Study on the Characteristics and Influence Factor of Methane and Coal Dust Gas/Solid Two-Phase Mixture Explosions
Abstract
:1. Induction
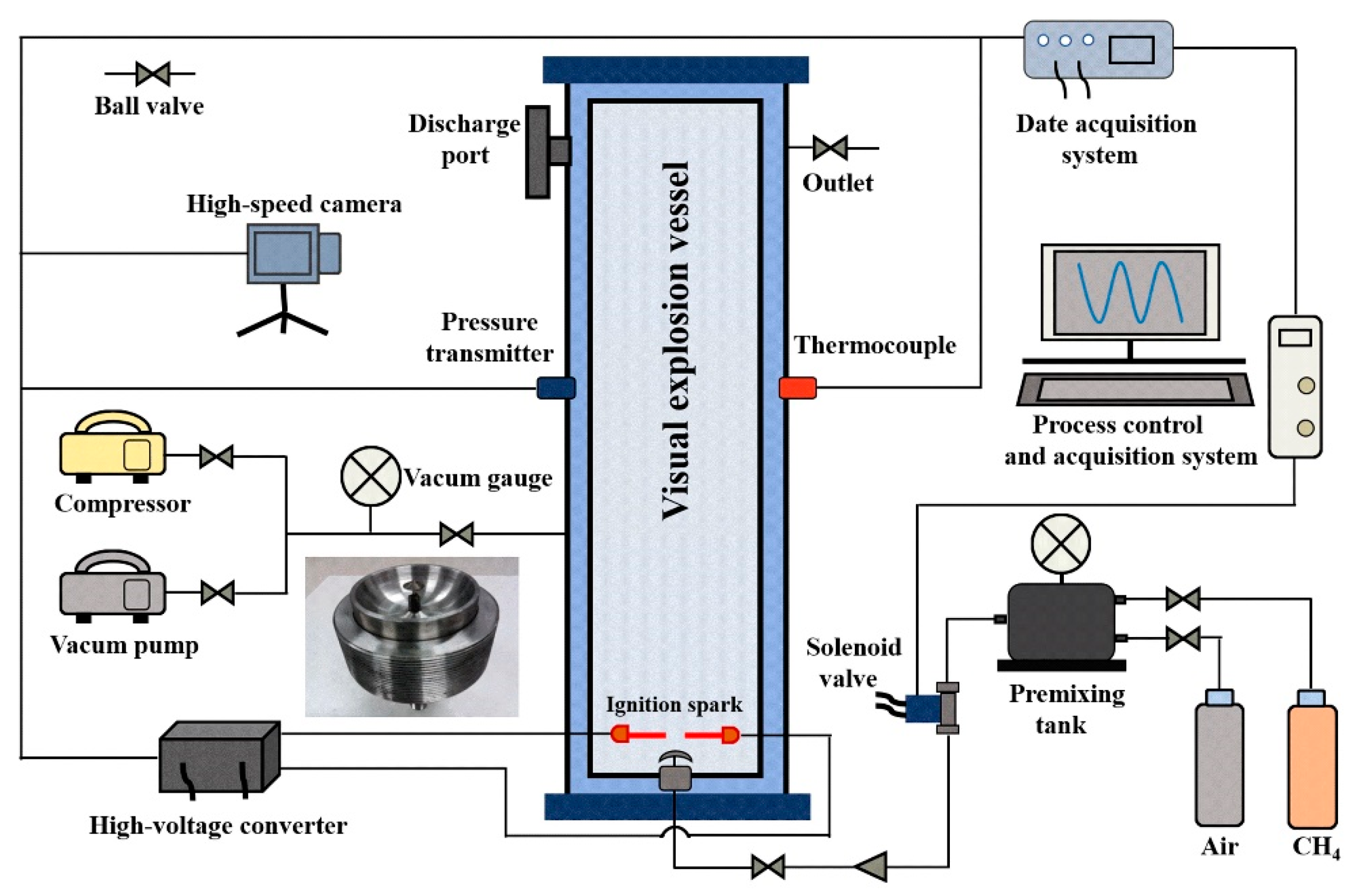
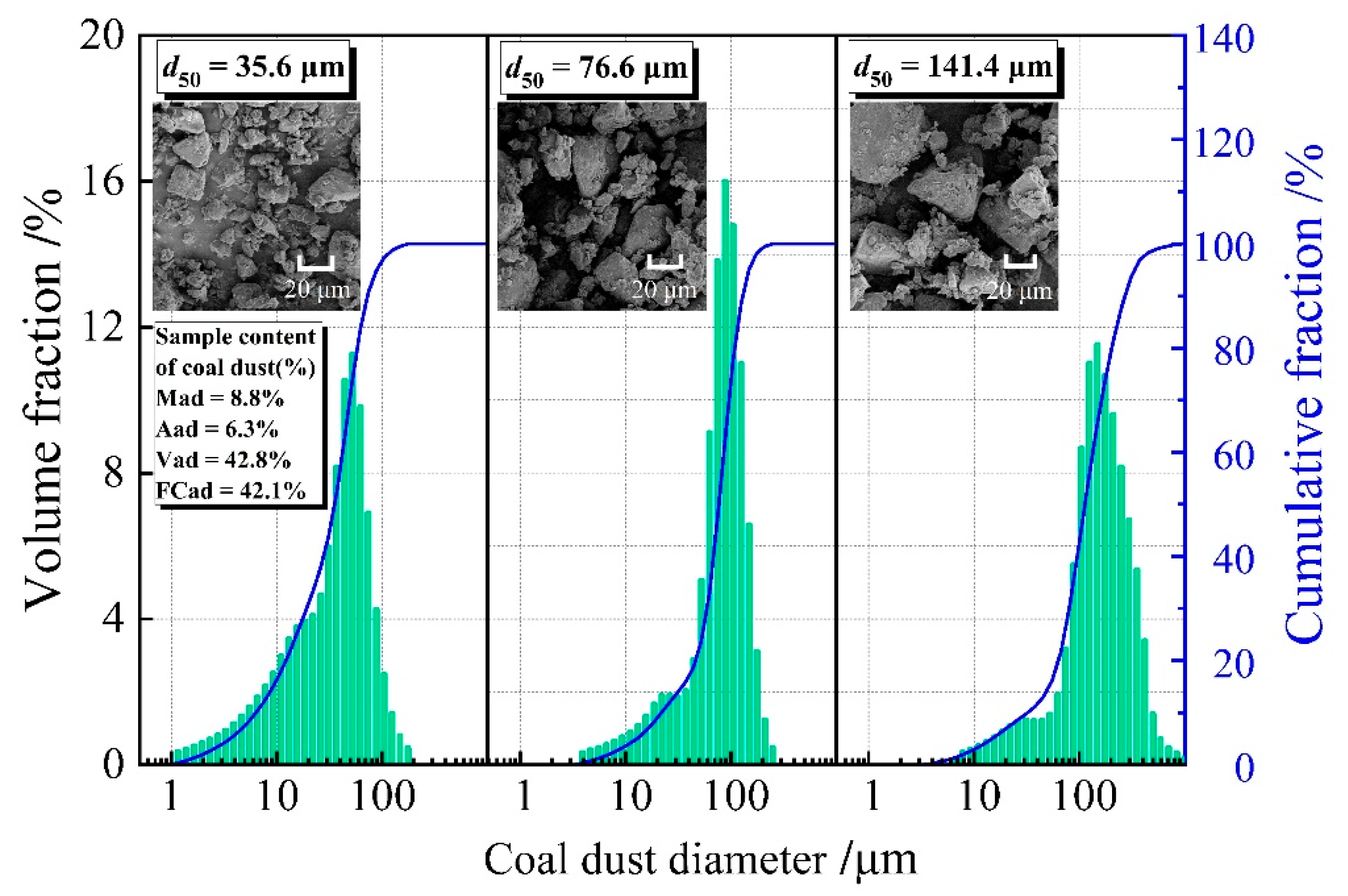
2. Experimental Setup
3. Results and Discussion
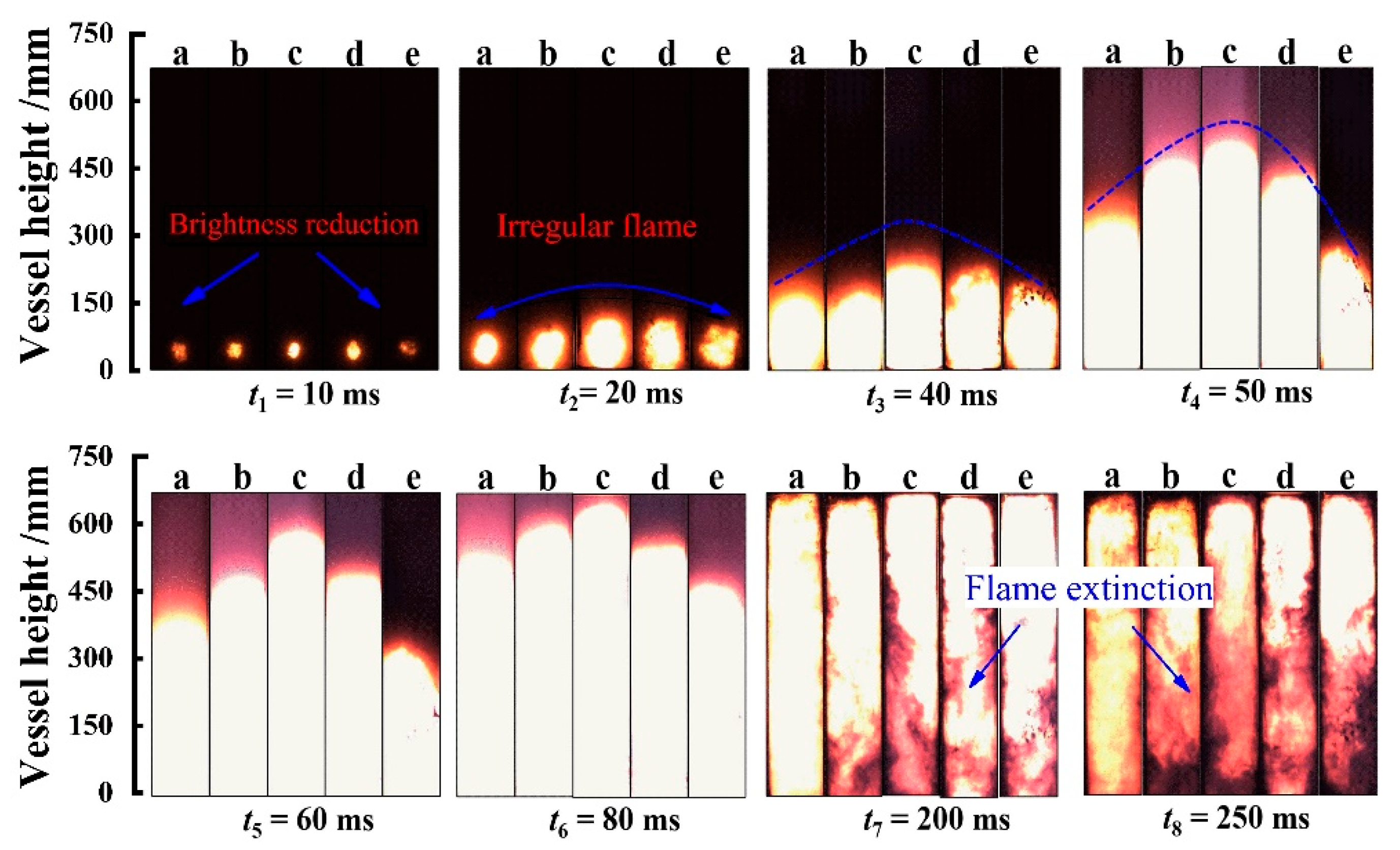
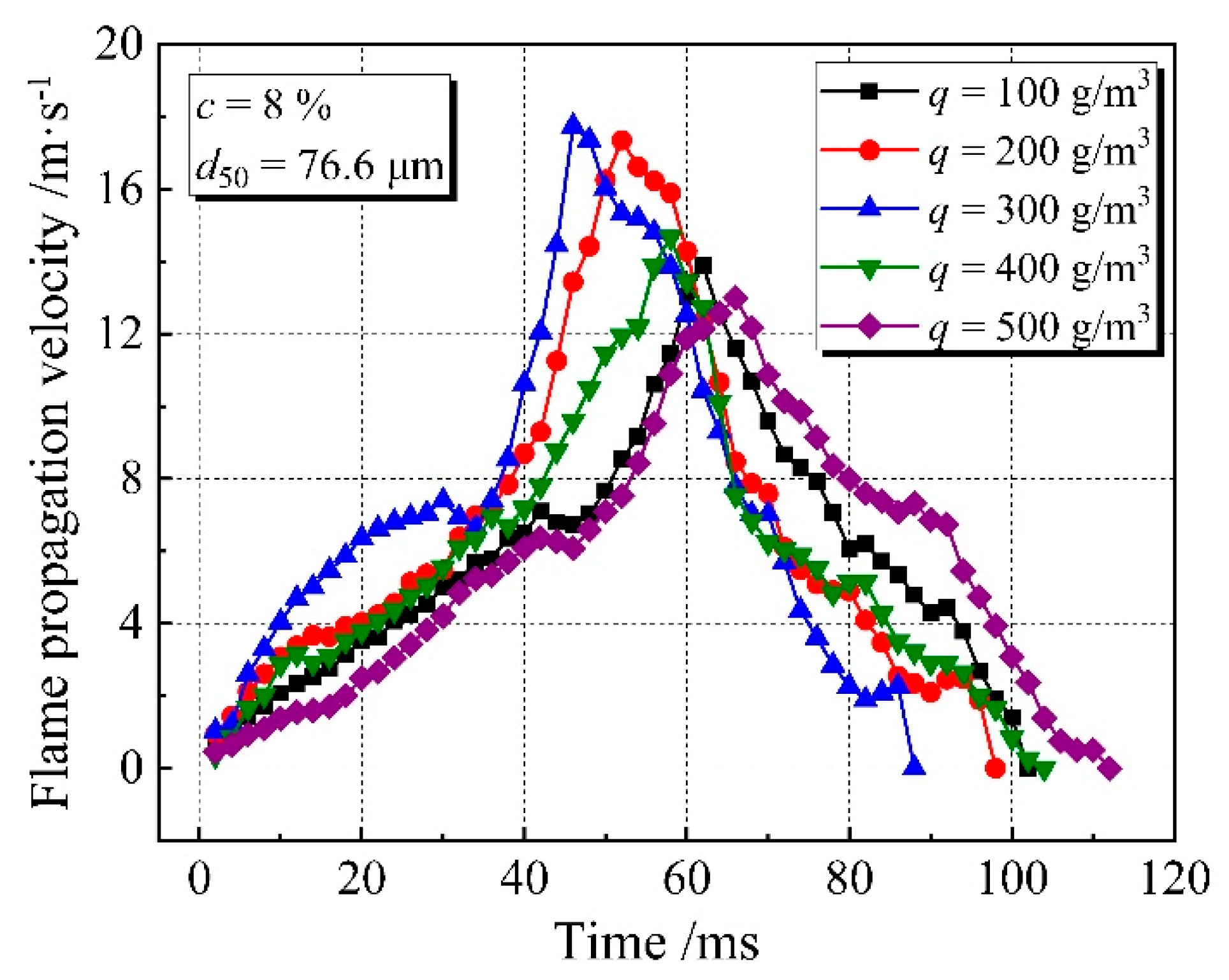
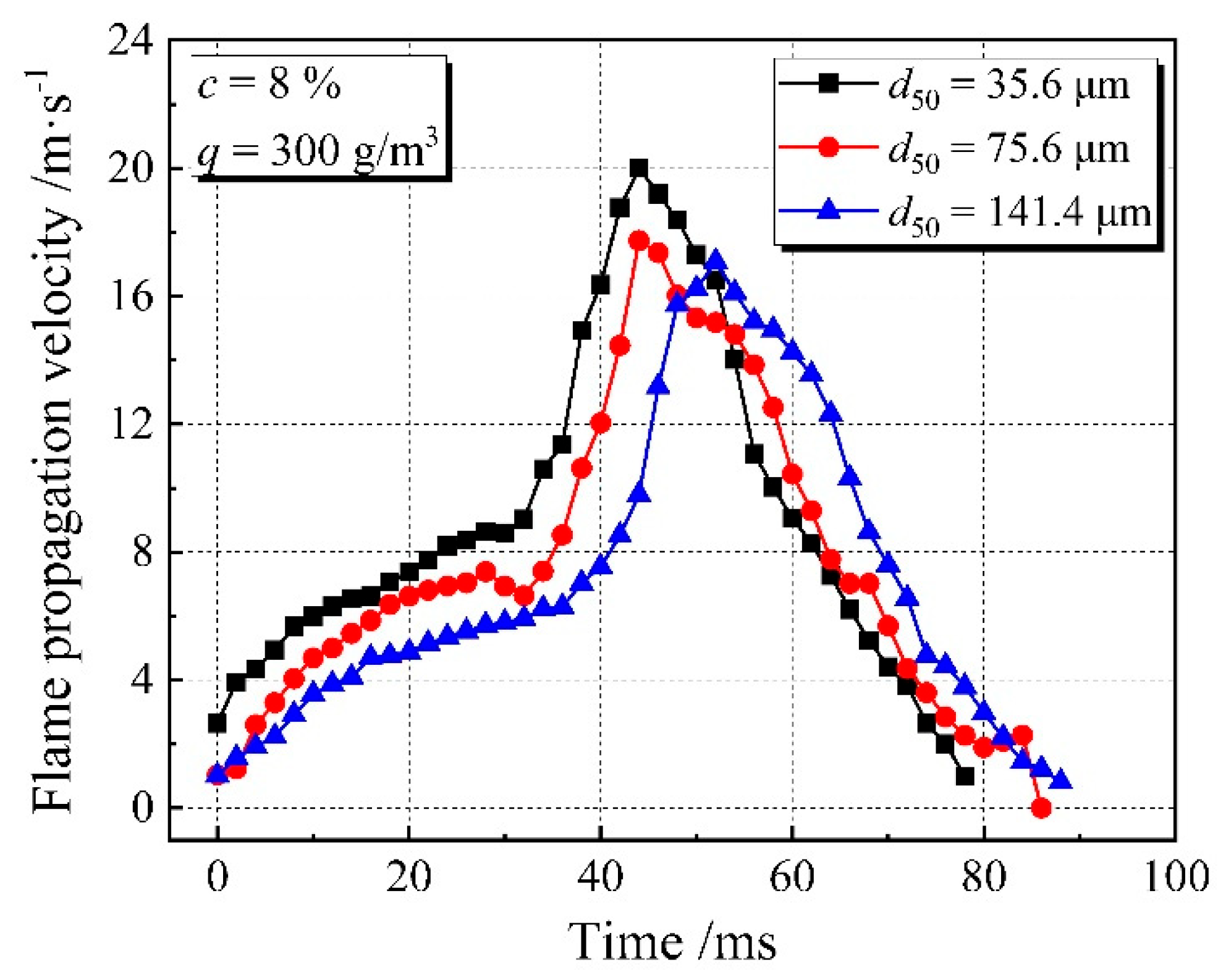
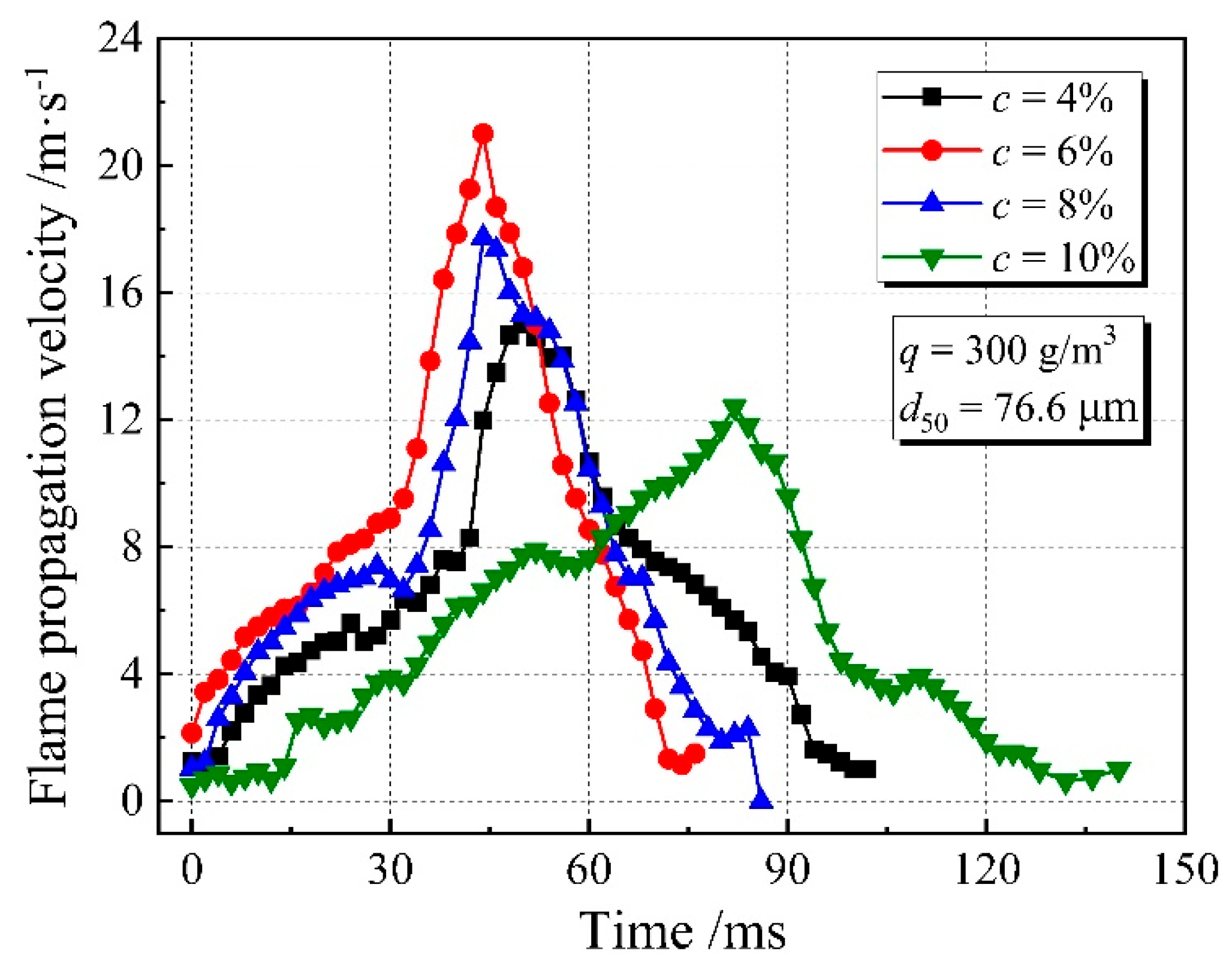
3.1. Flame Propagation Characteristics
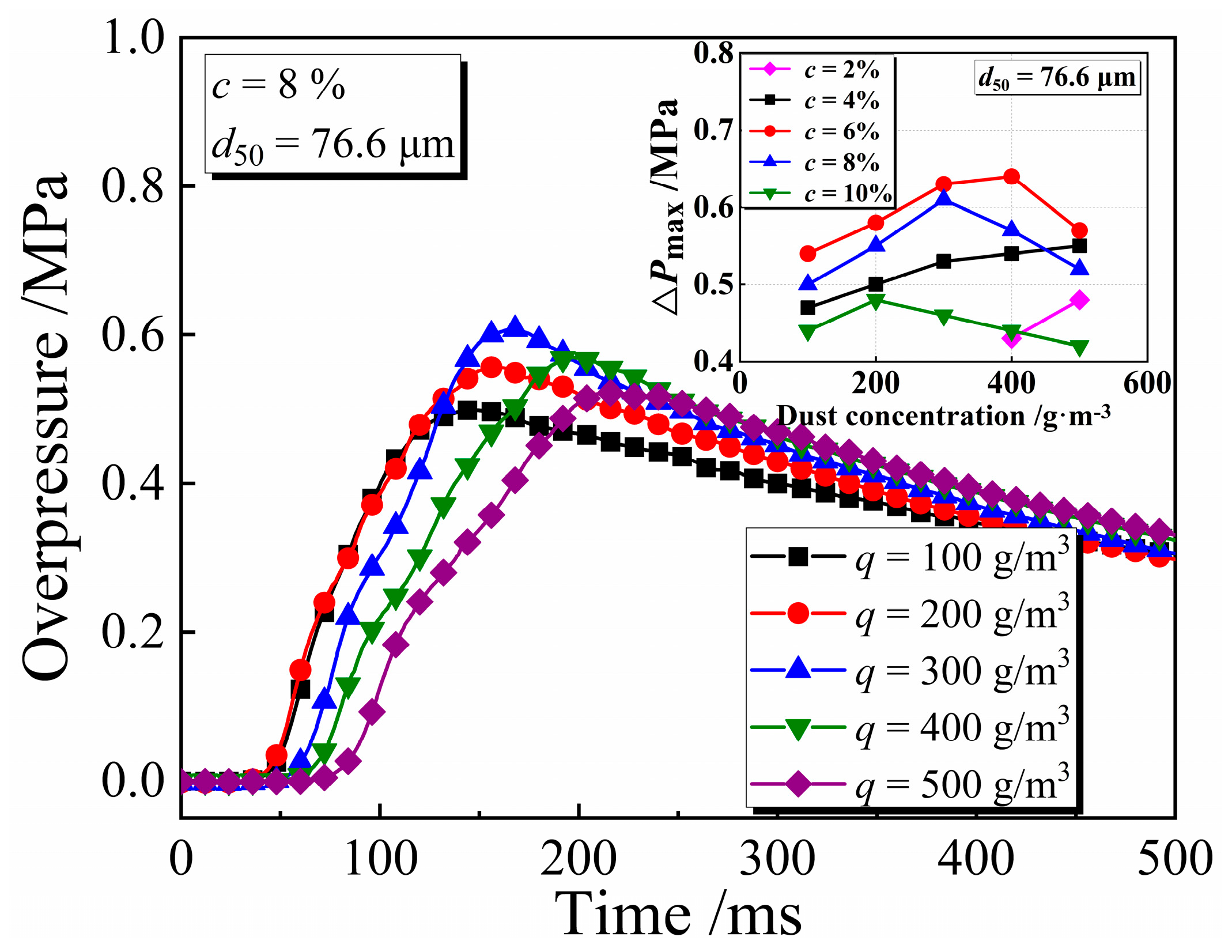
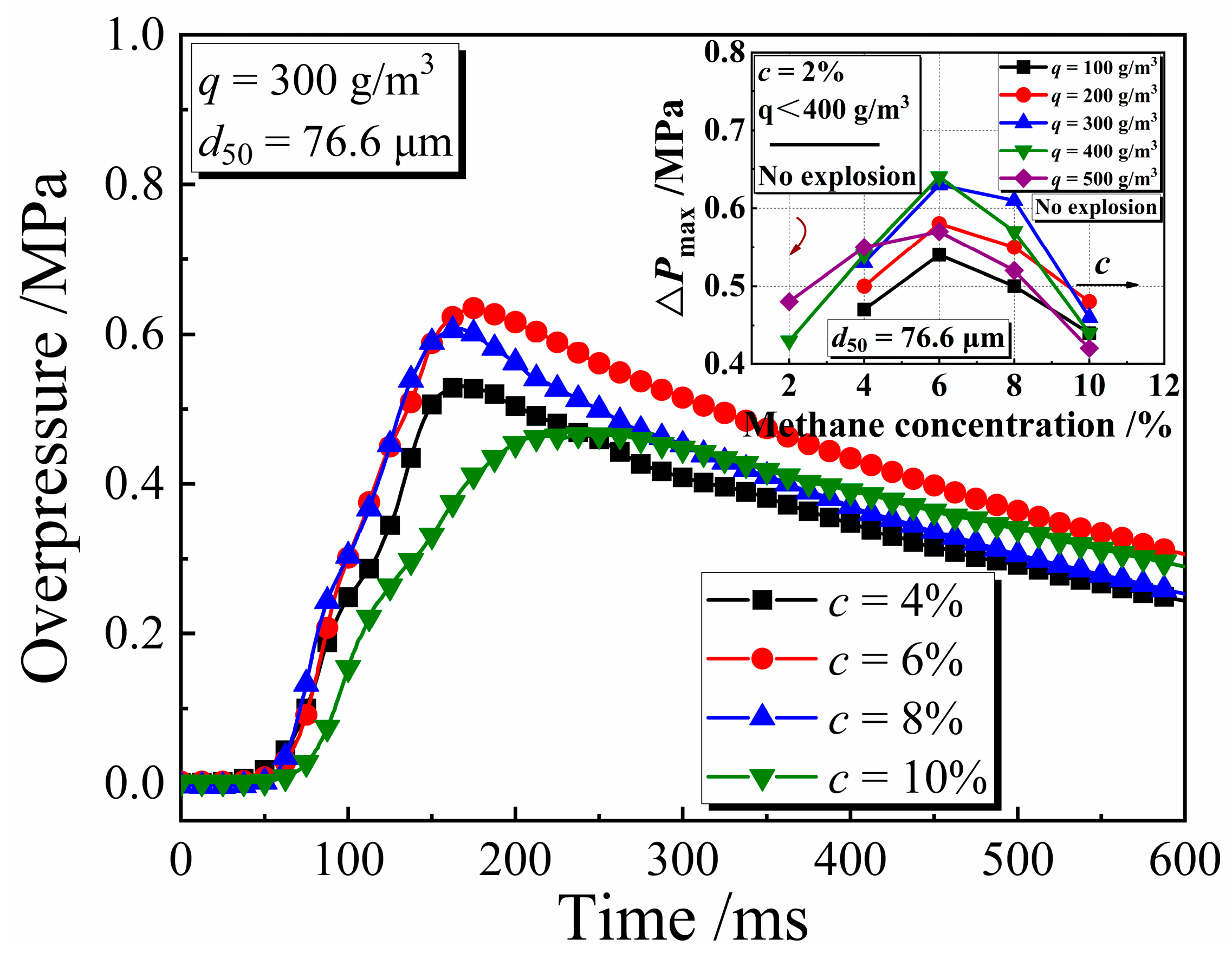
3.2. Explosion Pressure
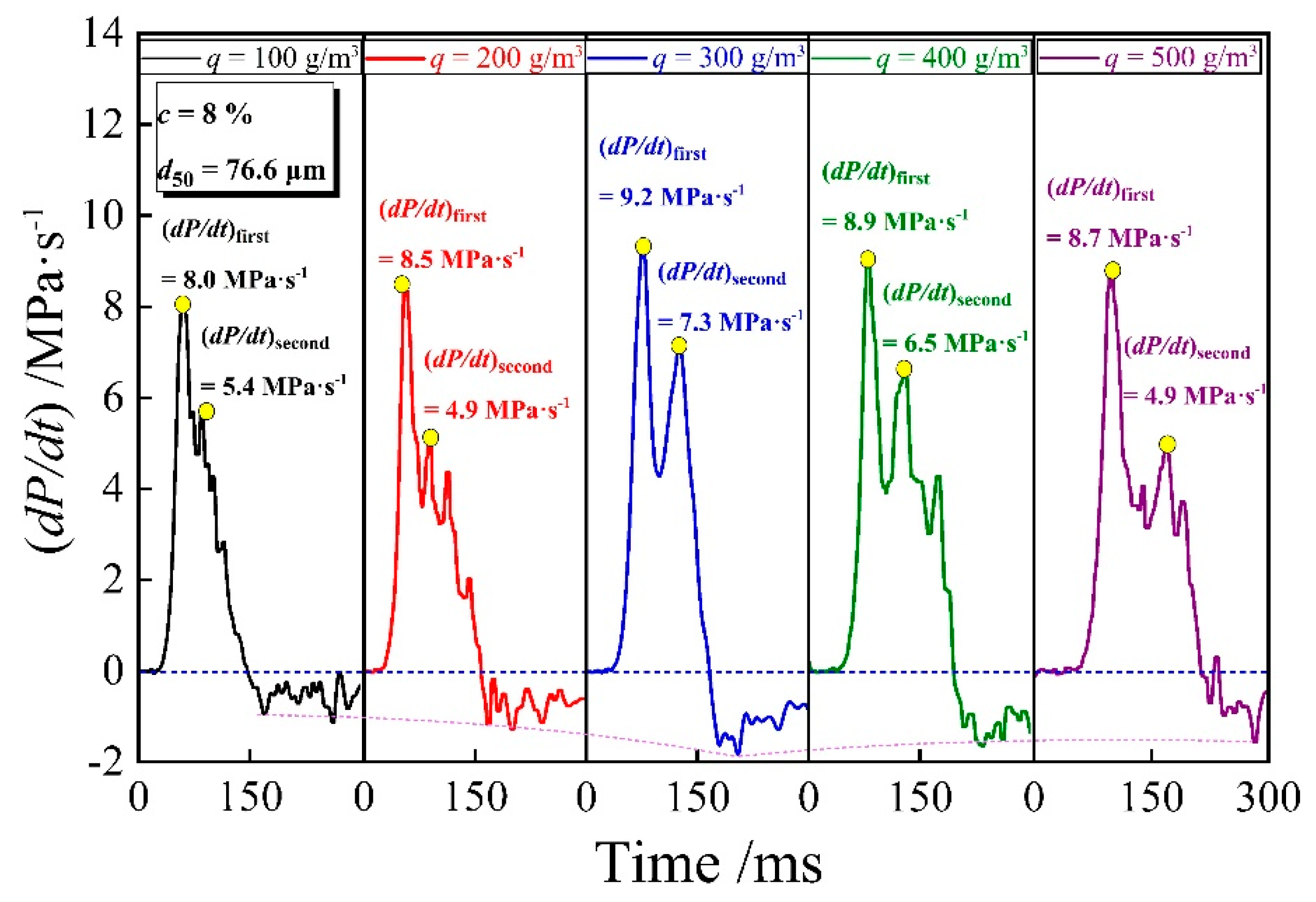
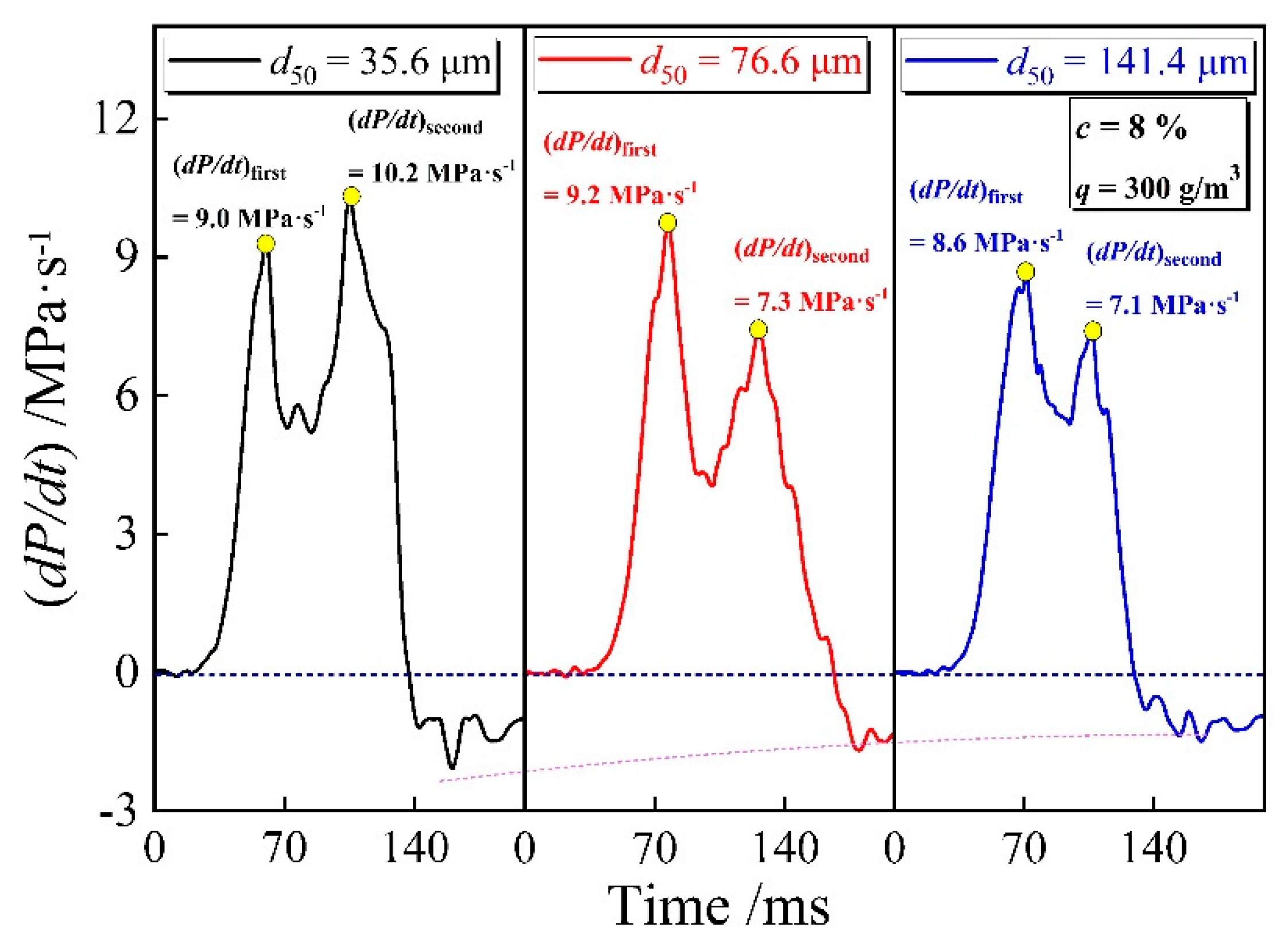
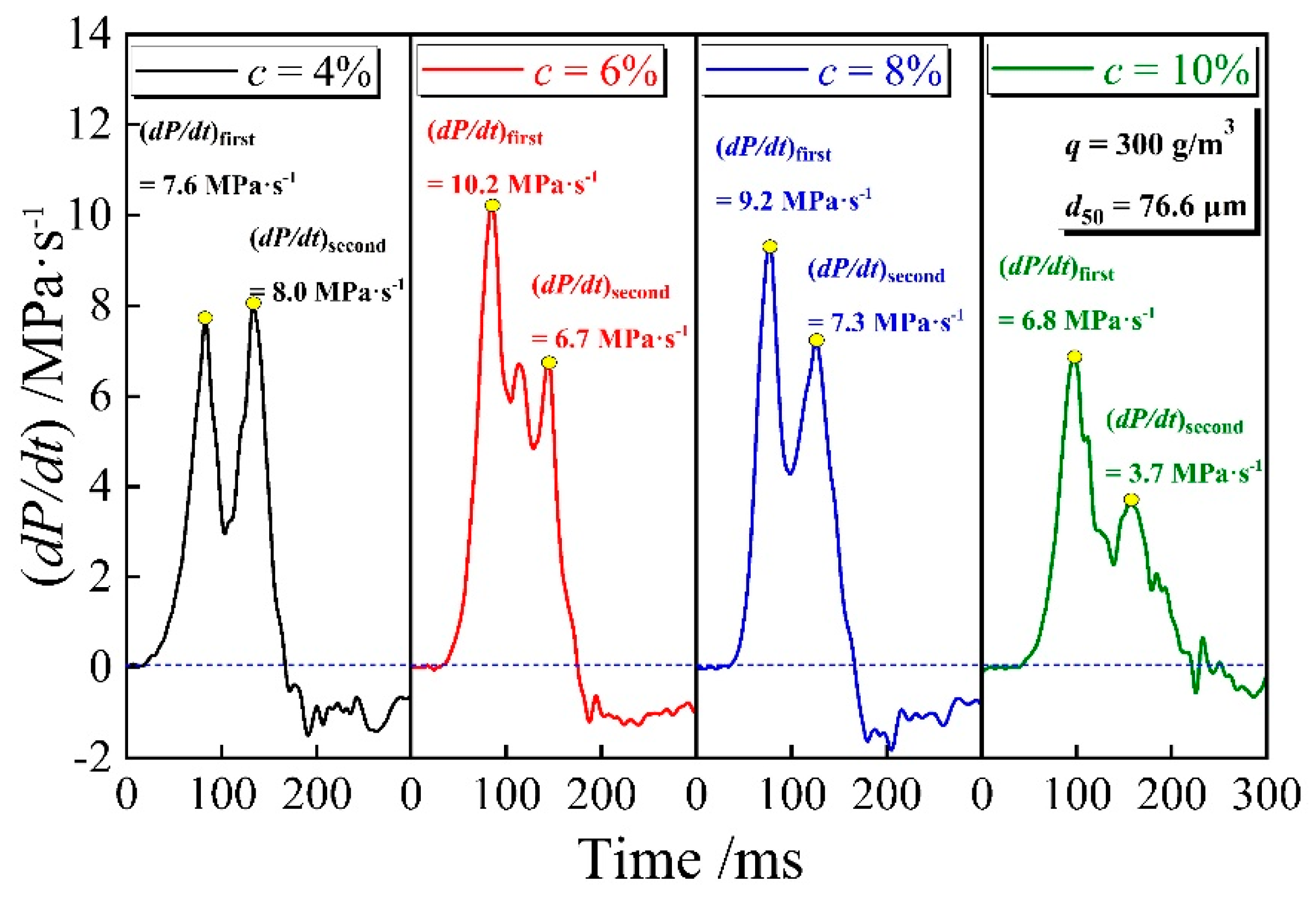
3.3. Explosion Pressure Rising Rate
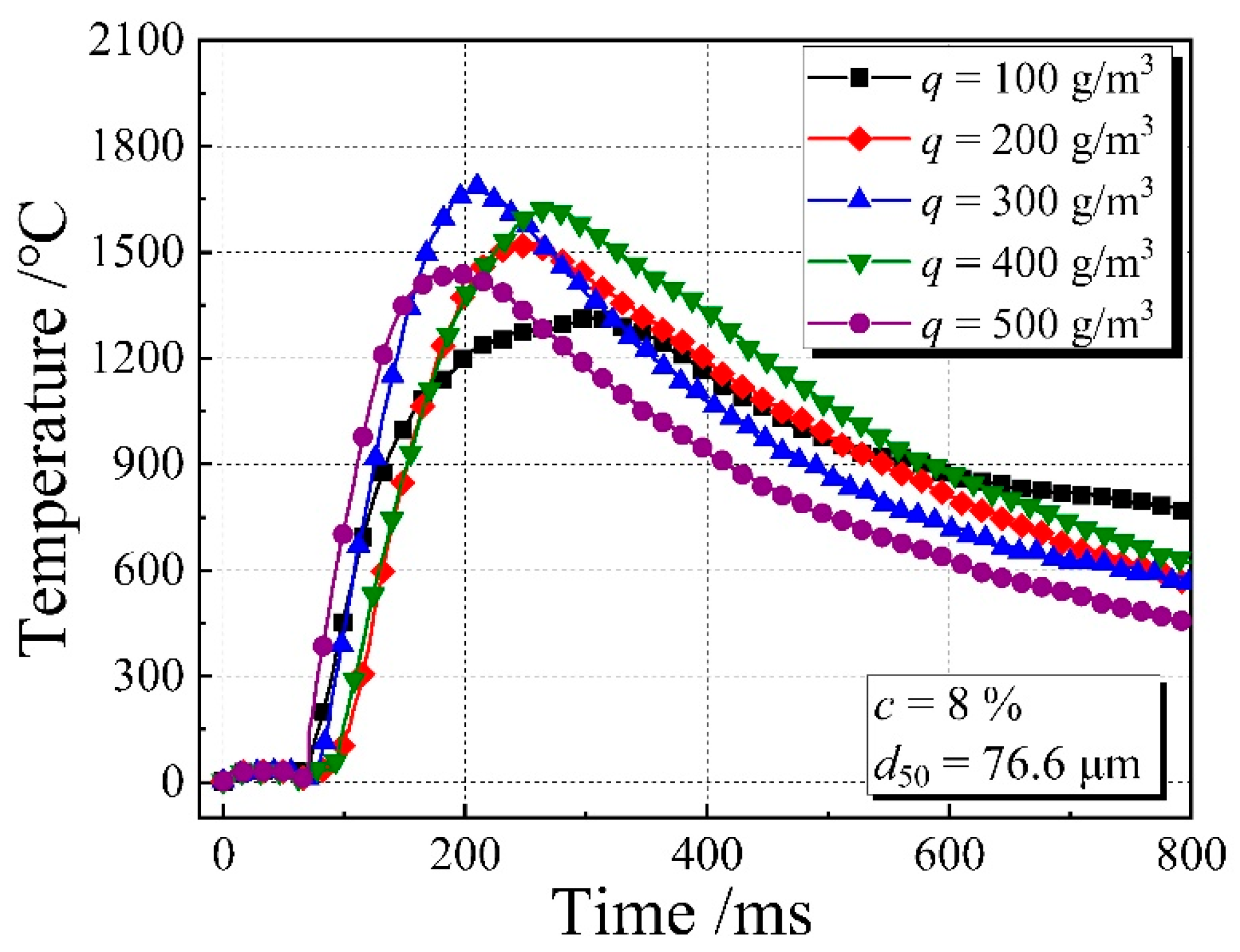
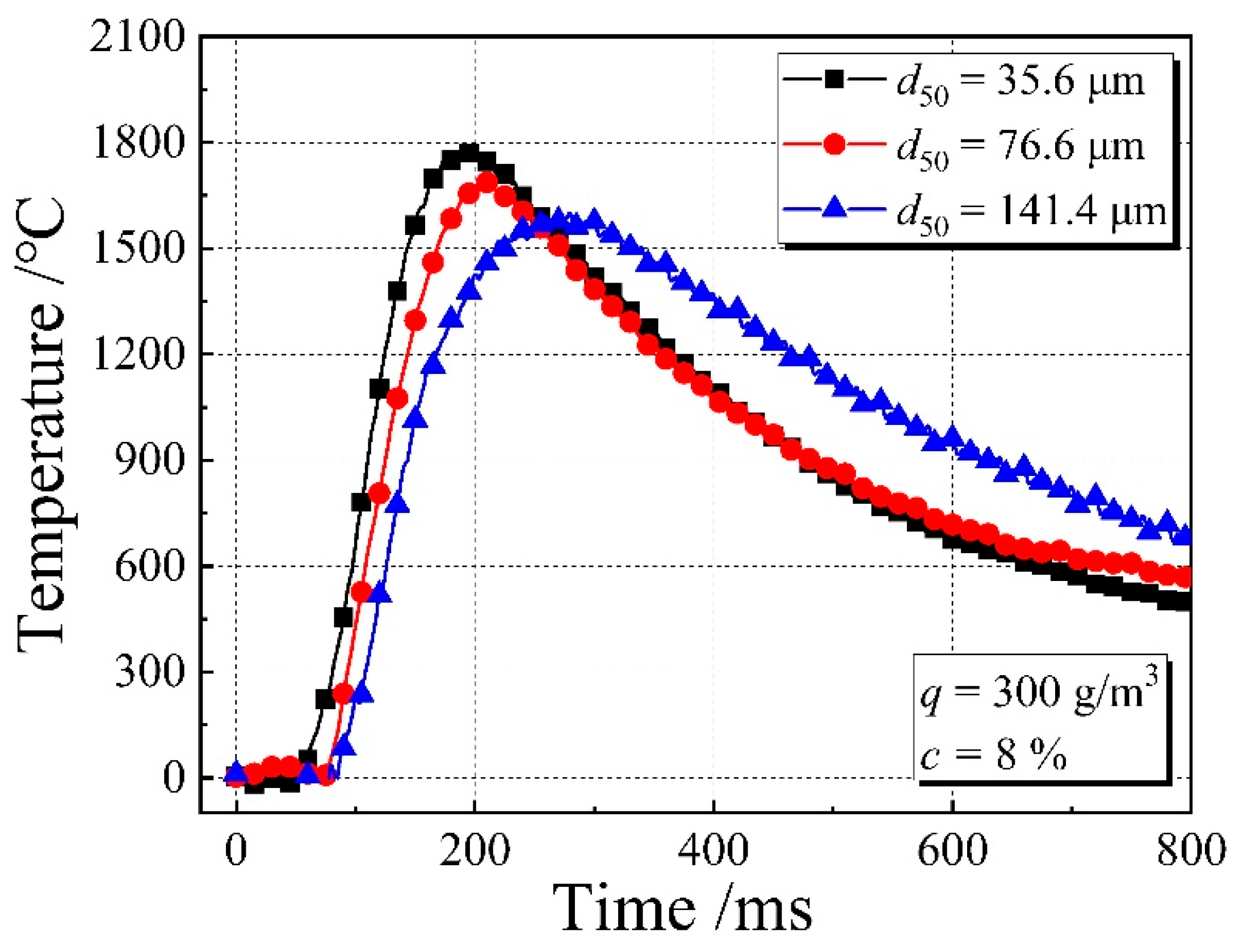
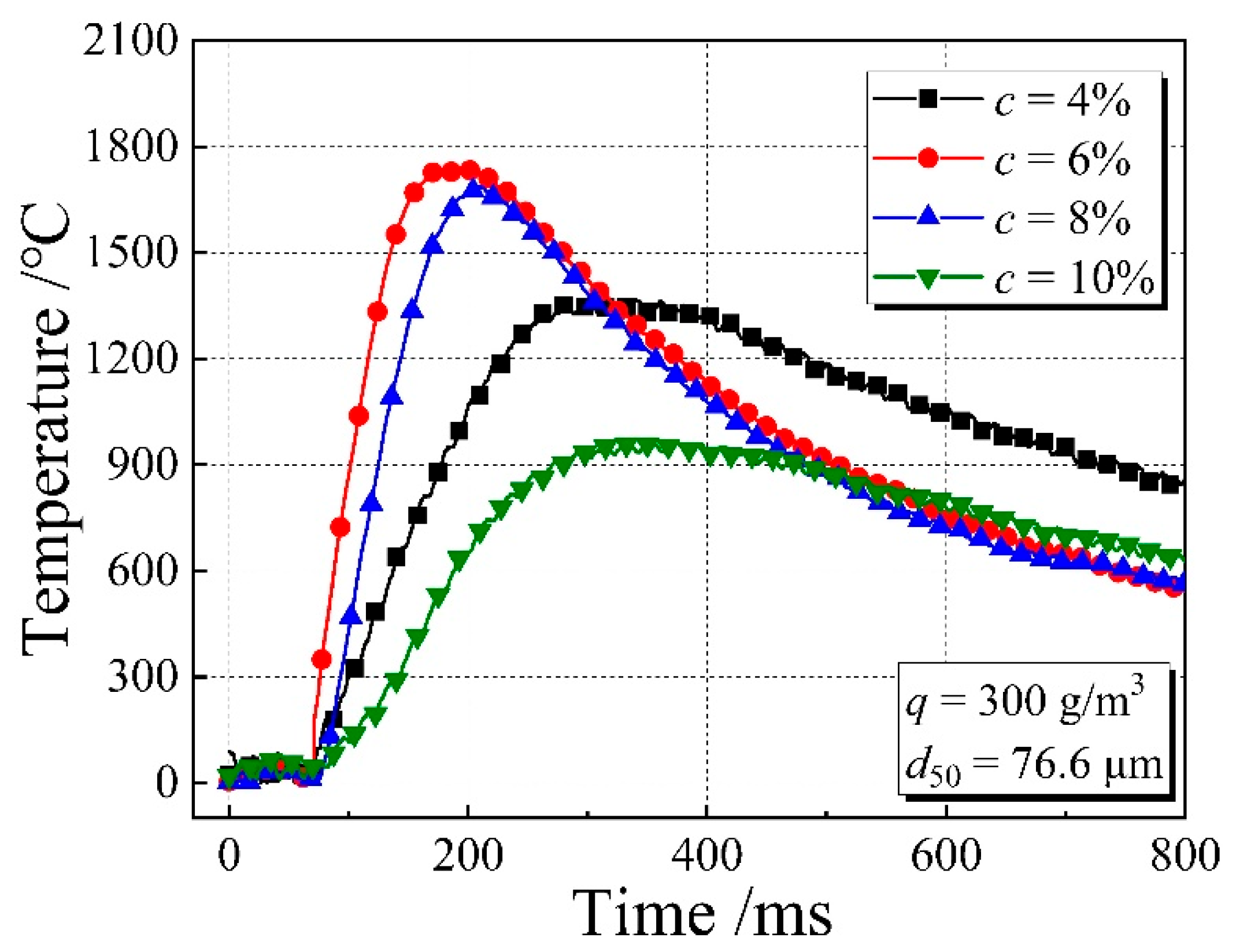
3.4. Flame Temperature
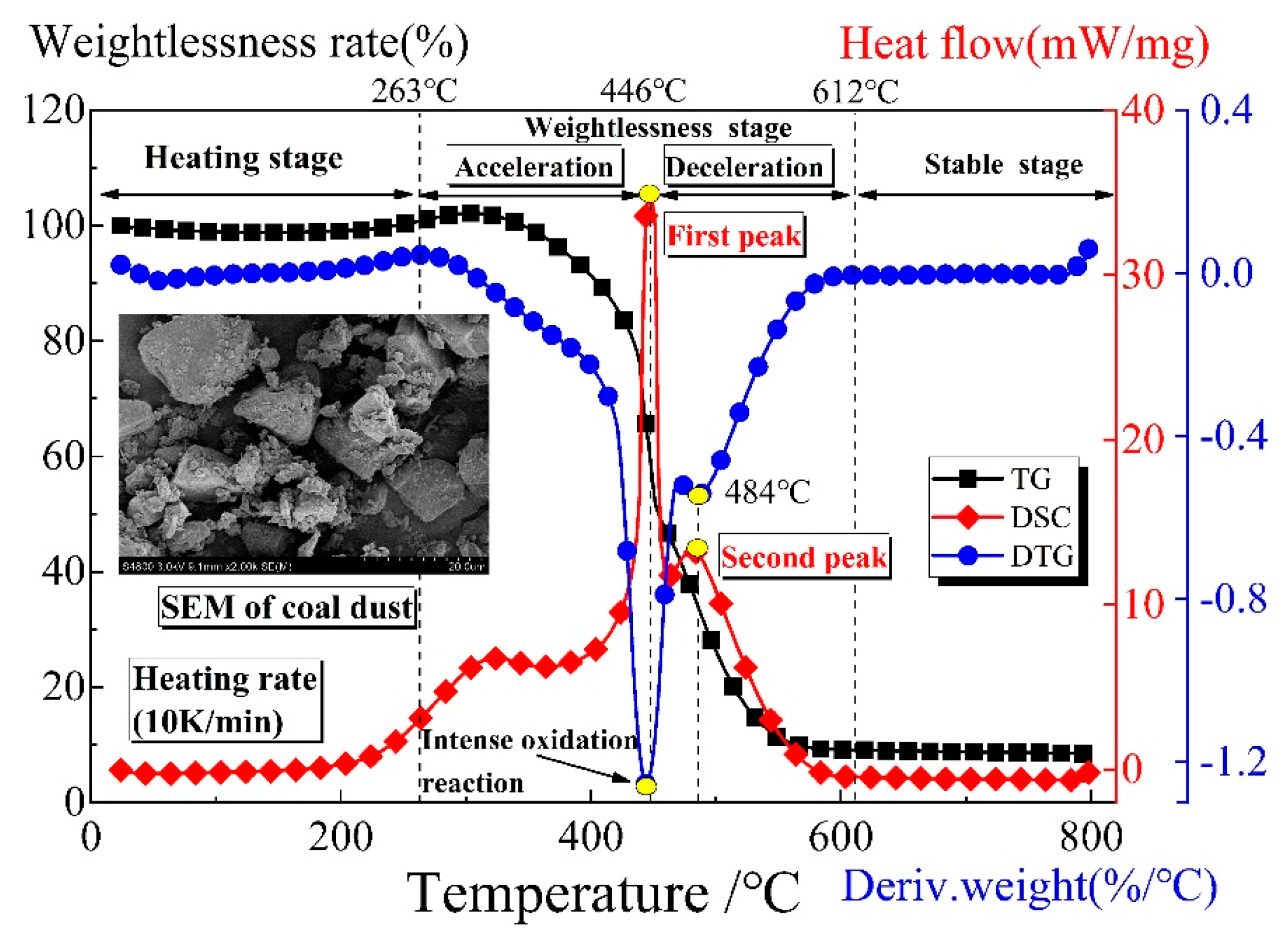
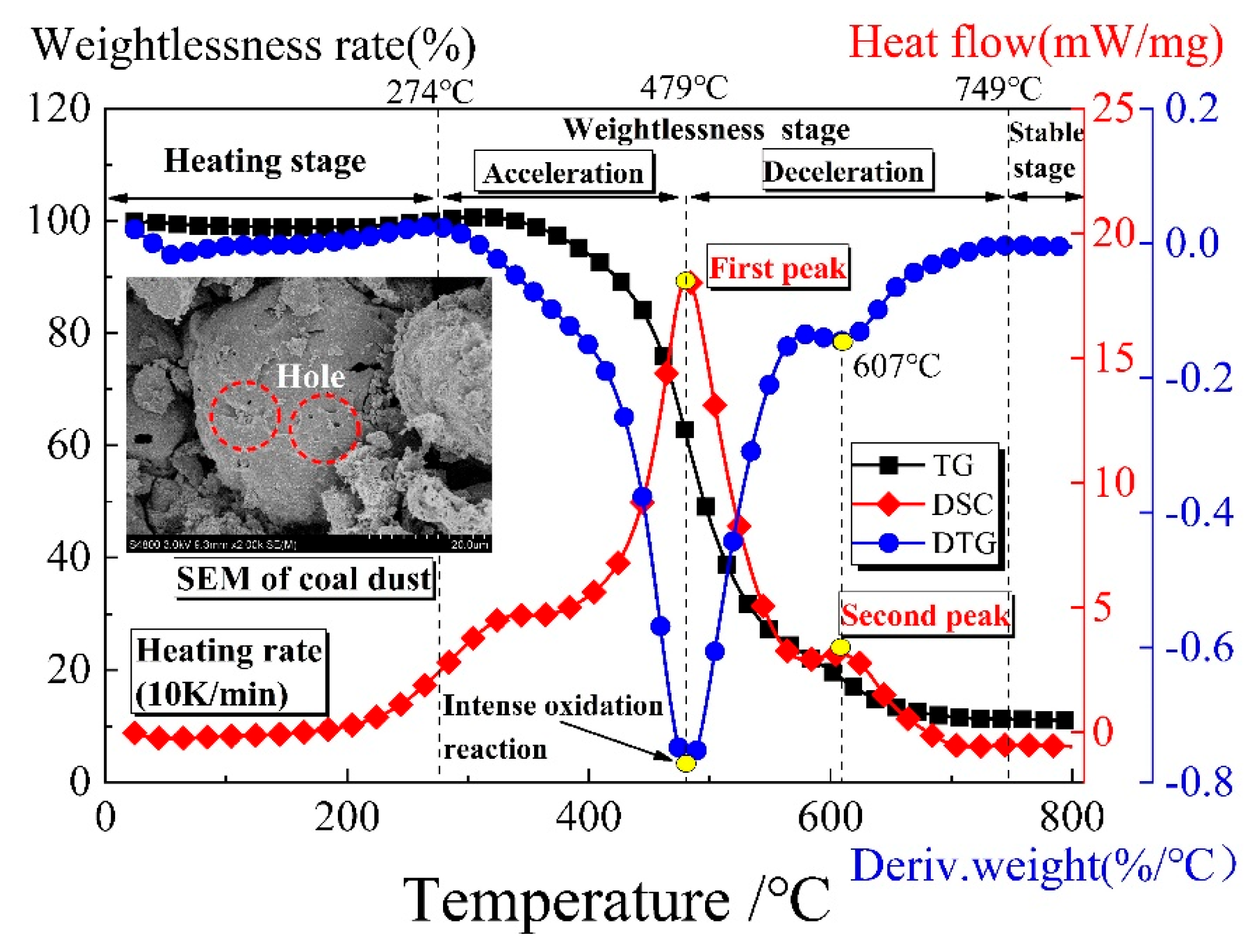
4. Product Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dufaud, O.; Perrin, L.; Traore, M. Dust/vapour explosions: Mixture behaviors. J. Loss Prev. Process Ind. 2008, 21, 481–484. [Google Scholar] [CrossRef]
- Rockwell, S.R.; Rangwala, A.S. Influence of coal dust on premixed turbulent methane air flames. Combust. Flame 2013, 160, 635–640. [Google Scholar] [CrossRef]
- Guo, C.W.; Shao, H.; Jiang, S.G.; Wang, Y.J.; Wang, K.; Wu, Z.Y. Effect of low-concentration coal dust on gas explosion propagation law. Powder Technol. 2020, 367, 243–252. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Z.T.; Qian, J.F.; Li, X.L. Comparison on the explosivity of coal dust and of its explosion solid residues to assess the severity of re-explosion. Fuel 2019, 251, 438–446. [Google Scholar] [CrossRef]
- Li, Q.Z.; Lin, B.Q.; Wang, K.; Zhao, M.Z.; Ruan, M.L. Surface properties of pulverized coal and its effects on coal mine methane adsorption behaviors under ambient conditions. Powder Technol. 2015, 270, 278–286. [Google Scholar] [CrossRef]
- Sazal, K.K.; Jafar, Z.; Daniel, E.; Behdad, M. Explosion severity of methane-coal dust mixture mixtures in a ducted spherical vessel. Powder Technol. 2018, 323, 95–105. [Google Scholar]
- Li, H.T.; Zhai, F.; Li, S.S.; Lou, R.Y.; Wang, F.C.; Chen, X.K.; Chu, C.M.; Yu, M.G. Macromorphological features and formation mechanism of particulate residues from methane/air/coal dust gas-solid two-phase mixture explosions: An approach for material evidence analysis in accident investigation. Fuel 2022, 315, 123209. [Google Scholar] [CrossRef]
- Jiang, H.P.; Bi, M.S.; Gao, Z.H.; Zhang, Z.L.; Gao, W. Effect of turbulence intensity on flame propagation and extinction limits of methane/coal dust explosions. Energy 2022, 239, 122246. [Google Scholar] [CrossRef]
- Li, L.; Qin, B.T.; Liu, J.S.; Leong, Y.K. Integrated experimentation and modeling of the formation processes underlying coal combustion-triggered methane explosions in a mined-out area. Energy 2020, 203, 117855. [Google Scholar] [CrossRef]
- Xiao, F.; Ren, J.J.; Pu, M.Z.; Chen, B.; Bi, M.S. Suppression effect of ultrafine water mist on methane-coal dust mixture explosion. Powder Technol. 2022, 406, 117590. [Google Scholar]
- Da, H.M.; Yin, H.P.; Liang, G.Q. Explosion inhibition of coal dust clouds under coal gasification atmosphere by talc powder. Process Saf. Environ Prot. 2022, 165, 286–294. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, X.F.; Yang, M.J.; Zhang, H.M.; Huang, C.Y.; Dai, H.M.; Li, Y.; Liu, J.; Zhu, H.W. Suppression characteristics and mechanisms of ABC powder on methane/coal dust compound deflagration. Fuel 2021, 298, 120831. [Google Scholar] [CrossRef]
- Wang, X.T.; Dai, H.M.; Liang, G.Q.; Zhu, H.W.; Zhang, B.Q.; He, S.; Zhao, Q.; Yin, H.P.; Chen, X.F. Flame propagation characteristics of mixed pulverized coal at the atmosphere of gasification. Fuel 2021, 300, 120954. [Google Scholar] [CrossRef]
- Sazal, K.K.; Jafar, Z.; Daniel, E.; Nader, M.; Behdad, M. Explosion characteristics of methane-air mixtures in a spherical vessel connected with a duct. Process Saf. Environ. Prot. 2017, 111, 85–93. [Google Scholar]
- Benedetto, A.D.; Garcia-agreda, A.; Russo, P.; Sanchirico, R. Combined effect of ignition energy and initial turbulence on the explosion behavior of lean gas/dust-air mixtures. Ind. Eng. Chem. Res. 2012, 51, 7663–7670. [Google Scholar] [CrossRef]
- Ji, W.T.; Wang, Y.; Yang, J.J.; He, J.; Wen, X.P.; Wang, Y. Methods to predict variations of lower explosion limit associated with mixture mixtures of flammable gas and dust. Fuel 2022, 310, 122138. [Google Scholar] [CrossRef]
- Zhao, P.; Tan, X.; Schmidt, M.; Wei, A.Z.; Huang, W.X.; Qian, X.M.; Wu, D.J. Minimum explosion concentration of coal dusts in air with small amount of CH4/H2/CO under 10-kJ ignition energy conditions. Fuel 2020, 260, 116401. [Google Scholar] [CrossRef]
- Chen, D.L.; Sun, J.H.; Liu, Y. Temperature profiles of the methane-coal dust mixture flame. J. Saf. Environ. 2008, 8, 123–125. [Google Scholar]
- Jing, G.X.; Shao, H.Y.; Wu, Y.L.; Guo, S.S.; Liu, C.; Zhang, S.Q. Experimental study on the influence of different coal species on gas and coal dust explosion. Saf. Coal Mines 2020, 51, 1–5. [Google Scholar]
- Cloney, C.T.; Ripley, R.C.; Pegg, M.J.; Amyotte, P.R. Role of particle diameter in laminar combustion regimes for mixture mixtures of coal dust and methane gas. Powder Technol. 2020, 362, 399–408. [Google Scholar] [CrossRef]
- Gao, C.; Li, H.; Su, D.; Huang, W.X. Explosion characteristics of coal dust in a sealed vessel. Expl Shock wave 2010, 30, 164. [Google Scholar]
- Wang, X.B. Effect of ignition delay time on explosion characteristics of methane coal dust mixture mixtures. Saf. Coal Mines 2020, 51, 23–27. [Google Scholar]
- Addai, E.K.; Gabel, D.; Kamal, M.; Krause, U. Minimum ignition energy of mixture mixtures of combustible dusts and gases. Process Saf. Environ. Prot. 2016, 102, 503–512. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Bi, M.S.; Qi, F. Experimental research into effects of obstacle on methane-coal dust mixture explosion. J. Loss Prev. Process Ind. 2012, 25, 127–130. [Google Scholar] [CrossRef]
- Yu, X.Z.; Zhang, Z.H.; Yan, X.Q.; Wang, Z.; Yu, J.L.; Gao, W. Explosion characteristics and combustion mechanism of hydrogen/tungsten dust hybrid mixtures. Fuel 2023, 332, 126017. [Google Scholar] [CrossRef]
- Ji, W.T.; Gan, X.Y.; Li, L.; Li, Z.; Wen, X.P.; Wang, Y. Prediction of the explosion severity of hybrid mixtures. Powder Technol. 2022, 400, 117273. [Google Scholar] [CrossRef]
- Xiong, X.X.; Gao, K.; Mu, J.; Li, B.; Zhang, D.; Xie, L.F. Study on explosion characteristic parameters and induction mechanism of magnesium powder/hydrogen hybrids. Fuel 2022, 326, 125077. [Google Scholar] [CrossRef]
- Cao, X.Y.; Lu, Y.W.; Jiang, J.C.; Wang, Z.R.; Wei, H.Y.; Li, Y.M. Experimental study on explosion inhibition by heptafluoropropane and its synergy with inert gas. J. Loss Prev. Process Ind. 2021, 71, 104440. [Google Scholar] [CrossRef]
- Cao, X.Y.; Bi, M.S.; Ren, J.J.; Chen, B. Experimental research on explosion suppression affected by ultrafine water mist containing different additives. J. Hazard. Mater. 2019, 368, 613–620. [Google Scholar] [CrossRef]
- Cao, X.Y.; Ren, J.J.; Zhou, Y.H.; Wang, Q.J.; Gao, X.L.; Bi, M.S. Suppression of methane/air explosion by ultrafine water mist containing sodium chloride additive. J. Hazard. Mater. 2015, 285, 311–318. [Google Scholar] [CrossRef]
- Cao, X.Y.; Wei, H.Y.; Wang, Z.R.; Fan, L.T.; Zhou, Y.Q.; Wang, Z. Experimental research on the inhibition of methane/coal dust mixture explosions by the ultrafine water mist. Fuel 2023, 331, 125937. [Google Scholar] [CrossRef]
- Cao, X.Y.; Wang, Z.; Wang, Z.R.; Zhou, Y.Q. Effect of wire mesh structure parameter on the flame propagation characteristics of syngas explosion. Fuel 2023, 334, 126658. [Google Scholar] [CrossRef]
- Cao, X.Y.; Zhou, Y.Q.; Wang, Z.R.; Fan, L.T.; Wang, Z. Experimental research on hydrogen/air explosion inhibition by the ultrafine water mist. Int. J. Hydrogen Energy 2022, 47, 23898–23908. [Google Scholar] [CrossRef]
- Castellanos, D.; Carreto-Vazquez, V.H.; Mashuga, C.V.; Trottier, R.; Mejia, A.F.; Mannan, M.S. The effect of particle size polydispersity on the explosibility characteristics of aluminum dust. Powder Technol. 2014, 254, 331–337. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Huang, C.Y.; Zhang, H.M.; Li, Y.; Chen, X.F. Characteristics of coal dust deflagration under the atmosphere of methane and their inhibition by coal ash. Fuel 2021, 291, 120121. [Google Scholar] [CrossRef]
- Chen, X.F.; Hou, Z.X.; Zhao, Q.; Li, Q.; Li, Y.; Huang, C.Y.; Dai, H.M. Suppression of methane/coal dust deflagration by Al(OH)3 based on flame propagation characteristics and thermal decomposition. Fuel 2022, 311, 122530. [Google Scholar] [CrossRef]
- Zhang, H.M.; Chen, X.F.; Zhang, Y.; Niu, Y.; Yuan, B.H.; Dai, H.M.; He, S. Effects of particle size on flame structures through corn starch dust explosions. J. Loss Prev. Process Ind. 2017, 50, 7–14. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.Y.; Chen, C.; Wang, P.P.; Xu, L.W. Experimental study to assess the explosion hazard of CH4/coal dust mixtures induced by high-temperature source surface. Process Saf. Environ. Prot. 2021, 154, 60–71. [Google Scholar] [CrossRef]
- Li, H.T.; Deng, J.; Chen, X.K.; Shu, C.M.; Kuo, C.H.; Zhai, X.W.; Wang, Q.H.; Hu, X.Y. Qualitative and quantitative characterisation for explosion severity and gaseous-solid residues during methane-coal particle mixture explosions: An approach to estimating the safety degree for underground coal mines. Process Saf. Environ. Prot. 2020, 141, 150–166. [Google Scholar] [CrossRef]
- Du, Y.Y.; Jiang, X.G.; Lv, G.J.; Ma, X.J.; Jin, Y.Q.; Wang, F.; Chi, Y.; Yan, J.H. Thermal behavior and kinetics of bio-ferment residue/coal blends during co-pyrolysis. Energ. Convers. Manag. 2014, 88, 459–463. [Google Scholar] [CrossRef]
- Wang, G.W.; Zhang, J.L.; Shao, J.G.; Liu, Z.J.; Zhang, G.H.; Xu, T.J.; Guo, H.Y.; Xu, R.S.; Lin, H. Thermal behavior and kinetic analysis of co-combustion of waste biomass/low rank coal blends. Energ. Convers. Manag. 2016, 124, 414–426. [Google Scholar] [CrossRef]
- Wang, J.H.; Du, J.; Chang, L.P.; Xie, K.C. Study on the structure and pyrolysis characteristics of Chinese western coals. Fuel Process Technol. 2010, 91, 430–433. [Google Scholar] [CrossRef]
- Meng, X.L.; Gao, M.Q.; Chu, R.Z.; Wu, G.G.; Fang, Q. Multiple linear equation of pore structure and coal-oxygen diffusion on low temperature oxidation process of lignite. Chin. J. Chem. Eng. 2016, 24, 818–823. [Google Scholar] [CrossRef]
- Liu, Z.T.; Zhang, S.S.; Xi, R.Z.; Guo, R.L.; Liu, B.B.; Lin, S. Analysis on residual gas characteristics of coal dust explosion in confined space. J. Coal Sci. 2015, 40, 1574–1579. [Google Scholar]
- Fu, W.B. Coal Combustion Theory and Its Macro General Law; Tsinghua University Press: Beijing, China, 2003. [Google Scholar]
- Nie, B.S.; Liu, X.F.; Yang, L.L.; Meng, J.Q.; Li, X.C. Pore structure characterization of different rank coals using gas adsorption and scanning electron microscopy. Fuel 2015, 158, 908–917. [Google Scholar] [CrossRef]
- Li, H.Y.; Ogawa, Y.; Shimada, S. Mechanism of methane flow through sheared coals and its role on methane recovery. Fuel 2003, 82, 1271–1279. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Gan, B.; Jiang, H.P.; Huang, L.; Gao, W. Investigations on the flame propagation characteristics in methane and coal dust mixture explosions. Shock Waves 2022, 42, 167–175. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Z.; Cao, X.; Wei, H. Study on the Characteristics and Influence Factor of Methane and Coal Dust Gas/Solid Two-Phase Mixture Explosions. Fire 2023, 6, 359. https://doi.org/10.3390/fire6090359
Wang Y, Wang Z, Cao X, Wei H. Study on the Characteristics and Influence Factor of Methane and Coal Dust Gas/Solid Two-Phase Mixture Explosions. Fire. 2023; 6(9):359. https://doi.org/10.3390/fire6090359
Chicago/Turabian StyleWang, Yue, Zhi Wang, Xingyan Cao, and Haoyue Wei. 2023. "Study on the Characteristics and Influence Factor of Methane and Coal Dust Gas/Solid Two-Phase Mixture Explosions" Fire 6, no. 9: 359. https://doi.org/10.3390/fire6090359
APA StyleWang, Y., Wang, Z., Cao, X., & Wei, H. (2023). Study on the Characteristics and Influence Factor of Methane and Coal Dust Gas/Solid Two-Phase Mixture Explosions. Fire, 6(9), 359. https://doi.org/10.3390/fire6090359





