Nitrated Phenols and PM2.5 Reduction of High-Sodium Coal Combustion by Diatomite Addition in a Typical Residential Stove
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Combustion Scheme
2.2. Sampling Method
2.3. Calculation Method of PM and Gaseous Pollutants EFs, and TE
2.4. Phenolics and Nitrated Phenols Analysis
2.5. Calculation Method of PM and Gaseous Pollutants EFs, and TE
3. Results
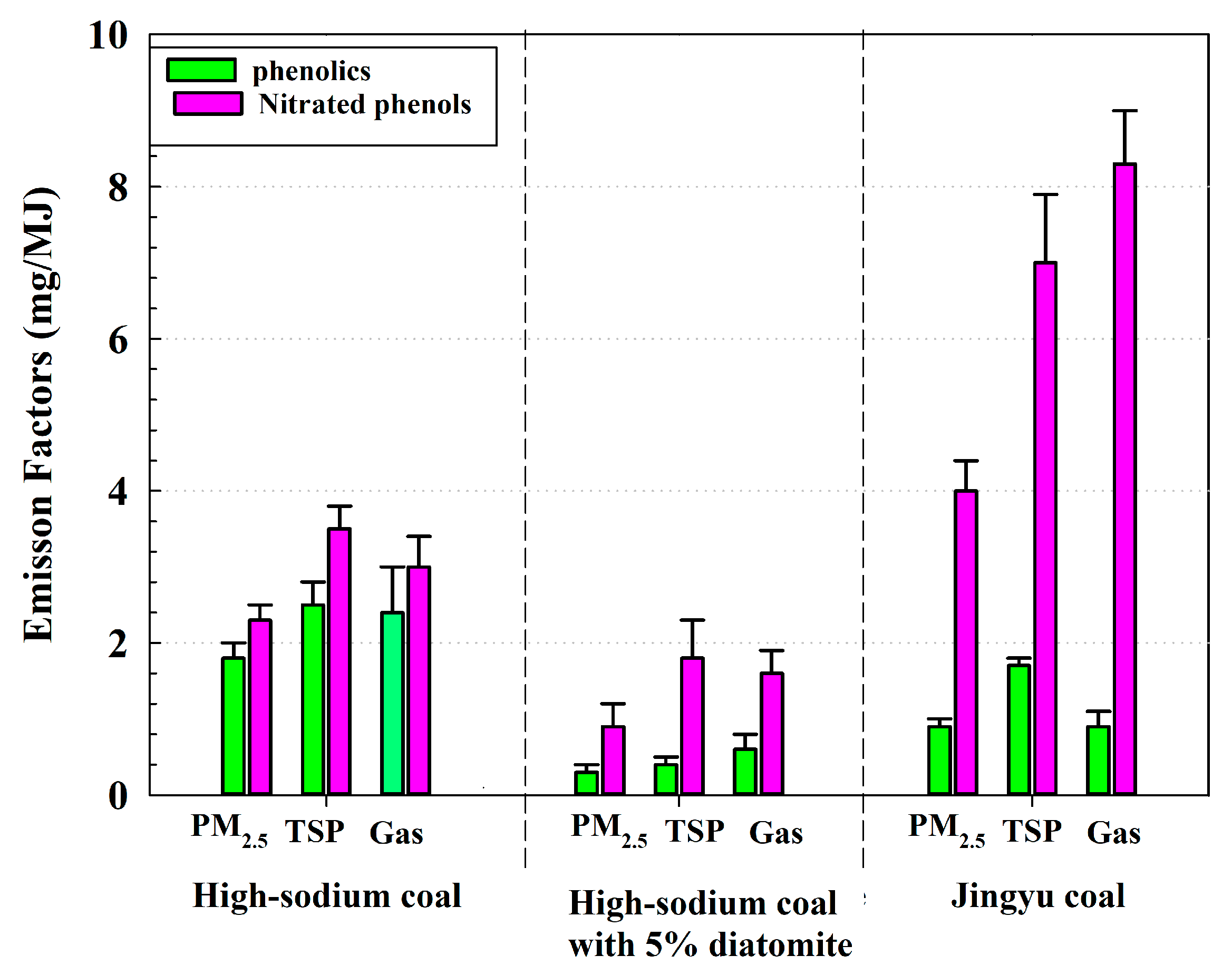
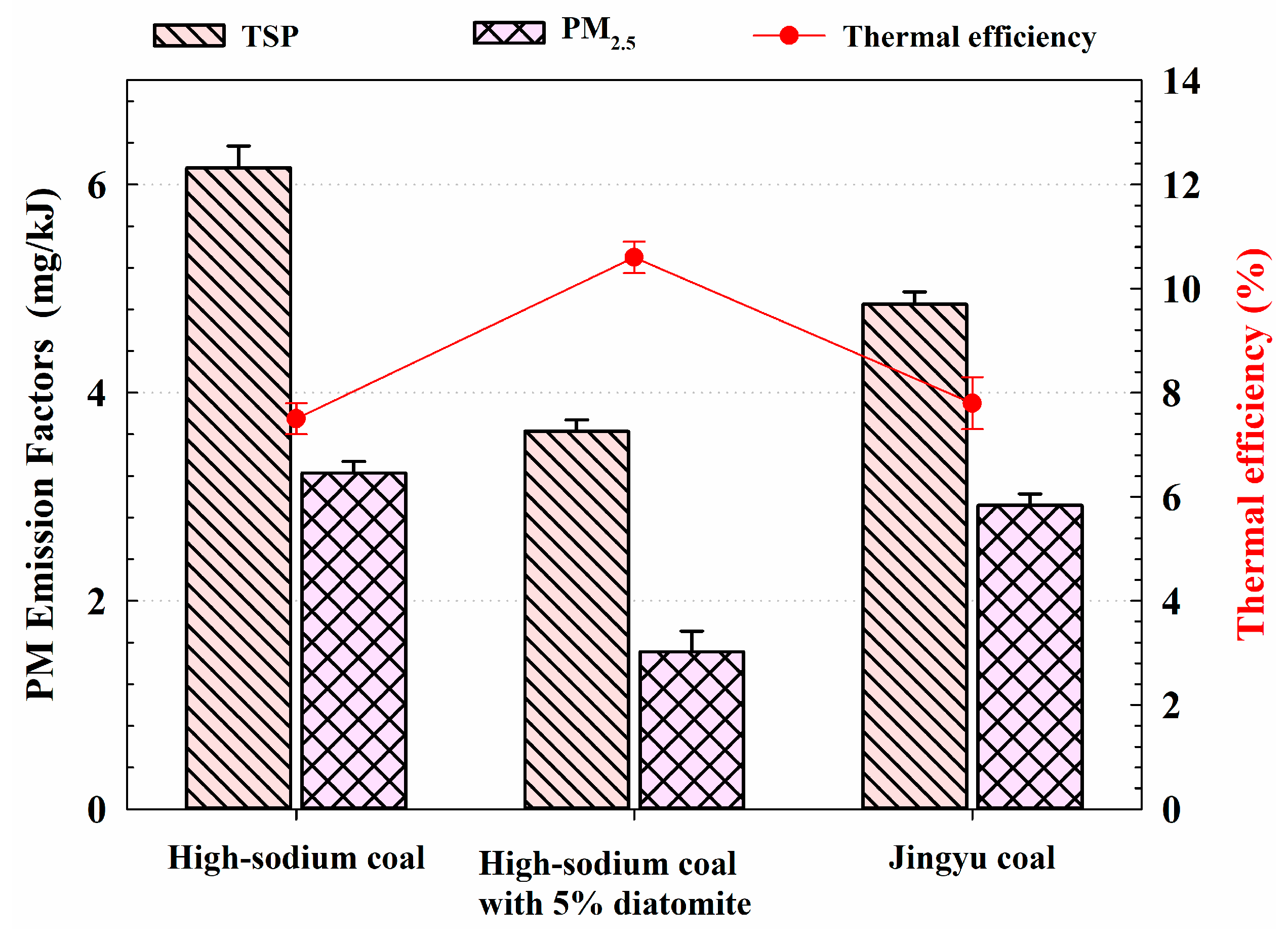
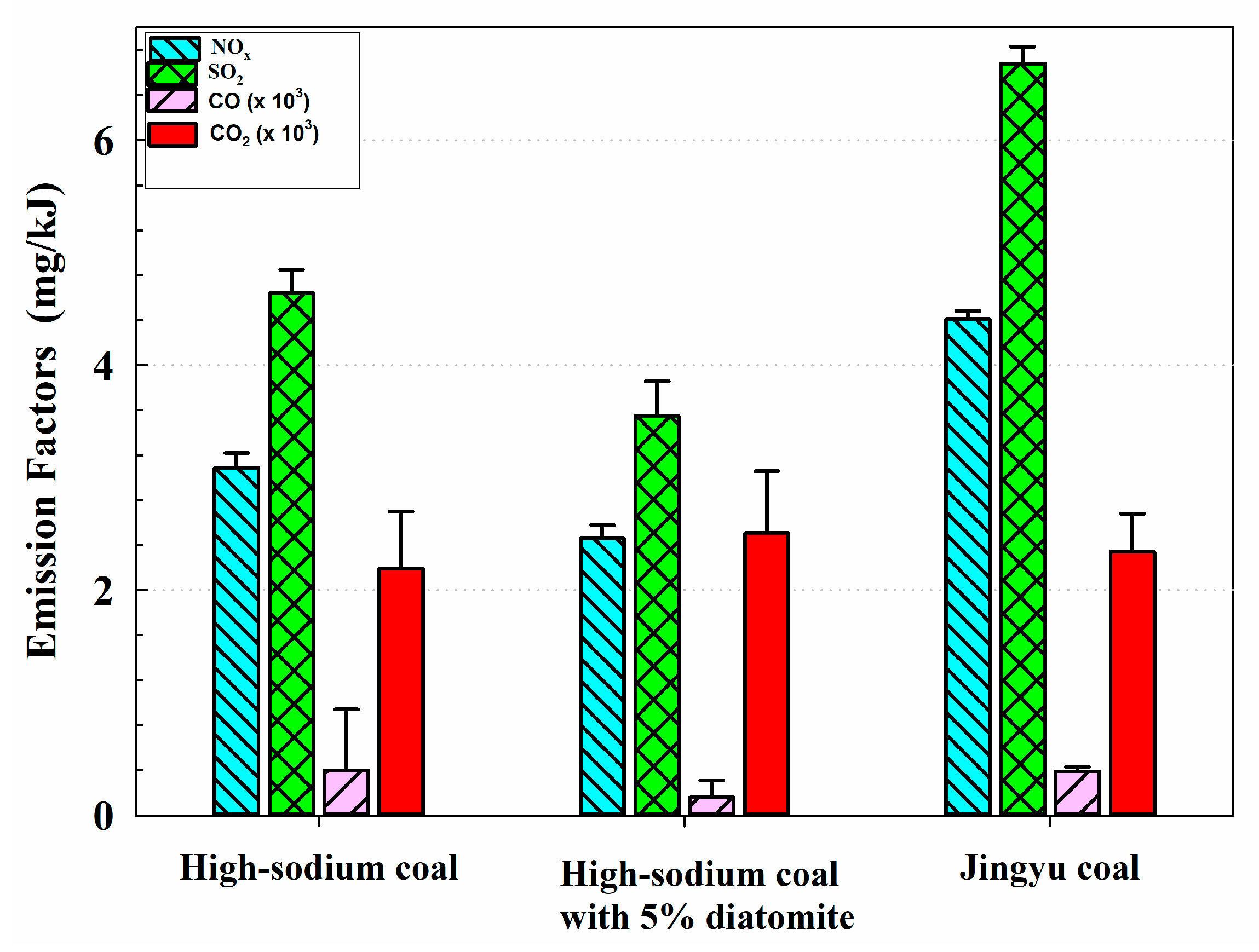
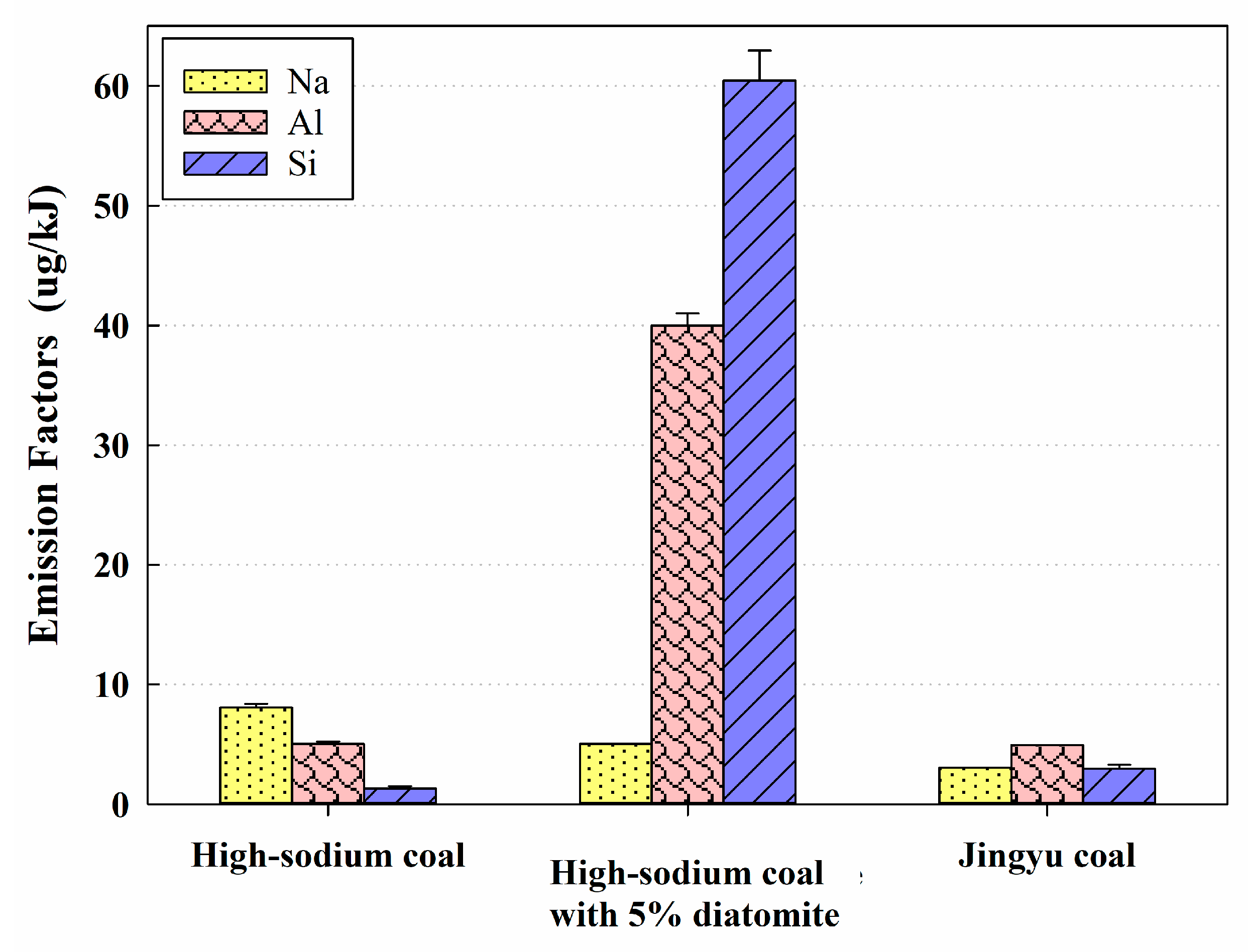
3.1. High-Sodium Coal: Lower EFs of Nitrated Phenols but Higher EFs of PM
3.2. High-Sodium Coal with 5% Diatomite: Effects of Diatomite on Reduction EFs of PM and Nitrated Phenols
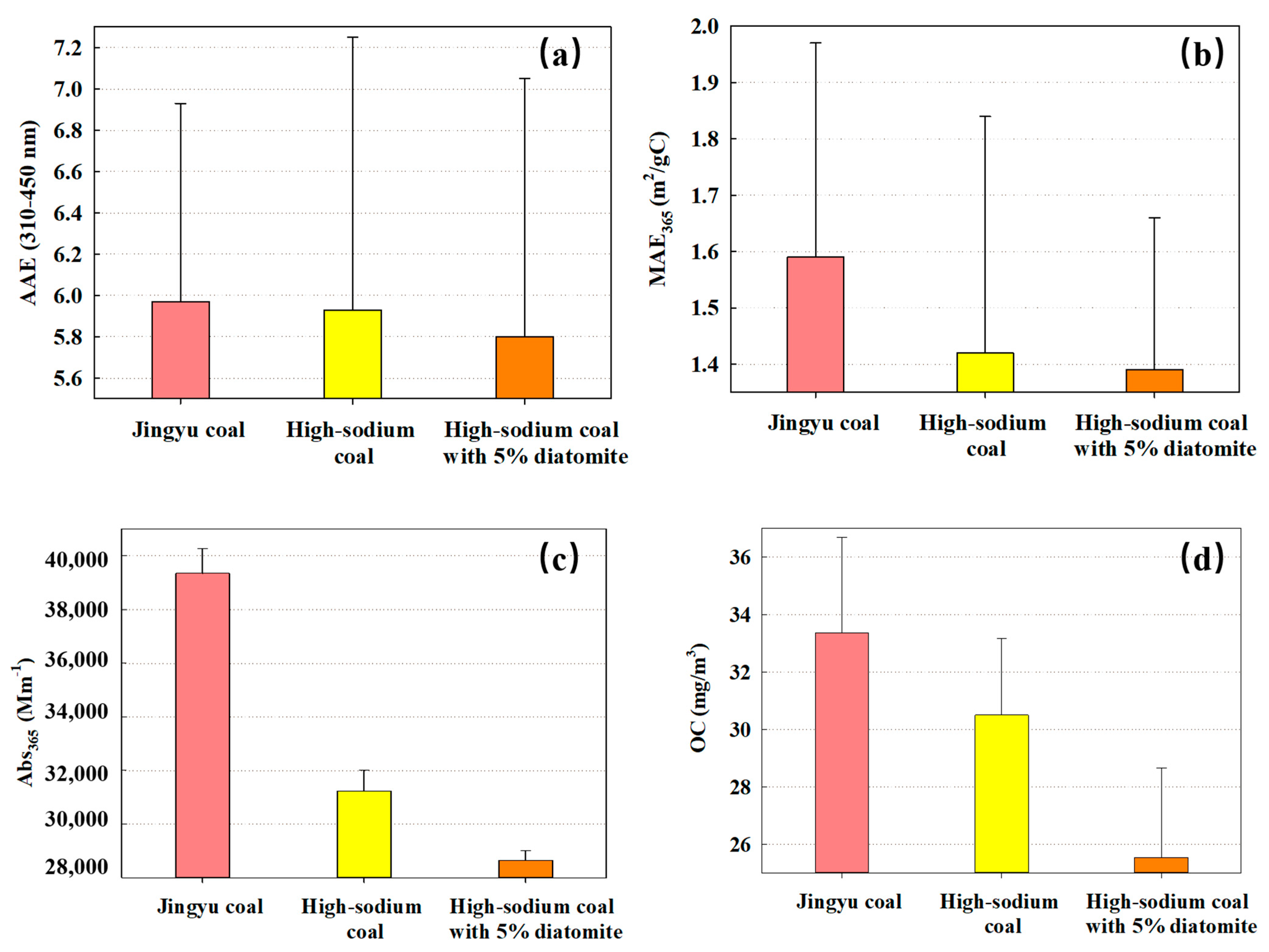
3.3. Optical Characteristics of Methanol Soluble Organic from PM2.5
4. Discussion
5. Conclusions
- (1)
- High-sodium coal discharged fewer nitrated phenols but higher PM EFs compared with Jinyu coal with less Na content. However, the PM of high-sodium coal was substantially promoted because the more easily vaporized component of Na became more condensation nuclei of particles.
- (2)
- Diatomite can dramatically decrease the PM emission caused by Na and improve the combustion performance of high-sodium coal. After 5% diatomite was added into the high-sodium coal, PM2.5 EFS sank to as low as 46.7%, and its EFs of phenolics and nitrated phenols also dropped significantly compared with high-sodium coal. The new mixture has therefore a superior combustion performance over Jingyu coal and high-sodium coal.
- (3)
- High-sodium coal with 5% diatomite is indeed an ideal solid fuel due to its advantages in nitrated phenols and PM reduction compared with Jingyu coal. Therefore, high-sodium coal is not only a conventional energy source in power plants, but also an environmentally friendly and promising fuel for residential combustion when mixed with 5% diatomite.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffer, A.; Gelencsér, A.; Guyon, P.; Kiss, G.; Schmid, O.; Frank, G.P.; Artaxo, P.; Andreae, M.O. Optical properties of humic-like substances (HULIS) in biomass-burning aerosols. Atmos. Chem. Phys. 2006, 6, 3563–3570. [Google Scholar] [CrossRef]
- Kirchstetter, T.W.; Novakov, T.; Hobbs, P.V. Evidence that the spectral dependence of light absorption by aerosols is affected by organic carbon. J. Geophys. Res. Atmos. 2004, 109, D21208. [Google Scholar] [CrossRef]
- Sun, J.; Xie, C.; Xu, W.; Chen, C.; Sun, Y. Light absorption of black carbon and brown carbon in winter in North China Plain: Comparisons between urban and rural sites. Sci. Total. Environ. 2021, 770, 144821. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Gu, R.; Hao, W.; Wang, W. Observations of fine particulate nitrated phenols in four sites in northern China: Concentrations, source apportionment, and secondary formation. Atmos. Chem. Phys 2018, 18, 4349–4359. [Google Scholar] [CrossRef]
- Teich, M.; Pinxteren, D.V.; Wang, M.; Kecorius, S.; Wang, Z.; Müller, T.; Mocnik, G.; Herrmann, H. Contributions of nitrated aromatic compounds to the light absorption of water-soluble and particulate brown carbon in different atmospheric environments in Germany and China. Atmos. Chem. Phys. 2017, 17, 1653–1672. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Lu, C.; Li, R.; Zhang, J.; Dong, S.; Yang, L.; Xue, L.; Chen, J.; Wang, W. Nitrated phenols and the phenolic precursors in the atmosphere in urban Jinan, China. Sci. Total. Environ. 2020, 714, 136760. [Google Scholar] [CrossRef]
- Xie, M.; Chen, X.; Hays, M.; Lewandowski, M.; Offenberg, J.H.; Kleindienst, T.E.; Holder, A.L. Light Absorption of Secondary Organic Aerosol: Composition and Contribution of Nitroaromatic Compounds. Environ. Sci. Technol. 2017, 51, 11607–11616. [Google Scholar] [CrossRef]
- Karim, K.; Gupta, S.K. Effects of alternative carbon sources on biological transformation of nitrophenols. Biodegradation 2002, 13, 353–360. [Google Scholar] [CrossRef]
- Natangelo, M.; Mangiapan, S.; Bagnati, R.; Benfenati, E.; Fanelli, R. Increased concentrations of nitrophenols in leaves from a damaged forestal site. Chemosphere 1999, 38, 1495–1503. [Google Scholar] [CrossRef]
- Bandowe, B.; Wei, C.; Han, Y.; Cao, J.; Zhan, C.; Wilcke, W. Polycyclic aromatic compounds (PAHs, oxygenated PAHs, nitrated PAHs and azaarenes) in soils from China and their relationship with geographic location, land use and soil carbon fractions. Sci. Total. Environ. 2019, 690, 1268–1276. [Google Scholar] [CrossRef]
- Lu, C.; Wang, X.; Dong, S.; Zhang, J.; Li, J.; Zhao, Y.; Liang, Y.; Xue, L.; Xie, H.; Zhang, Q.; et al. Emissions of fine particulate nitrated phenols from various on-road vehicles in China. Environ. Res. 2019, 179, 108709. [Google Scholar] [CrossRef]
- Bond; Tami, C. Primary particle emissions from residential coal burning: Optical properties and size distributions. J. Geophys. Res. 2002, 107, 8347. [Google Scholar]
- Li, Q.; Qi, J.; Jiang, J.; Wu, J.; Duan, L.; Wang, S.; Hao, J. Significant reduction in air pollutant emissions from household cooking stoves by replacing raw solid fuels with their carbonized products. Sci. Total. Environ. 2019, 650, 653–660. [Google Scholar] [CrossRef]
- Sun, J.; Zhi, G.; Hitzenberger, R.; Chen, Y.; Tian, C.; Zhang, Y.; Feng, Y.; Cheng, M.; Zhang, Y.; Cai, J. Emission factors and light absorption properties of brown carbon from household coal combustion in China. Atmos. Chem. Phys. 2017, 17, 4769–4780. [Google Scholar] [CrossRef]
- Li, M.; Fan, X.; Zhu, M.; Zou, C.; Song, J.; Wei, S.; Jia, W.; Peng, P.A. Abundance and Light Absorption Properties of Brown Carbon Emitted from Residential Coal Combustion in China. Environ. Sci. Technol. 2019, 53, 595–603. [Google Scholar] [CrossRef]
- Cui, M.; Xu, Y.; Yu, B.; Liu, L.; Li, J.; Chen, Y. Characterization of carbonaceous substances emitted from residential solid fuel combustion using real-world data from the Beijing-Tianjin-Hebei region. Sci. Total. Environ. 2022, 837, 155529. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, G.; Shi, L.; Li, H.; Lang, D.; Zhang, L.; Pan, B.; Tao, S. Real-World Emission Characteristics of Environmentally Persistent Free Radicals in PM2.5 from Residential Solid Fuel Combustion. Environ. Sci. Technol. 2022, 56, 3997–4004. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Qi, J.; Li, Q.; Chen, J.; Chen, J. Extreme Exposure Levels of PCDD/Fs Inhaled from Biomass Burning Activity for Cooking in Typical Rural Households. Environ. Sci. Technol. 2021, 55, 7299–7306. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zheng, M.; Bosch, C.; Andersson, A.; Desyaterik, Y.; Sullivan, A.P.; Collett, J.L.; Zhao, B.; Wang, S.; He, K. Important fossil source contribution to brown carbon in Beijing during winter. Sci. Rep. 2017, 7, 43182. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, X.; Li, R.; Gu, R.; Zhang, Y.; Li, W.; Gao, R.; Chen, B.; Xue, L.; Wang, W. Emissions of fine particulate nitrated phenols from residential coal combustion in China. Atmos. Environ. 2019, 203, 10–17. [Google Scholar] [CrossRef]
- Bolzacchini, E.; Bruschi, M.; Hjorth, J.; Meinardi, S.; Rosenbohm, E. Gas-phase reaction of phenol with NO3. Environ. Sci. Technol. 2001, 35, 1791–1797. [Google Scholar] [CrossRef]
- Kelly, J.L.; Michelangeli, D.V.; Makar, P.A.; Hastie, D.R.; Mozurkewich, M.; Auld, J. Aerosol speciation and mass prediction from toluene oxidation under high NOx conditions. Atmos. Environ. 2010, 44, 361–369. [Google Scholar] [CrossRef]
- Heal, M.R.; Harrison, M.; Cape, J.N. Aqueous-phase nitration of phenol by N2O5 and ClNO2. Atmos. Environ. 2007, 41, 3515–3520. [Google Scholar] [CrossRef]
- Jacobson; Mark, Z. Isolating nitrated and aromatic aerosols and nitrated aromatic gases as sources of ultraviolet light absorption. J. Geophys. Res. Atmos. 1999, 104, 3527–3542. [Google Scholar] [CrossRef]
- Harrison, M.; Barra, S.; Borghesi, D.; Vione, D.; Arsene, C.; Olariu, R.I. Nitrated phenols in the atmosphere: A review. Atmos. Environ. 2005, 39, 231–248. [Google Scholar] [CrossRef]
- Lu, J.W.; Flores, J.M.; Lavi, A.; Abo-Riziq, A.; Rudich, Y. Changes in the optical properties of benzo[a]pyrene-coated aerosols upon heterogeneous reactions with NO2 and NO3. Phys. Chem. Chem. Phys. 2011, 13, 6484–6492. [Google Scholar] [CrossRef] [PubMed]
- Lüttke, J.; Levsen, K. Phase partitioning of phenol and nitrophenols in clouds. Atmos. Environ. 1997, 31, 2649–2655. [Google Scholar] [CrossRef]
- Song, G.; Song, W.; Qi, X.; Lu, Q. Transformation Characteristics of Sodium of Zhundong Coal Combustion/Gasification in Circulating Fluidized Bed. Energy Fuels 2016, 30, 3473–3478. [Google Scholar] [CrossRef]
- Yu, K.; Chen, X.; Cai, T.; Tang, H.; Liang, C. The effect of Kaolinite’s structure on migration and release characteristics of sodium under oxy-fuel combustion condition. Fuel 2020, 277, 118154. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Xing, H.; Wang, G.; Deng, H.; Hu, H.; Li, X.; Yao, H. Correlations between the sodium adsorption capacity and the thermal behavior of modified kaolinite during the combustion of Zhundong coal. Fuel 2019, 237, 170–177. [Google Scholar] [CrossRef]
- Zheng, L.; Jin, J.; Liu, Z.; Kou, X.; He, X.; Shen, L. Ash formation characteristics in co-combusting coagulation sludge and Zhundong coal. Fuel 2022, 311, 122571. [Google Scholar] [CrossRef]
- Yang, T.; Wang, X.; Tan, H.; Wei, B.; Deng, S.; Zhang, L.; Zhang, H. Existence and release of sodium in Zhundong coal: Effects of treating temperature and silica additives. Int. J. Oil Gas Coal Technol. 2016, 11, 63–74. [Google Scholar] [CrossRef]
- Wang, X.; Ruan, R.; Yang, T.; Adeosun, A.; Zhang, L.; Wei, B.; Tan, H.; Axelbaum, R.L. Sulfate Removal by Kaolin Addition To Address Fouling in a Full-Scale Furnace Burning High-Alkaline Zhundong Coal. Energy Fuels 2017, 31, 12823–12830. [Google Scholar] [CrossRef]
- Qi, J.; Li, Q.; Wu, J.; Jiang, J.; Miao, Z.; Li, D. Biocoal Briquettes Combusted in a Household Cooking Stove: Improved Thermal Efficiencies and Reduced Pollutant Emissions. Environ. Sci. Technol. 2017, 51, 1886–1892. [Google Scholar] [CrossRef]
- Qi, J.; Wu, J.; Zhang, L. Influence of Molding Technology on Thermal Efficiencies and Pollutant Emissions from Household Solid Fuel Combustion during Cooking Activities in Chinese Rural Areas. Symmetry 2021, 13, 2223. [Google Scholar] [CrossRef]
- Qi, J.; Liu, L.; Wu, J. Improving Combustion Technology for Cooking Activities for Pollutant Emission Reduction and Carbon Neutrality. Atmosphere 2022, 13, 561. [Google Scholar] [CrossRef]
- Liu, Y.; Che, D.F.; Xu, T.M. Effects of minerals and sodium addition on nitrogen release during coal combustion. Zhongguo Dianji Gongcheng Xuebao/Proc. Chin. Soc. Electr. Eng. 2005, 25, 136–141. [Google Scholar]
- Ma, P.; Huang, Q.; Gao, Q.; Li, S. Effects of Na and Fe on the formation of coal-derived soot in a two-stage flat-flame burner. Fuel 2020, 265, 116914. [Google Scholar] [CrossRef]
- Castoldi, L.; Matarrese, R.; Lietti, L.; Forzatti, P. Intrinsic reactivity of alkaline and alkaline-earth metal oxide catalysts for oxidation of soot. Appl. Catal. B 2009, 90, 278–285. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.a.; Yan, Y.; Jin, X.; Liu, Y.; Che, D. Release and transformation of sodium during combustion of Zhundong coals. J. Energy Inst. 2016, 89, 48–56. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, L.; Zhao, Y.; Ji, J.; Wang, Q.; Luo, Z.; Bai, Y. Transformation behavior of alkali metals in high-alkali coals. Fuel Process. Technol. 2018, 169, 288–294. [Google Scholar] [CrossRef]
- Li, X.; Tsona, N.T.; Lin, D. Relative Humidity Changes the Role of SO2 in Biogenic Secondary Organic Aerosol Formation. J. Phys. Chem. Lett. 2021, 12, 7365–7372. [Google Scholar]
- Laiman, V.; Hsiao, T.-C.; Wang, Y.-H.; Young, L.-H.; Chao, H.-R.; Lin, T.-H.; Heriyanto, D.S.; Chuang, H.-C. Contributions of acidic ions in secondary aerosol to PM2.5 bioreactivity in an urban area. Atmos. Environ. 2022, 275, 119001. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Q.; Cheng, X.; Mohr, C.; Cai, J.; Huang, W.; Shrivastava, M.; Ye, P.; Fu, P.; Shi, X. Precursors and Pathways Leading to Enhanced Secondary Organic Aerosol Formation during Severe Haze Episodes. Environ. Sci. Technol. 2021, 55, 15680–15693. [Google Scholar] [CrossRef] [PubMed]
- Köpsel, R.F.W.; Halang, S. Catalytic influence of ash elements on NOx formation in char combustion under fluidized bed conditions. Fuel 1997, 76, 345–351. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Sheng, X.; Li, N.; Ping, Q. Adsorption and Release Kinetics, Equilibrium, and Thermodynamic Studies of Hymexazol onto Diatomite. ACS Omega 2020, 5, 29504–29512. [Google Scholar] [CrossRef]
- Yu, W.; Deng, L.; Yuan, P.; Liu, D.; Yuan, W.; Liu, P.; He, H.; Li, Z.; Chen, F. Surface silylation of natural mesoporous/macroporous diatomite for adsorption of benzene. J. Colloid Interface Sci. 2015, 448, 545–552. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Yu, M.; Huang, X.; Zhong, J.; Huang, W.; Chen, R. Elaborate control over the morphology and pore structure of porous silicas for VOCs removal with high efficiency and stability. Adsorption 2017, 23, 37–50. [Google Scholar] [CrossRef]
- Shadman, F.; Punjak, W.A. Thermochemistry of alkali interactions with refractory adsorbents. Thermochim. Acta 1988, 131, 141–152. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, S.Y.; Liu, D.H.; Guo, X.; Dong, A.X.; Xiong, S.W.; Shi, D.Z.; Lü, J.F. Existence form of sodium in high sodium coals from Xinjiang and its effect on combustion process. J. Fuel Chem. Technol. 2013, 41, 832–838. [Google Scholar]
- Ma, D.; Jia, S.; Hu, Z.; Wang, X.; Li, L.; Tan, H.; ur Rahman, Z. Experimental investigation of water washing effect on high-chlorine coal properties. Fuel 2022, 319, 123838. [Google Scholar] [CrossRef]
| Parameter | Mad a (%) | Mt a (%) | Ad b (%) | Vd b (%) | FCd b (%) | St, d b (%) | Nad c (%) | Cad c (%) | Oad c (%) | Had c (%) | Qnet, ar a (MJ/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| High-sodium coal | 15.30 | 20.42 | 11.19 | 41.51 | 42.18 | 0.49 | 0.76 | 53.91 | 16.80 | 4.27 | 24.58 |
| Jingyu coal | 1.92 | 1.19 | 39.68 | 23.36 | 36.23 | 1.69 | 0.89 | 48.45 | 7.69 | 3.35 | 17.18 |
| Coal | SiO2(%) | Al2O3 (%) | Fe2O3 (%) | CaO (%) | MgO (%) | Na2O (%) | K2O (%) | TiO2 (%) | SO3 (%) |
|---|---|---|---|---|---|---|---|---|---|
| High-sodium coal | 20.85 | 13.44 | 4.55 | 36.70 | 6.43 | 6.76 | 0.66 | 0.31 | 8.75 |
| Jingyu coal | 56.78 | 27.43 | 1.60 | 2.2 | 0.86 | 0.18 | 0.38 | 1.92 | 1.35 |
| Diatomite | 78.56 | 9.98 | 5.87 | 0 | 0.87 | 0.58 | 1.36 | 0.92 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Wu, J. Nitrated Phenols and PM2.5 Reduction of High-Sodium Coal Combustion by Diatomite Addition in a Typical Residential Stove. Fire 2023, 6, 89. https://doi.org/10.3390/fire6030089
Qi J, Wu J. Nitrated Phenols and PM2.5 Reduction of High-Sodium Coal Combustion by Diatomite Addition in a Typical Residential Stove. Fire. 2023; 6(3):89. https://doi.org/10.3390/fire6030089
Chicago/Turabian StyleQi, Juan, and Jianjun Wu. 2023. "Nitrated Phenols and PM2.5 Reduction of High-Sodium Coal Combustion by Diatomite Addition in a Typical Residential Stove" Fire 6, no. 3: 89. https://doi.org/10.3390/fire6030089
APA StyleQi, J., & Wu, J. (2023). Nitrated Phenols and PM2.5 Reduction of High-Sodium Coal Combustion by Diatomite Addition in a Typical Residential Stove. Fire, 6(3), 89. https://doi.org/10.3390/fire6030089





