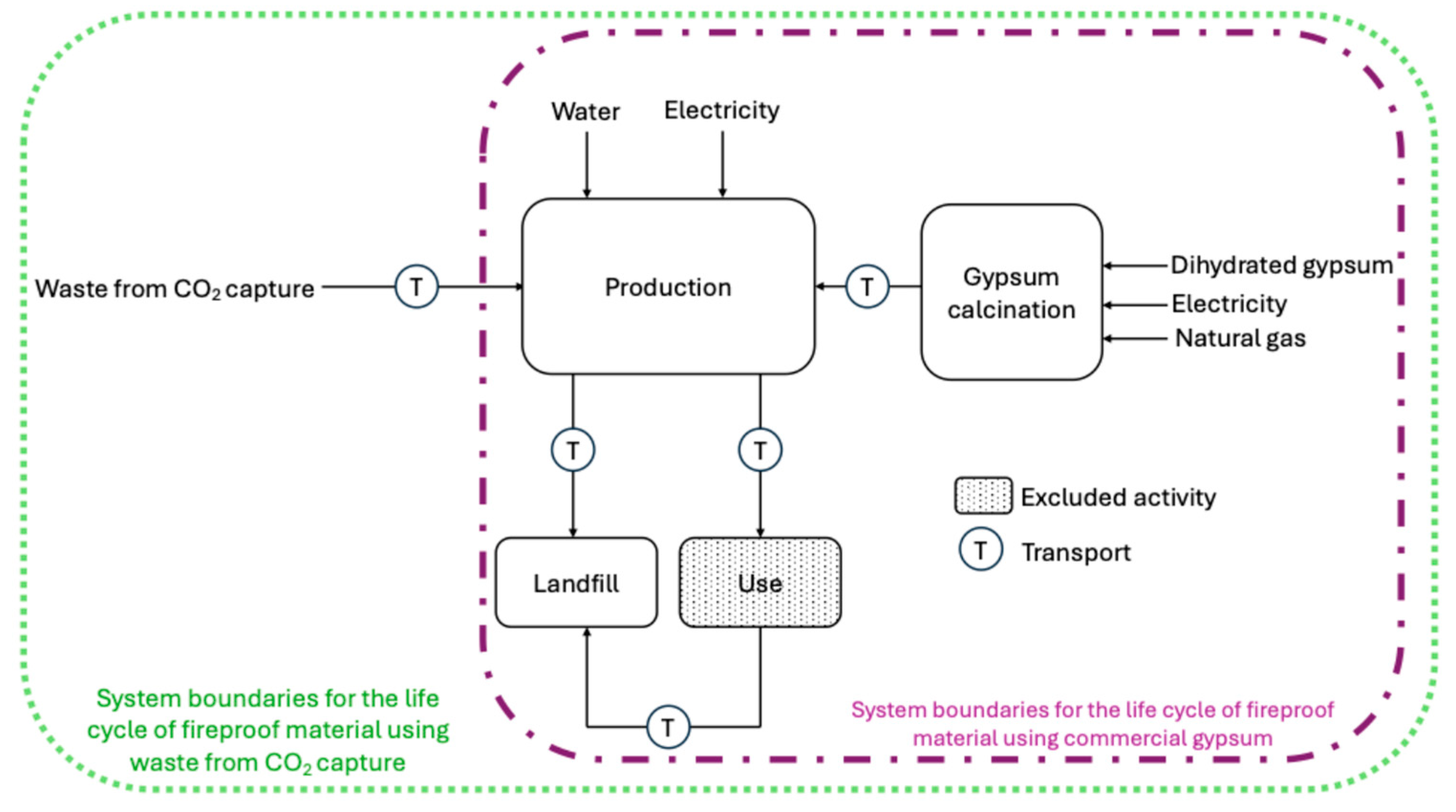
2.5. Life Cycle Assessment
2.5.1. Life Cycle Inventory
2.5.2. Environmental Impact Assessment
3. Results and Discussion
3.1. Characterization of the Waste after the CO2 Capture
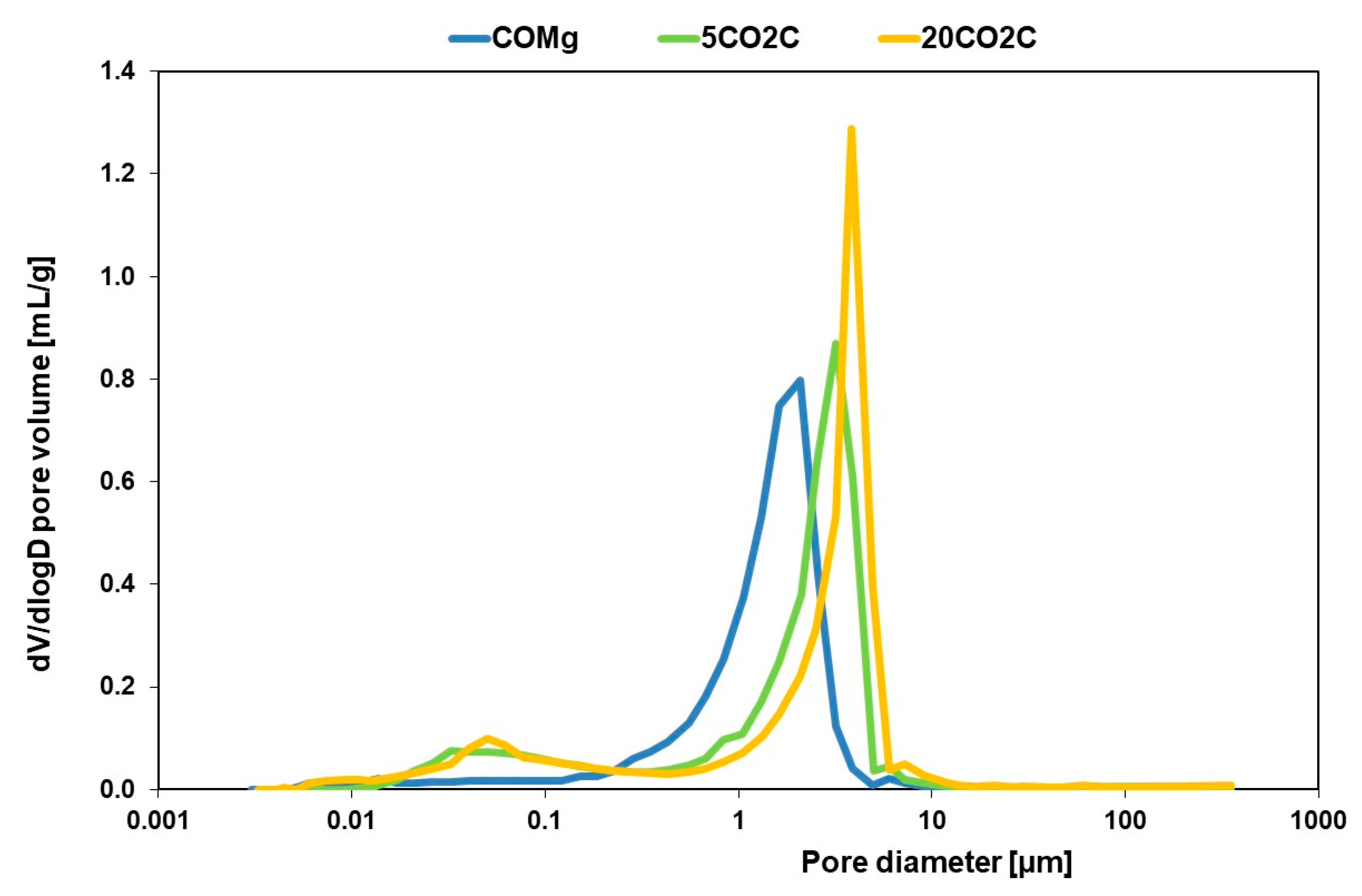
3.2. Particle Size Distribution
3.3. Physical and Mechanical Properties of Products
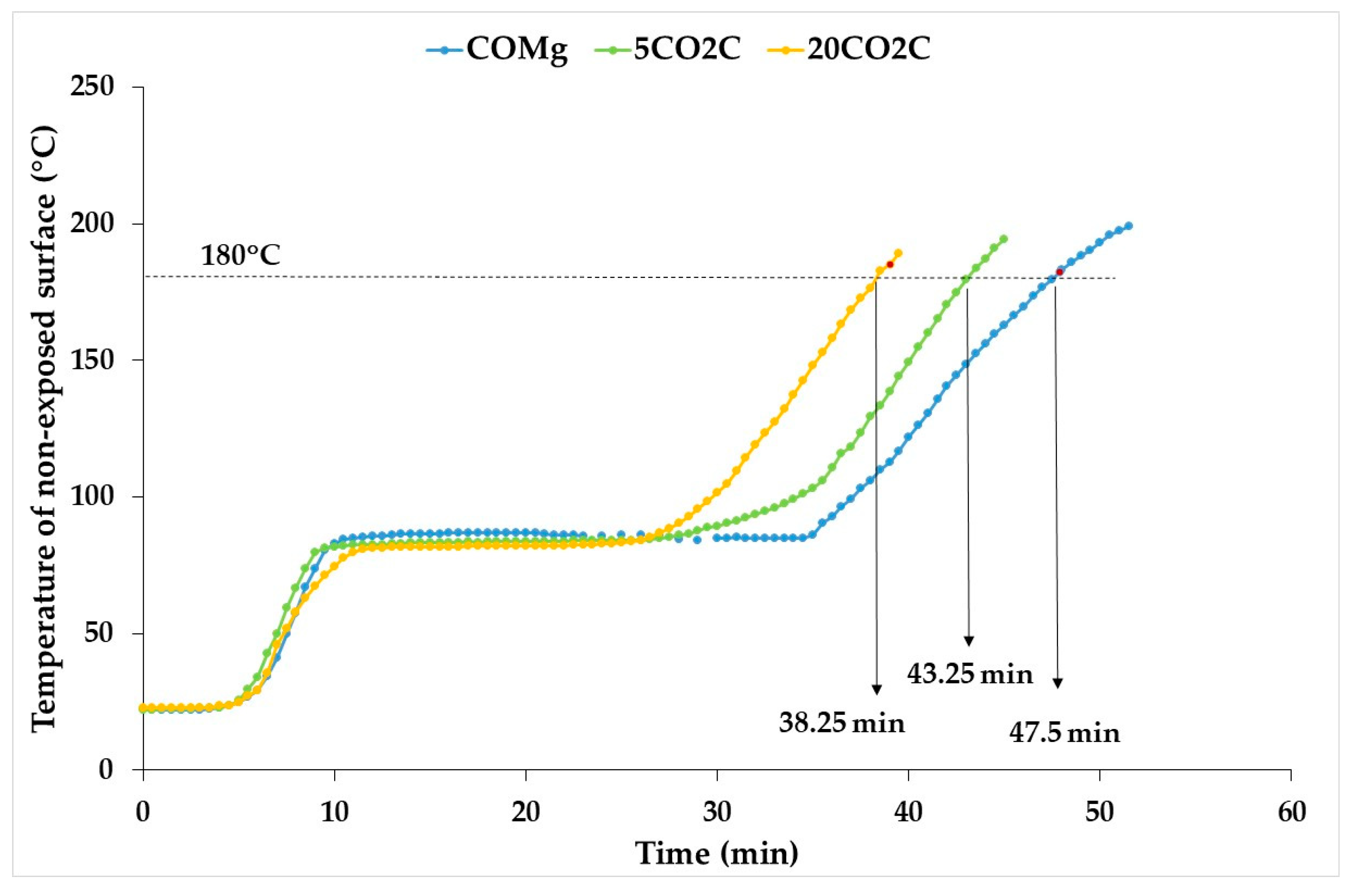
3.4. Thermal Conductivity and Fire Resistance
4. Life Cycle Assessment Analysis
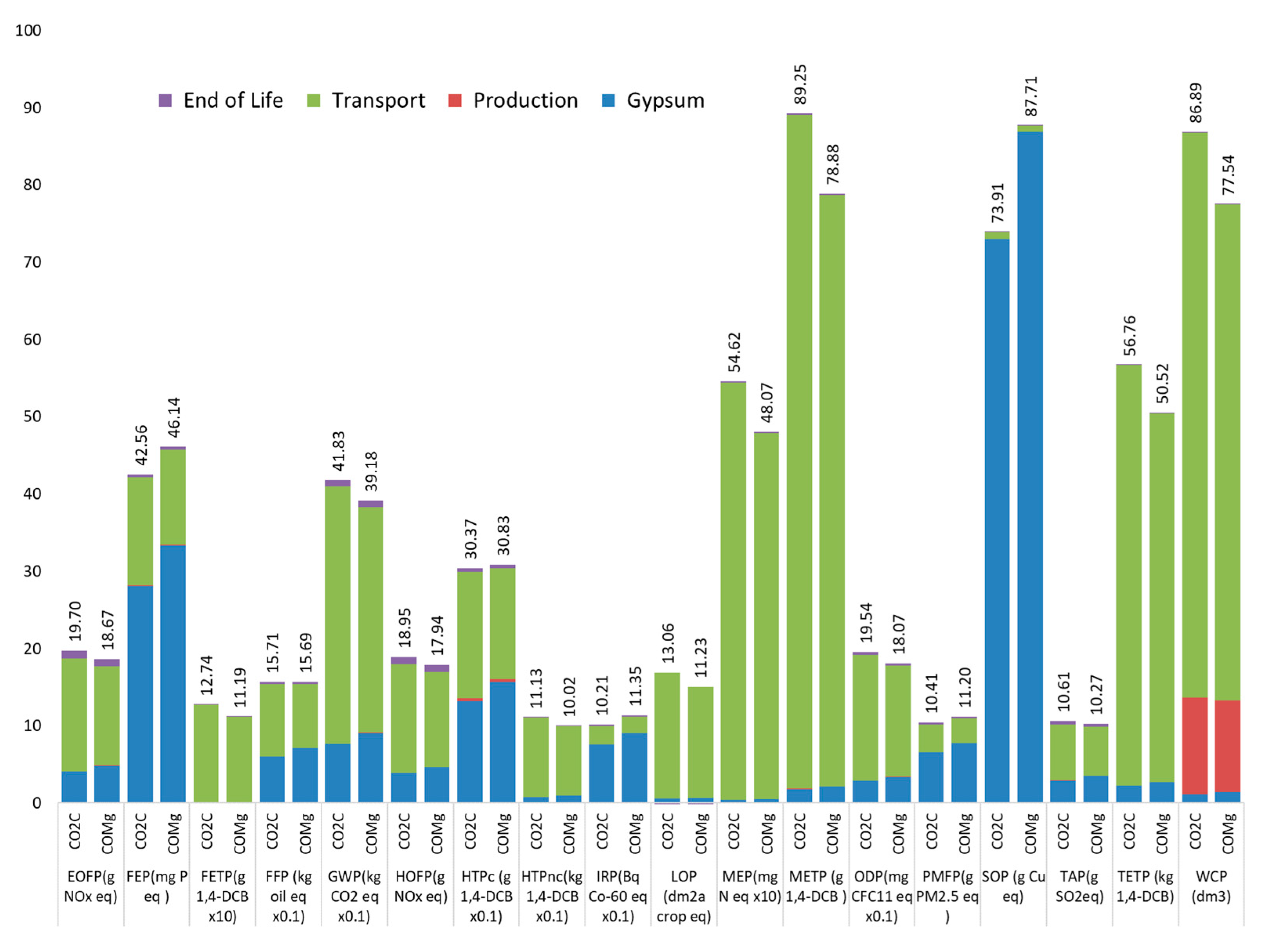
4.1. Impact Categories
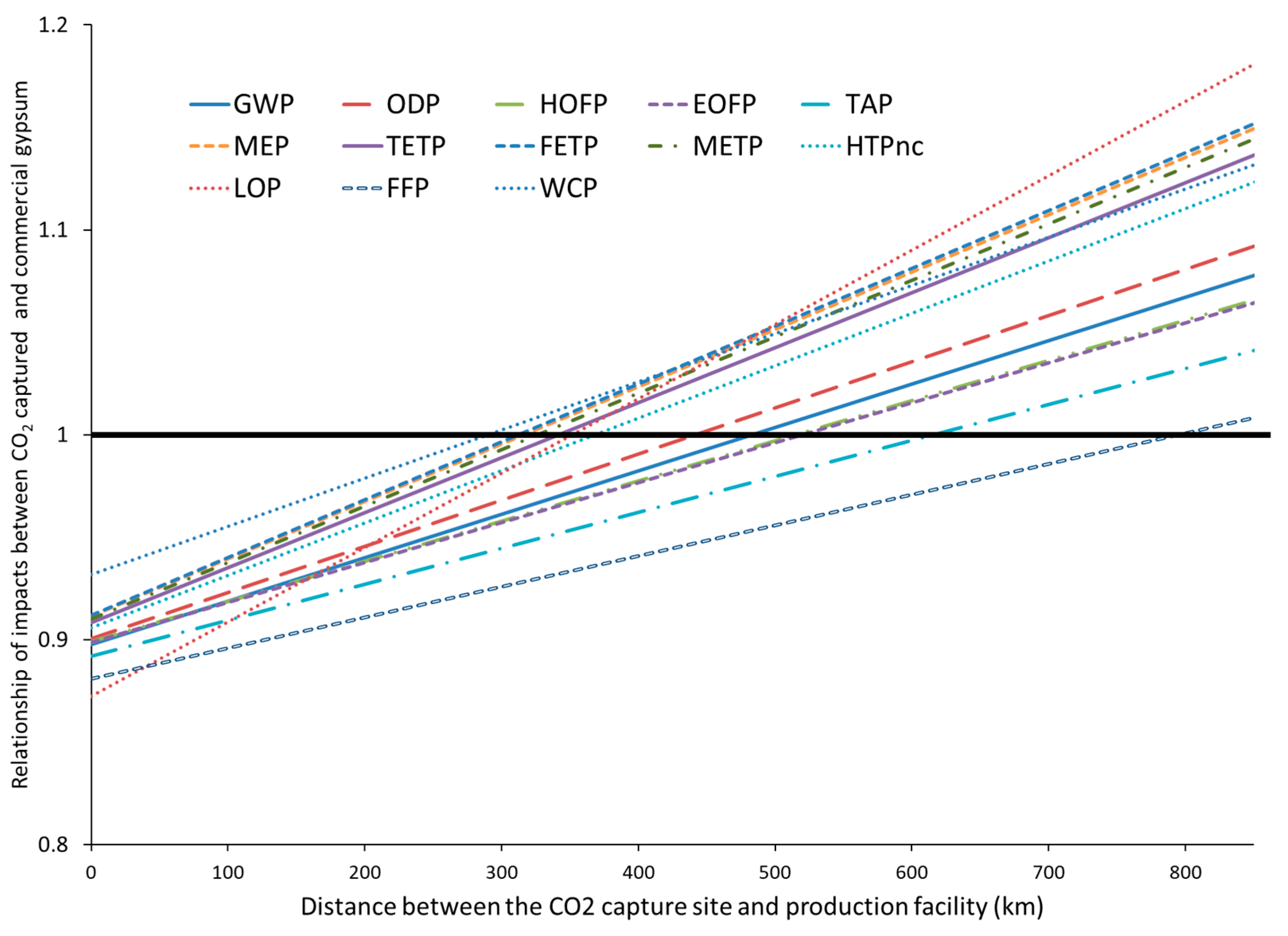
4.2. Sensitivity Analysis
5. Conclusions
- -
- The use of waste from CO2 capture produces a decrease in the density of the material, although in the ranges used, the material can be classified as high density.
- -
- The increase in the dosage of waste from CO2 capture produces a decrease in the flexural and compressive properties, although a dosage of up to 20%wt by weight satisfies all the mechanical requirements.
- -
- The replacement of commercial gypsum with waste from CO2 capture decreases the thermal conductivity and fire resistance, but this fire resistance decrease is less than that of the water chemically bound to the gypsum since the calcium carbonate present in the waste decomposes endothermically, absorbing part of the fire energy.
- -
- From the life cycle assessment, in the analyzed scenario, the material made with CO2 capture waste does not perform better in most environmental impacts compared to gypsum. Transportation becomes a critical factor for the sustainability of the recycling of this waste. The material made with CO2 capture waste presents environmental advantages over gypsum only if the distance between the gypsum production plant and the waste source is lower than 200 km compared with the distance between the gypsum extraction point and the gypsum production plant.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maiti, S.; Jain, N.; Malik, J. A Comprehensive Review of Flue Gas Desulphurized Gypsum: Production, Properties, and Applications. Constr. Build. Mater. 2023, 393, 131918. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, X.; Zhang, Z.; Wei, C.; Gu, J. Application of the Industrial Byproduct Gypsum in Building Materials: A Review. Materials 2024, 17, 1837. [Google Scholar] [CrossRef] [PubMed]
- Maichin, P.; Jitsangiam, P.; Nongnuang, T.; Boonserm, K.; Nusit, K.; Pra-ai, S.; Binaree, T.; Aryupong, C. Stabilized High Clay Content Lateritic Soil Using Cement-FGD Gypsum Mixtures for Road Subbase Applications. Materials 2021, 14, 1858. [Google Scholar] [CrossRef]
- Phutthimethakul, L.; Kumpueng, P.; Supakata, N. Use of Flue Gas Desulfurization Gypsum, Construction and Demolition Waste, and Oil Palm Waste Trunks to Produce Concrete Bricks. Crystals 2020, 10, 709. [Google Scholar] [CrossRef]
- Watts, D.B.; Dick, W.A. Sustainable Uses of FGD Gypsum in Agricultural Systems: Introduction. J. Environ. Qual. 2014, 43, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Abdolvand, Y.; Sadeghiamirshahidi, M. Soil Stabilization with Gypsum: A Review. J. Rock Mech. Geotech. 2024, in press. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Hekmatmehr, H.; Esmaeili, A.; Pourmahdi, M.; Atashrouz, S.; Abedi, A.; Abuswer, M.A.; Nedeljkovic, D.; Latifi, M.; Farag, S.; Mohaddespour, A. Carbon Capture Technologies: A Review on Technology Readiness Level. Fuel 2024, 363, 130898. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-Combustion Carbon Capture. Renew. Sust. Energy Rev. 2021, 138, 110490. [Google Scholar] [CrossRef]
- Luis, P. Use of Monoethanolamine (MEA) for CO2 Capture in a Global Scenario: Consequences and Alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Leventaki, E.; Riddell, A.; Wojtasz-Mucha, J.; Bernin, D. Effluents and Residues from Industrial Sites for Carbon Dioxide Capture: A Review. Environ. Chem. Lett. 2023, 21, 319–337. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of Fly Ash for CO2 Capture, Utilization, and Storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Leventaki, E.; Baena-Moreno, F.M.; Wojtasz-Mucha, J.; Sjöstedt, N.; Tajik, A.R.; Sardina, G.; Ström, H.; Bernin, D. Experimental Evaluation of Black Liquor Carbonation for Carbon Dioxide Capture. J. CO2 Util. 2023, 72, 102516. [Google Scholar] [CrossRef]
- Li, L.; Yu, H.; Ji, L.; Zhou, S.; Dao, V.; Feron, P.; Benhelal, E. Integrated CO2 Capture and Mineralization Approach Based on KOH and Cement-Based Wastes. J. Environ. Chem. Eng. 2024, 12, 113382. [Google Scholar] [CrossRef]
- Sun, X.; Xu, B.; Yi, Y. Effects of Accelerated Carbonation on Fine Incineration Bottom Ash: CO2 Uptake, Strength Improvement, Densification, and Heavy Metal Immobilization. J. Clean. Prod. 2024, 475, 143714. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, P.; Shen, X.; Lu, J.; Ye, S.; Wang, H.; Ling, T.; Ran, Q. Utilization of Solid Wastes to Sequestrate Carbon Dioxide in Cement-Based Materials and Methods to Improve Carbonation Degree: A Review. J. CO2 Util. 2023, 72, 102502. [Google Scholar] [CrossRef]
- Moreno, V.; González-Arias, J.; Ruiz-Martinez, J.D.; Balart-Gimeno, R.; Baena-Moreno, F.M.; Leiva, C. FGD-Gypsum Waste to Capture CO2 and to Recycle in Building Materials: Optimal Reaction Yield and Preliminary Mechanical Properties. Materials 2024, 17, 3774. [Google Scholar] [CrossRef]
- Yew, M.C.; Yew, M.K.; Yuen, R.K.K. Experimental Analysis of Lightweight Fire-Rated Board on Fire Resistance, Mechanical, and Acoustic Properties. Fire 2023, 6, 221. [Google Scholar] [CrossRef]
- Gravit, M.; Shabunina, D.; Nedryshkin, O. The Fire Resistance of Transformable Barriers: Influence of the Large-Scale Factor. Fire 2023, 6, 294. [Google Scholar] [CrossRef]
- Romero-Gómez, M.I.; Silva, R.V.; Flores-Colen, I.; de Brito, J. Influence of Polypropylene Residues on the Physico-Mechanical and Water-Resistance Properties of Gypsum Plasters. J. Clean. Prod. 2022, 371, 133674. [Google Scholar] [CrossRef]
- Leiva, C.; Vilches, L.F.; Vale, J.; Fernández-Pereira, C. Fire Resistance of Biomass Ash Panels Used for Internal Partitions in Buildings. Fire Saf. J. 2009, 44, 622–628. [Google Scholar] [CrossRef]
- Galiano, Y.L.; Leiva, C.; Arenas, C.; Arroyo, F.; Vilches, L.; Pereira, C.F.; Villegas, R. Behaviour of Fly Ash-Based Geopolymer Panels Under Fire. Waste Biomass Valorization 2017, 8, 2485–2494. [Google Scholar] [CrossRef]
- Jiang, Z.; Qin, B.; Shi, Q.; Ma, Z.; Shao, X.; Xu, Y.; Hao, M.; Yang, Y. Effect of Ball Milling Activation on CO2 Mineralization Performance in Fly Ash and Fire Resistance Capabilities of Mineralized Product. J. Environ. Chem. Eng. 2024, 12, 113954. [Google Scholar] [CrossRef]
- Mussa, M.H.; Radzi, N.A.M.; Hamid, R.; Mutalib, A.A. Fire Resistance of High-Volume Fly Ash RC Slab Inclusion with Nano-Silica. Materials 2021, 14, 3311. [Google Scholar] [CrossRef] [PubMed]
- Peceño, B.; Alonso-Fariñas, B.; Vilches, L.F.; Leiva, C. Study of Seashell Waste Recycling in Fireproofing Material: Technical, Environmental, and Economic Assessment. Sci. Total Environ. 2021, 790, 148102. [Google Scholar] [CrossRef]
- Batistella, M.; Roux, J.-C.; Masarra, N.-A.; le Saout, G.; Xenopoulos, C.; Lopez-Cuesta, J.-M. Incorporation of Fly Ash in Flame-Retardant Systems of Biopolyesters. Polymers 2023, 15, 2771. [Google Scholar] [CrossRef]
- Zanoletti, A.; Ciacci, L. The Reuse of Municipal Solid Waste Fly Ash as Flame Retardant Filler: A Preliminary Study. Sustainability 2022, 14, 2038. [Google Scholar] [CrossRef]
- Peceño, B.; Luna-Galiano, Y.; Varela, F.; Alonso-Fariñas, B.; Leiva, C. Study of a Fire-Resistant Plate Containing Fly Ashes Generated from Municipal Waste Incinerator: Fire and Mechanical Characteristics and Environmental Life Cycle Assessment. Materials 2024, 17, 1813. [Google Scholar] [CrossRef]
- Guedri, A.; Abdallah, F.; Mefteh, N.; Hamdi, N.; Baeza-Urrea, O.; Wagner, J.-F.; Zagrarni, M.F. Addition of Phosphogypsum to Fire-Resistant Plaster Panels: A Physic–Mechanical Investigation. Inorganics 2023, 11, 35. [Google Scholar] [CrossRef]
- Salazar, P.A.; Fernández, C.L.; Luna-Galiano, Y.; Sánchez, R.V.; Fernández-Pereira, C. Physical, Mechanical and Radiological Characteristics of a Fly Ash Geopolymer Incorporating Titanium Dioxide Waste as Passive Fire Insulating Material in Steel Structures. Materials 2022, 15, 8493. [Google Scholar] [CrossRef]
- Leiva, C.; Arenas, C.; Vilches, L.F.; Arroyo, F.; Luna-Galiano, Y.; Villegas, R.; Fernández-Pereira, C. Use of Zeolitized Coal Fly Ash as Main Component in Panels with High Fire Resistance. ACI Mater. J. 2018, 115, 393–399. [Google Scholar] [CrossRef]
- Kielė, A.; Vaičiukynienė, D.; Bertašius, Š.; Krivenko, P.; Bistrickaitė, R.; Jocius, V.; Ramukevičius, D. Alkali-Activated Slag Coatings for Fire Protection of OPC Concrete. Materials 2023, 16, 7477. [Google Scholar] [CrossRef]
- Sakkas, K.; Nomikos, P.; Sofianos, A.; Panias, D. Utilisation of FeNi-Slag for the Production of Inorganic Polymeric Materials for Construction or for Passive Fire Protection. Waste Biomass Valorization 2014, 5, 403–410. [Google Scholar] [CrossRef]
- Gutiérrez-González, S.; Gadea, J.; Rodríguez, A.; Junco, C.; Calderón, V. Lightweight Plaster Materials with Enhanced Thermal Properties Made with Polyurethane Foam Wastes. Constr. Build. Mater. 2012, 28, 653–658. [Google Scholar] [CrossRef]
- EN 13279-1:2008; Gypsum Binders and Gypsum Plasters—Part 1: Definitions and Requirements. European Committee for Standardization (CEN): Brussels, Belgium, 2008.
- EN 12859; Gypsum Blocks Definitions, Requirements and Test Methods. European Committee for Standardization (CEN): Brussels, Belgium, 2012.
- EN 1015-11; Methods of Test for Mortar for Masonary—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar. European Committee for Standardization (CEN): Brussels, Belgium, 2000.
- EN 1363-1; Fire Resistance Tests—Part 1: General Requirements. European Committee for Standardization (CEN): Brussels, Belgium, 2021.
- Vilches, L.F.; Leiva, C.; Vale, J.; Olivares, J.; Fernández-Pereira, C. Fire Resistance Characteristics of Plates Containing a High Biomass−Ash Proportion. Ind. Eng. Chem. Res. 2007, 46, 4824–4829. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 14044; Environmental Management—Life Cycle Assessment—Requirement and Guidelines—International. Organization for Standardization: Geneva, Switzerland, 2006.
- Fundación Labein. Guía Técnica Para La Medición, Estimación y Cálculo de Las Emisiones al Aire. Soc. Pública De Gestión Ambiental del Gobierno Vasco. 2005. Available online: https://www.euskadi.eus/contenidos/documentacion/eprtr/es_guia/adjuntos/residuos.pdf (accessed on 11 September 2024).
- Gálvez-Martos, J.L.; Styles, D.; Schoenberger, H.; Zeschmar-Lahl, B. Construction and Demolition Waste Best Management Practice in Europe. Resour. Conserv. Recycl. 2018, 136, 166–178. [Google Scholar] [CrossRef]
- Mercante, I.T.; Bovea, M.D.; Ibáñez-Forés, V.; Arena, A.P. Life Cycle Assessment of Construction and Demolition Waste Management Systems: A Spanish Case Study. Int. J. Life Cycle Assess. 2012, 17, 232–241. [Google Scholar] [CrossRef]
- Google Maps. Distances. 2024. Available online: https://www.google.com/maps/ (accessed on 10 September 2024).
- European Commission. Regulation (EC) No 715/2007—Of the European Parliament and of the Council of 20 June 2007 on Type Approval of Motor Vehicles with Respect to Emissions from Light Passenger and Commercial Vehicles (Euro 5 and Euro 6) and on Access to Vehicle Repair and Maintenance Information; European Commission: Strasbourg, France, 2007.
- PRé Consultants, B.V. PRé Consultants PRé Consultants SimaPro 9.6.0.1. LCA Software and Database Manual; PRé Consultants, B.V.: Amersfoort, The Netherlands, 2024. [Google Scholar]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A Harmonised Life Cycle Impact Assessment Method at Midpoint and Endpoint Level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Ríos, J.D.; Leiva, C.; de la Concha, A.; Ariza, M.P.; Cifuentes, H. Influence of Graphene Oxide Concentration and Ultrasonication Energy on Fracture Behavior of Nano-Reinforced Cement Pastes. Crystals 2024, 14, 707. [Google Scholar] [CrossRef]
- Chen, C.; Wang, H.; Wang, Y.; Chen, Y.; Jiu, S. Mechanical and Thermal Properties of Thermally Conductive Enhanced Paraffin/Gypsum Composites. Processes 2023, 11, 999. [Google Scholar] [CrossRef]
- Prałat, K.; Ciemnicka, J.; Koper, A.; Buczkowska, K.E.; Łoś, P. Comparison of the Thermal Properties of Geopolymer and Modified Gypsum. Polymers 2021, 13, 1220. [Google Scholar] [CrossRef]
- Leiva, C.; Vilches, L.F.; Vale, J.; Fernández-Pereira, C. Influence of the Type of Ash on the Fire Resistance Characteristics of Ash-Enriched Mortars. Fuel 2005, 84, 1433–1439. [Google Scholar] [CrossRef]



| Sample | Density (kg/m3) | Superficial Hardness (Shore C) | Compressive Strength (MPa) | Flexural Strength (MPa) |
|---|---|---|---|---|
| COMg | 1380 ± 15 | 92 ± 3 | 8.3 ± 1.2 | 1.2 ± 0.2 |
| 5CO2C | 1349 ± 12 | 89 ± 3 | 6.6 ± 0.9 | 0.8 ± 0.1 |
| 20CO2C | 1296 ± 10 | 82 ± 2 | 3.0 ±0.4 | 0.2 ± 0.1 |
| Sample (Ratio Gypsum/Waste) | Cp (J/g·K) | Thermal Diffusivity (α) (cm2/s) | Thermal Conductivity (W/m·K) |
|---|---|---|---|
| COMg | 0.32 ± 0.03 | 0.0035 ± 0.0002 | 0.155 ± 0.03 |
| 20CO2C | 0.21 ± 0.02 | 0.0025 ± 0.0001 | 0.068 ± 0.01 |
| Section | Input | Output | Unit | COMg | 20CO2C |
|---|---|---|---|---|---|
| Thickness | cm | 2.00 | 2.36 | ||
| Volume (dm3) | 20.00 | 23.60 | |||
| Gypsum calcination | Gypsum di-hydrate | kg | 30.67 | 28.07 | |
| Natural gas | MJ | 21.22 | 19.42 | ||
| Electricity | kWh | 0.72 | 0.66 | ||
| Gypsum hemi-hydrate2 | kg | 25.86 | 23.67 | ||
| Emissions to air | |||||
| Methane | mg | 0.03 | 0.03 | ||
| Carbon Dioxide | mg | 1.18 | 1.08 | ||
| Carbon Monoxide | mg | 0.21 | 0.19 | ||
| Volatile Organic Compounds | mg | 0.11 | 0.10 | ||
| Nitrogen Oxides | mg | 1.10 | 1.01 | ||
| Nitrogen Dioxide | mg | 0.02 | 0.02 | ||
| CO2 capture waste | kg | - | 5.92 | ||
| Production | Water | kg | 11.64 | 13.31 | |
| Electricity | Wh | 1.17 | 1.21 | ||
| Waste to Landfill | kg | 3.75 | 4.17 | ||
| End of life | Waste to Landfill | kg | 27.60 | 30.59 | |
| Transport | Transport | tkm | 19.48 | 24.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Martinez, J.D.; Moreno, V.; González-Arias, J.; Peceño Capilla, B.; Baena-Moreno, F.M.; Leiva, C. A Circular Economy Perspective: Recycling Wastes through the CO2 Capture Process in Gypsum Products. Fire Resistance, Mechanical Properties, and Life Cycle Analysis. Fire 2024, 7, 365. https://doi.org/10.3390/fire7100365
Ruiz-Martinez JD, Moreno V, González-Arias J, Peceño Capilla B, Baena-Moreno FM, Leiva C. A Circular Economy Perspective: Recycling Wastes through the CO2 Capture Process in Gypsum Products. Fire Resistance, Mechanical Properties, and Life Cycle Analysis. Fire. 2024; 7(10):365. https://doi.org/10.3390/fire7100365
Chicago/Turabian StyleRuiz-Martinez, Jaime D., Virginia Moreno, Judith González-Arias, Begoña Peceño Capilla, Francisco M. Baena-Moreno, and Carlos Leiva. 2024. "A Circular Economy Perspective: Recycling Wastes through the CO2 Capture Process in Gypsum Products. Fire Resistance, Mechanical Properties, and Life Cycle Analysis" Fire 7, no. 10: 365. https://doi.org/10.3390/fire7100365
APA StyleRuiz-Martinez, J. D., Moreno, V., González-Arias, J., Peceño Capilla, B., Baena-Moreno, F. M., & Leiva, C. (2024). A Circular Economy Perspective: Recycling Wastes through the CO2 Capture Process in Gypsum Products. Fire Resistance, Mechanical Properties, and Life Cycle Analysis. Fire, 7(10), 365. https://doi.org/10.3390/fire7100365







