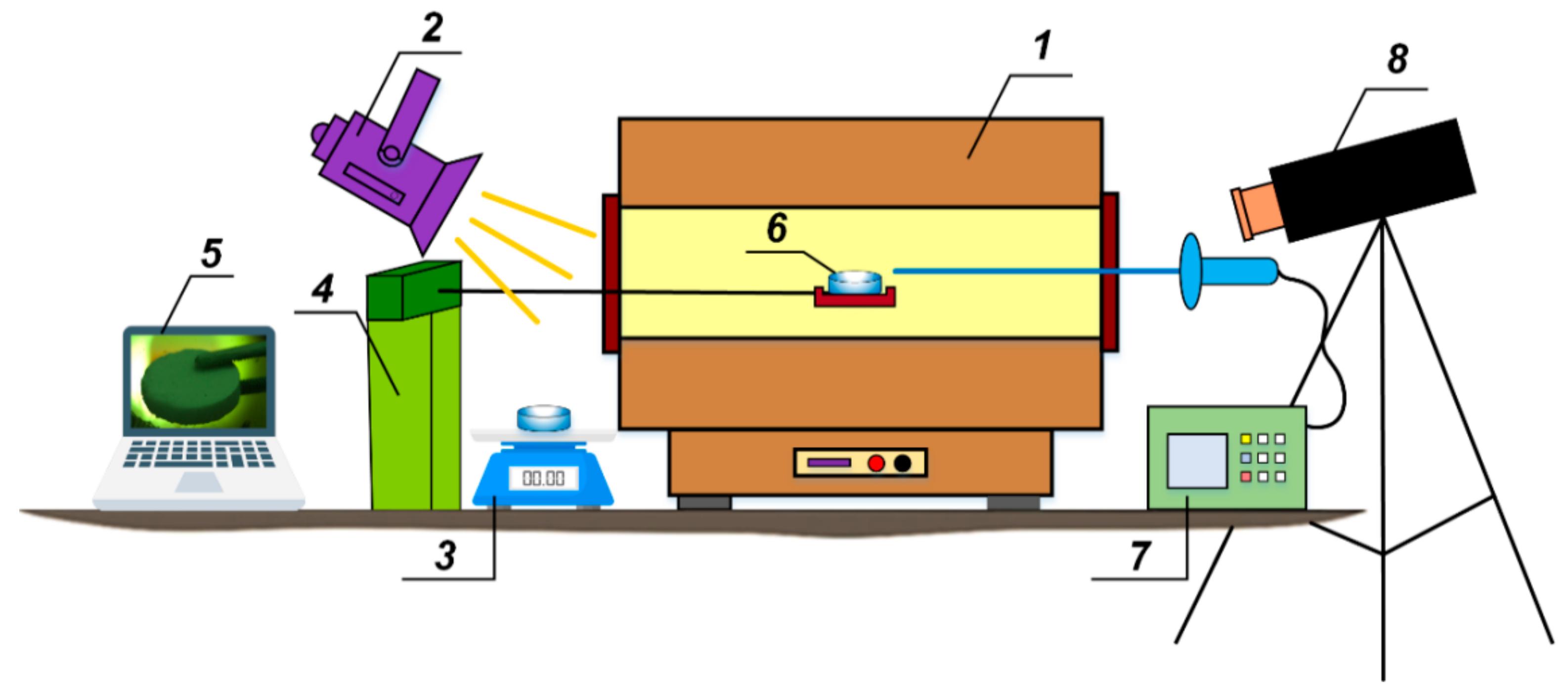
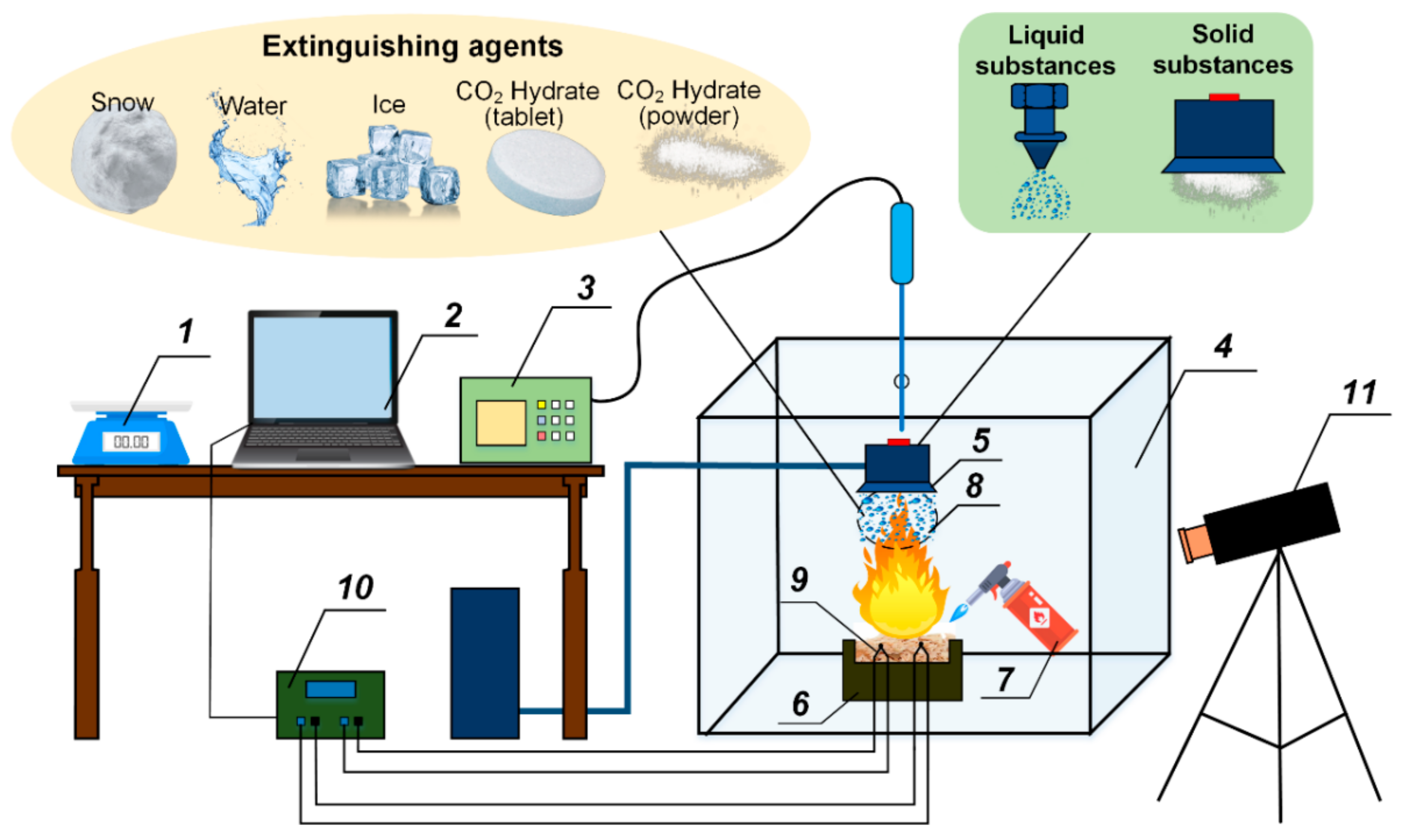
3.1. Patterns of CO2 Hydrate Dissociation
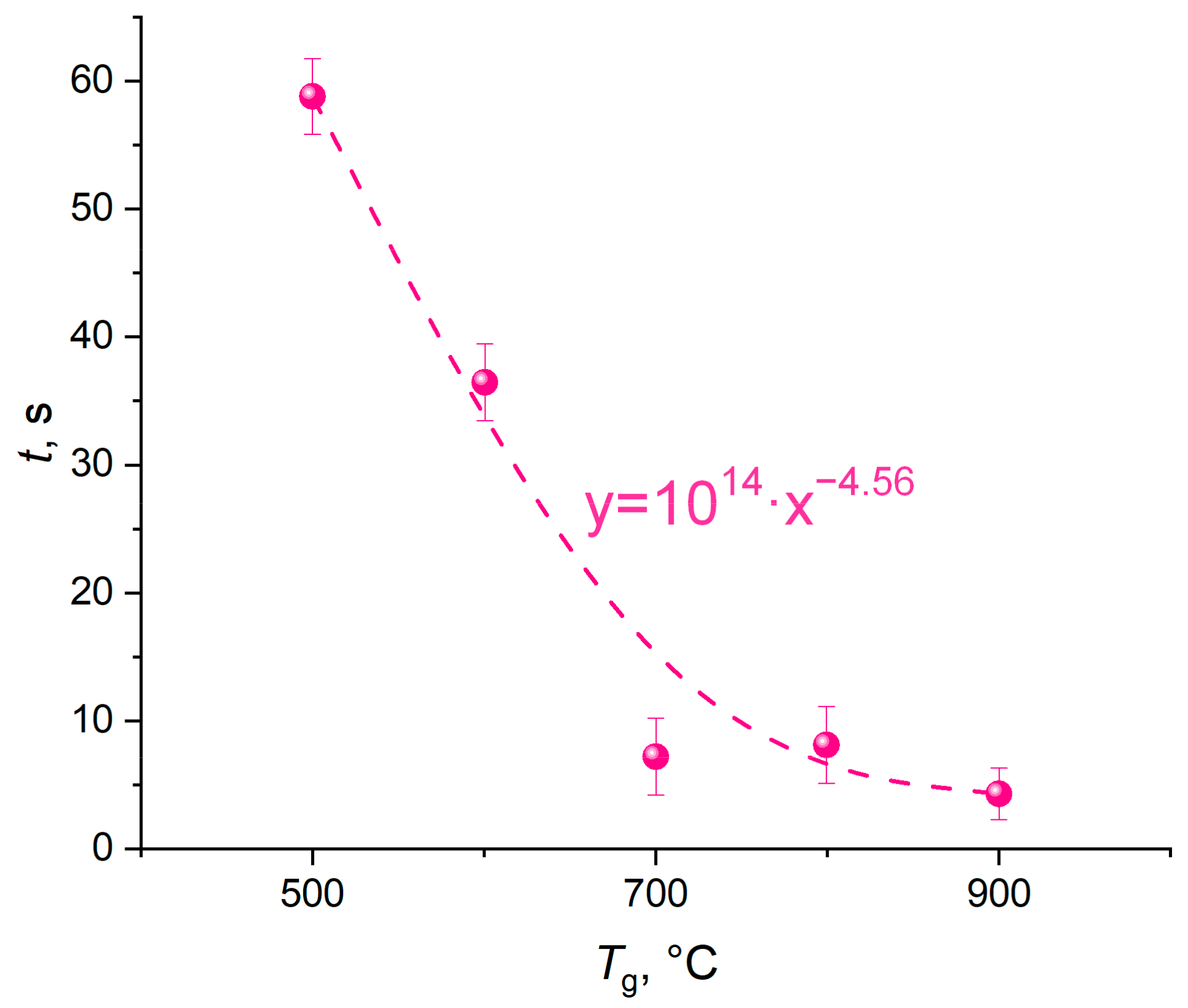
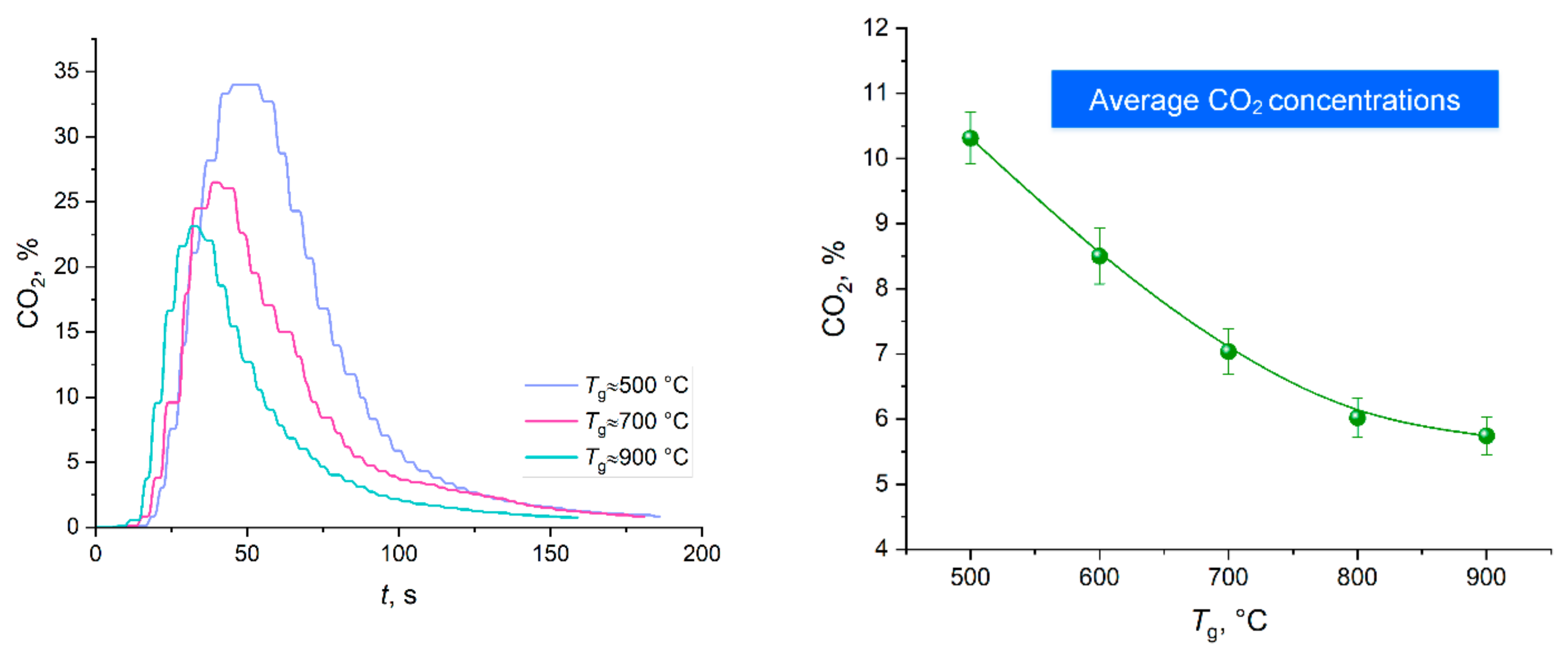
Figure 3 shows the thermal decomposition times of a CO
2 hydrate tablet when varying the ambient gas temperature in the range of 500–900 °C. The error bars in the figure illustrate the random error in a series of measurements of thermal decomposition time (confidence intervals). To process the results (including gross error identification and elimination), standard approaches were used [
39]. These involved calculating the mathematical expectation (Equation (1)), variance of a random variable (Equation (2)), and standard deviation for each series (Equation (3)). Then the width of the error bars was calculated (Equation (4)).
The following nomenclature was taken in Equations (1)–(4): Mx—mathematical expectation; Xi—measurement result; n—number of measurements; V—variance; σ—standard deviation; Δ—width of error bar; tαn—Student’s coefficient.
When choosing the values of
tαn, the confidence coefficient was taken as equal to 0.95. In
Section 3, all the main figures show the error bars that illustrate the range of possible values of the measured parameter (with a 95% probability). If error bars are not shown, this indicates that either their values are too small, or the results of continuous/instantaneous measurements of the corresponding parameter are presented (for example, droplet velocity fields, velocity profiles, droplet size distributions). The accuracy of the latter is described by systematic measurement errors of the corresponding parameters (
Section 2).
The thermal decomposition time was found to decrease to about 1/9 of the initial value with an increase in the furnace temperature from 500 to 700 °C. A temperature increase from 700 °C to 900 °C caused a 35% time reduction (
Figure 3). This result indicates that the gas–vapor area near the dissociating powder surface has a certain degree of saturation with gases. The gas hydrates used in the experiments are 70–75% water. This factor is the key to the patterns recognized in the experiments. Clearly, the higher the gas temperature, the more intense the ice melting and water evaporation. The intensification of these processes accelerates the self-preservation of hydrate granules, leading to the clogging of some pores in the near-surface layer. The release of carbon dioxide is inhibited. Thus, the dissociation rates reach a certain asymptotic value. This suggests that both low and extremely high ambient gas temperatures are not effective at catalyzing the hydrate dissociation. It is important to choose the right average temperature of the gas environment.
The ambient gas temperature (
T) was found to affect the dissociation time (
τdis) of carbon dioxide hydrate. The carbon dioxide hydrate was placed into a muffle furnace with a constant ambient gas temperature. The kinetic equation for the dissociation as well as kinetic constants are given in [
40]. A model of gas hydrate dissociation at negative temperatures (beyond the self-preservation region) controlling for the dissociation kinetics and gas filtration through pores is considered in [
41]:
where
Y is the degree of carbon dioxide hydrate particle conversion to ice, parameter
parameter
R0 is the radius of the sphere,
kR and
kF are the kinetic and filtration coefficients,
ρH is the CO
2 hydrate density,
b is the initial carbon dioxide concentration C
0,
µ is the dynamic viscosity of gas,
pEq is the equilibrium pressure in the CO
2 hydrate,
D0 is particle diameter and
p0 is the ambient pressure. Modeling also involved the calculation of thermal balance controlling for the dissociation, ice melting, and water evaporation [
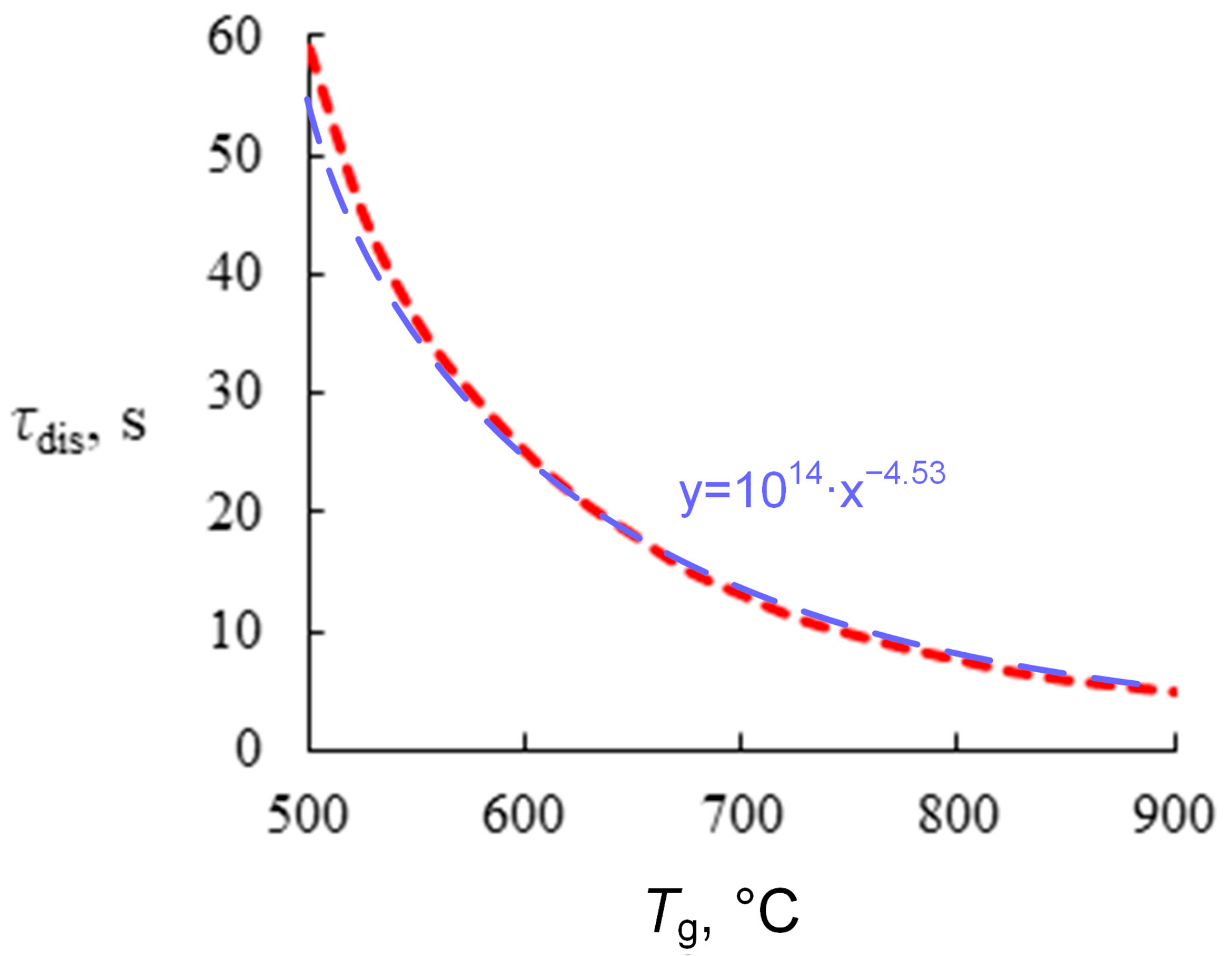
41]. The results of predictive calculations are given in
Figure 4. The curve is nonlinear. At above 700 °C, the impact of temperature (slope of the curve) is much lower than in the temperature range under 600 °C. The computational findings are in acceptable agreement with experimental data given in
Figure 3.
When analyzing
Figure 3 and
Figure 4, we singled out important patterns of gas dissociation from the hydrate. First, the relationship of hydrate dissociation rate and full dissociation time (gas release) versus temperature is exponential. This aspect can predict the gas release time for different applications. In particular, gas–vapor mixtures are produced in reactors at an ambient gas temperature of less than 600 °C. The relationships obtained in this research show that the gas mixing times (i.e., the preparation times of gas–vapor mixtures) should be several dozens of seconds. In this case, all of the gas will leave the hydrate, and complete miscibility will be achieved. The temperature range of 500 °C to 700 °C corresponds to the typical technologies of composite fuel pyrolysis and gasification as well as the co-combustion of several components, in particular, hydrocarbons, coal and oil processing wastes, biomass, municipal wastes, etc. Carbon dioxide and water vapor are commonly regarded as promising gas environments for effective pyrolysis and gasification of composite fuels. Thermal conversion of composite fuels in carbon dioxide and water vapor proceeds with an intense release of carbon monoxide, methane, and hydrogen. The concentrations of sulfur and nitrogen oxides are minimized due to minimum oxygen concentrations. The systems of the so-called low-temperature fuel combustion and high-temperature gasification are triggered at ambient gas temperatures of over 700 °C. The CO
2 added to the gas–vapor mixture provides control of oxidation reactions, and other reactions also become more controllable under oxygen deficiency. As a result, the concentrations of unspecified gas emissions decrease. The curves of hydrate dissociation time against
T show that the durations of the typical processes remain practically the same (several seconds) at ambient gas temperatures of over 800 °C. Thus, it is advisable to set the limit at 800 °C for the commercial implementation of the processes in reactors and chambers. Small-size hydrate heating units would suffice to generate the entire volume of gas when preparing a gas–vapor mixture with the required CO
2 concentration.
Another important pattern identified during the analysis of
Figure 3 and
Figure 4 is that the times of complete hydrate dissociation determined in a series of experiments were quite well reproducible. This is crucial for the use of the data obtained for predicting the duration of commercial production processes. The experimental data were obtained for fixed sizes of hydrate tablets. As these samples are similar to natural hydrate layers in shape and structure, and these layers dissociate consecutively, it is possible to predict the complete hydrate dissociation times at identical temperatures with different layer thicknesses.
The third pattern is related to a more intense hydrate dissociation in an experimental chamber as compared to typical commercial systems in which hydrates are delivered in layers. Such layers are heated in production units in the same way as in a tubular muffle furnace in the experiments—all around—but substrates for samples have different structures. In the experiments, the hydrate samples were placed on a perforated mesh to make it similar to tablets suspended in a chamber or free-falling granules. Such meshes are often used in commercial production processes. The heat is supplied to the hydrate sample surface through this mesh, and water drains from the surface of a tablet through the mesh as well. With a non-perforated substrate, the heat supply to the lower surface of the hydrate tablet proceeds in a different way, and there is no drainage. In this case, the complete hydrate dissociation will last longer than it was established in this research. It is more efficient to heat hydrate granules and tablets from all sides and let water drain from their surface. This will minimize the self-preservation of hydrate pores, thus accelerating hydrate decomposition and gas dissociation.
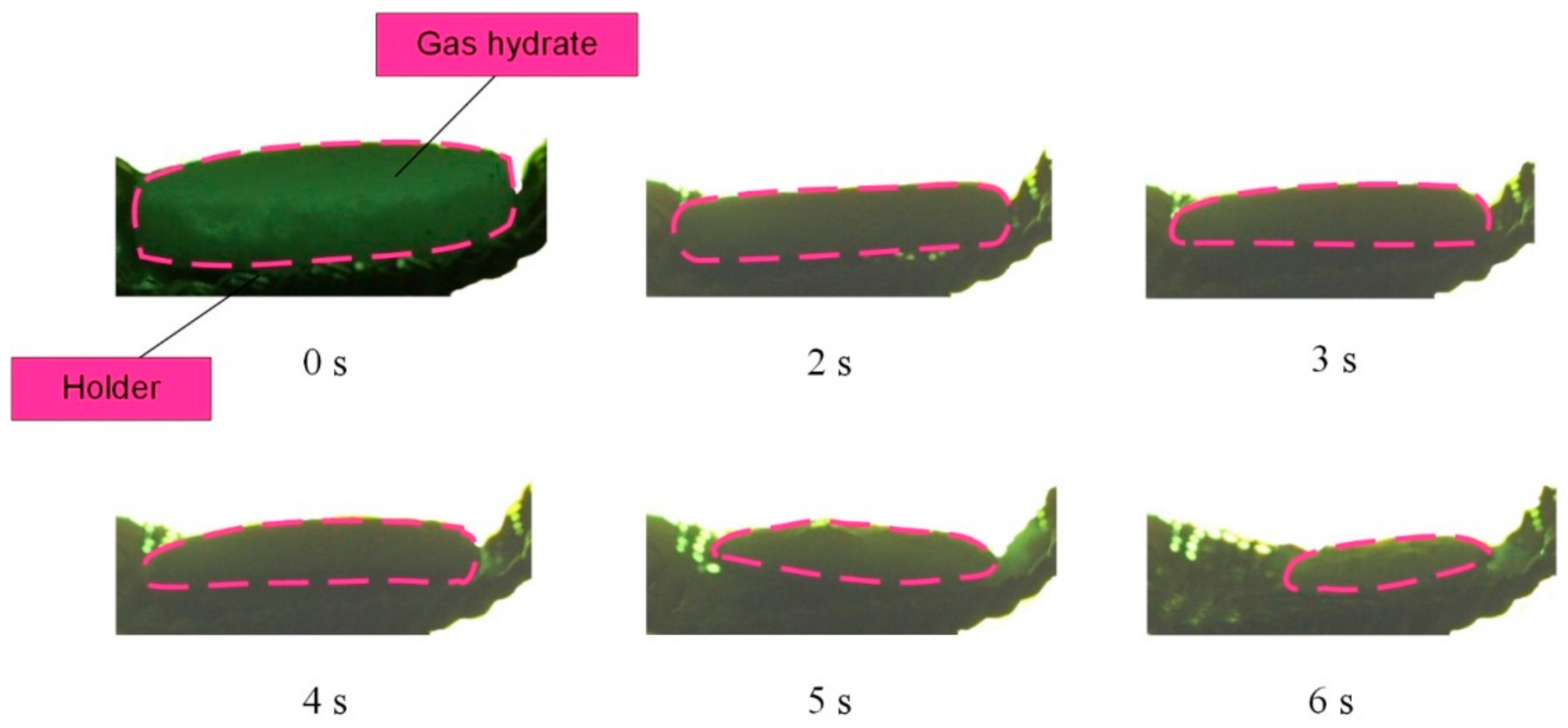
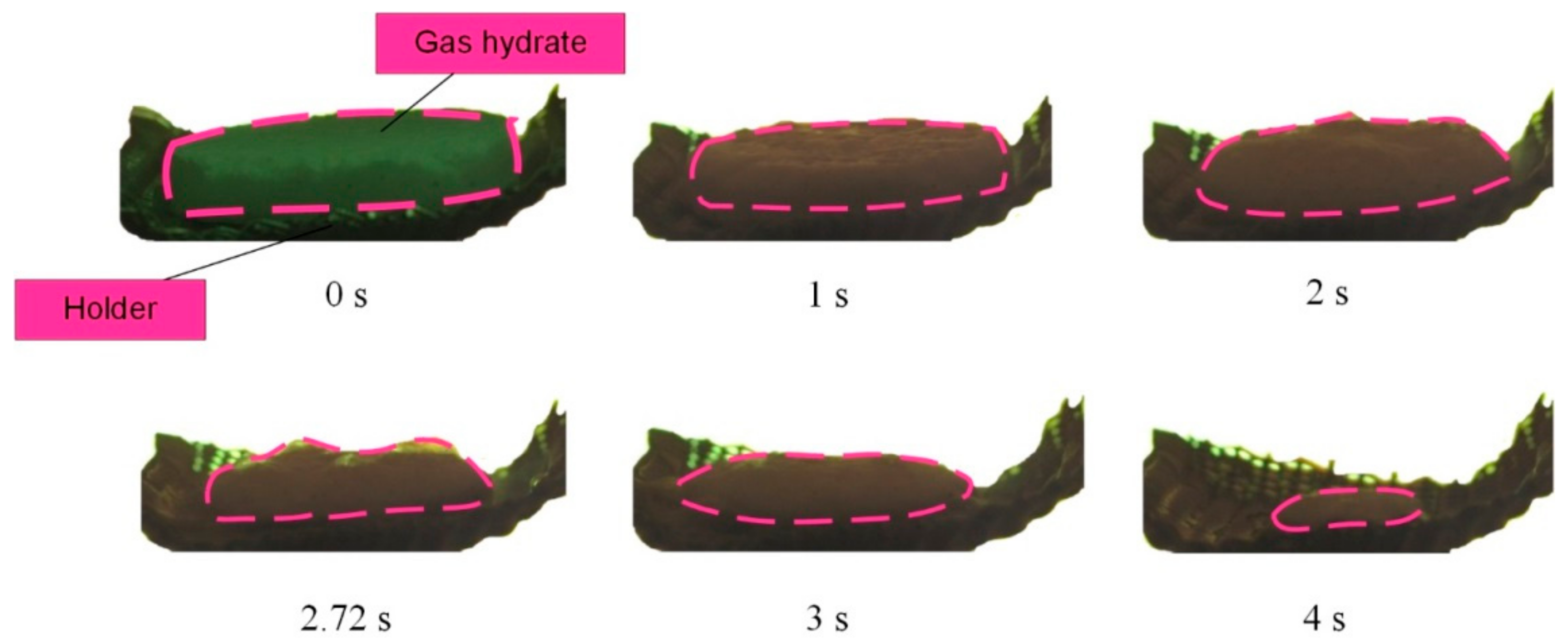
Figure 5,
Figure 6 and
Figure 7 present the images showing the thermal decomposition of a carbon dioxide hydrate tablet in a muffle furnace at
Tg ≈ 500–900 °C. Gas and vapor release from the surface was recorded in the form of the outflow of a gas–vapor mixture with vortices. These processes had interesting and distinct stages. At first, we recorded a gas release in the form of almost transparent tracks from the hydrate surface. This stage was relatively short. The higher the ambient gas temperature was, the faster this stage finished. At the second stage, a water film was formed on the hydrate surface. The vapor outflow was difficult to identify on the images at this stage because the formation of a water film on the surface of the hydrate particle triggered the self-preservation of pores, so gases passed through pores only partially. After that, the water film served as a membrane of sorts and only let a small fraction through. After a short time, bubbles started to form on the surface of the water film. The surface of the hydrate particle became uneven. When the critical pressure was exceeded, bubbles imploded, and the gas–vapor mixture was released from the hydrate surface. These processes were not monotonous. It was only when the deep layers were heated that the final stage started, notable for the irreversible gas and vapor release from the hydrate surface causing the dispersion of the near-surface layer and increase in the hydrate surface area. The hydrate sample dispersion led to the expansion of the channels of gas release from the depth of the hydrate. The physical dispersion mechanism is related to water boiling, water film retaining vapor and gas, and the third phase in the form of ice reinforcing the vapor-water frame. Under such conditions, the vapor and gas pressure in the deep layers increased rapidly, but their release from the surface was suppressed by the reinforced frame. Thus, when the critical pressure was exceeded, the vapor and gas release caused the ice particles to break off with a new portion of gas inside. The release of this gas from the newly formed hydrate fragments (commonly known as secondary or child fragments) accelerated after that. This is how the cascade dissociation was triggered for the primary gas hydrate sample and its secondary fragments.
3.2. Anthropogenic Emissions from the Decomposition of Gas Hydrates
Water vapor is known to reduce the harmful emissions from the combustion of a wide range of materials and substances, which is important from the environmental perspective. Compartment fires involve various materials and produce a large amount of anthropogenic emissions that are harmful to respiratory organs. That is why it is important to evaluate how water and temperature of the gas environment temperature affect the concentration of emissions. Water is formed when gas hydrate decomposes and ice melts at a high ambient temperature.
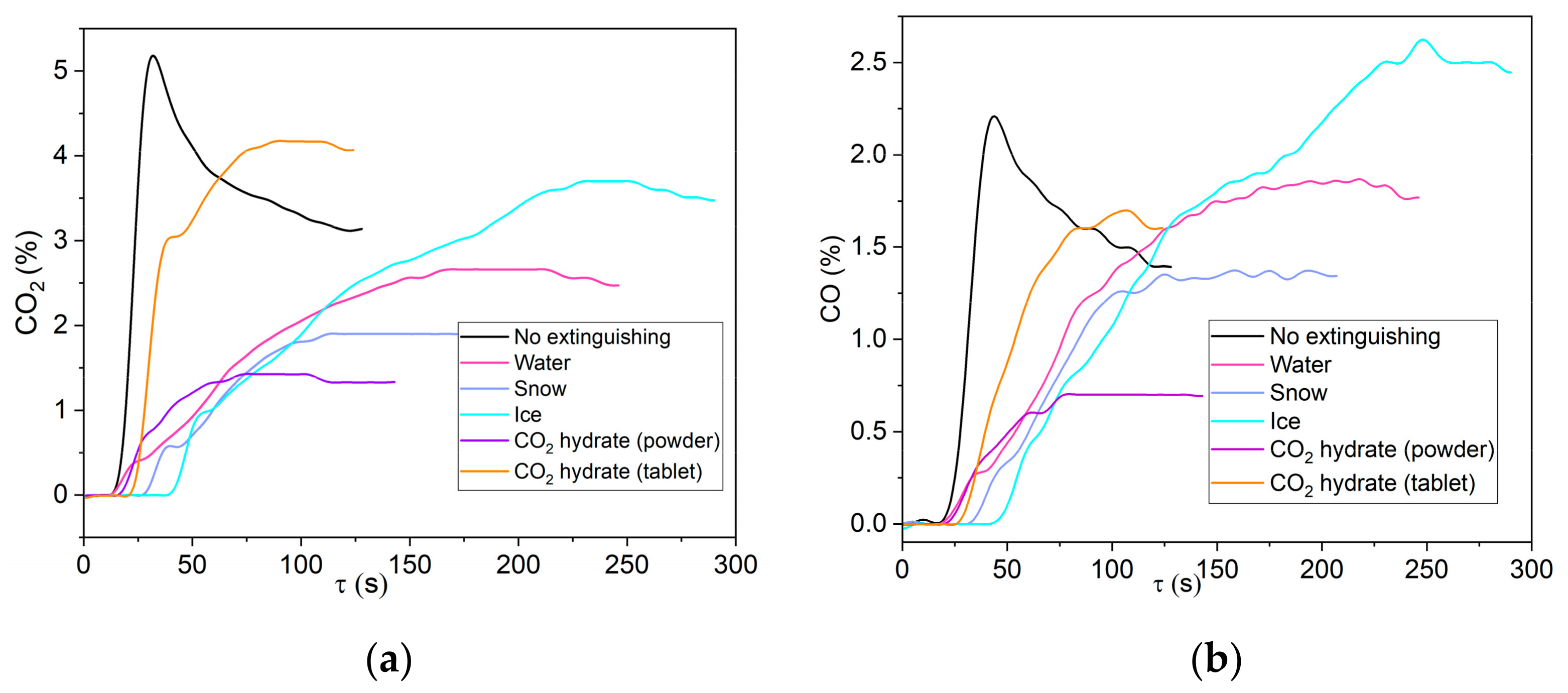
Figure 8 and
Figure 9 show the measured concentrations of CO
2 and CO emitted during the thermal decomposition of carbon dioxide hydrate. The error bars given in
Figure 8 and
Figure 9 (on the right) were calculated according to the procedure described in Ref. [
39] and illustrate the random error in the average gas component concentration (confidence intervals) in a series of five measurements. As the temperature increases, the gas concentration drops. Both the maximum concentration and the gas release time decrease. An increase in the concentration with a higher ambient temperature stems from a more intense interaction between the decomposition products and water vapor (i.e., radicals responsible for the reactions with carbon are formed more rapidly). A slight increase in the CH
4 concentrations was recorded at high temperatures in the furnace (
Tg ≥ 700 °C). In particular, the methane concentration was 0.01% at
Tg ≈ 700 °C and 0.02% at
Tg ≈ 900 °C.
Table 3 presents the maximum concentrations of CO
2 and CO from the combustion and extinguishing of a fire involving flammable materials with varying extinguishing agents.
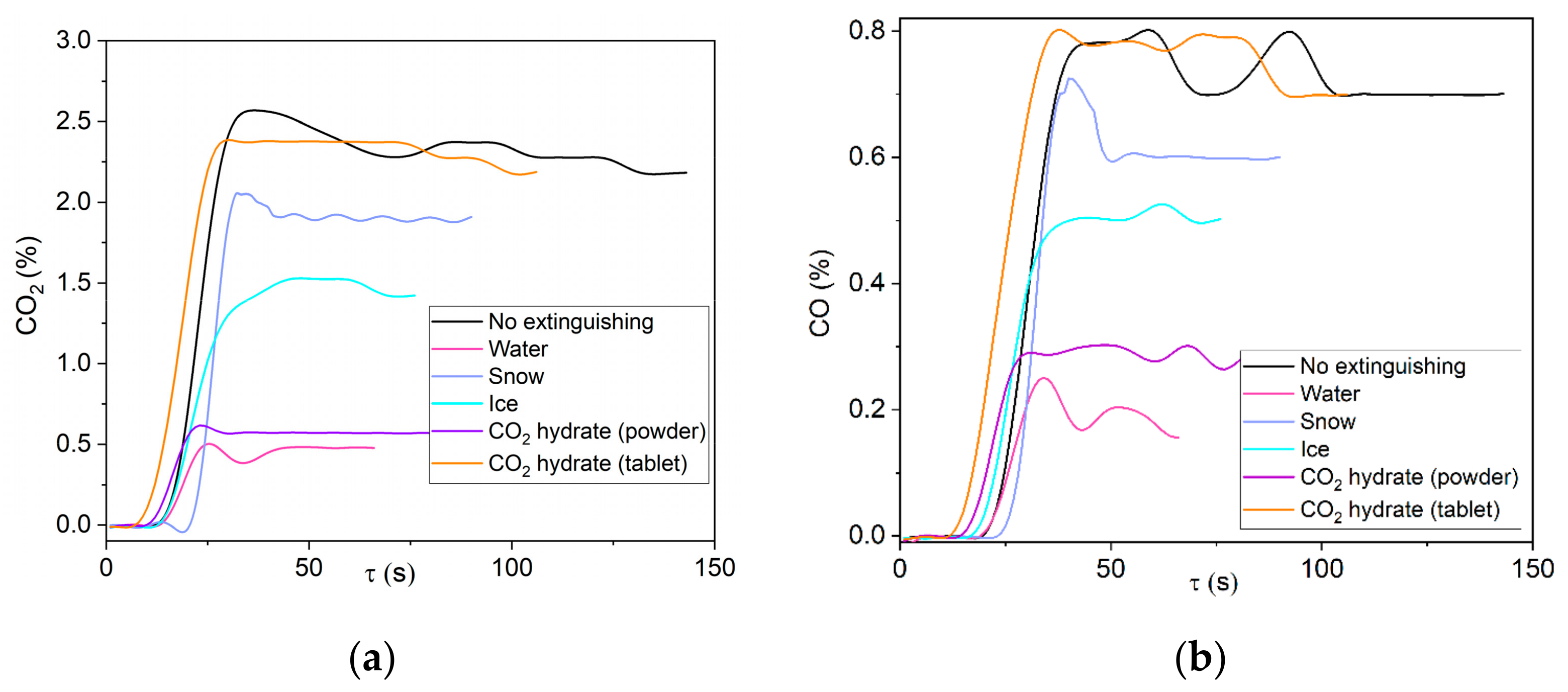
Figure 10 presents the concentrations of the main gases emitted from the combustion and extinguishing of a fire involving wood with the help of several extinguishing agents. The fire was deemed extinguished when the thermocouple measuring the temperature in the internal layer of the material (at a depth of 2–3 mm from the upper free surface) read ≤ 100 °C (minimum pyrolysis temperature of the combustible materials under study). The gas component concentrations were no longer recorded at this temperature. According to the data obtained, the maximum CO
2 concentrations were observed from the combustion of wood without extinguishing and equaled 5.9 vol%. The use of any of the extinguishing agents provided a 42% to 82% reduction in CO
2 emissions into the atmosphere. Water and CO
2 hydrate showed the highest suppression performance for wood. Hydrated CO
2 powder triggered two main mechanisms necessary for fire containment. The temperature in the gas phase decreased due to the gas hydrate dissociation, ice shell melting, and water evaporation. The decrease led to the deceleration of chain-branching oxidation reactions with a high activation energy. The inert gas—CO
2—rapidly released from the hydrate displaced the oxygen from the combustion zone, thus inhibiting the oxidation of wood-based material. Fire is suppressed by a gas hydrate in an air–vapor environment due to ice crust melting and water evaporation. A certain fraction of carbon monoxide is spent in the water gas shift reaction: OH + CO→H + CO
2. The lower production rate of H and OH radicals in the reaction zone leads to a significant deceleration of the combustion front propagation and flame quenching. The experimental findings obtained for snow and ice confirm the positive effect of CO
2 hydrate powder. Snow and ice provide a significant decrease in the flame temperature, but the fire containment takes longer because the gas hydrate dissociation requires some time as well. The delay of the carbon dioxide hydrate decomposition is determined by the filtration and kinetic resistance of the hydrate [
41], as well as the heat of dissociation, which reduces the velocity of the thermal front within the powder particle. Due to longer extinguishing time, more material burns out, increasing the CO
2 emissions into the atmosphere. However, this drawback (longer extinguishing time) is typical of fire suppression on a quasi-flat surface of the fuel. In real-life conditions, however, materials are located at different levels, hence the high altitude of flame propagation. Further, we will prove that the fire suppression performance of gas hydrate powder is significantly higher than that of water spray.
All the trends with the recorded component concentrations of a gas–vapor–air mixture show significantly different durations. This effect stems from the different durations of flame combustion and thermal decomposition of materials exposed to different extinguishing agents. When research findings are presented in this format, it is possible to analyze how fast the corresponding physicochemical processes slow down in the depth of the material and in the close vicinity of its surface. Especially valuable are the recorded extrema on the trends as well as their number, because they reflect the cyclic and cascade manner of the heat exchange processes and chemical reactions. With the physical mechanisms of fire suppression described above, it is possible to reliably predict the amount and type of extinguishing agent required for effective firefighting. On average, it took 50 to 200 s for the concentrations of all the components detected in the gas–vapor–air mixture to go down to zero. The time depended on the mass of the pyrolyzing material, type of extinguishing agent, and operation of the exhaust system. If a gas hydrate powder remained on the surface of the material by the time the concentration of pyrolysis products started to decrease, this decrease remained monotonic. However, if the film of the extinguishing agent based on water, ice, or hydrate granules became thinner and had gaps, the trends of the decrease in the concentration of pyrolysis products were not fully monotonic. This stems from the unstable heat exchange conditions across the material surface.
The combustion of wood without extinguishing produced the maximum CO concentrations. The use of extinguishing agents reduced the CO emission by 3.5 times. The extinguishing agents under study were ranked as follows in terms of the efficiency of carbon monoxide emission decrease (from least effective to most effective): snow, CO2 hydrate tablet, ice, water, and CO2 hydrate powder. Taking the CO2 emissions into account, the hydrate powder turned out to be the most effective agent under the conditions of the CO concentration decrease, mainly because the rate of the reaction OH + CO→H + CO2 decreased. The reaction was inhibited by the release of more CO2 from the hydrate, which led to a decrease in the CO and CO2 production.
The comparison of experimental findings on hydrate powder and tablets gave an interesting result. Wood fire suppression using a hydrate tablet took a longer time and produced higher CO2 and CO concentrations. This can be explained by the differences in the surface areas of the hydrate and burning solid material. When hydrate was applied in the powder form, it spread evenly across the wood surface, so the combustion reaction was also suppressed evenly across the entire surface of the material. The size of the hydrate tablet (in particular, its diameter of 20 mm) prevented it from covering the entire free surface of the reacting material. As a result, the thermal decomposition of the sample was not suppressed evenly: pyrolysis and combustion continued on the edges. The central part, however, covered by the hydrate tablet, stopped burning almost instantaneously after the suppression started. Thus, it was experimentally proven that the class A fire suppression efficiency using CO2 hydrate is largely defined by its dissociation rate and free surface area (hydrate/fire contact area).
The trends obtained for cardboard (
Figure 11) agree overall agree with the data on wood fire suppression. The minimum emissions of carbon oxides were produced during the suppression of a cardboard fire with CO
2 hydrate powder. The difference in the threshold concentrations of CO
2max and CO
max from the combustion of cardboard without suppression and with suppression using CO
2 powder was 70%. The maximum gas release time and rather high concentrations of carbon oxides indicate that ice is the least effective extinguishing material of those considered in this research. When used to extinguish burning cardboard, water exhibited lower efficiency in decreasing the CO
2 and CO emissions as compared to wood fire suppression (
Figure 10). Water quickly evaporated from the cardboard surface and got absorbed into cardboard without penetrating into the depth of the porous cardboard layer, several centimeters high. As a result, pyrolysis continued in the depth, and local combustion continued over the surface of the material.
Figure 12 presents the concentrations of the main gaseous emissions from the combustion of linoleum with and without extinguishing. The minimum concentrations of carbon oxides were recorded when water was used for extinguishing. The concentrations of CO
2 and CO emitted during the suppression with carbon dioxide hydrate are somewhat higher than those for water. The volume of CO
2 hydrate contained in one tablet turned out to be insufficient for the complete containment of a fire involving linoleum.
Experimental research into the suppression of combustible materials must identify the minimum (critical) masses of extinguishing agents. When the ratio of the combustible material mass to the extinguishing agent mass changes, the concentration of pyrolysis and combustion products changes as well.
Figure 13 shows the data on CO
2 and CO concentrations as a function of the CO
2 hydrate mass. The functions are nonlinear. When the mass increases from 7 g to 15 g, the emission decreases by three times for CO and by eight times for CO
2. Further changes in the mass do not affect the volume and maximum concentrations of emitted gases that much. The minimum extremum is observed for CO
2 and CO concentrations. The concentrations of CO
2 and CO increase when a certain mass of carbon dioxide hydrate is exceeded. This nonlinear behavior is associated not only with the combustion temperature but also with the water vapor concentration in the combustion and pyrolysis zones. The water temperature and concentration govern both the elementary reactions and the oxidation rate.
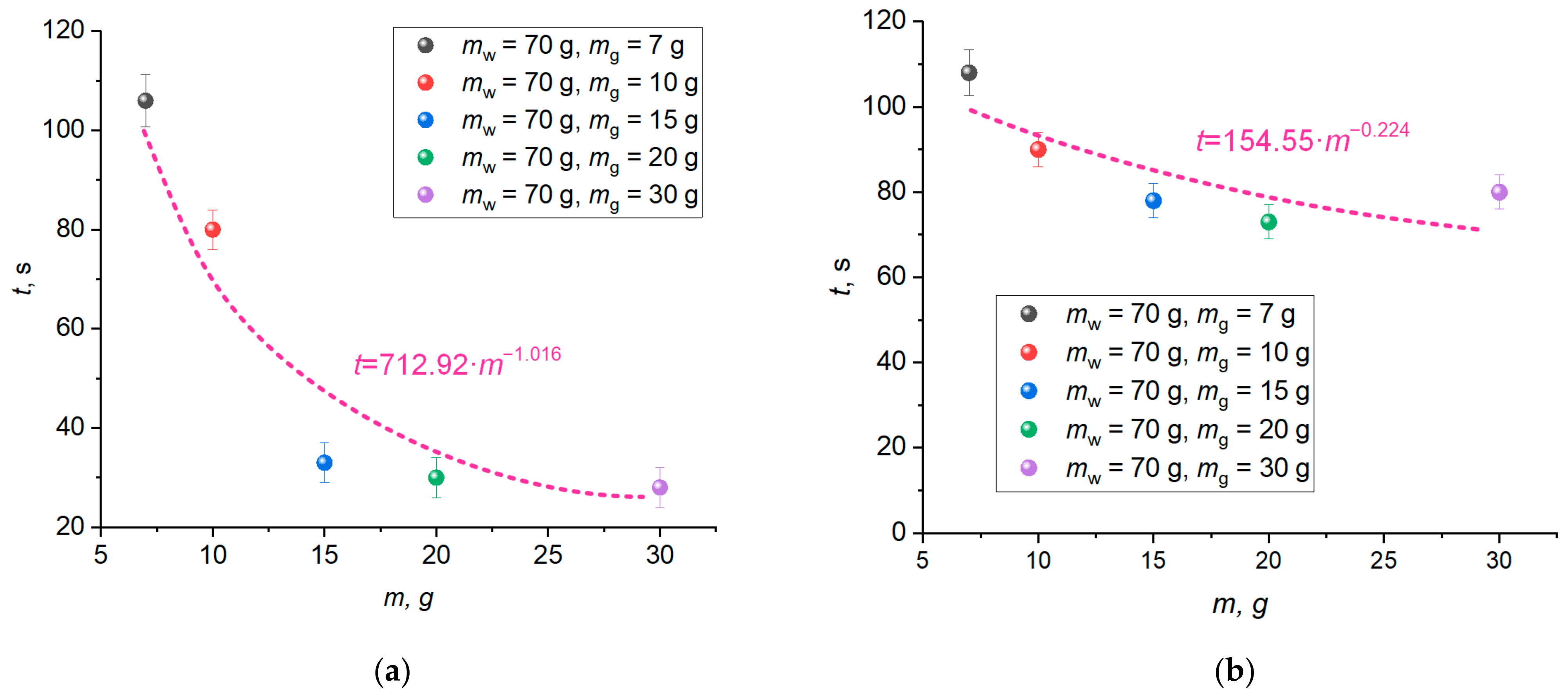
Figure 14a,b shows the curve of the time when the CO
2 and CO emissions started to decrease versus the mass of hydrate (
mg = 7–30 g) with the mass of wood remaining constant (
mw = 70 g). It is clear that the time until the gas concentrations begin to decrease shortens with an increase in the mass of the extinguishing hydrate. A change in the mass of carbon dioxide hydrate from 7 g to 15 g reduces the time until the CO
2 and CO concentrations start to go down by 71% and 28%, respectively. An increase in the hydrate mass from 15 g to 30 g leads to a negligible change (up to 10%) in the time before the concentrations begin to decrease.
Figure 14a,b presents the approximation curves of the exponential (CO
2) and polynomial (CO) nature with the mathematical expressions describing these curves. The resulting mathematical expressions allow the extrapolation to greater masses of extinguishing agents and larger fire areas.
3.3. Conditions for the Effective Fire Containment and Suppression Using Gas Hydrates
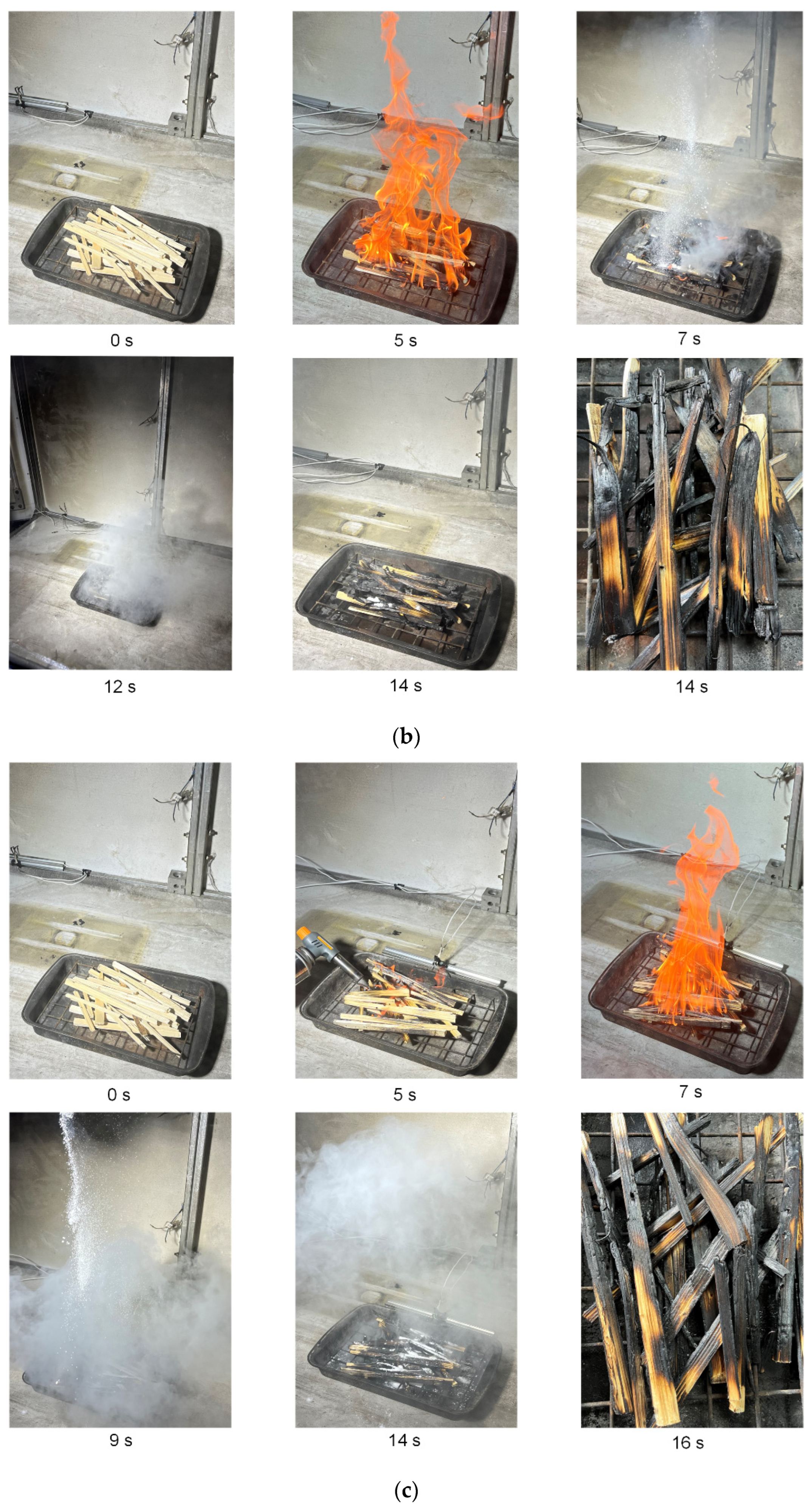
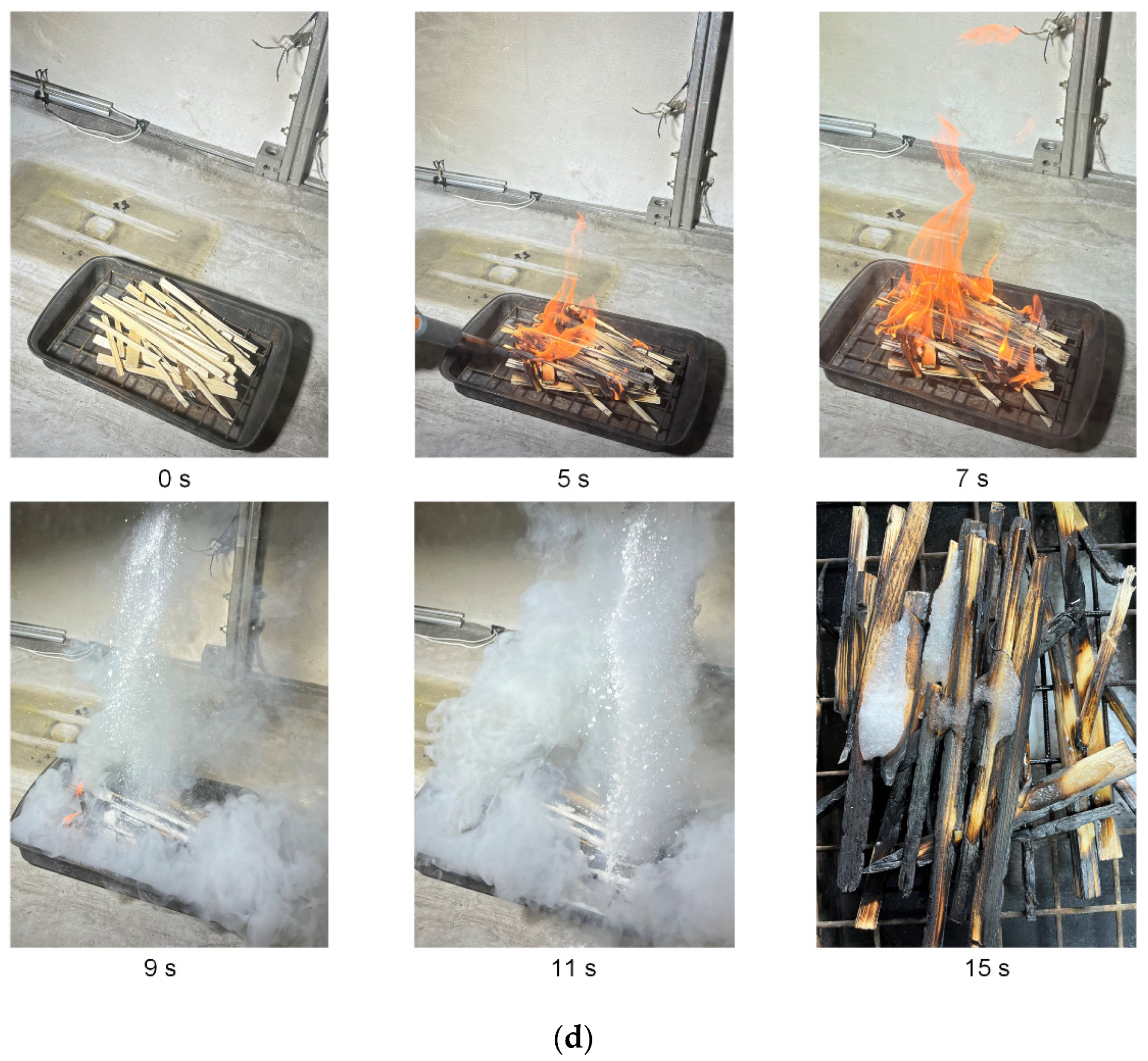
Figure 15 presents the typical images showing a fire comprised of pine rods being extinguished with carbon dioxide hydrate powder. The mass of timber remained constant (
mw = 70 g), and the mass of gas hydrate was varied. The footage of wood fire suppression using hydrate powder with a mass of 15 g and 30 g is given. According to the data obtained, no more than 15 g of hydrate powder is needed to fully extinguish a laboratory-scale wood fire of this mass. A further increase in the hydrate mass is impractical (superfluous). Thus, 5/1 is the optimal ratio of the burning material mass to the CO
2 hydrate powder mass. However, experiments with a sample of greater mass (180 g) show that more hydrate is needed to extinguish the fire and achieve the same CO
2 and CO concentrations as with a small sample. In this case, the mass ratio was equal to 2.25/1 (sample mass/hydrate mass). This result indicates that the relationship between the size of the sample and the mass of the gas hydrate used for fire suppression is nonlinear. Therefore, this nonlinearity needs to be considered when extending the results to larger fires.
In real conditions of compartment fire suppression, combustible materials are located in ties, cascades, and layers. In this case, it is necessary to switch to total flooding. There are quite large air gaps between the layers of burning material, so a different mechanism is required for the suppression. When upper layers are extinguished, middle and lower layers continue to burn because air (oxidizer) can still access them. Moreover, extinguishing agents may fail to reach lower layers. We performed preliminary experiments with multi-tier structures (wood pieces were arranged in several layers) and found that most of the extinguishing agent failed to reach lower layers with this type of structures. Just a small volume of sprayed water reached the lower layers as it drained down the wood pieces. Isolated zones were formed where water interacted with the lower layers of the fire. As part of the experiments, we extinguished a fire comprised of several tiers of wood (
Figure 16). The fire suppression footage is presented. Pine rods were arranged in several tiers. The rods were 150–200 mm long and 5–7 mm thick; the total height of the tiers was 50–70 mm. Water spray and carbon dioxide hydrate were used for fire suppression. The mass of the extinguishing agent was varied. The minimum critical mass of the carbon dioxide hydrate powder required for fire suppression was 80 g. The wood to gas hydrate mass ratio was 2/1.
It is a known fact that water spray provides effective fire suppression due to the large surface area of droplets. This factor leads to a large vapor flow and a vapor cloud forming. The temperature in the flame combustion zone drops sharply, and the access of the oxidizer to the pyrolysis and combustion products becomes limited. The experiments have shown that water spray only suppresses the fire in the upper tier, which has a vapor cloud over it. Water droplets evaporate too fast to reach the lower tiers of wood pieces. Water vapor goes up due to gravitational convection, which blocks the access to the lower part of the combustion zone not only for vapor but also for small water droplets. Carbon dioxide hydrate powder, however, shows high efficiency in suppression of flame combustion and temperature reduction below the pyrolysis temperature. The dissociation of gas hydrate and its melting to form water took 0.1–1 s, and this time was enough for most of the powder granules to fall on different tiers and reach the base of the fire. The fall took less than 0.1 s. As a result, a high concentration of the inert mixture components—carbon dioxide and water vapors—was provided in the entire wood crib. Due to the heat of the gas hydrate dissociation, ice melting, and water evaporation, as well as due to the low initial temperature of the granules (about −30 °C), the temperature inside the fire crib quickly fell lower than the wood pyrolysis temperature. The main factors providing the suppression of flame combustion using the carbon dioxide hydrate are as follows: (i) solid particles falling to the bottom of the burning wood crib, (ii) phase transitions leading to a dramatic decrease in the temperature of the material and gas–vapor mixture, (iii) a large amount of carbon dioxide and vapor released from the hydrate preventing the access of the oxidizer. In addition, the density of carbon dioxide is much higher than that of air or vapor, which slows down the diffusion and convection of carbon dioxide from the combustion zone.
It is also important to note that total flooding with unsprayed water requires a large volume of the extinguishing agent and does not provide fire suppression. In this case, it is impossible to provide the even supply of water to the tiers of wood pieces in a wood crib. Local combustion zones are formed that water cannot reach. However, it is possible to distribute carbon dioxide hydrate powder (when it falls by gravity) evenly throughout the wood crib.
Figure 17 shows the images of materials (
mw ≈ 6 g) used in the experiments with and without extinguishing using water, snow, ice, CO
2 hydrate powder, and CO
2 hydrate tablet.
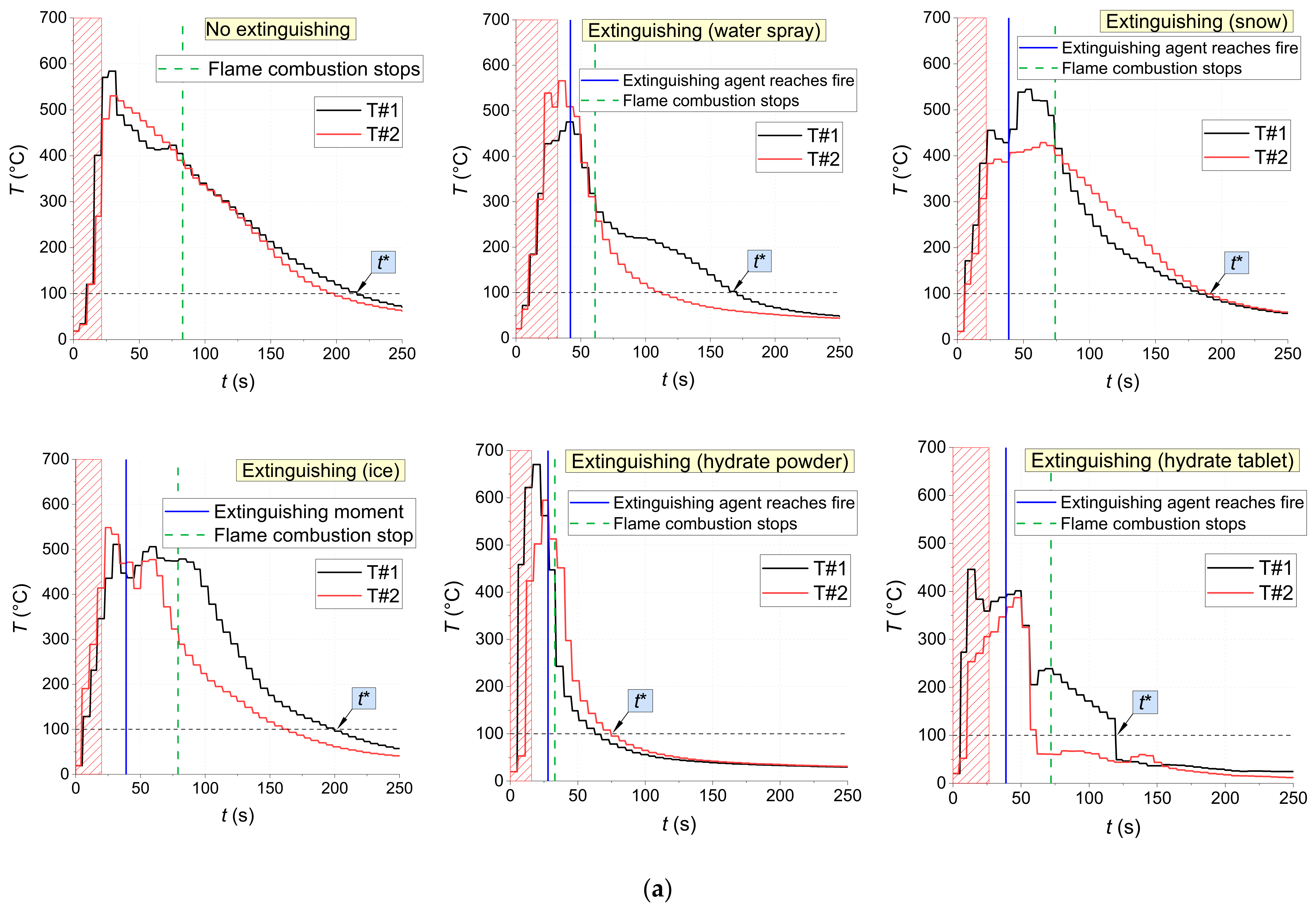
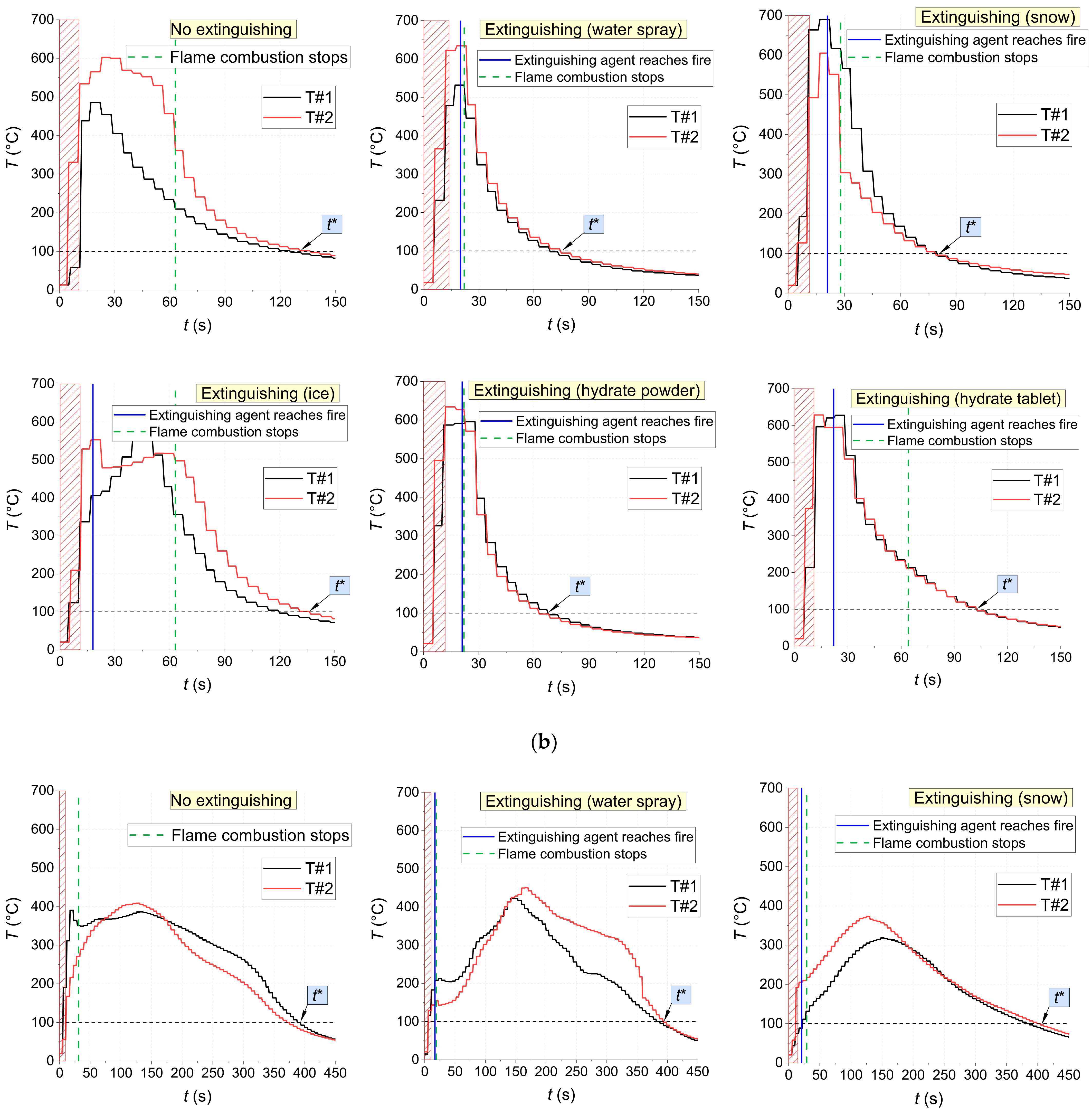
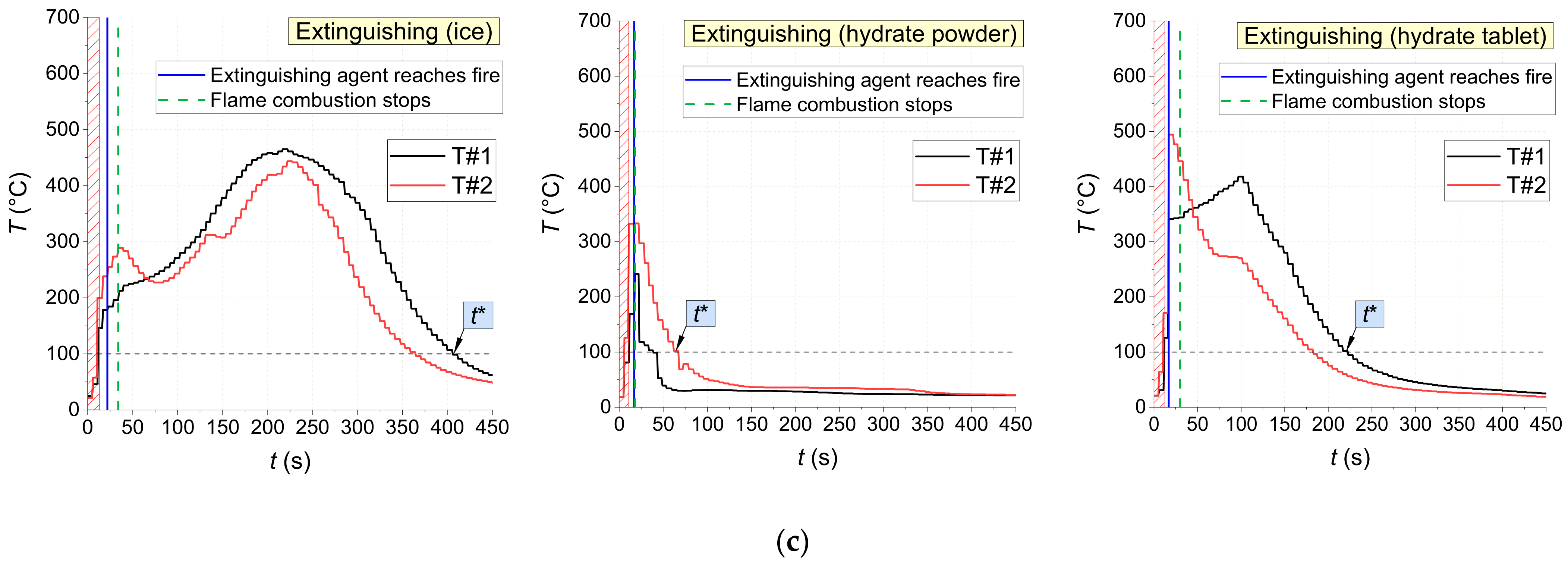
Figure 18 shows the temperature trends obtained using thermocouples in the center of fires with and without being exposed to extinguishing agents (water spray, snow, ice, and CO
2 hydrate tablet). There are distinct intervals of intense pyrolysis and combustion of materials, supply of extinguishing agents, as well as the fire containment and suppression. The non-monotonic sectors on the trends illustrate the highly unsteady nature of the combustion after ignition and suppression using a wide range of agents. The conditions of fire containment and suppression were provided in all the experiments: a temperature decrease below 100 °C in the near-surface and deep layers. The analysis of
Figure 18 shows that CO
2 hydrate powder provided the shortest time between the supply of the extinguishing agent and the moment when the flame combustion of the material stopped for all the laboratory-scale fires under study (1–5 s). Water aerosol was the second best extinguishing agent with 2–19 s. The worst result was obtained using ice: here, the flame quenching time was comparable to the one without extinguishing.
The gas burner application time was determined by the type of fuel and the specific nature of its flaming. For instance, laboratory-scale fires made of wood were exposed to the burner until the temperature inside it reached 450–500 °C as recorded by at least one thermocouple (for about 25–30 s on average) (
Figure 18). For fires made of linoleum, these values were 500–600 °C and 10–15 s, and for those made of cardboard, 200–250 °C and 15–20 s, respectively.
Figure 18 reports the thermocouple measurements inside the fuel layer. The flame temperature does not play a major role in terms of laboratory-scale fire suppression because pyrolysis proceeds in the depth of the fuel even when no flame combustion is observed.
Table 4 presents the data illustrating the action of extinguishing agents on the laboratory-scale fires based on the above-mentioned fuels. The experimental findings (
Figure 18,
Table 4) demonstrate the dynamics of the physical and chemical processes occurring in the structure of the pyrolyzing fuel. The analysis of dynamic processes is what makes it possible to confirm the guaranteed conditions of the full suppression of a chemical reaction. Fires exposed to suppression systems often reignite because fuel continues to pyrolyze even when flame combustion is contained. The experimental data show principal differences between the physics and chemistry of pyrolysis and fire containment using snow, ice, water, and inert gas hydrates in the form of powder and tablets. Differences in the temperature variation trends between different layers of pyrolyzing fuel exposed to extinguishing agents are the basis for extending the research findings to thicker samples. According to our conclusion, the thermal decomposition of materials arranged in a thick layer cannot be suppressed effectively only using the high heat capacity of the extinguishing agent. Phase transitions play an important part as they provide fast heat removal. The intense release of the inert gas during the hydrate dissociation improves the efficiency of flame fire suppression through diluting the gas–air mixture and displacing the oxidizer.
Snow and ice were selected as alternative extinguishing agents to determine how the extinguishing agent temperature and phase condition affected the suppression process. The use of snow and ice allowed us to evaluate the effect of dissociation (CO
2 formation). According to the experimental findings (
Table 4), when ice and snow are used as extinguishing agents, the time until flame combustion stops (
tb) is 2–4 times longer compared to fire suppression with water aerosol, while extinction times (
t) are comparable. Flame quenching time depends more on the contact area between the extinguishing agent and the pyrolyzing material, while complete fire suppression time largely depends on the volume of the extinguishing agent (the volumes of all the agents were comparable in the experiments). The temperature of the extinguishing agent has a negligible effect on the times
tb and
t (
Table 4), as well as on the temperatures of the laboratory-scale fires (
Figure 18). With the phase condition and free surface temperatures being similar, the flame quenching time is on average 85% shorter when hydrate powder is used as an extinguishing agent compared to snow (irrespective of the type of fuel), and the complete fire suppression time is 15–70% shorter (depending on the type of fuel) (
Table 4). A similar comparison of suppression by ice and a hydrate tablet shows overall similar values of
tb. However,
t* is on average 50% shorter for hydrate tablets than for ice. The results indicate that hydrate dissociation (CO
2 formation and oxidizer displacement from the combustion zone) has the decisive influence on suppression characteristics when CO
2 hydrates are used as extinguishing agents (in the form of both powder and tablets), and all the other conditions are similar.
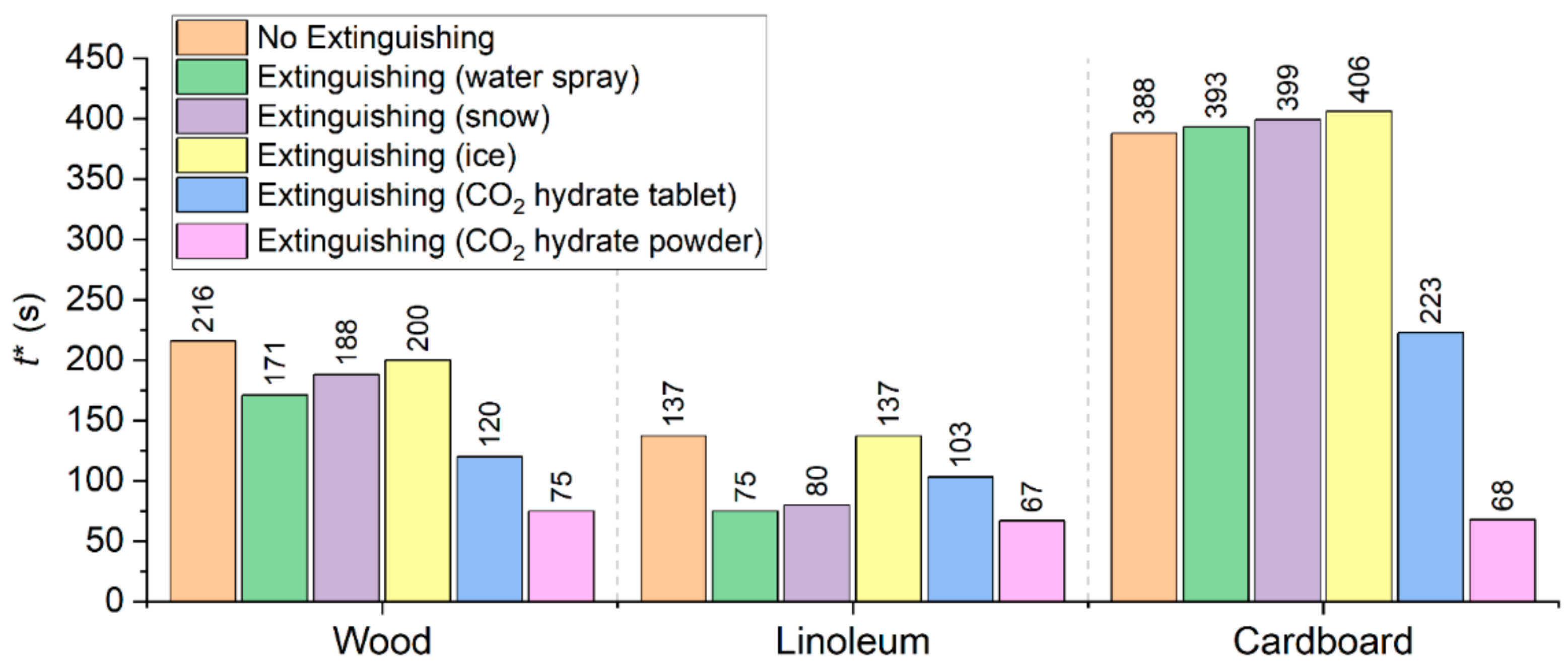
Figure 19 gives the times of complete burnout/decay of the fires used in the experiments. We considered the conditions of their combustion without suppression by extinguishing agents and with suppression using water spray, snow, ice, as well as CO
2 hydrate tablet and powder. The principal physical differences in the conditions of fire suppression using different extinguishing agents were recorded quite consistently. In particular, the interaction of water spray with the surface of almost all the fires was notable for the bounce of some of the droplets and their repeated collision with burning and pyrolyzing fragments of the material. In addition, the ascending flue gases entrained small droplets of water. The fire suppression using water spray took quite a long time in the case of burst injection. Burst injection of liquid into the combustion zone reduced the consumption of the extinguishing agent but increased the fire containment and suppression time. Unlike water droplets, the interaction of gas hydrate granules with the burning surface was notable for their sticking to the surface, so the agglomeration of granules and material was the dominating regime. This enhanced the important mechanism of fire containment and suppression based on cooling the surface. Hydrate granules formed a film on the surface of the burning material as part of the heat exchange with it. Due to the high heat capacity and vaporization heat of water, there was a significant heat sink from the surface to the depth of the hydrate. This enhanced the CO
2 release. The hydrate layer became heterogeneous: it formed a composition with ice particles, water, as well as vapor and gas bubbles. Such structures are more effective than homogeneous ones during heat exchange. This is because the heat sink was intensified not only during the heating of liquids but also during their evaporation and boiling. The drop of snow and ice on the surface of the reacting materials in the form of powder also provided a denser coverage of the surface, but the presence of vaporization centers in hydrates in the form of ice particles, water droplets, and gas bubbles ensured better cooling of the surfaces of the burning samples. The principal differences between the mechanisms of fire containment and suppression used in the experiments were especially noticeable at high temperature in the reaction zone. An important benefit of gas hydrates used as extinguishing agents is the rapid displacement of the oxidizer from the combustion and pyrolysis zone. This does not only inhibit the oxidation reactions but also triggers the reactions stifling the growth of carbon monoxide, hydrogen, and methane concentrations typical of a certain stage of fire suppression by water.
Interesting physical differences in the fire containment and suppression have been revealed between the materials used in this study: wood, linoleum, and cardboard. Wood burns with a stable and rather large flame zone due to the active release of pyrolysis gases and gas-phase reactions. It is impossible to contain wood fire by replacing oxygen with inert gas alone. Fire suppression can only be effective if several mechanisms are used, in particular, cooling the gas phase and wood surface as well as preventing the pyrolysis products, oxidizer, and combustion products from mixing. Cardboard and paper burn out very fast in the gas phase to form a fragile solid frame. The fire containment and suppression in this case is possible by cooling and destroying this frame but in a reserved manner. In particular, if the water droplets, ice and snow particles, hydrate tablets and powder granules did not cause the formation of firebrands, (i.e., actively pyrolyzing fragments of material), the combustion stopped rather quickly. However, if the burning material broke up after contact with the extinguishing agent, the fire area inevitably grew. The supply of hydrate tablets and granules caused the minimum fragmentation of the reacting material. The intense diffusion of gas from the hydrate surface prevents the particles of burning material from breaking off and covering long distances. Linoleum only has a flame combustion zone during the uninterrupted supply of the oxidizer and energy to its surface. Therefore, it is possible to effectively suppress the combustion of this material by blocking these two flows.
The acceptable repeatability of the experimental results suggests that they can be extended to other materials and systems as a whole because the mechanisms of combustion and, hence, of fire containment and suppression for most materials are the same as those considered here. For instance, rubber goods, particle board, and fiberboard are close to wood, and plastics are close to linoleum. Therefore, the interaction patterns of hydrate tablets and granules with the materials under study will mostly be identical for the most common materials, substances, and systems found in compartments.
The analysis of experimental footage allowed us to hypothesize the feasibility of using a set of hydrated gases and implementing alternative approaches to fire suppression. In particular, it is possible to effectively control the composition of pyrolysis gases by using specialized gaseous environments in reactors and chambers. We have established experimentally that the composition of pyrolysis and combustion products may vary in a wide range when different gasifying agents are used. Combustible gases in hydrates can be used to intensify the so-called back fire that is initiated in front of an active fire front. When these fronts meet, the fire gets contained (this solution is often used when combating wildfires). The main limitations of using multiple gases as part of hydrates stem from different critical (threshold) volumes of gases that a crystal lattice of a hydrate can hold. As a rule, the maximum concentrations in the hydrate structure range from 15 to 40% for most gases. This should be considered when using gas hydrates for fire containment and suppression. For instance, a hydrate lattice can hold up to 40% of carbon dioxide, but no more than 20% of methane or propane. Thus, double or triple hydrates can be effective in terms of controlling certain reactions in the pyrolysis, gasification, and flame combustion zone, but the amount of gases is limited by the hydrate structure. The obtained concentrations of gases in the pyrolysis and gasification products as well as gas–vapor mixtures in the reaction zone with and without suppression show the impact of the initial gas content within the material and hydrate, as well as the thermal conditions. These findings can be used to predict the necessary amount (volume of gas and mass of water) and type of hydrate for fire suppression.
The experimental findings (
Figure 18 and
Figure 19) show that the CO
2 hydrate powder is the most effective extinguishing agent in terms of fire suppression time when the mass of the extinguishing agent approximates 1.5 g (
Figure 19). The experiments established the necessary and sufficient volumes of water to extinguish the combustion of the materials under study. When analyzing the research findings, we took into account the discharge density specified in [
42] that a firefighting system must be able to provide. Thus, according to [
42], the required discharge density for the first and second groups of facilities is in the range of 0.08–0.12 l/(m
2s) with a maximum duration of 30–60 min. Thus, with the above parameters, the maximum specified discharge density reaches 144–432 l/m
2. For the spray nozzle used in the experiments with a water droplet size ranging from 5 to 120 μm (with the specific discharge density set at 0.3 l/(m
2s) and spraying time of 4–10 s), the specific water volume per unit area of the fire required to extinguish a fire is 8.7 l/m
2 for wood, 7.2 l/m
2 for cardboard, 3.6 l/m
2 for paper, and 0.9 l/m
2 for linoleum. The specific water volume required to extinguish a fire can increase by 1.5–2 times if the average droplet radius is increased to 250–300 μm. These values would be 17.4 l/m
2 for wood, 14.4 l/m
2 for cardboard, 7.2 l/m
2 for paper, and 1.8 l/m
2 for linoleum. These values are considerably lower than the required 144–432 l/m
2 [
42]. This confirms the efficiency of the approach used in this research. With gas hydrates as extinguishing agents, a much smaller amount is necessary for fire containment and suppression. The calculations show that wood requires more CO
2 hydrate than cardboard, paper, or linoleum do due to the nuances of thermal decomposition and flame combustion described above. In particular, the minimum (threshold) mass of the hydrate powder is 20 g/m
2 for wood, 10 g/m
2 for cardboard, 5 g/m
2 for linoleum, and 4 g/m
2 for paper. The conditions of hydrate powder distribution over the surface of pyrolyzing material play an important role. Tablets effectively contain and suppress combustion only when the reacting materials have a small area and there is no inflow of oxygen. Granulated powder is more versatile in this respect. It can effectively cover quite a large surface area of the material. In this case, the gas release from the hydrate will be quite considerable and fast. The fire energy is also extensively spent on heating ice and water as well as their crystallization and evaporation, respectively. In the case of high temperatures in the combustion zone, water boiling begins to play an important part as well. Moreover, due to the multi-phase frame and heterogeneous structure of hydrates, we observe film and bubble boiling. Any application needs reliable experimental data on the dissociation rates and complete decomposition times to describe these patterns. Such data as well as the approximations and formulas obtained in this research can be used to extend the results to different sizes of fires, reacting materials, and temperatures of the gaseous environment in the flame combustion zone and intense pyrolysis area.
As gas hydrates are a multi-phase system, it is quite difficult to describe their heating, gas decomposition, changes in the phase structure and component proportions (gas, vapor, water, and ice) using the known dimensionless similarity criteria. Clearly, that would require large-scale and long-term research involving leading teams of specialists from different countries to obtain the criterial proportions that could be used to reliably predict the fire suppression characteristics for a wide range of materials and substances as well as single, double, triple, and multi-component hydrates with different compositions and gas types. The experimental data made it possible to analyze the patterns of physical and chemical processes as well as phase transitions during the interaction of gas hydrate samples and burning materials with a variable free surface area.
The numerical data were scaled to the areas of compartment fires. The necessary and sufficient masses of gas hydrate were determined for extinguishing fires on different areas. In particular, burning materials with an area ranging from 10 to 20 m
2 can be extinguished using 0.2–0.4 kg of gas hydrate powder, which is approximately 45 wt% less than water spray. Moreover, the fire containment and suppression time is 11–82% shorter for hydrate powder than for water spray. Gas dissociation, ice melting, as well as water evaporation and boiling play a major role in the effective fire suppression and containment. That is why it is important to know the relationships between these characteristics and temperature in the combustion and pyrolysis zone as well as consider the complete gas dissociation and liquid evaporation times. The latter can be used to calculate the relative spraying densities for the surface of reacting materials. The experiments involved much smaller fire areas than those observed in real-life conditions. In a simplified statement, an increase in the fire surface area was proportional to an increase in the mass of gas hydrate required for fire containment and suppression. A more precise simulation would significantly complicate the prediction model. There is good reason to do that in the future as an independent work. Simple prediction of the necessary amount of gas hydrate is necessary to provide a quick response to fire outbreaks. For a detailed description of the estimation model, its limitations and deviations from the experiments, please see [
40,
41,
43]. These models describe the dissociation of gas hydrate with varying heat exchange conditions (for instance, ambient temperature). The models do not describe the pyrolysis or chemical reactions during gas-phase combustion. Such a simulation would require further research. A simulation that would factor in the gas hydrate dissociation time depending on the heat exchange conditions is also a major scientific objective. The results of simplified modeling here can be used for scaling—selecting the mass of an extinguishing agent (carbon dioxide hydrate)—with varying fire surface areas.
The key barrier to the large-scale use of gas fire suppression systems is the complicated evacuation of people. As a rule, gas fire suppression is launched when no living organisms are present in the fire-affected compartments, buildings, and structures. Due to their controllable dissociation rate, gas hydrates coupled with water vapor injection make it possible to evacuate people even in the course of fire containment and suppression. One can estimate the time for human evacuation from compartments given the known hydrate dissociation time, gas-to-water ratio in hydrates, as well as the gas and steam concentrations as functions of the interaction time with the reacting materials. At the same time, if the temperature in the combustion zone, pyrolysis gas concentration, and type of burning material are known, the fire containment and suppression can be optimized in terms of suppression time, volume of the extinguishing agent involved, and conditions of the agent supply. In particular, systems of CO2 hydrate granules with different initial temperatures and, hence, different initial dissociation rates can be regarded as especially promising. It is possible to vary not only the initial concentrations of the hydrated gas and water vapors but also their rheological characteristics (fluidity, viscosity, and structure of layers). This will allow firefighters to employ each of the three fire suppression mechanisms separately or together: cooling the surface and gas environment, displacement of oxidizer and pyrolysis products, and intensification of endothermic phase transitions to control chemical reactions.
We propose that future research should focus on using gas hydrate as an extinguishing agent for liquid and composite systems. Fires involving such systems are difficult to combat due to their high reactivity, and composite systems are even more challenging because they can react both in the gas phase and on the surface (heterogeneous combustion). However, where conventional water, gas, and foam firefighting may struggle or fail, gas hydrates bring new benefits to the table. According to the experiments, these benefits improve the fire-extinguishing performance for a wide range of materials and substances. It is important to adapt the proposed engineering solutions based on gas hydrates to challenging operating conditions.
Carbon dioxide hydrates have not yet been used for fire containment and suppression. Here, we have, for the first time, shown the experimental findings explaining the physical effects emerging during the suppression of class A fires using carbon dioxide hydrates. The data obtained are extremely important as they confirm that hydrates with inert gases can be used for fire suppression. The data will also be helpful for developing physical and mathematical models to predict the effective conditions of fire containment by exposure to a gas hydrate. The physicochemical processes behind this are extremely complex, as the research has shown. Therefore, we concentrated on performing experiments with small-size fires. Due to the small size and limited mass of the gas hydrate powder and tablets, we managed to determine the critical ratios between the mass of pyrolyzing and burning materials and gas hydrate. We also determined the factors, processes, and effects exerting the most significant influence on the conditions of combustion front propagation. When processing the experimental findings, we obtained enough data to extend them to large-scale fires. These aspects define the scientific novelty and practical value of the research findings. The new knowledge on hydrate dissociation under the conditions considered in this study can serve as a basis for the development of the theory of changes in the hydrate structure and component composition of the gas–vapor–air mixture during their dissociation under limited energy supply. Using this new knowledge, balance models can be constructed that will quickly estimate the required ratios of the hydrate mass and volume of gases to limit the growth of heat fluxes not just for firefighting but also in heat exchange and cooling systems, which are widely used in direct-contact selective technologies. Using the data obtained, it is advisable to develop a summarized model simulating the heat exchange of inert gas hydrate with chemically active materials and substances.