Post-Fire Recovery of Plant Biodiversity Changes Depending on Time Intervals since Last Fire in Semiarid Shrublands
Abstract
1. Introduction
2. Materials and Methods
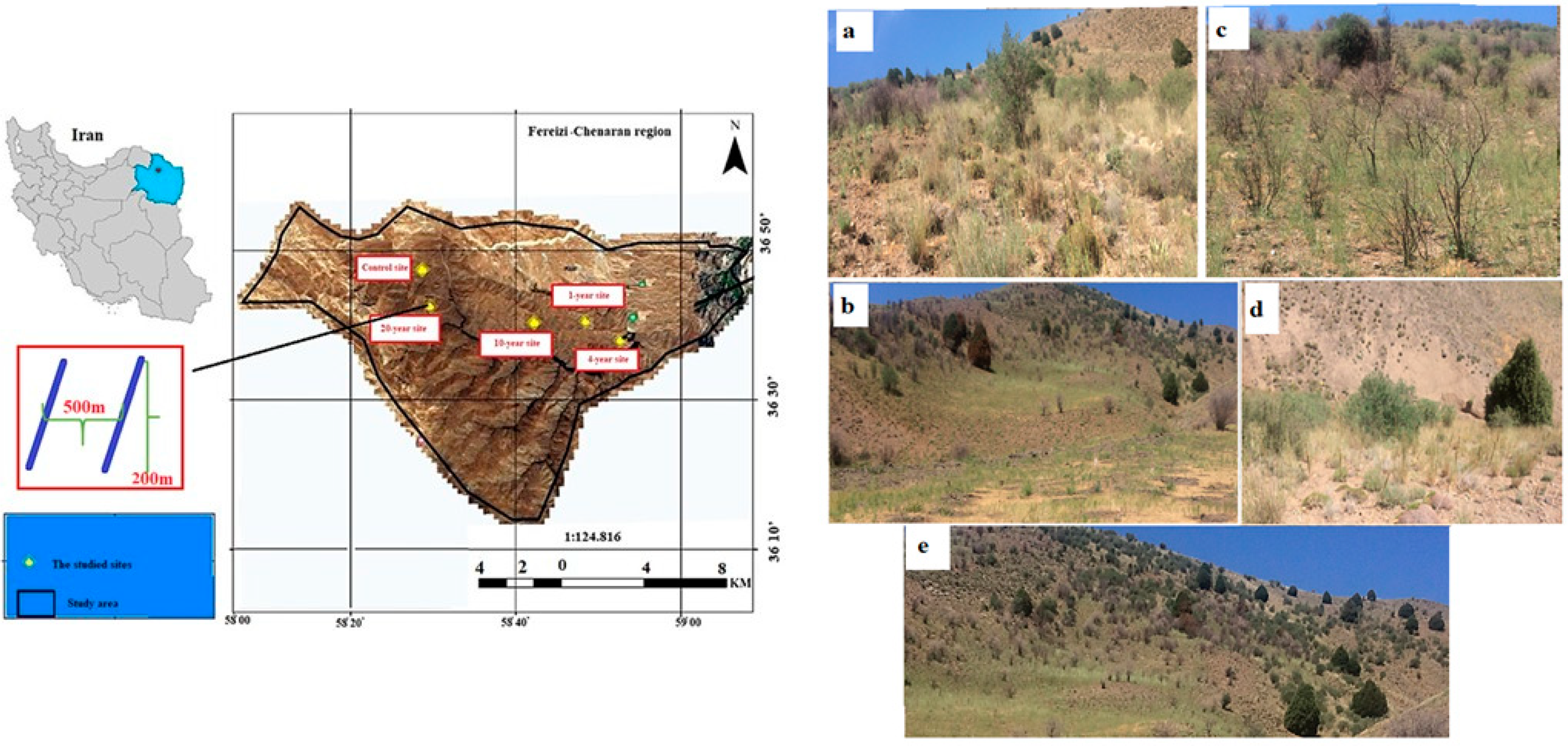
2.1. Study Area
2.2. Data Collection
2.2.1. Site Selection and Vegetation Sampling
2.2.2. Plant Functional Traits
2.2.3. Phylogenetic Information
2.2.4. Measures of Taxonomic, Functional, and Phylogenetic Diversity
2.3. Statistical Analyses
3. Results
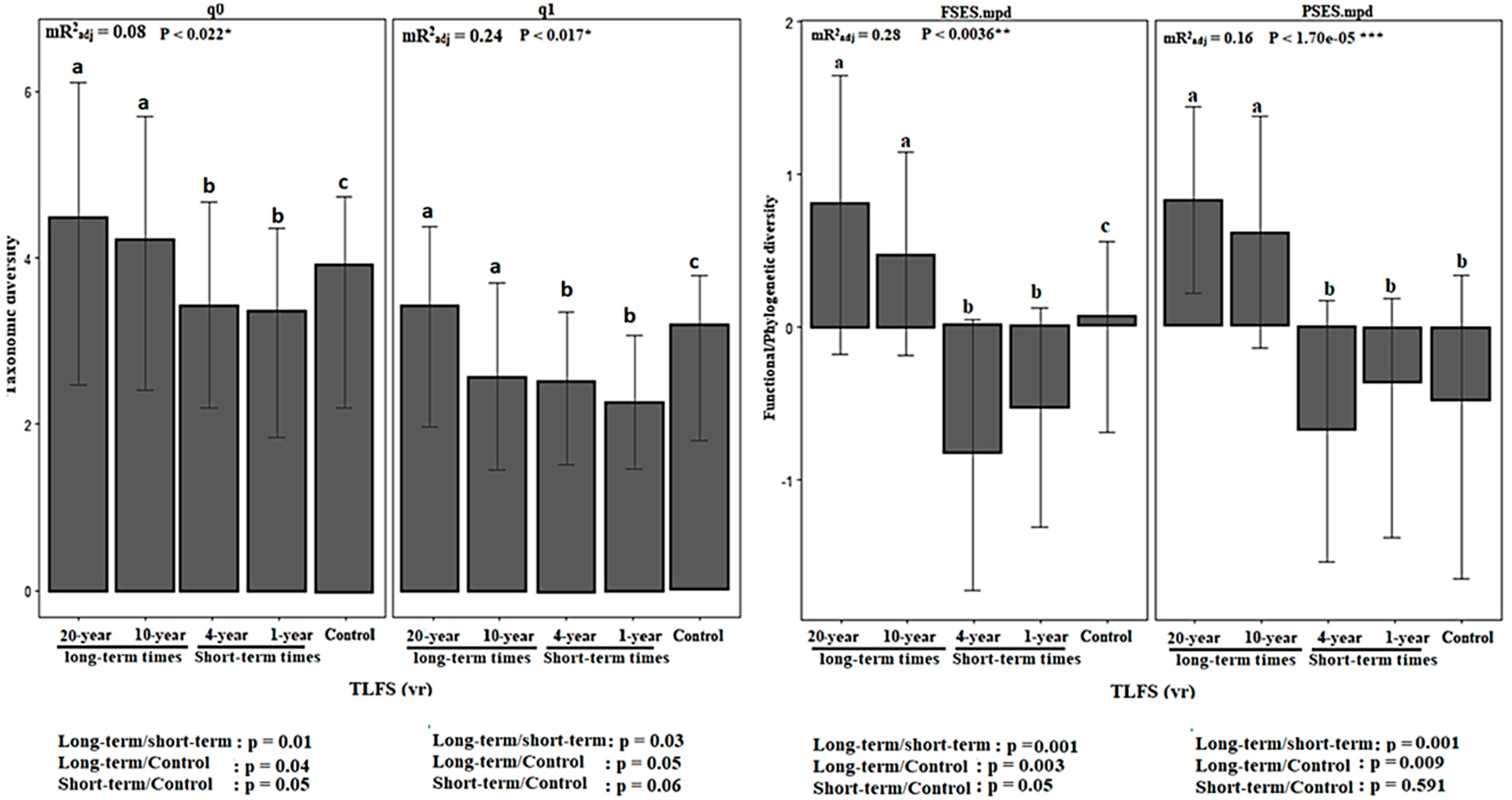
3.1. Plant Biodiversity Changes across Time after Wildfire
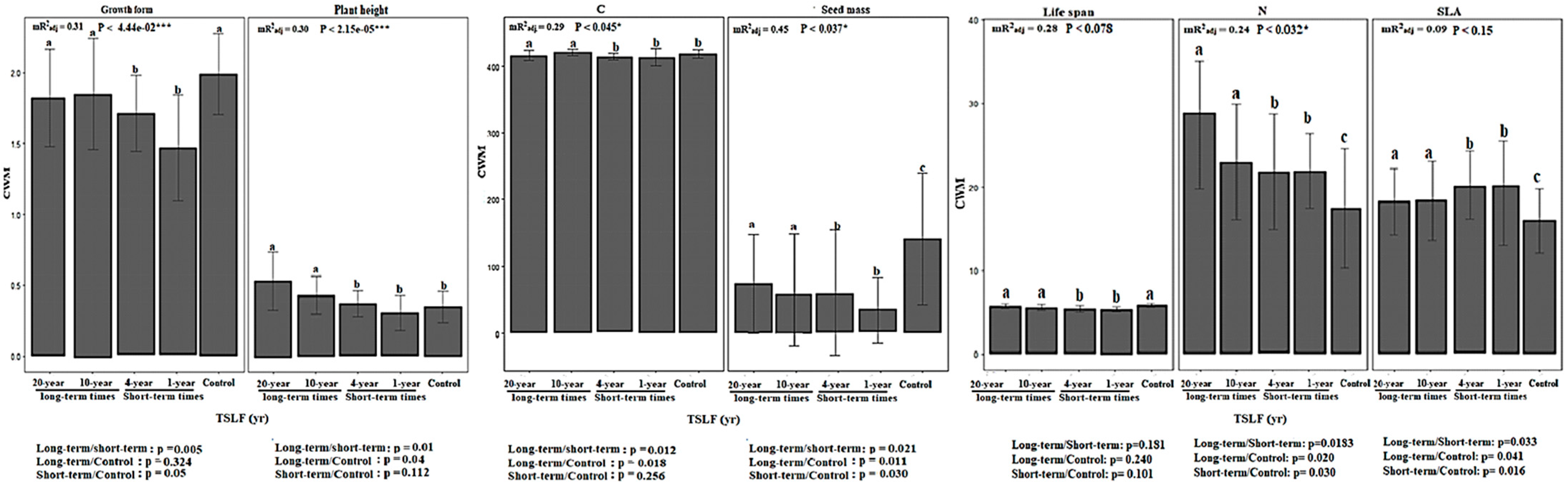
3.2. Diversity of Functional Traits across Time Scales
4. Discussion
4.1. Post-Fire Recovery of Plant Biodiversity Facets across Time since Last Fire
4.2. Post-Fire Recovery of Plant Biodiversity Consistent with Changes in Trait, Evolution, and Ecosystem Stability
4.3. Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badano, E.I.; Samour-Nieva, O.R.; Flores, J.; Flores-Flores, J.L.; Flores-Cano, J.A.; Rodas-Ortíz, J.P. Facilitation by nurse plants contributes to vegetation recovery in human-disturbed desert ecosystems. J. Plant Ecol. 2016, 9, 485–497. [Google Scholar] [CrossRef]
- Ren, H.; Yang, L.; Liu, N. Nurse plant theory and its application in ecological restoration in lower subtropics in China. Prog. Nat. Sci. 2008, 18, 137–142. [Google Scholar] [CrossRef]
- Swenson, N.G.; Enquist, B.J.; Pither, J.; Thompson, J.; Zimmerman, J.K. The problem and promise of scale dependency in community phylogenetics. Ecology 2006, 87, 2418–2424. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Davies, T.J. Phylogenies in Ecology: A Guide to Concepts and Methods; Princeton University Press: Princeton, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Kozak, K.H.; Fine, P.V.; Kembel, S.W. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009, 12, 693–715. [Google Scholar] [CrossRef]
- Yang, J.; Swenson, N.J.; Zhang, G.; Ci, X.; Cao, M.; Sha, L.; Lin, L. Local-scale partitioning of functional and phylogenetic beta diversity in a tropical tree assemblage. Sci. Rep. 2015, 5, 12731. [Google Scholar] [CrossRef]
- Piston, N.; Schob, C.; Armas, C.; Prieto, I. Contribution of co-occurring shrub species to community richness and phylogenetic diversity along an environmental gradient. Perspect. Plant Ecol. Evol. Syst. 2016, 19, 30–39. [Google Scholar] [CrossRef]
- Keesstra, S.D.; Bouma, J.; Wallinga, J.; Tittonell, P.; Smith, P.; Cerdà, A.; Quinton, J.N.; Pachepsky, Y.; van der Putten, W.H.; Bardgett, R.D. The significance of soils and soil science towards realization of the United Nations Sustainable Development Goals. Soil 2016, 2, 111–128. [Google Scholar] [CrossRef]
- Bowd, E.J.; Lindenmayer, D.B.; Banks, S.C.; Blair, D.P. Logging and fire regimes alter plant communities. Ecol. Applicat. 2018, 28, 826–841. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.M.J.S.; Perry, G.L.; Higgins, S.I.; Johnson, C.N.; Fuhlendorf, S.D.; Murphy, B.P. Pyrodiversity is the coupling of biodiversity and fire regimes in food webs. Philos. Trans. R. Soc. B. 2016, 371, 20150169. [Google Scholar] [CrossRef] [PubMed]
- Hurteau, M.D.; Bradford, J.B.; Fulé, P.Z.; Taylor, A.H.; Martin, K.L. Climate change, fire management, and ecological services in the southwestern US. For. Ecol. Manag. [CrossRef]
- Pausas, J.G.; Ribeiro, E. The global fire-productivity relationship. Glob. Ecol. Biogeog. 2013, 22, 728–736. [Google Scholar] [CrossRef]
- Vallego, V.R.; Arianoutsou, M.; Moreira, F. Fire Ecology and Post-Fire Restoration Approaches in Southern European Forest Types. Manag. For. Ecosyst. 2012, 24, 93–119. [Google Scholar] [CrossRef]
- Bahalkeh, K.; Abedi, M.; Tilaki, D.; Michalet, R. Fire slightly decreases on the short-term the competitive effects of a thorny cushion shrub in a semi-arid mountain steppe. Appl. Veg. Sci. 2021, 24, e12575. [Google Scholar] [CrossRef]
- Abedi, M.; Omidipour, R.; Hosseini, S.V.; Bahalkeh, K.; Gross, N. Fire disturbance effects on plant taxonomic and functional β-diversity mediated by topographic exposure. Ecol. Evol. 2022, 12, e8552. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2016, 16, 406–411. [Google Scholar] [CrossRef]
- Hernandez-Serrano, A.; Verdu, M.; Gonzalez-Martínez, S.C.; Pausas, J.G. Fire structures pine serotiny at different scales. Am. J. Bot. 2013, 100, 2349–2356. [Google Scholar] [CrossRef]
- Jankju, M.; Delavari, A.; Ganjali, A. Interseeding Bromus kopetdaghensis, in shrublands. Iran. J. Range Sci. 2008, 2, 314–328. [Google Scholar]
- Huston, M.A. Disturbance, productivity, and species diversity: Empiricism vs. logic in ecological theory. Ecology 2014, 95, 2382–2396. [Google Scholar] [CrossRef]
- Omidipour, R.; Tahmasebi, P.; Faizabadi, M.F.; Faramarzi, M.; Ebrahimi, A. Does β diversity predict ecosystem productivity better than species diversity? Ecol. Indic. 2021, 122, 107212. [Google Scholar] [CrossRef]
- Bashirzadeh, M.; Soliveres, S.; Farzam, M.; Ejtehadi, H. Plant–plant interactions determine taxonomic, functional and phylogenetic diversity in severe ecosystems. Glob. Ecol. Biogeog. 2022, 31, 649–662. [Google Scholar] [CrossRef]
- Pashirzad, M.; Ejtehadi, H.; Vaezi, J.; Sheferson, R.P. Spatial scale dependent phylogenetic signal in species distributions along geo graphic and elevation gradients in a mountainous rangeland. Ecol. Evol. 2018, 8, 10364–10373. [Google Scholar] [CrossRef]
- Soliveres, S.; Maestre, F.T.; Bowker, M.A.; Torices, R.; Quero, J.L.; Garcia-Gomez, M.; Noumi, Z. Functional traits determine plant co-occurrence more than environment or evolutionary relatedness in global drylands. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Soliveres, S.; Torices, R.; Maestre, F.T. Environmental conditions and biotic interactions acting together promote phylogenetic randomness in semi-arid plant communities: New methods help to avoid misleading conclusions. J. Veg. Sci. 2012, 23, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Kembel, S.W.; Hubbell, S.P. The phylogenetic structure of a neotropical forest tree community. Ecology 2006, 87, S86–S99. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, Y.J.; Zhang, J.; Wang, X. Latitudinal gradients in phylogenetic relatedness of angiosperm trees in North America. Glob. Ecol. Biogeog. 2013, 22, 1183–1191. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Verdu, M. Facilitation can increase the phylogenetic diversity of plant communities. Ecol. Lett. 2007, 10, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Vega-Alvarez, J.; Garcia-Rodriguez, J.; Cayuela, L. Facilitation beyond specie richness. J. Ecol. 2019, 107, 722–734. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Barbosa, N.P.U.; Alberton, B.; Barbieri, A.; Dirzo, R.; Goulart, F.; Guerra, T.J.; Morellato, L.P.C.; Solar, R.R.C. The deadly route to collapse and the uncertain fate of Brazilian rupestrian grasslands. Biodiv. Conser. 2018, 27, 2587–2603. [Google Scholar] [CrossRef]
- Memariani, F.; Joharchi, M.R.; Ejtehadi, H.; Emadzadeh, K.H. A cintribution of the flora and vegetation of Binalood mountain range. NE Iran: Floristic and Chrological studies in Fereizi. Ferdowsi Univ. Int. J. Biol. Sci. 2009, 1, 1–17. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Inter. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Bashirzadeh, M.; Shefferson, R.P.; Farzam, M. Plant–plant interactions determine natural restoration of plant biodiversity over time, in a degraded mined land. Ecol. Evol. 2022, 12, e8878. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Omidipour, R.; Abedi, M.; Baskin, C. Effects of fire disturbance on alpha and beta diversity and on beta diversity components of soil seed banks and aboveground vegetation. Plant Ecol. Evol. 2017, 150, 247–256. [Google Scholar] [CrossRef]
- Lienin, P.; Kleyer, M. Plant trait responses to the environment and effects on ecosystem properties. Basic Appl. Ecol. 2012, 13, 301–311. [Google Scholar] [CrossRef]
- Lavorel, S.; Díaz, S.; Cornelissen, J.H.C.; Garnier, E.; Harrison, S.P.; McIntyre, S.; Pausas, J.G.; Pérez-Harguindeguy, N.; Roumet, C.; Urcelay, C. Plant functional types: Are we getting any closer to the Holy Grail? In Terrestrial Ecosystems in a Changing World; Canadell, J., Pitelka, L.F., Pataki, D., Eds.; Springer: Berlin, Germany, 2007; pp. 149–164. [Google Scholar]
- Davies, K.W.; Svejcar, T.J.; Bates, J.D. Interaction of historical and nonhistorical disturbances maintains native plant communities. Ecol. Applic. 2009, 19, 1536–1545. [Google Scholar] [CrossRef]
- Rahmanian, S.; Hejda, M.; Ejtehadi, H.; Farzam, M.; Memariani, F.; Pyšek, P. Effects of livestock grazing on soil, plant functional diversity, and ecological traits vary between regions with different climates in northeastern Iran. Ecol. Evol. 2019, 9, 8225–8237. [Google Scholar] [CrossRef]
- Anacker, B.; Rajakaruna, N.; Ackerly, D.; Harrison, S.; Keeley, J.; Vasey, M. Ecological strategies in California chaparral: Interacting effects of soils, climate, and fire on specific leaf area. Plant Ecol. Div. 2011, 4, 179–188. [Google Scholar] [CrossRef]
- Sakschewski, B.; von Bloh, W.; Boit, A.; Rammig, A.; Kattge, J.; Poorter, L.; Peñuelas, J.; Thonicke, K. Leaf and stem economics spectra drive diversity of functional plant traits in a dynamic global vegetation model. Glob. Change Biol. 2015, 21, 2711–2725. [Google Scholar] [CrossRef]
- Maitner, B.S.; Boyle, B.; Casler, N.; Condit, R.; Enquist, B.J. The BIEN R package: A tool to access the Botanical Information and Ecology Network (BIEN) database. Method Ecol. Evol. 2018, 9, 373–379. [Google Scholar] [CrossRef]
- Kattge, J.; Diaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY—A global database of plant traits. Glob. Change Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Kleyer, M.; Bekker, R.M.; Knevel, I.C.; Bakker, J.P.; Sonnenschein, P.; Poschlod, P.; Van Groenendael, J.M.; Klimeš, L.; Klimešová, J.; Klotz, S.; et al. The LEDA Traitbase: A database of life-history traits of the Northwest European flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Bocci, G. TR8: An R package for easily retrieving plant species traits. Methods Ecol. Evol. 2015, 6, 347–450. [Google Scholar] [CrossRef]
- Lamanna, C.; Blonder, B.; Violle, C.; Kraft, N.J.B.; Sandel, B.; Imova, I.; Donoghue, J.C.; Svenning, J.C.; McGill, B.J.; Boyle, B.; et al. Functional trait space and the latitudinal diversity gradient. Proc. Natl. Acad. Sci. USA 2014, 111, 13745–13750. [Google Scholar] [CrossRef] [PubMed]
- Cavieres, L.A.; Brooker, R.W.; Butterfield, B.J.; Cook, B.J.; Kikvidze, Z.; Lortie, C.J.; Michalet, R.; Pugnaire, F.I.; Schöb, C.; Xiao, S.; et al. Facilitative plant interactions and climate simultaneously drive alpine plant diversity. Ecol. Lett. 2013, 17, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qian, H.V. PhyloMaker: An R package that can generate very large phylogenies for vascular plants. Ecography 2019, 42, 1353–1359. [Google Scholar] [CrossRef]
- Cayuela, L.; Granzow-de la Cerda, Í.; Albuquerque, F.S.; Golicher, D.J. taxonstand: An r package for species names standardization in vegetation databases. Methods Ecol. Evol. 2012, 3, 1078–1083. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Tucker, C.M.; Cadotte, M.W.; Carvalho, S.B.; Davies, T.J.; Ferrier, S.; Fritz, S.A.; Grenyer, R.; Helmus, M.R.; Jin, L.S.; Mooers, A.O.; et al. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev. 2017, 92, 698–715. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Ann. Rev. Ecol. Syst. 2012, 33, 475–505. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Burkle, L.A.; Simanonok, M.P.; Durney, J.S.; Myers, J.A.; Belote, R.T. Wildfires Influence Abundance, Diversity, and Intraspecific and Interspecific Trait Variation of Native Bees and Flowering Plants Across Burned and Unburned Landscapes. Front. Ecol. Evol. 2019, 7, 252. [Google Scholar] [CrossRef]
- Barton, K. Package ‘MuMIn’. Model Selection and Model Averaging Based on Information Criteria. R Package Version 1.15.11. 2013. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 2 January 2023).
- Blomberg, S.P.; Garland, T.J.r.; Ives, A.R. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar] [CrossRef]
- Herath, D.N.; Lamont, B.B.; Enright, N.J.; Miller, B.P. Impact of fire on plant-species persistence in post-mine restored and natural shrubland communities in southwestern Australia. Biol. Conserv. 2009, 142, 2175–2180. [Google Scholar] [CrossRef]
- Hanes, T.L. Succession after fire in the chaparral of southern California. Ecol. Monogr. 1971, 41, 27–52. [Google Scholar] [CrossRef]
- Enright, N.J.; Mosner, E.; Miller, B.P.; Johnson, N.; Lamont, B.B. Soil versus canopy seed storage and plant species coexistence in species-rich shrublands of southwestern Australia. Ecology 2007, 88, 2292–2304. [Google Scholar] [CrossRef]
- McLauchlan, K.K.; Higuera, P.E.; Miesel, J.; Rogers, B.M.; Schweitzer, J.; Shuman, J.K.; Tepley, A.J.; Varner, J.M.; Veblen, T.T.; Adalsteinsson, S.A.; et al. Fire as a fundamental ecological process: Research advances and frontiers. J. Ecol. 2020, 108, 2047–2069. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. The role of nurse plants in the restoration of degraded environments. Front. Ecol. Environ. 2006, 4, 196–202. [Google Scholar] [CrossRef]
- Badano, E.I.; Cavieres, L.A. Impacts of ecosystem engineers on community attributes: Effects of cushion plants at different elevations of the Chilean Andes. Div. Distr. 2006, 12, 388–396. [Google Scholar] [CrossRef]
- Bannister, J.A.; Travieso, N.; Acevedo, M.; Acevedo, M.; Puettmann, K.; Salas-Eljatib, C. Shrub influences on seedling performance when restoring the slow-growing conifer Pilgerodendron uviferum in southern bog forests. Restor. Ecol. 2019, 28, 396–407. [Google Scholar] [CrossRef]
- Gomez-Aparicio, L. The role of plant interactions in the restoration of degraded ecosystems: A meta-analysis across life-forms and ecosystems. J. Ecol. 2009, 97, 1202–1214. [Google Scholar] [CrossRef]
- Gómez-González, S.; Torres-Díaz, C.; Bustos-Schindler, C.; Gianoli, E. Anthropogenic fire drives the evolution of seed traits. Proc. Natl. Acad. Sci. USA 2011, 108, 18743–18747. [Google Scholar] [CrossRef] [PubMed]
- Le Bagousse-Pinguet, Y.; Soliveres, S.; Gross, N.; Torices, R.; Berdugo, M.; Maestre, F.T. Phylogenetic, functional, and taxonomic richness have both positive and negative effects on ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2019, 116, 8419–8424. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Cano, J.A.; Goberna, M.; Verdú, M. Data from: Trait based selection of nurse plants to restore ecosystem functions in mine tailings. J. Appl. Ecol. 2018, 55, 1195–1206. [Google Scholar] [CrossRef]
- Svriz, M.; Damascos, M.A.; Zimmermann, H.; Hensen, I. The exotic shrub Rosa rubiginosa as a nurse plant. Implications for the restoration of disturbed temperate forests in Patagonia, Argentina. For. Ecol. Manag. 2013, 289, 234–242. [Google Scholar] [CrossRef]
- Wilgan, B.W.V.; Govender, V.; Forsyth, G.G.; Krajii, T. Towards adaptive fire management for biodiversity conservation: Experience in South African National Parks. Koedoe-Afr. Prot. Area Conserv. Sci. 2011, 53, 1–9. [Google Scholar] [CrossRef]
- Bond, W.J.; Woodward, F.I.; Midgley, G.F. The global distribution of ecosystems in a world without fire. New Phytol. 2005, 165, 525–538. [Google Scholar] [CrossRef]
- UN. Transforming Our World: The 2030 Agenda for Sustainable Development; Governmental Document A/RES/70/1; General Assembly in September 2015; United Nations: New York, NY, USA, 2015; 35p. [Google Scholar]
- Visser, S.; Keesstra, S.; Maas, G.; De Cleen, M. Soil as a basis to create enabling conditions for transitions towards sustainable land management as a key to achieve the SDGs by 2030. Sustainability 2019, 11, 6792. [Google Scholar] [CrossRef]
| Functional Traits | Blomberg’s K-Statistic K p-Value |
|---|---|
| Seed mass | 0.48 0.003 ** |
| Plant height | 0.37 0.05 * |
| Specific Leaf Area (SLA) | 0.29 0.009 ** |
| Life span | 0.11 0.60 ns |
| Leaf Carbon content | 0.55 0.005 ** |
| Leaf Nitrogen content | 0.09 0.11 ns |
| Growth form | 0.51 0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashirzadeh, M.; Abedi, M.; Shefferson, R.P.; Farzam, M. Post-Fire Recovery of Plant Biodiversity Changes Depending on Time Intervals since Last Fire in Semiarid Shrublands. Fire 2023, 6, 103. https://doi.org/10.3390/fire6030103
Bashirzadeh M, Abedi M, Shefferson RP, Farzam M. Post-Fire Recovery of Plant Biodiversity Changes Depending on Time Intervals since Last Fire in Semiarid Shrublands. Fire. 2023; 6(3):103. https://doi.org/10.3390/fire6030103
Chicago/Turabian StyleBashirzadeh, Maral, Mehdi Abedi, Richard P. Shefferson, and Mohammad Farzam. 2023. "Post-Fire Recovery of Plant Biodiversity Changes Depending on Time Intervals since Last Fire in Semiarid Shrublands" Fire 6, no. 3: 103. https://doi.org/10.3390/fire6030103
APA StyleBashirzadeh, M., Abedi, M., Shefferson, R. P., & Farzam, M. (2023). Post-Fire Recovery of Plant Biodiversity Changes Depending on Time Intervals since Last Fire in Semiarid Shrublands. Fire, 6(3), 103. https://doi.org/10.3390/fire6030103






