Abstract
Environmental and energy sustainability concerns have catalyzed a global transition toward renewable biofuel alternatives. Among these, biodiesel stands out as a promising substitute for conventional diesel in compression-ignition engines, providing compatibility without requiring modifications to engine design. A comprehensive understanding of biodiesel’s physical properties is crucial for accurately modeling fuel spray, atomization, combustion, and emissions in diesel engines. This study focuses on predicting the physical properties of PODL20 and EB100, including liquid viscosity, density, vapor pressure, latent heat of vaporization, thermal conductivity, gas diffusion coefficients, and surface tension, all integrated into the CONVERGE CFD fuel library for improved combustion simulations. Subsequently, numerical simulations were conducted using the predicted properties of the biodiesels, validated by experimental in-cylinder pressure data. The prediction models demonstrated excellent alignment with the experimental results, confirming their accuracy in simulating spray dynamics, combustion processes, turbulence, ignition, and emissions. Notably, significant improvements in key combustion parameters, such as cylinder pressure and heat release rate, were recorded with the use of biodiesels. Specifically, the heat release rates for PODL20 and EB100 reached 165.74 J/CA and 140.08 J/CA, respectively, compared to 60.2 J/CA for conventional diesel fuel. Furthermore, when evaluating both soot and NOx emissions, EB100 displayed a more balanced performance, achieving a significant reduction in soot emissions of 34.21% alongside a moderate increase in NOx emissions of 45.5% compared to diesel fuel. In comparison to PODL20, reductions of 20.4% in soot emissions and 3% in NOx emissions were also noted.
1. Introduction
The widespread use of diesel engines is primarily due to their exceptional efficiency and durability, making them indispensable in the industrial, transportation, and agricultural sectors. However, diesel engines are also significant contributors to smoke and nitrogen oxide (NOx) emissions, which present substantial environmental and health challenges [1,2]. In recent years, rising diesel fuel prices, decreasing supply, stringent pollution regulations, and the rapid depletion of petroleum reserves have intensified the search for alternative fuels. It is widely recognized that enhancing engine design, reformulating fuels, and adopting alternative fuels are essential strategies for achieving clean combustion in diesel engines [3].
Alternative fuels for internal combustion engines include hydrogen, vegetable oils, alcohol, and natural gas. Modified vegetable oils exhibit diesel-like characteristics and can be used to power compression-ignition engines with minimal modifications [4,5]. Over the past 35 years, research has focused on biodiesel formulation, leading to the utilization of refined vegetable oils, animal fats, and microalgae as viable fuel sources [6].
The esterification of fatty acids significantly influences several key characteristics of biodiesel fuels [7]. Indicators of biodiesel quality, such as specific heat capacity, density, viscosity, vapor pressure, thermal conductivity, surface tension, latent heat of vaporization, and latent heat of combustion, play crucial roles in combustion and emission performance. For accurate combustion simulations, software programs like KIVA and CONVERGE require comprehensive data on various grades of biodiesel, including both liquid and vapor phases. However, many of these fuel properties are either unavailable or insufficiently documented in the literature.
In response to this gap, several researchers have developed models to predict the physical parameters of diesel fuel and biodiesel using various mathematical approaches. For instance, An et al. [8] conducted a study that focused on predicting biodiesel’s physical properties, including boiling point, critical attributes, density, latent heat of vaporization, viscosity, thermal conductivity, temperature, and gas diffusion coefficients, to model their combustion process, establishing optimal prediction models for each parameter and, thereby, providing valuable standards for predicting biodiesel combustion behavior.
Similarly, Ruan et al. [9] investigated methods for evaluating the physical parameters of used cooking oil, focusing on viscosity, density, and surface tension under varying temperatures, by developing a predictive program for biodiesel physical properties, which was validated against empirical measurements. On the other hand, Cheng et al. [10] used computational fluid dynamics (CFD) modeling to study the effects of liquid density, vapor pressure, surface tension, viscosity, and vapor diffusivity on fuel pulverization and axial vapor penetration for the following three biofuels: palm oil methyl ester (PME), soybean methyl ester (SME), and coconut methyl ester (CME). Their results indicate that liquid surface tension and vapor pressure are the most sensitive parameters for fuel pulverization.
Recent studies have extensively investigated diesel engines using biodiesel blends and additives, focusing on performance, combustion, and emissions through both experimental and numerical methods. Padmanabha and Mohanty [11] enhanced Jatropha–Karanja biodiesel with ethylene glycol monoacetate (EGM) and tri-ethylene glycol mono-methyl ether (TGME) additives, resulting in a 4.1–4.7% increase in the brake thermal efficiency (BTE), a 0.18–0.2 kg/kWh reduction in brake-specific fuel consumption (BSFC), a 29.8–40% decrease in hydrocarbon (HC) and carbon monoxide (CO) emissions, and a 12.6% rise in the heat release rate (HRR), with only a 5.2% increase in NOx emissions. Similarly, Fareed et al. [12] studied the effects of waste-cooking-oil biodiesel blends (WB10 and WB20) and castor biodiesel blends (CB10 and CB20) on diesel engine emissions and performance at 3000 rpm. Compared to diesel fuel, the specific fuel consumption increased by 2–8.5% and the thermal efficiency decreased by 2.5–9.5% for the blends; in addition, CO emissions decreased by 0.5–3.5%, HC by 6–14%, and smoke by 6–11%, while NOx emissions increased by 1.5–6.5%. The peak heat release rate dropped by 1.5–5%. WB10 + CB10 was identified as the optimal blend for improving diesel engine performance and emissions.
Furthermore, El-Adawy [13] found that adding 50 ppm zinc oxide nanoparticles to waste cooking vegetable oil improved engine performance. Engine torque increased by 6.74%, 4.9%, and 3.69%, while BSFC decreased by 5.6%, 6.44%, and 2.5% for the blends B0ZnO, B20ZnO, and B40ZnO, respectively, compared to their non-nanoparticle counterparts. In addition, Winangun et al. [14] and Sarıdemir et al. [15] investigated the combustion behavior of palm oil biodiesel and waste-cooking-oil biodiesel mixed with hydrogen at flows of 2.5, 5, 7.5, and 10 lpm. Hydrogen enrichment was shown to optimize biodiesel use in diesel engines. Winangun’s study found that adding hydrogen at 2.5 lpm resulted in a 27.38% increase in BTE and a 47.61% decrease in BSFC compared to biodiesel alone. Sarıdemir’s study showed that adding 15 and 30 lpm of hydrogen to B25 fuel decreased BSFC by 17.58% and 30.75% and improved BTE by 17% and 10.19%, respectively.
Moreover, Simhadri et al. [16] studied the effects of titanium dioxide (TiO2) nanoparticle additives on Mahua Biodiesel B20 blends at different injection pressures. They found a 5.3% higher HRR with B20T50 at 180 bars and improved BTE and fuel consumption at higher pressures. TiO2 also reduced emissions, with notable decreases in smoke opacity and NOx at 240 bars. In a related context, Kunchi et al. [17] investigated the impact of adding zinc and manganese nanoparticles (50 ppm and 75 ppm) to Terminalia bellirica biodiesel B20 on diesel engine performance, combustion, and emissions at injection timings of 21° BTDC, 23° BTDC, and 25° BTDC, finding that the nanoparticle dispersion and advanced injection timings significantly enhanced performance and combustion characteristics while reducing emissions.
Meng et al. [18] conducted an experimental study on the effects of waste cooking oil biodiesel blends at 0%, 10%, 20%, and 30% on combustion and emissions in a common-rail diesel engine. Their findings showed that adding biodiesel improved engine efficiency and emission profiles, especially at 50% and 70% loads. Moreover, Ergen [19] analyzed the impacts of adding 2.5%, 5%, and 7.5% diethyl ether to corn-oil biodiesel with exhaust gas recirculation (EGR) on diesel engine performance, finding that a 10% EGR and 5% diethyl ether blend reduced engine torque by 3% and NO emissions by up to 70% compared to diesel fuel. Additionally, Azad et al. [20] investigated the impact of two new ternary biodiesel blends, ManCr_Pa (Mandarin, Crambe biodiesel, and paraffin) and AvBn_Pa (Avocado, Bush nut biodiesel, and paraffin), finding that these blends significantly reduced CO by 33.3%, HC by 33.3–73.3%, and PM by 17.8–28.8%, with a slight increase in BSFC by 0.52% and a minor decrease in BTE by 0.25–0.42%, showing a nearly similar engine performance to that of diesel fuel at high engine RPMs. In contrast, Krishnan et al. [21] conducted a comprehensive review of the synergistic potential of alcohols and biodiesel in dual-fuel diesel engines, indicating that fuel performance and emissions depend on the blend ratios, with BTE ranging from 1.5% to 15% and NOx and PM emissions decreasing by 6% to 50% and 7% to 90%, respectively.
Similarly, Ooi et al. [22] examined the impact of adding multiwalled carbon nanotubes (MWCNTs) to a palm-oil biodiesel–diesel blend B20, finding that MWCNTs reduced ignition delay, shortened combustion length, and accelerated combustion phasing by up to 17.6%, 12.9%, and 18.5%, respectively, while enhancing BSFC and BTE by up to 15.7% and 16.3% and reducing CO and HC emissions by up to 34.7% and 16.0%, respectively, but increasing NOx emissions by up to 43.5%, suggesting overall improvements in performance, combustion, and emission characteristics. Li et al. [23] conducted a modeling study on blending n-octanol with biodiesel, using a reaction mechanism with 115 species and 489 reactions to explore its impact on combustion quality and emissions in diesel engines. By progressively increasing n-octanol from 0% to 100% in 10% increments, their simulations demonstrated that blending n-octanol with B20 can enhance power output and reduce soot emissions.
Abramek et al. [24] analyzed corrosion causes and impacts of common rail fuel injectors, identifying vulnerable components, evaluating corrosive wear with a unique classification and detailing the repair efficiency and typical issues of leading manufacturers. Lastly, Ramachandran et al. [25] investigated the performance and emissions of a diesel engine using a reactivity controlled compression ignition (RCCI) with blends of microalgae biodiesel and compressed natural gas (CNG) at ratios of 10%, 20%, 30%, and 40%. Their findings revealed that a 30% CNG blend significantly improved thermal efficiency by 4.35% and reduced NOx and smoke emissions by 25% and 31%, respectively, compared to standard biodiesel combustion.
Accurately predicting the physical properties of biodiesel is paramount for simulating key processes, such as pulverization, atomization, and combustion, within diesel engine cylinders. This foundational step is essential for the comprehensive modeling of biodiesel behavior. The current research focuses on the theoretical prediction of the physical properties of the following two specific biodiesels: neat Eucalyptus biodiesel (EB100) and a blend of palm oil and 20% D-limonene (PODL20). Using advanced mathematical methodologies, this study aims to establish a reliable predictive framework for these biodiesels. To validate these predictions, experimental tests were conducted on a diesel engine powered by the selected biodiesels. The commercial CFD software CONVERGE 3.2 was utilized to model intricate in-cylinder phenomena, including fuel atomization, auto-ignition, combustion, and pollutant formation.
2. Physical Properties Prediction Models of Bio-Diesel Fuel
The fuel spray exerts a considerable influence on ignition, combustion, and pollutant production in diesel engines. Moreover, it significantly impacts vapor pressure, surface tension, heat of vaporization, and viscosity. Therefore, accurately predicting these characteristics is essential before modeling combustion processes. Notably, some of these characteristics vary with temperature, necessitating relevance from 0 K to the fuel’s critical temperature. The physical and chemical properties of biodiesel, as detailed in Table 1, will be employed to forecast these physical attributes. This section introduces several predictive algorithms tailored to each specific characteristic, ensuring a comprehensive and precise modeling approach.

Table 1.
Physical and chemical characteristics of neat biodiesel (EB100-PODL20) and neat diesel fuel.
2.1. Typical Boiling Point
Typically, the boiling point is defined as the temperature at which a compound’s vapor pressure equals one atmosphere of pressure, expressed in Kelvins. By applying specific correlations, such as those reported by Yuan et al. [26], it is possible to predict the boiling point for various compounds. For methyl esters, the boiling point (Tb) can be estimated using the following equation:
where, for methyl esters, CN indicates how many carbon atoms are present, and Tb represents the average boiling temperature.
Additionally, Reid et al. [27] proposed a model based on the group contributions method, which predicts the boiling point as follows:
There is evidence, provided in [27], that this quantity can be obtained by adding the individual contributions of atoms to the total quantity, m.
2.2. Important Characteristics
The three most frequently cited pure component constants that are challenging to determine experimentally are critical temperature, critical volume, and critical pressure. These constants are essential inputs for accurately predicting various physical properties.
2.2.1. Method of Ambrose
The method of Ambrose [28] provides formulas to estimate critical properties based on empirical correlations, offering a robust approach to determine these essential constants.
where M stands for the molar mass, in g/mol; Pc, Tc, and Vc represent the critical pressure, temperature, and volume, measured in [Pa], [K], and [cm3/mol], respectively. According to [28], the values for ∆T, ∆P, and ∆V can be calculated by summing the contributions of the individual atoms or groups of atoms.
2.2.2. Joback Technique
The Joback technique [29] employs the same units as the Ambrose method [28] for estimating the following critical properties:
In this case, n denotes the number of carbon atoms within the molecule.
2.3. Vapor Pressure Behavior
2.3.1. Lee–Kesler Method
This approach [30] is one of the best techniques for predicting vapor pressure, necessitating the acentric factor, as well as the fluid’s critical pressure and temperature.
The vapor pressure equation is expressed as follows:
where
The reduced temperature (Tr) and reduced vapor pressure (Pvpr) are calculated as and , respectively. Additionally, ω is the acentric factor, determined by the following equation:
where θ is the ratio of Tb to Tc, as follows: .
2.3.2. Gamez–Thodos Method
The Gamez–Thodos method [26] for estimating vapor pressure is expressed by the following equation:
where
with
2.4. Liquid Density
2.4.1. Rackett Equation
The Rackett equation for predicting the specific volume (Vs) of a substance is given by [31] the following:
where
where VS–R represents the investigational value of VS at the temperature of reference (TR); and Tr–R represents the lowered temperature at the specified temperature (TR); and VS–R is a compound-specific constant. The overhead formula may be rewritten in the following scenario:
At reference temperature (TR), ρR represents the experimental density [g/cm3]. Additionally, a second experimental density value is needed at a different temperature to determine ZRA other than what was observed at the reference temperature (TR).
2.4.2. Handbook Data
The following formula can be used to estimate the density of a liquid (ρ) [32]:
where A, B, C, and D are constants provided in the Handbook Data.
2.5. Liquid Viscosity
2.5.1. Method of Orrick and Erbar (Low Temperature) [8]
This method uses a group contribution approach to analyze fluid viscosities at low temperatures (Tr < 0.75). It is assumed that there exists a direct connection between the reciprocal of temperature and the logarithm of viscosity. The relationship is expressed as:
The liquid viscosity (ηL) is expressed in centipoise [cP] and the liquid density (ρL) at 20 °C is expressed in [g/cm3]. M is the molar mass. The unified participation technique can be used to determine the model parameters A and B, as outlined in Table 2.

Table 2.
Group contribution parameters using the Joback method.
2.5.2. Letsou and Stiel Method (High Temperature) [33]
At temperatures beyond the limited range of 0.7 to 1.0 Tr, the assumption that the logarithm of liquid viscosity (lnηL) has a linear relationship with the reciprocal of absolute temperature becoming inaccurate. Consequently, an alternative approach is required. Letsou and Stiel [33] proposed the following expression to estimate viscosity at high temperatures:
where
Here, ηSL represents the liquid viscosity in centipoise (cP). Another viscosity prediction correlation for this high-temperature range (in mPa·s) is proposed by Sastri and Rao [34]:
where
- ✓
- α is a constant (0.1175 for alcohols and 0.248 for other substances),
- ✓
- B is the boiling-point viscosity (ηB),
- ✓
- ,
- ✓
- .
2.5.3. Handbook Data [32]
Liquid viscosity can also be determined using values provided in handbook data, where the viscosity (ηL) is expressed in pascal-seconds (Pa·s). The equation used for this purpose is as follows:
2.6. Thermal Conductivity of Liquids
2.6.1. Latini et al.’s Method [35]
The thermal conductivity, denoted as λL, can be expressed using the following equation:
where the coefficient A is defined as follows:
where A*, α, β, and γ are determined as specified in Reference [35]; λL represents the thermal conductivity. The constants for the various organic compounds, including their corresponding parameters, are detailed in Table 3.

Table 3.
Constants for various organic compounds.
2.6.2. Sastri’s Method [36]
The following equation [36] is used to calculate the thermal conductivity of a fluid:
where the exponent m is given as follows:
(Tb) represents the ability of a liquid to conduct heat at its normal boiling temperature, expressed in [W/m K]. The constants a = 0.16 and n = 0.2 are empirical parameters.
2.6.3. Handbook Data [32]
The λL conductivity can be represented as follows:
The constant values A, B, and C can be obtained from the Handbook Data [32].
2.7. Latent Heat of Vaporization
2.7.1. Fish and Lielmez’s Method [37]
Fish and Lielmez [37] proposed an alternative formulation for predicting the latent heat of vaporization at low temperatures, presented as follows:
with
The vaporization latent heat (∆Hvb) at the boiling point for organic and inorganic liquids is determined using the formulae below, where p and q are set to 0.13856 and 0.35298 for inorganic and organic liquids, respectively.
2.7.2. Correlation of the Pitzer A Centric Factor [27]
The equation below can be used to correlate ΔHv with Tc, Tr, and x:
where R is the gas constant. The formula is applicable for elevated-temperature conditions, where 0.6 < Tr ≪ 1.0, as noted in Reference [27].
2.7.3. Riedel Method
According to Riedel’s equation [38], ΔHv is correlated with the Tc, Tbr, and Pc, as follows:
2.7.4. Handbook Data [27]
The ΔHv can be estimated using the following formula:
It is noted that for accurate results, only high-temperature forecasts should be made using the formula, when 0.6 < Tr << 1.0, as stated in [27].
2.8. Surface Tension
2.8.1. MacLeod–Sugden Correlation [39]
The MacLeod–Sugden equation can be used to relate the surface tension (σ):
In this context, ρLb represents the molar density of the liquid at its normal boiling point. The parameter [P] can be obtained in [39], while σ denotes the surface tension. Additionally, the value 4n = 1.24 is applied to other organic compounds.
2.8.2. Correlational States
The surface tension (σ) can be correlated using the following equation [27]:
where αc is a parameter defined by Riedel and given by the following:
2.8.3. Handbook Data [27]
Surface tension (σ) can also be estimated using data from handbooks, with the following equation:
where A, B, C, D, and E are constants obtained from the Handbook Data [27].
2.9. Specific Heat Capacity
To calculate the heat capacity of liquids at 293.15 K, the collective effort approach developed in [40,41] is used. To predict the specific heat capacity at 293.15 K, Chueh and Swanson suggested the following formulation [40,41]:
where ΔCp is the contribution of element i (see Table 4). In the case of methyl esters containing only fatty acids, the factor m corresponds to the following: tom = 1 for oleic acid, m = 2 for linoleic and erucic acids, and m = 3 for linolenic acid.

Table 4.
Group contribution values for Chueh and Swanson’s method.
3. Analysis and Interpretation of Results
3.1. Prediction Methods for Physical Properties of Biodiesels
In this part, a comprehensive prediction of the physical properties of neat eucalyptus (EB100) and PODL20 biodiesels was conducted. The most accurate prediction model was determined for each property, providing results that can assist in biodiesel combustion modeling.
Table 5 presents the predicted normal boiling temperatures of EB100 (C9H16O) and PODL20 (C8.44H16O) biodiesels compared to the results of Yuan et al. [26], Reid et al. [27], and the Handbook Data [32]. The calculations revealed that Yuan et al.’s method closely aligned with the Handbook Data, exhibiting only minor deviations.

Table 5.
Normal boiling points of neat eucalyptus biodiesel.
Table 6 compares the results of using the Joback method and Ambrose’s method for estimating the critical properties of EB100 and PODL20 biodiesels, as reported in the Handbook Data. The Ambrose method showed slight differences in the predictions of critical volume and critical temperature, while the Joback method exhibited minor discrepancies in the estimation of critical pressure.

Table 6.
Estimated critical properties using Ambrose’s method and Joback’s method.
To predict the physical properties of neat EB100 and PODL20 biodiesels, the best prediction model was selected for each property. The normal boiling point was estimated using Yuan et al.’s method, which demonstrated slight deviations from the Handbook Data. This method proved to be suitable for accurately predicting the boiling points of the biodiesels studied, as corroborated by other references [42].
For critical properties, both the Joback and Ambrose methods were employed and compared with the Handbook Data. Ambrose’s method reported small variations in critical temperature and volume, while the Joback method exhibited minor variations in critical pressure. These differences were relatively minor, indicating both methods are reliable for predicting critical properties, as supported by Refs. [8,42].
Liquid viscosity was predicted using the correlation of Orrick and Erbar, which closely matched the Handbook Data, confirming its accuracy for viscosity predictions. The Rackett method was validated as precise for estimating liquid density, showing strong agreements with both the Handbook Data and experimental data obtained at 288 K. This finding is corroborated by other researchers [9,43].
The vapor pressure characteristics were estimated using the Lee–Kesler and Gamez–Thodos methods, both of which produced satisfactory results. These methods effectively captured the vapor pressure characteristics of the biodiesels, as supported by Refs. [8,42].
The liquid thermal conductivity was predicted using the methods of Latini and Sastri, with the results aligning closely with the Handbook Data, thus validating the accuracy of these methods for this property. This finding is consistent with other research [8].
The latent heat of vaporization was compared using the following three methods: Fish and Lielmezs, Riedel, and Pitzer. Of these, Riedel’s method demonstrated the closest alignment with the Handbook Data, making it the preferred model for this property, as noted in Ref. [42].
Surface tension was evaluated using the Macleod–Sugden correlation and the corresponding states model, with the latter showing better agreement and, thus, being the more accurate model for surface tension predictions. This outcome is consistent with findings from other studies [8].
Finally, the specific heat capacity was assessed with respect to temperature. This approach effectively captured the temperature dependence of specific heat capacity, aligning closely with the established data. Figure 1 compares the physical characteristics of hexadecane (C16H34), a primary component of diesel, with neat eucalyptus and PODL20 biodiesels. The comparison highlights the necessity of carefully selecting methods for determining fuel properties. All data are organized by temperature, and the minimal variations observed among the properties suggest that using EB100 and PODL20 biodiesels in diesel engines does not necessitate hardware modifications.
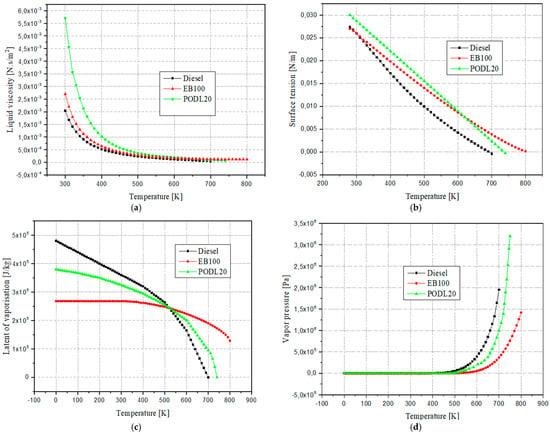
Figure 1.
Comparative analysis of physical properties between diesel fuel, EB100, and PODL20 biodiesels: (a) Liquid viscosity, (b) Surface tension, (c) Latent of vaporization, (d) Vapor pressure, (e) Thermal conductivity, (f) Specific heat capacity, and (g) Density.
3.2. Engine Specifications and Mesh
This study comprehensively analyzed the engine performances and exhaust emissions of EB100 and PODL20 biodiesels compared to traditional diesel fuel.
The evaluations were conducted using a single-cylinder, air-cooled Lister Petter ST1 diesel engine, as shown in Figure 2, with the physical characteristics of eucalyptus-derived biodiesel measured and compared to those of conventional diesel.
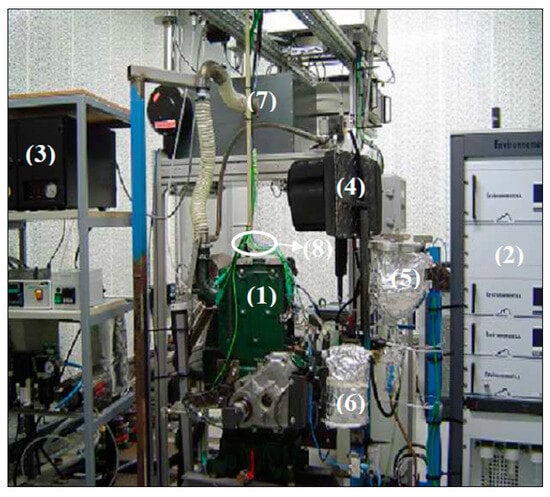
Figure 2.
Engine test bench setup: (1) diesel engine; (2) gas analyzer; (3) particle analyzer; (4) diesel tank; (5) biodiesels tank; (6) flow meter; (7) tranquilization system; (8) cable of the various sensors.
Subsequent performance testing was conducted with the engine operating at its standard speed of 1500 revolutions per minute, with the injection timing precisely calibrated to 20° before top dead center.
During the tests, injection timing and pressure profiles were systematically recorded for EB100, PODL20, and diesel fuel, with particular emphasis on engine operation under a 50% load condition.
The detailed specifications and profiles pertinent to the engine’s operation and fuel characteristics are comprehensively presented in Table 7, which outlines the engine specifications, and Table 8, which describes the fuel injection system specifications. Furthermore, Figure 3 illustrates the fuel injection profiles for neat diesel, EB100, and PODL20 under a 50% load condition, providing a clear comparative analysis of their performance characteristics.

Table 7.
Engine specifications.

Table 8.
Fuel injection system specifications.
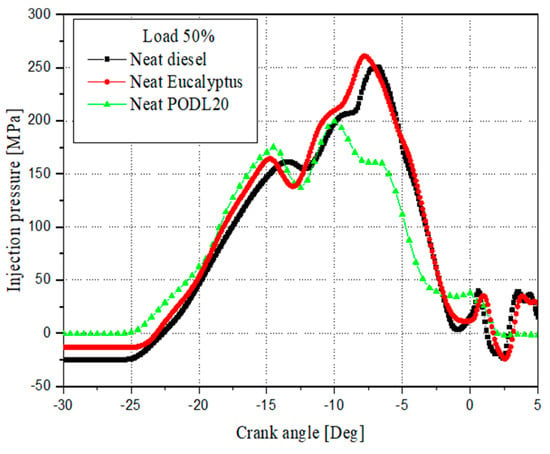
Figure 3.
Fuel injection profiles for neat diesel, EB100, and PODL20 at 50% loads.
Eucalyptus biodiesel exhibits distinct physical and chemical properties compared to petroleum-based diesel fuel. While its viscosity can be similar, it is often higher, potentially impacting the precision of the fuel injection system in delivering the correct amount of fuel to the engine. The biodiesels contain oxygen in their chemical structure, unlike conventional diesel fuel, which leads to more complete combustion and different emission profiles.
Closed-cycle combustion simulations for both diesel and biodiesel fuels were performed using CFD CONVERGE 3.2. These simulations employed user-supplied combustion chamber surface data and a novel boundary technique that dynamically modifies the intersecting cells (cut-cell) during runtime. This method significantly enhances grid generation and computational efficiency while enabling local grid refinement through fixed embedding and adaptive mesh refinement (AMR). The mesh was designed to accurately represent the engine geometry, with boundary adjustments ensuring precision (e.g., bore and stroke). An AMR approach was utilized to adjust the grid based on velocity and temperature criteria. Figure 4 illustrates the computational grid domain at 65° CA ATDC, showcasing the detailed and refined mesh structure.
Figure 4.
Computational grid view.
3.3. Computational Framework
The numerical analysis for this study was conducted using the CONVERGE simulation program, specifically version 3.2, which operates on a Windows XP 64-bit system. This advanced three-dimensional fluid dynamics tool is designed for chemically reactive flow analysis and was initially developed to model fuels such as gasoline and diesel. This CFD allows for solving the governing equations of mass transport, represented by compressible equations, as detailed in Refs. [43,44]. To address these equations, CONVERGE incorporates various physical and chemical models, including the KH-RT breakup model for spray and atomization, the RNG k–ε model for turbulence, the CTC-Shell model for combustion, the Zel’dovich mechanism for NO formation, and a soot emission model based on the Hiroyasu formation model, with detailed descriptions provided in Refs. [44,45,46,47]. For accurate simulation of complex fluid and chemically reacting flows, specific thermodynamic properties, such as viscosity, latent heat of vaporization, vapor pressure, and density, of EB100 and PODL20 biodiesels have been integrated into CONVERGE’s fuel library (liquid.dat).
3.4. Combustion Modeling of a Diesel Engine
3.4.1. Combustion Analysis
Combustion characteristics are rigorously evaluated through the analysis of cylinder pressure as a function of the crank angle, providing insights into the performances and efficiencies of different fuels. Figure 5 illustrates a comparative analysis of the in-cylinder pressure profiles for the EB100 and PODL20 biodiesels, as well as conventional diesel fuel. The results show distinct differences in peak cylinder pressures among the fuels:
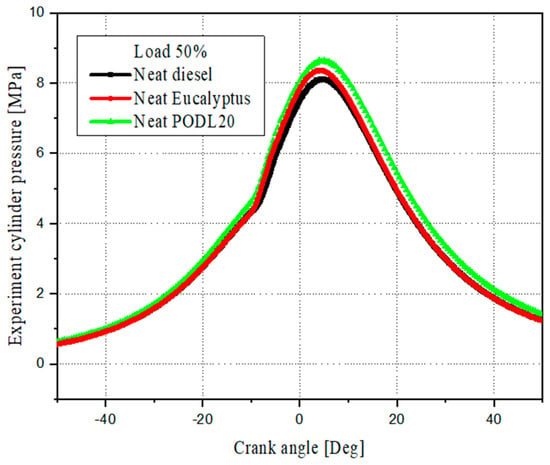
Figure 5.
Cylinder pressure profiles: diesel vs. biodiesel fuels.
- ▪
- Conventional diesel fuel: Has the lowest peak cylinder pressure at 8.11 MPa. While conventional diesel provides reliable performance, its lower peak pressure compared to the biodiesels suggests slightly less efficient combustion. This might be attributed to the lack of oxygen in its chemical composition, which can affect the completeness of combustion;
- ▪
- EB100 biodiesel: Shows a peak cylinder pressure of 8.38 MPa, which is 3.3% higher than conventional diesel. This indicates that EB100 has good combustion properties, likely due to the esterified eucalyptus oil’s chemical structure, which promotes efficient combustion;
- ▪
- PODL20 biodiesel: Exhibits the highest peak cylinder pressure at 8.65 MPa, which is 6.7% higher than conventional diesel and 3.2% higher than EB100. This suggests that PODL20 biodiesel has a strong combustion performance, potentially leading to higher engine efficiency and power output. The higher peak pressure might be due to the optimized blend of palm oil and D-limonene, enhancing the ignition quality and combustion efficiency.
To ensure accuracy, the CONVERGE code 3.2 was validated against experimental data. This validation process involved comparing experimental and simulated cylinder pressures at 1500 rpm for different fuels. Figure 6, Figure 7 and Figure 8 illustrate a detailed comparison of simulation results with the experimental data, demonstrating a high degree of alignment. The numerical results closely match the experimental measurements, underscoring the reliability of the simulation model parameters, boundary conditions, and the predicted physical characteristics of EB100 and PODL20 biodiesels. These characteristics include viscosity, surface tension, latent heat of vaporization, vapor pressure, thermal conductivity, specific heat capacity, and density. The successful validation confirms that the CONVERGE CFD 3.2 software is well-suited for accurately modeling the combustion dynamics of these biodiesels.
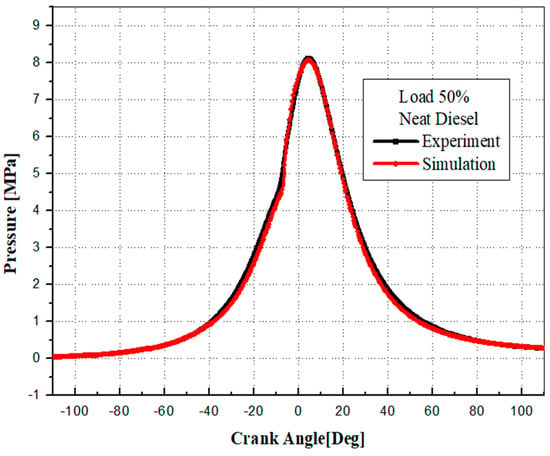
Figure 6.
Cylinder pressure: simulation vs. experimental data for DF.
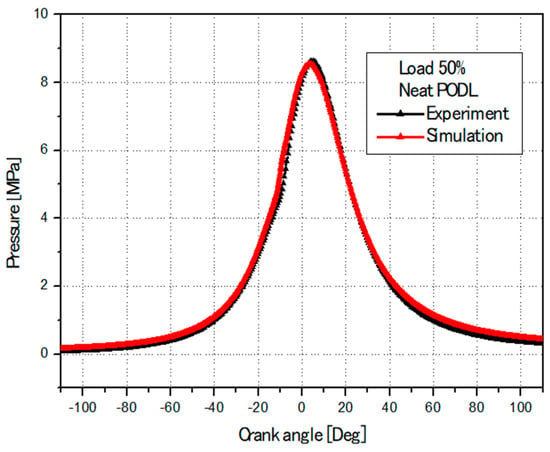
Figure 7.
Comparison between the simulation’s and experiment’s cylinder pressures (PODL).
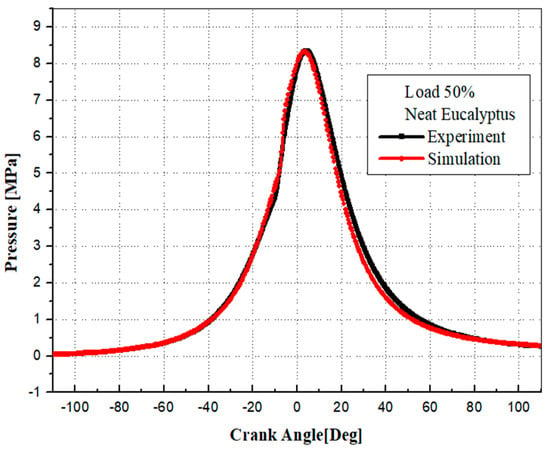
Figure 8.
Comparison between the simulation’s and experiment’s cylinder pressures (EB100).
Figure 9 illustrates the heat release rate (HRR) for eucalyptus biodiesel (EB100), PODL20 biodiesel, and conventional diesel fuel under typical load conditions. The results provide a comprehensive comparison of the combustion characteristics of these fuels, highlighting significant differences in combustion phasing and heat release rates.
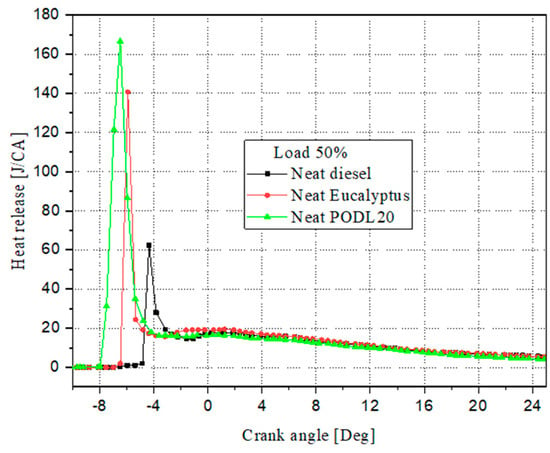
Figure 9.
Evolution of the heat release rate during combustion.
- Combustion phasing:
- ▪
- Conventional diesel fuel: the combustion phasing for diesel fuel occurs at 4.95° CA;
- ▪
- EB100 biodiesel: Advances the combustion phasing by 26.15% compared to diesel fuel, with combustion occurring at −6.5° CA. This advancement is likely due to the oxygenated nature of EB100, which promotes faster ignition and combustion processes;
- ▪
- PODL20 biodiesel: Exhibits the most advanced combustion phasing, with a 40.29% advancement compared to diesel fuel, occurring at −8.04° CA. The blend of palm oil and D-limonene in PODL20 likely contributes to this significant advancement by enhancing ignition quality and combustion efficiency.
- Heat release rate:
- ▪
- Conventional diesel fuel: Exhibits the lowest HRR at 60.2 J/CA. This relatively lower HRR can be attributed to the absence of oxygen in its chemical composition, resulting in less efficient combustion;
- ▪
- EB100 biodiesel: Shows an HRR of 140.08 J/CA, which is 132.65% higher than that of conventional diesel. The oxygen content in the esterified eucalyptus oil structure enhances the combustion process, leading to a significantly higher HRR compared to diesel fuel;
- ▪
- PODL20 biodiesel: Demonstrates the highest HRR at 165.74 J/CA, which is 175.36% higher than conventional diesel and 18.31% higher than EB100. The optimized blend of palm oil and D-limonene not only advances the combustion phasing but also maximizes the energy release during combustion, resulting in the highest HRR among the tested fuels.
The advanced combustion phasing and higher HRR of EB100 and PODL20 biodiesels compared to conventional diesel fuel underscore the benefits of using oxygenated biodiesel fuels. These fuels not only promote faster ignition and more efficient combustion but also result in significantly higher energy output, which is beneficial for improving engine performance. PODL20, in particular, demonstrates the most substantial improvements in both the combustion phasing and HRR, highlighting its potential as an optimal alternative to conventional diesel fuel.
Figure 10 depicts the variations in the average in-cylinder temperatures throughout the combustion cycles for the investigated fuels: PODL20, EB100, and conventional diesel fuel. The maximum average in-cylinder temperatures observed were 1451.62 K for PODL20, 1392.43 K for EB100, and 1230.17 K for conventional diesel fuel. Expressed in percentage terms relative to conventional diesel fuel, PODL20’s maximum temperature was approximately 18.0% higher, while EB100’s was about 13.2% higher. These higher temperatures for PODL20 and EB100 can be explained by their higher oxygen contents. PODL20’s extra oxygen promotes more complete combustion, resulting in higher temperatures. Similarly, EB100’s higher oxygen content also leads to more complete combustion compared to conventional diesel fuel. In contrast, conventional diesel fuel lacks inherent oxygen and relies on external oxygen during combustion. Therefore, the higher maximum in-cylinder temperatures for PODL20 and EB100 reflect their better combustion characteristics compared to conventional diesel fuel.
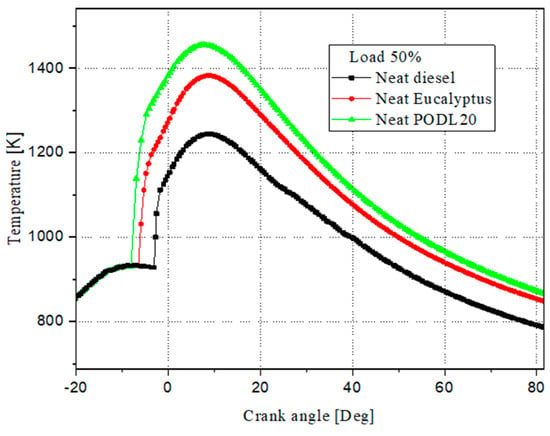
Figure 10.
Temperature evolution during combustion.
Figure 11 highlights the ignition delay for the fuels at 1500 rpm. Neat diesel exhibits a longer delay of 15° CA, compared to 12 CA for PODL20 and 14.02 CA for EB100. These data underscore the combustion efficiencies and enhanced performances of biodiesel fuels over conventional diesel. Specifically, PODL20 showed a 20% shorter ignition delay compared to neat diesel, while EB100’s ignition delay was approximately 6.5% shorter. These shorter ignition delays for biodiesel fuels indicate quicker combustion initiation, leading to more efficient and effective combustion processes.
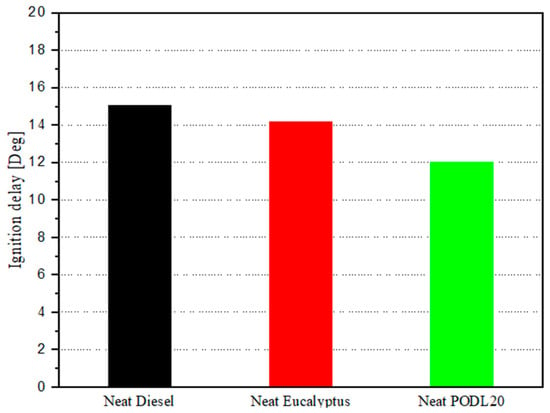
Figure 11.
Ignition delay with different fuels at 1500 rpm.
3.4.2. Emission Characteristics Analysis
Figure 12 illustrates the variations in soot emissions for the EB100 and PODL20 biodiesels and diesel fuel as a function of the crank angle under 50% load conditions. The data indicate that soot emissions peaked near the end of the premixed combustion phase and decreased during the non-premixed combustion phase. Notably, the EB100 and PODL20 biodiesels showed significantly lower soot emissions compared to diesel fuel. EB100, with soot emissions of 1.194 × 10−7 kg, achieved a reduction of approximately 34.21% compared to diesel fuel, which had emissions of 1.815 × 10−7 kg. Similarly, PODL20, with soot emissions of 1.5 × 10−7 kg, showed a reduction of approximately 21.05%. This reduction in soot emissions for biodiesels can be attributed to their higher oxygen contents, which promote more complete fuel oxidation and, consequently, reduces soot levels in the exhaust gases.
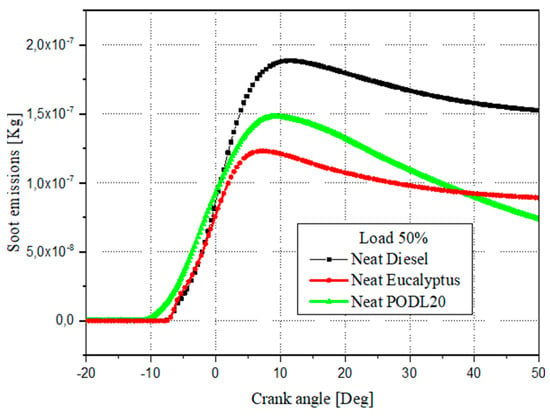
Figure 12.
Soot emissions formation across crank angle variations.
Figure 13 demonstrates the variation in NOx emissions with respect to the crank angles for both the biodiesel fuels and the diesel fuel. The maximum NOx emissions observed were 1.2 × 10−6 kg for PODL20, 1.164 × 10−6 kg for EB100, and 8 × 10−7 kg for conventional diesel fuel. PODL20’s NOx emissions were 50% higher, while EB100’s were 45.5% higher, indicating that both biodiesel fuels produced more NOx than the diesel, with PODL20 having the highest emissions. The increased NOx emissions in biodiesels can be attributed to their higher combustion temperatures and increased oxygen content, which favor the formation of nitrogen oxides. Additionally, referring to Figure 12, the soot emissions data show that PODL20 emits 1.5 × 10−7 kg of soot (a 21.05% reduction compared to diesel fuel), and EB100 emits 1.194 × 10−7 kg (a 34.21% reduction), whereas conventional diesel fuel emits 1.815 × 10−7 kg of soot. Considering both soot and NOx emissions, EB100 demonstrates a more balanced performance, achieving a significant reduction in soot emissions by 34.21% and a moderate increase in NOx emissions by 45.5% compared to diesel fuel and reductions of 20.4% and 3% in soot and NOx emissions respectively, compared to PODL20.
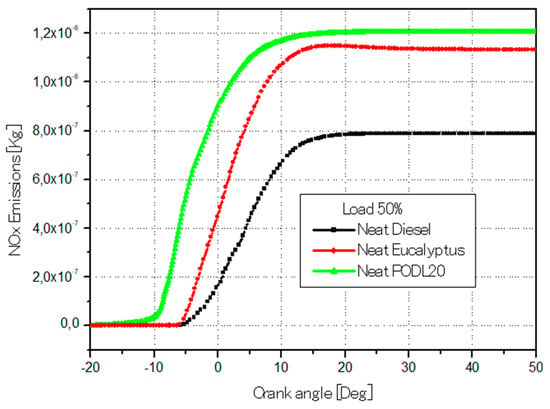
Figure 13.
NOx emissions formation across the crank angle variations.
4. Conclusions
This study highlights the superior quality and efficiency of biodiesels compared to conventional diesel. Various models based on EB100 and PODL20 biodiesels were developed to predict their physical properties. These predicted properties were then utilized in a combustion simulation of a four-stroke compression engine using CONVERGE CFD 3.2 software. The simulations focused on analyzing spray characteristics, combustion behavior, and pollutant emissions for both biodiesels and diesel fuel. The computational analysis provided the following key results:
- ▪
- The analysis showed that Yuan’s method had the highest accuracy in predicting the average boiling temperature of biodiesels;
- ▪
- When comparing the predicted biodiesel properties with those in the Handbook Data, it was found that the Ambrose method produced slight errors in critical volume and temperature estimates, while the Joback method resulted in smaller errors for the critical pressure estimations;
- ▪
- Vapor pressure predictions using the Lee-Kesler technique and the Gamez–Thodos approach showed good agreement;
- ▪
- The Riedel approach proved more accurate than other methods in predicting the latent heat of vaporization;
- ▪
- The Rackett model provided accurate predictions of the liquid density;
- ▪
- The Orrick and Erbar technique was more suitable for estimating liquid viscosity across a wide temperature range;
- ▪
- The Latini and Sastri methods produced predictions for liquid thermal conductivity that aligned well with the Handbook Data;
- ▪
- Surface tension was more accurately predicted using corresponding states correlation compared to the Macleod–Sugden correlation;
- ▪
- A high level of agreement between predicted and experimental values confirmed the accuracy of the numerical predictions obtained in this study for properties such as viscosity and density;
- ▪
- The engine heat release rate, ignition delay period, and in-cylinder pressure were improved. Biodiesels like PODL20 and EB100 demonstrate potential for enhancing combustion and performance characteristics in diesel engine applications;
- ▪
- EB100 showed a more balanced performance with a significant reduction in soot emissions by 34.21% and a moderate increase in NOx emissions by 45.5% compared to conventional diesel fuel. On the other hand, PODL20 also reduced soot emissions by 21.05% but had a higher increase in NOx emissions, at 50%, compared to EB100.
Author Contributions
Conceptualization, H.B., S.A., N.K., C.M., K.N., Y.M. and L.K.; methodology, H.B., S.A., N.K., C.M., K.N., Y.M. and L.K.; software, H.B., S.A., N.K., C.M., K.N., Y.M. and L.K.; validation, H.B., S.A., N.K., C.M., K.N., Y.M. and L.K.; investigation, H.B., S.A., N.K., C.M., K.N., Y.M. and L.K.; resources, H.B., S.A., N.K., C.M., K.N., Y.M. and L.K.; writing—original draft preparation, H.B., S.A., N.K., C.M., K.N., Y.M. and L.K.; writing—review and editing, H.B., S.A., N.K., C.M., K.N., Y.M. and L.K.; supervision, Y.M. and L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Research and Graduate Studies at King Khalid University through Large Research Project, under grant number: RGP2/352/45.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.
Acknowledgments
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project, under grant number: RGP2/352/45.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AvBn_Pa | Avocado, bush nut biodiesel, and paraffin |
| AMR | Adaptive mesh refinement |
| ASTM | American Society for Testing and Material |
| ATDC | After top dead center |
| BSFC | Brake specific fuel consumption |
| BTDC | Before top dead center |
| BTE | Brake thermal efficiency |
| CA | Crank angle |
| CB | Castor biodiesel |
| CFD | Computational fluid dynamics. |
| CME | Coconut methyl ester |
| CN | Cetane number |
| CNG | Compressed natural gas |
| CO | Carbon monoxide |
| CTC | Characteristic time combustion |
| EB100 | Neat eucalyptus biodiesel |
| EGM | Ethylene glycol monoacetate |
| EGR | Exhaust gas recirculation |
| HC | hydrocarbon |
| HRR | Heat release rate |
| KH-RT | Kelvin–Helmholtz/Rayleigh–Taylor |
| lpm | Liter per million |
| ManCr_Pa | Mandarin, Crambe biodiesel, and paraffin |
| MWCNTs | Multiwalled carbon nanotubes |
| NOx | Nitrogen oxide |
| PM | Particulate matter (PM) |
| PME | Palm oil methyl ester |
| PODL20 | Palm oil and 20% D-limonene |
| ppm | Particle per million |
| RNG | Renormalization group |
| SME | Soybean methyl ester |
| TGME | Tri-ethylene glycol mono-methyl ether |
| TiO2 | Titanium dioxide |
| WB | Waste-cooking-oil biodiesel |
References
- Larsen, C.; Oey, F.; Levendis, Y.A. An Optimization Study on the Control of NOx and Particulate Emissions from Diesel Engines; SAE Technical Paper 960473; SAE International: Warrendale, PE, USA, 1996. [Google Scholar] [CrossRef]
- Summers, J.C.; Van Houtte, S.; Psaras, D. Simultaneous control of particulate and NOx emissions from diesel engines. Appl. Catal. B Environ. 1996, 10, 139–156. [Google Scholar] [CrossRef]
- Lu, X.-C.; Yang, J.-G.; Zhang, W.G.; Huang, Z. Effect of cetane number improver on heat release rate and emissions of high speed diesel engine fueled with ethanol–diesel blend fuel. Fuel 2004, 83, 2013–2020. [Google Scholar] [CrossRef]
- Humke, A.L.; Barsic, N.J. Performance and Emissions Characteristics of a Naturally Aspirated Diesel Engine with Vegetable Oil Fuels; SAE Technical Paper 810262; SAE International: Warrendale, PE, USA, 1981. [Google Scholar] [CrossRef]
- Kumar, M.S.; Ramesh, A.; Nagalingam, B. An experimental comparison of methods to use methanol and Jatropha oil in a compression ignition engine. Biomass Bioenergy 2003, 25, 309–318. [Google Scholar] [CrossRef]
- Ballerini, D. Les Biocarburants: Repondre Aux Defis Energetiques Et Environnementaux Des Transports; Éditions Technip: Paris, France, 2011. [Google Scholar]
- Demirbas, A.; Biodiesel, A. A Realistic Fuel Alternative for Diesel Engines; Springer: London, UK, 2008; ISBN 10-1846289947. [Google Scholar] [CrossRef]
- An, H.; Yang, W.M.; Maghbouli, A.; Chou, S.K.; Chua, K.J. Detailed physical properties prediction of pure methyl esters for biodiesel combustion modeling. Appl. Energy 2013, 102, 647–656. [Google Scholar] [CrossRef]
- Ruan, D.F.; Chen, Z.H.; Wang, K.F.; Chen, Y.; Yang, F. Physical property prediction for waste cooking oil biodiesel. Open Fuels Energy Sci. J. 2014, 7, 62–68. [Google Scholar] [CrossRef][Green Version]
- Cheng, X.; M Ismail, H.; Ng, K.H.; Gan, S.; Lucchini, T. Effects of fuel thermo-physical properties on spray characteristics of biodiesel fuels. In Proceedings of the FISITA 2012 World Automotive Congress; Lecture Notes in Electrical Engineering; Springer: Berlin/Heidelberg, Germany, 2013; Volume 191, pp. 117–126. [Google Scholar] [CrossRef]
- Padmanabha, H.S.A.; Mohanty, D.K. Enhancement of combustion, performance and emission characteristics of diesel engines fuelled with jatropha-karanja biodiesel using EGM and TGME as additive. Energy 2024, 300, 131523. [Google Scholar] [CrossRef]
- Fareed, A.F.; El-Shafay, A.S.; Mujtaba, M.A.; Riaz, F.; Gad, M.S. Investigation of waste cooking and castor biodiesel blends effects on diesel engine performance, emissions, and combustion characteristics. Case Stud. Therm. Eng. 2024, 60, 104721. [Google Scholar] [CrossRef]
- El-Adawy, M. Effects of diesel-biodiesel fuel blends doped with zinc oxide nanoparticles on performance and combustion attributes of a diesel engine. Alex. Eng. J. 2023, 80, 269–281. [Google Scholar] [CrossRef]
- Winangun, K.; Setiyawan, A.; Sudarmanta, B.; Puspitasari, I.; Dewi, E.L. Investigation on the properties of a biodiesel-hydrogen mixture on the combustion characteristics of a diesel engine. Case Stud. Chem. Environ. Eng. 2023, 8, 100445. [Google Scholar] [CrossRef]
- Sarıdemir, S.; Polat, F.; Ağbulut, Ü. Improvement of worsened diesel and waste biodiesel fuelled-engine characteristics with hydrogen enrichment: A deep discussion on combustion, performance, and emission analyses. Process Saf. Environ. Prot. 2024, 184, 637–649. [Google Scholar] [CrossRef]
- Simhadri, K.; Rao, P.S.; Paswan, M. Improving the combustion and emission performance of a diesel engine with TiO2 nanoparticle blended Mahua biodiesel at different injection pressures. Int. J. Thermofluids 2024, 21, 100563. [Google Scholar] [CrossRef]
- Kunchi, L.R.; Bhatti, S.K.; Lankapalli, S.V.P.; Sagari, J. Effect of m lti ferrites nanoparticles added Terminalia bellirica biodiesel on diesel engine: Combustion, performance, and emission studies. Int. J. Thermofluids 2024, 22, 100652. [Google Scholar] [CrossRef]
- Meng, J.; Xu, W.; Meng, F.; Wang, B.; Zhao, P.; Wang, Z.; Ji, H.; Yang, Y. Effects of waste cooking oil biodiesel addition on combustion, regulated and unregulated emission characteristics of common-rail diesel engine. Process Saf. Environ. Prot. 2023, 178, 1094–1106. [Google Scholar] [CrossRef]
- Ergen, G. Comprehensive analysis of the effects of alternative fuels on diesel engine performance combustion and exhaust emissions: Role of biodiesel, diethyl ether, and EGR. Therm. Sci. Eng. Prog. 2024, 47, 102307. [Google Scholar] [CrossRef]
- Azad, A.K.; Halder, P.; Wu, Q.; Rasul, M.G.; Hassan, N.M.S.; Karthickeyan, V. Experimental investigation of ternary biodiesel blends combustion in a diesel engine to reduce emissions. Energy Convers. Manag. X 2023, 20, 100499. [Google Scholar] [CrossRef]
- Krishnan, M.G.; Rajkumar, S.; Thangaraja, J.; Devarajan, Y. Exploring the synergistic potential of higher alcohols and biodiesel in blended and dual fuel combustion modes in diesel engines: A comprehensive review. Sustain. Chem. Pharm. 2023, 35, 101180. [Google Scholar] [CrossRef]
- Ooi, J.B.; Kau, C.C.; Manoharan, D.N.; Wang, X.; Tran, M.V.; Hung, Y.M. Effects of multi-walled carbon nanotubes on the combustion, performance, and emission characteristics of a single-cylinder diesel engine fueled with palm-oil biodiesel-diesel blend. Energy 2023, 281, 128350. [Google Scholar] [CrossRef]
- Li, J.; Liang, Y.; Wang, S.; Wu, S.; Yang, W.; Liu, R. Blending n-octanol with biodiesel for more efficient and cleaner combustion in diesel engines: A modeling study. J. Clean. Prod. 2023, 403, 136877. [Google Scholar] [CrossRef]
- Abramek, K.F.; Stoeck, T.; Osipowicz, T. Statistical evaluation of the corrosive wear of fuel injector elements used in common rail systems. Stroj. Vestn. -J. Mech. Eng. 2015, 61, 91–98. [Google Scholar] [CrossRef][Green Version]
- Ramachandran, E.; Krishnaiah, R.; Venkatesan, E.P.; Parida, S.; Dwarshala, S.K.R.; Khan, S.A.; Linul, E. Prediction of RCCI combustion fueled with CNG and algal biodiesel to sustain efficient diesel engines using machine learning techniques. Case Stud. Therm. Eng. 2023, 51, 103630. [Google Scholar] [CrossRef]
- Yuan, W.; Hansen, A.C.; Zhang, Q. Vapor pressure and normal boiling point predictions for pure methyl esters and biodiesel fuels. Fuel 2005, 84, 943–950. [Google Scholar] [CrossRef]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids, 4th ed.; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Ambrose, D.; Connett, J.E.; Green, J.H.S.; Hales, J.L.; Head, A.J.; Martin, J.F. Thermodynamic properties of organic oxygen compounds 42. Physical and thermodynamic properties of benzaldehyde. J. Chem. Thermodyn. 1975, 7, 1143–1157. [Google Scholar] [CrossRef]
- Joback, K.G.; Reid, R.C. Estimation of pure-component properties from group-contributions. Chem. Eng. Commun. 1987, 57, 233–243. [Google Scholar] [CrossRef]
- Lee, B.I.; Kesler, M.G. A generalized thermodynamic correlation based on three-parameter corresponding states. AIChE J. 1975, 21, 510–527. [Google Scholar] [CrossRef]
- Rackett, H.G. Equation of state for saturated liquids. J. Chem. Eng. Data 1970, 15, 514–517. [Google Scholar] [CrossRef]
- Yaws, C.L. Handbook of Transport Property Data: Viscosity, Thermal Conductivity, and Diffusion Coefficients of Liquids and Gases; Gulf Publishing Company: Houston, TX, USA, 1995. [Google Scholar]
- Yuan, W.; Hansen, A.C.; Zhang, Q. Predicting the physical properties of biodiesel for combustion modeling. Trans. ASAE 2003, 46, 1487–1493. [Google Scholar] [CrossRef]
- Sastri, S.R.S.; Rao, K.K. A new group contribution method for predicting viscosity of organic liquids. Chem. Eng. J. 1992, 50, 9–25. [Google Scholar] [CrossRef]
- Latini, G.; Passerini, G.; Polonara, F. A prediction method for thermal conductivity of alternative refrigerants in the liquid phase. Int. J. Thermophys. 1996, 17, 85–98. [Google Scholar] [CrossRef]
- Sastri, S.R.S.; Rao, K.K. A new temperature–thermal conductivity relationship for predicting saturated liquid thermal conductivity. Chem. Eng. J. 1999, 74, 161–169. [Google Scholar] [CrossRef]
- Fish, L.W.; Lielmezs, J. General method for predicting the latent heat of vaporization. Ind. Eng. Chem. Fundam. 1975, 14, 248–256. [Google Scholar] [CrossRef]
- Vetere, A. Again the Riedel equation. Fluid Phase Equilibria 2006, 240, 155–160. [Google Scholar] [CrossRef]
- Phankosol, S.; Sudaprasert, K.; Lilitchan, S.; Aryusuk, K.; Krisnangkura, K. Estimation of surface tension of fatty acid methyl ester and biodiesel at different temperatures. Fuel 2014, 126, 162–168. [Google Scholar] [CrossRef]
- Chem, C.A.D. Physical Properties. In User Guide Chemstations; INC.2901 Wilcrest Drive, Suite 305 HOUSTON, TX, USA. Available online: https://www.accessengineeringlibrary.com/content/book/9780070116825 (accessed on 15 August 2024).
- Poling, B.E.; Prausnitz, J.M.; O’connell, J.P. Properties of Gases and Liquids, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- Arvelos, S.; Romanielo, L.L. Prediction the physical properties of pure esters for modeling biodiesel combustion. Rev. Virtual Química 2018, 10, 1355–1372. [Google Scholar] [CrossRef]
- Bousbaa, H.; Sary, A.; Tazerout, M.; Liazid, A. Investigations on a compression ignition engine using animal fats and vegetable oil as fuels. J. Energy Resour. Technol. 2012, 134, 022202. [Google Scholar] [CrossRef]
- Richards, K.; Senecal, P.; Pomraning, E. CONVERGE 2.1.0 Theory Manual; Convergent Science Inc.: Madison, WI, USA, 2013; Volume 579. [Google Scholar]
- Bousbaa, H.; Benayad, Z.; Naima, K. Numerical analysis of fuels type effect on combustion and emissions of turbo-charged direct injection engine. Energy Thermofluids Eng. 2022, 2, 31–38. [Google Scholar] [CrossRef]
- Ramos, J.I. Internal Combustion Engine Modeling; Hemisphere Publishing Corporation: London, UK, 1989. [Google Scholar]
- Hiroyasu, H.; Kadota, T. Models for combustion and formation of nitric oxide and soot in direct injection diesel engines. SAE Tech. Pap. 1976, 760129. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).