Abstract
Fire is an important disturbance factor in shrublands, grasslands, and savannas. It alters the habitat of a multitude of species and, under natural dynamics, is a major determinant of landscape vegetation patterns. Here, we evaluate the effects of different wildfire regimes on the abundance of sun spiders in the Cerrado-Pantanal ecotone. To study how different fire regimes affect the number of individual sun spiders, we considered the frequency of fire occurrences in the last 20 years and classified locations as high frequency or low frequency. We also classified the time of the last fire in 2020 as occurring in the first or second half of the year. In addition, we compared the number of individual sun spiders before and after fire. We found no effects of fire frequency and period when the fire occurred in 2020, but the number of individual sun spiders was higher after wildfires. Although ground-dwelling are considered fire sensitive, some can employ strategies to tolerate fire so that they are able to not only survive, but also reproduce in fire-prone landscapes. Thus, we suggest that sun spiders are resilient, can explore sites under different fire regimes, and can be considered pyrophilous species.
1. Introduction
Fire plays a key ecological role in many terrestrial environments, particularly in flammable shrublands, grasslands, and savannas [1]. These ecosystems evolved under periodically occurring natural fires that start at the beginning or at the end of the rainy season and are typically caused by lightning [2]. At these times, the air and fuel layers are both wet, the vegetation is green, and fire events are usually followed by rain [2].Therefore, natural fires are more irregular, less severe, and cause less damage to biodiversity than human-initiated wildfires which are more difficult to control and expensive to fight [3,4].
The Pampa, the Cerrado, and the Pantanal are considered fire-dependent biomes [5,6]. Fires are recurrent in these environments, as they accumulate large amounts of combustible dry plant material during the dry season [6]. Fires can occur naturally in the Cerrado and the Pantanal and have historical relevance in the maintenance of these biomes [6]. In addition, the traditional people, such as the Kadiwéu indigenous people, also use fire as a management tool for various purposes, such as a weapon of war, hunting, cultivation, and mainly to prevent large fires [7,8].
In 2019−2020, the incidence of fires increased in Brazil. The Pantanal in particular recorded the largest area of fire in 2020 in the last 20 years, with about 30% of its area burned [6,9]. As the frequency of high-severity fires is expected to increase with global warming, understanding the impact of fires on biodiversity is critical [10]. The alteration of the frequency, intensity, and pattern of wildfire regimes by man is estimated to have endangered at least 4400 terrestrial and freshwater species and their habitats worldwide [11]. In general, tiny soil-dwelling organisms are among the least studied with regard to the effects of fire, in spite of their role in ecosystem functions [12]. The scarcity of studies on the effects of fire on fauna results from the difficulty of conducting large-scale controlled experiments to reproduce effects due to the risks of fire spreading outside the experimental areas and the ethical concerns with regard to this elective disturbance in such systems [13].
Solifugae (also known as camel spiders or sun spiders) is a mesodiverse order of ground-dwelling predators arachnids, with approximately 1200 species distributed across 144 genera and 12 families [14]. Sun spiders mainly inhabit arid and semi-arid regions worldwide [15,16]. Among the seven Arachnida orders, Solifugae are usually referred to as ‘the neglected cousins’ [17] due to their high diversity and systematic shortfalls. For instance, in the Neotropics, most studies focused on systematics and the taxonomy of taxa from two Solifugae families [18,19,20,21,22,23,24,25,26], while the ecology and natural history of these organisms remain poorly known [27,28,29]. The number of sun spiders may follow a well-defined seasonal pattern as their abundance has been described to decrease with the increasing monthly mean temperature [27], but was observed to increase soon after fires [27]. Furthermore, wildfires will increase the abundance of herbivores for a short post-fire period because most plants show rapid regrowth after fires [30,31], consequently increasing the availability of resources for predators such as sun spiders.
In this study, we evaluate the effects of different wildfire regimes on the abundance of individual sun spiders belonging to the order Solifugae. We aim to answer the questions: (1) Do locations with high fire frequency have reduced numbers of sun spiders? (2) Considering that early fires (i.e., at the end of the rainy season) have lower intensity than late fires (at the end of the dry season), is there a difference in the abundance of sun spiders one year after the fire event? (3) Considering that soon after the fire event there is plant regrowth and colonization by herbivorous insects, will the abundance of sun spiders, which are predators, increase compared to the before-fire period?
We test three alternative hypotheses: (1) The number of individual sun spiders will be higher with low fire frequency, as these sites have higher habitat complexity than sites with high fire frequency. (2) Sites where the last fire event occurred at the end of the rainy season tend to have low amounts of biomass available as fuel, so here we expect that fire will have a lower effect on the abundance of sun spiders than at sites where the last event occurred at the end of the dry season. (3) After burning, owing to the increase in herbivorous insects, we will have an increase in sun spiders [30,31,32,33].
2. Materials and Methods
2.1. Study Area
This study was conducted in the Kadiwéu Indigenous Reserve (20°37′ S, 57°03′ W), a 540,000 ha reserve located in the north of Porto Murtinho Municipality, southwestern Mato Grosso do Sul State, Brazil (Figure 1). The reserve is located in a ecotone between two Brazilian biomes, the Pantanal (wetland) and the Cerrado (savanna), encompassing heterogeneous vegetation formed by different physiognomies [7]. These complex vegetation mosaics are characterized by patches of closed tree formations interspersed with a herbaceous-grassy matrix, which is subject to different fire regimes (Figure 2 and Figure 3) [8,34,35].
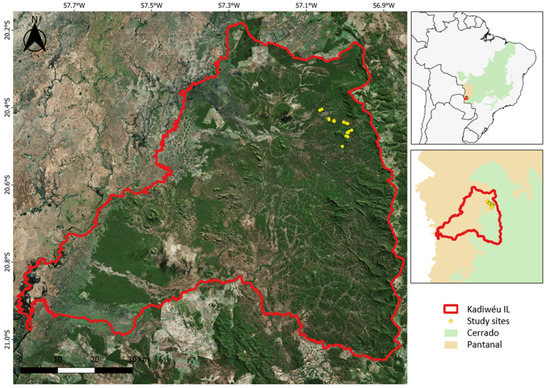
Figure 1.
Location of the study sites where sun spiders were sampled in the Kadiwéu Indigenous Reserve, Porto Murtinho, Mato Grosso do Sul, Brazil.

Figure 2.
The aspect of the study sites in the Kadiwéu Indigenous Reserve changes throughout the year, as seen at the beginning of the dry season in June 2021 (a), at the end of the dry season in August 2021 (b), and at the beginning of the wet season in October 2021 (c).

Figure 3.
Landscape view of the Kadiwéu Indigenous Reserve at the beginning of the dry season in June 2021 (a), the end of the dry season in August 2021 (b), with several fire foci visible in the background, and at the beginning of the wet season in February 2021 (c).
2.2. Experimental Design
We collected data eight times at 17 study sites monthly between June 2021 and February 2022, except for January 2022. We had five high frequency/late sites, four high frequency/early sites, four low frequency/early sites, and four low frequency/late sites. The high frequency/late treatment had one more replicate than the others, which we included in the final analyses, as randomly excluding one of the five sites did not affect the results.
We defined fire frequency as the number of times fire events occurred between 2001 and 2020. We categorized the sites as high fire frequency (more than seven fire events in 2001–2020, with an event approximately every 1–2 years) or low fire frequency (seven fire events or less in the same period, with an event approximately every 3–4 years) [36,37]. We classified the last occurrence of fire at the sampling site in the year 2020 as early when the event occurred in the first half of the year (in the early dry season) or late when the event occurred in the second half of the year (i.e., late dry season).
To test the short-term effect of fire on the abundance of sun spiders, we considered the timing of fire events during the sampling period: before fire, after fire (max. one month), and after fire (two months or more).
We calculated fire frequency by mapping the burn scars for 2001–2020 obtained from images by the MODIS-Terra satellite of the Vegetation Indices product (MOD13Q1 v.6) of the United States Geological Survey [38]. We preprocessed the images and then classified them using Spring v. 5.5 software [39]. We used non-supervised classification with pixel-to-pixel K-means classifier, configured for nine themes and 100 interactions. We opted for this configuration, as after several tests it proved to be the most suitable for our objective. This process resulted in yearly maps of burned areas, which we merged to generate the fire frequency map for the whole 20-year period.
To estimate landscape parameters after the occurrence of wildfires, we used the Normalized Difference Vegetation Index (NDVI). We calculated NDVI from Sentinel 2 satellite images, with a 10 m spatial resolution. We considered all images that were available for the 30-day interval after the fire in study sites and calculated NDVI using the Google Earth Engine platform [40]. The values of NDVI range from −1 to 1, with higher index values indicating higher vegetation cover [41].
2.3. Sun Spiders Sampling
Sun spiders were captured using 500 mL pitfall traps, installed at ground level. We selected pitfall traps based on the recommendations of Muma [42], as they were found to be a reliable collection method to estimate population size and seasonal variation in sun spiders. Our traps consist of a 7 cm high plastic cup with a circular opening of 5 to 6 cm radius, filled up half (approximately 250 mL) with soap and water solution to break the surface tension. In total, we employed 1360 traps at 17 sites during eight visits. We divided each site into five 50 m2-plots, each containing two traps 10 m apart. After 48 h the pitfall traps were emptied and the specimens were sorted and stored in 70% ethanol.
2.4. Data Analysis
We modeled the effects of fire frequency (low or high) and the timing of fire in 2020 (early or late) on the abundance of sun spiders using two generalized linear models (GLM) with negative binomial distribution and log-link function due to the high variance (overdispersion). In both models, the abundance of sun spiders was the response variable while the independent variable was fire frequency and timing in the second one. All data were analyzed in the R version 4.2.0 [43] using the glm.nb function of the MASS package [44] and calculated pseudo-R2 and p-values using the nagelkerke function of the rcompanion package [45].
To compare the differences in the number of sun spiders among sampling periods in 2021 (before, one month after, and two months after fires), we performed a Kruskal–Wallis rank sum test using the kruskal.test function, and in case of significant results, a post-hoc Dunn test to determine which sampling periods differed from each other [46].
To verify whether there was an increase in the NDVI at different times after the wildfires, we performed a one-way repeated measures ANOVA using the ezANOVA function of the ez package [47], followed by a paired t-test with Bonferroni correction to verify the differences between the sampling times using the pairwise.t.test function of the same package.
3. Results
We collected 120 sun spiders specimens, all of an undescribed species of the Genus Gaucha Mello-Leitão (Mummucidae), to be described later (L.S. Carvalho, in prep.). Low frequency/late fire plots had the highest number (37 individuals; 31%), followed by low frequency/early fire (33 ind.; 28%), high frequency/late fire (26 ind.; 22%), and high frequency and early fire plots (24 ind.; 20%). In 2021, the fires occurred in August and September at the end of the dry season. Fire frequency (X2fire_frequency = 1.30; pseudo-R2 = 0.011; p = 0.25) and the timing of the fire event in 2020 (X2period_fire = 0.30; pseudo-R2 = 0.002; p = 0.58) did not affect spider abundance (Figure 4).
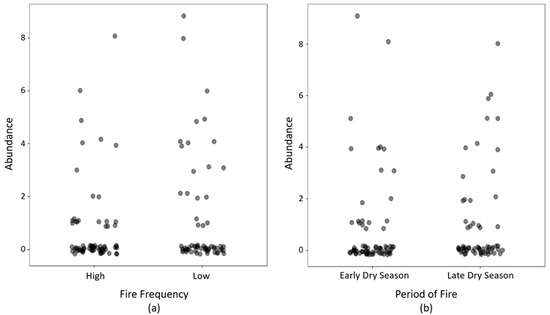
Figure 4.
(a) The number of sun spiders collected by pitfall traps at sites with different fire frequency (a) and early vs late in the season (b) in the Kadiwéu Indigenous Reserve, Porto Murtinho, Mato Grosso do Sul State, Brazil. High fire frequency means more than seven fire events in 2001–2020, while low fire frequency is seven or less. A fire is considered an early dry season event if it occurred between January and June in 2020, and late in July or after.
We found differences in the abundance of sun spiders before, one month after, and two months or more after a fire event in 2021 (X2 = 33.331; df = 2, p < 0.001; Figure 5), with a significant increase one month after fires. From the second month onwards, the abundance did not differ from the samples taken before the fires.
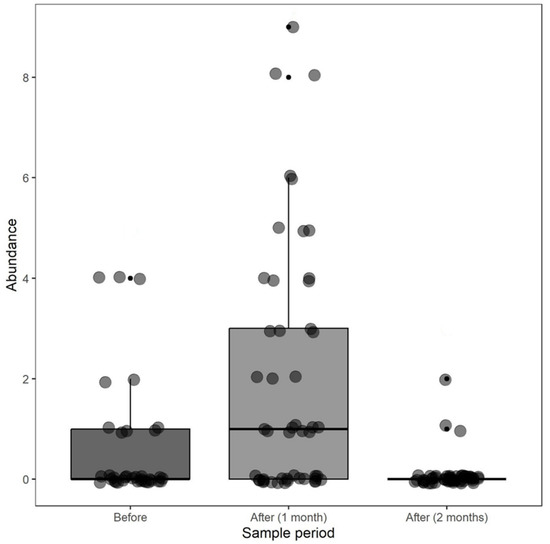
Figure 5.
Abundance of sun spiders before, one month after, and two months after fires in 2021 in the Kadiwéu Indigenous Reserve, Porto Murtinho, Mato Grosso do Sul State, Brazil.
We found differences in the NDVI as a function of the days after the fires at the sampling sites in 2021 (one-way repeated measures ANOVA, F = (2, 32) 13.19, p < 0.001), with the lowest index values at 10–25 days after fire (comparison between pairs with Bonferroni correction (0.299 ± 0.065). The NDVI increased after 30 days after fires (Table 1).

Table 1.
Study site locations and Normalized Difference Vegetation Index (NDVI) Values during, 10–25 after, and 30 or more days after fire.
4. Discussion
In this study, we assessed the effects of different frequencies and timings of fire occurrence on the abundance of sun spiders. Even though ground-dwelling predators abundance has been reported to decrease after fire [48], this pattern was not observed in our study. We found that the differences in fire frequency and the timing of the fire did not affect the abundance of sun spiders. Arthropod responses to fire depend on a variety of factors, including the species studied, their stage of development at the time of fire, and their responses to changes in habitat and community characteristics [49].
In a study of the effects of prescribed fire for fuel reduction and manual fuel removal and control, in general, no differences were detected in the richness, composition, or biomass of soil macroarthropods at the order and family level in oak and hickory forests [50]. The few taxa (e.g., Araneae: Coriniidae and Cyrtaucheniidae, Coleoptera: Scarabaeidae, and Hymenoptera: Formicidae) that showed a response increased in biomass or relative abundance in response to manual fuel removal combined with prescribed burning or prescribed burning only relative to at least some of the other treatments or controls [50].
Similar results were obtained in a study of spiders in South African grasslands, with fire frequency having no measurable effects on terrestrial spider abundance nor on the structure of the assemblage [51]. Similarly, ants were found not to be substantially affected in fire-prone environments [52,53].
Our results suggest that sun spiders are resilient and can survive at sites with different fire regimes, as their numbers remained unchanged for two or more months after the fire. Spider communities have been reported to be functionally resilient to fire disturbance in grassland ecosystems, recovering within a year after fire [54]. Fire resistance and resilience are closely related to the history of fire in the ecosystem and the respective taxa [55]. Spider communities in deciduous forests have also demonstrated resilience to disturbance by fire and possibly as a consequence of frequent fires that occurred in these forests historically [56]. In fire-prone regions, resistance and resilience are generally positively correlated with fire frequency and such regions are considered fire dependent [5]. However, ecosystems with no natural fire occurrence are affected by an increase in fire frequency and are highly sensitive to wildfires [5].
Soil arthropods in general are considered extremely resilient to different fire regimes [57,58]. These organisms have the ability to exploit burned environments because a few centimeters below the surface, soil temperatures are generally relatively low even during the fire event and these invertebrates are likely to escape underground or take advantage of refuges within or near the burned area [59,60]. However, the response of arthropods to wildfires can vary, since their abundance and diversity can increase, decrease, or remain unchanged [48]. According to a review on the effects of fire on arthropods, the orders Araneae, Lepidoptera, and the suborder Homoptera respond negatively to the effects of fire [48]. On the other hand, Orthoptera (grasshoppers and crickets) and Coleoptera (beetles) generally respond positively to fire [48,61].
Similarly to what has been reported in the literature from the Brazilian Cerrado [27], we found that the abundance of sun spiders was highest immediately after fires, at the end of the dry season. The same study found that numbers declined 3–4 months later [27]. A similar increase was recorded months after the occurrence of a wildfire in desert grassland in New Mexico, in the number of sun spiders, scorpions, grasshoppers, and beetles [62]. The effects of fire in natural ecosystems largely depend on the co-evolution of the given ecosystem with fire, i.e., how natural fires have shaped speciation, species composition, and vegetation structure, as well as animal populations over time [6]. The biota of these landscapes is adapted to a diversity of fire regimes and many species depend on fire to complete their life cycles or are benefited by habitat modifications and resource availability induced by wildfires [63]. Therefore, our results suggest that the Gaucha sp. Evaluated in our study can be considered pyrophilous, as their abundance increased soon after fire and used habitats or substrates created by fire [64].
Here we found that NDVI significally increased 30 days after fire, supporting our third hypothesis, which implies that plant biomass increases with time after fires [65]. Savanna vegetation is resilient to frequent fire and most plant species regenerate vigorously after burning [66]. This rapid regrowth increases the availability of resources for herbivorous insects that prefer younger leaves [32,33]. Therefore, wildfire can increase the abundance of the main herbivorous insect orders for a short post-fire period [30,31]. Phytophages become the main component of the soil fauna community once the vegetation has recovered slightly [67]. In a successive phase, soil-dwelling predators enter the burned area [68]. Considering that sun spiders are excellent predators, the regrowth of plants after fire indirectly increases the availability of resources for them, since there is an increase in the abundance of insects that can serve as food [62].
Predators respond to fire depending on the guild to which they belong [69,70]. The abundance of hunting spiders, for instance, can increase after fire in response to the higher abundance of potential preys [54,70,71]. The abundance of web-building spiders, on the other hand, can decline due to the lack of microhabitats and dense foliage to build their webs [54]. Lycosid spiders are known to tolerate changes in microclimate, as they are relatively unaffected by vegetation structure [71].
While vegetation, temperature, precipitation, and other factors also affect the seasonal dynamics of soil fauna, their relative importance is not clear [72,73]. Fire intensity and severity as well as the season of the burning all affect the direct mortality of soil invertebrates, particularly if the fire event occurs during a critical phase of the invertebrates’ phenology, such as diapause or the reproductive period [12]. Apparently, this is not the case for our studied species. Sun spiders had a more clustered distribution during the dry season, which seems to be a characteristic of the taxon, as described in the literature [27,74]. The emergence and reproductive activity of adults of different species determine the increase in the number of sun spiders [75]. Oviposition at the end of the cold and dry season ensures the most favorable conditions for larval hatching and nymphal development with the onset of the warm and wet season, when small arthropods appear in large numbers [75].
Identifying a general pattern in the effects of fire on arachnids is very difficult, mainly due to the differences between fire regimes, ecosystem characteristics, and the biology of the species that make up each assemblage [76]. Even though all ground-dwelling can be considered “fire intolerant”, many populations survive and reproduce in fire-prone landscapes and have strategies to tolerate fire (e.g., resistance, refuge seeking, or exogenous recolonization) [76]. During combustion, spiders can survive if they find refuge in the ground or under non-flammable debris [77]. It is also likely that most of the spider fauna will colonize a burned area immediately after the fire [71]. The Cerrado and the Pantanal are biomes with large knowledge gaps with regard to the occurrence of these organisms [78]. Therefore, our study expands the amount of information on the occurrence of sun spiders, presents the effects of fire on their abundance, and demonstrates the importance of fire for the maintenance of this taxon.
Author Contributions
Conceptualization, B.A.A., L.S.C. and D.B.R.; investigation, B.A.A. and D.B.R.; formal analysis, T.S.T.; writing—original draft preparation, B.A.A., T.S.T., L.S.C., M.d.R.O. and D.B.R.; writing—review and editing, B.A.A., T.S.T., L.S.C. and D.B.R.; supervision, D.B.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by CNPQ (Edital 33/2018; Grant number: 441948/2018-9). We thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) for B.A.A’s grant (CAPES n. 88887.470100/2019-00) and T.S.T.’s grant (CAPES/COFECUB n. 88887.647558/2021-00).
Data Availability Statement
Data are available from the authors upon request.
Acknowledgments
We are grateful to the leaders of the Kadiwéu Indigenous Nation, as well as all inhabitants of Aldeia Alves de Barros for the authorization of access to the Kadiwéu Indigenous Territory; firefighter members and Integrated Fire Management agents of the Associação de Brigadistas Indígena da Nação Kadiwéu (ABINK) for their assistance in the field; Centro Nacional de Prevenção e Combate aos Incêndios Florestais (Prevfogo)/Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) for technical and logistical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bowman, D.M.J.S.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P.; et al. Fire in the Earth System. Science (80-) 2009, 324, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, C.N.; Batista, E.K.L. Good Fire, Bad Fire: It Depends on Who Burns. Flora Morphol. Distrib. Funct. Ecol. Plants 2020, 268, 151610. [Google Scholar] [CrossRef]
- Balch, J.K.; Bradley, B.A.; Abatzoglou, J.T.; Chelsea Nagy, R.; Fusco, E.J.; Mahood, A.L. Human-Started Wildfires Expand the Fire Niche across the United States. Proc. Natl. Acad. Sci. USA 2017, 114, 2946–2951. [Google Scholar] [CrossRef]
- Hantson, S.; Andela, N.; Goulden, M.L.; Randerson, J.T. Human-Ignited Fires Result in More Extreme Fire Behavior and Ecosystem Impacts. Nat. Commun. 2022, 13, 2717. [Google Scholar] [CrossRef] [PubMed]
- Hardesty, J.; Myers, R.; Fulks, W. Fire, Ecosystems and People: A Preliminary Assessment of Fire as a Global Conservation Issue. Fire Manag. 2005, 22, 78–87. [Google Scholar]
- Pivello, V.R.; Vieira, I.; Christianini, A.V.; Ribeiro, D.B.; Menezes, L.S.; Berlinck, C.N.; Melo, F.P.L.; Marengo, J.A.; Tornquist, C.G.; Tomas, W.M.; et al. Understanding Brazil’s Catastrophic Fires: Causes, Consequences and Policy Needed to Prevent Future Tragedies. Perspect. Ecol. Conserv. 2021, 19, 233–255. [Google Scholar] [CrossRef]
- Ferreira, B.H.d.S.; Guerra, A.; Oliveira, M.d.R.; Reis, L.K.; Aptroot, A.; Ribeiro, D.B.; Garcia, L.C. Fire Damage on Seeds of Calliandra Parviflora Benth. (Fabaceae), a Facultative Seeder in a Brazilian Flooding Savanna. Plant Species Biol. 2021, 36, 523–534. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Ferreira, B.H.S.; Souza, E.B.; Lopes, A.A.; Bolzan, F.P.; Roque, F.O.; Pott, A.; Pereira, A.M.M.; Garcia, L.C.; Damasceno, G.A.; et al. Indigenous Brigades Change the Spatial Patterns of Wildfires, and the Influence of Climate on Fire Regimes. J. Appl. Ecol. 2022, 59, 1279–1290. [Google Scholar] [CrossRef]
- Damasceno-Junior, G.A.; Roque, F.O.; Garcia, L.C.; Ribeiro, D.B.; Tomas, W.M.; Scremin-Dias, E.; Dias, F.A.; Libonati, R.; Rodrigues, J.A.; Santos, F.L.M.; et al. Lessons to Be Learned from the Wildfire Catastrophe of 2020 in the Pantanal Wetland. Wetl. Sci. Pract. 2021, 38, 107–115. [Google Scholar]
- Buckingham, S.; Murphy, N.; Gibb, H. Effects of Fire Severity on the Composition and Functional Traits of Litter-Dwelling Macroinvertebrates in a Temperate Forest. For. Ecol. Manag. 2019, 434, 279–288. [Google Scholar] [CrossRef]
- Kelly, L.T.; Giljohann, K.M.; Duane, A.; Aquilué, N.; Archibald, S.; Batllori, E.; Bennett, A.F.; Buckland, S.T.; Canelles, Q.; Clarke, M.F.; et al. Fire and Biodiversity in the Anthropocene. Science (80-) 2020, 370, eabb0355. [Google Scholar] [CrossRef]
- Certini, G.; Moya, D.; Lucas-Borja, M.E.; Mastrolonardo, G. The Impact of Fire on Soil-Dwelling Biota: A Review. For. Ecol. Manag. 2021, 488, 118989. [Google Scholar] [CrossRef]
- Duarte, M.H.L.; Sousa-Lima, R.S.S.; Young, R.J.; Vasconcelos, M.F.; Bittencourt, E.; Scarpelli, M.D.A.; Farina, A.; Pieretti, N. Changes on Soundscapes Reveal Impacts of Wildfires in the Fauna of a Brazilian Savanna. Sci. Total Environ. 2021, 769, 144988. [Google Scholar] [CrossRef] [PubMed]
- Word Solifugae Catalog. Available online: https://wac.nmbe.ch/order/solifugae/6 (accessed on 21 November 2022).
- Cloudsley-Thompson, J.L. Adaptational Biology of Solifugae (Solpugida). Bull. Br. Arachnol. Soc. 1977, 4, 61–71. [Google Scholar]
- Punzo, F. Dispersion, Temporal Patterns of Activity, and the Phenology of Feeding and Mating Behaviour in Eremobates Palpisetulosus Fichter (Solifugae, Eremobatidae). Bull. Br. Arachnol. Soc. 1997, 10, 303–307. [Google Scholar]
- Harvey, M.S. The Neglected Cousins: What Do We Know about the Smaller Arachnid Orders? J. Arachnol. 2002, 30, 357–372. [Google Scholar] [CrossRef]
- Carvalho, L.S.; Candiani, D.F.; Bonaldo, A.B.; Suesdek, L.; Silva, P.R.R. A New Species of the Sun-Spider Genus Mummucia(Arachnida: Solifugae: Mummucidae) from Piauí, Northeastern Brazil. Zootaxa 2010, 2690, 19–31. [Google Scholar] [CrossRef]
- Iuri, H.A.; Iglesias, M.S.; Ojanguren Affilastro, A.A. A New Species of Chileotrecha Maury, 1987 (Solifugae: Ammotrechidae) from Argentina with Notes on the Genus. Zootaxa 2014, 3827, 20–30. [Google Scholar] [CrossRef]
- Botero-Trujillo, R.; Iuri, H.A. Chileotrecha Romero (Kraus, 1966) Comb. Nov. And Pseudocleobis Patagonicus (Roewer, 1934) Comb. Nov. Transferral from Mummuciidae to Ammotrechidae (Arachnida, Solifugae). Zootaxa 2015, 3990, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Botero-Trujillo, R. The Smallest Known Solifuge: Vempironiella Aguilari, New Genus and Species of Sun-Spider (Solifugae: Mummuciidae) from the Coastal Desert of Peru. J. Arachnol. 2016, 44, 218–226. [Google Scholar] [CrossRef]
- Botero-Trujillo, R.; Ott, R.; Carvalho, L.S. Systematic Revision and Phylogeny of the South American Sun-Spider Genus Gaucha Mello-Leitão (Solifugae: Mummuciidae), with Description of Four New Species and Two New Generic Synonymies. Arthropod Syst. Phylogeny 2017, 75, 3–44. [Google Scholar]
- Botero-Trujillo, R.; Ott, R.; Mattoni, C.I.; Nime, M.F.; Ojanguren-Affilastro, A.A. Two New Species of the Sun-Spider Genus Gaucha from Argentina and Brazil (Solifugae, Mummuciidae). Zootaxa 2019, 4551, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.S.; Botero-Trujillo, R. On the Sun-Spiders of the Ibirapemussu Species-Group of the Genus Gaucha Mello-Leitão, 1924 (Solifugae, Mummuciidae), with Description of a New Species. Zootaxa 2019, 4700, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.F.V.R.; Ferreira, R.L.; Carvalho, L.S. A New Species of the Genus Gaucha Mello-Leito, 1924 from Minas Gerais, Brazil (Solifugae, Mummuciidae). Zootaxa 2021, 5061, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Iuri, H.A.; Ramírez, M.J.; Mattoni, C.I.; Ojanguren-Affilastro, A.A. Revision and Cladistic Analysis of Subfamily Nothopuginae (Solifugae, Ammotrechidae). Zool. Anz. 2021, 295, 126–155. [Google Scholar] [CrossRef]
- Martins, E.G.; Bonato, V.; Machado, G.; Pinto-Da-Rocha, R.; Rocha, L.S. Description and Ecology of a New Species of Sun Spider (Arachnida: Solifugae) from the Brazilian Cerrado. J. Nat. Hist. 2004, 38, 2361–2375. [Google Scholar] [CrossRef]
- Peretti, A.V.; Willemart, R.H. Sexual Coercion Does Not Exclude Luring Behavior in the Climbing Camel-Spider Oltacola Chacoensis (Arachnida, Solifugae, Ammotrechidae). J. Ethol. 2007, 25, 29–39. [Google Scholar] [CrossRef]
- Peretti, A.V.; Vrech, D.E.; Hebets, E.A. Solifuge (Camel Spider) Reproductive Biology: An Untapped Taxon for Exploring Sexual Selection. J. Arachnol. 2021, 49, 299–316. [Google Scholar] [CrossRef]
- Diniz, I.R.; Morais, H.C. Efeitos Do Fogo Sobre Os Insetos Do Cerrado: Consensos e Controvérsias. In Efeitos do Regime do Fogo Sobre a Estrutura de Comunidades de Cerrado: Resultados do Projeto Fogo; Ibama: Brasília, Brazil, 2010; pp. 121–131. [Google Scholar]
- Diniz, I.R.; Higgins, B.; Morais, H.C. How Do Frequent Fires in the Cerrado Alter the Lepidopteran Community? Biodivers. Conserv. 2011, 20, 1415–1426. [Google Scholar] [CrossRef]
- Seyffarth, J.A.; Calouro, A.M.; Price, P.W. Leaf Rollers in Ouratea Hexasperma (Ochnaceae): Fire Effect and the Plant Vigor Hypothesis. Rev. Bras. Biol. 1996, 56, 135–137. [Google Scholar]
- Marquis, R.J.; Diniz, I.R.; Morais, H.C. Patterns and Correlates of Interspecific Variation in Foliar Insect Herbivory and Pathogen Attack in Brazilian Cerrado. J. Trop. Ecol. 2001, 17, 127–148. [Google Scholar] [CrossRef]
- Pott, A.; Oliveira, A.K.M.; Damasceno-Junior, G.A.; Silva, J.S.V. Plant Diversity of the Pantanal Wetland. Braz. J. Biol. 2011, 71, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.S.; Gamarra, R.M.; Mioto, C.L.; Silva, N.M.; Conceição Filho, A.P.; Pott, A. Analysis of the Landscape Complexity and Heterogeneity of the Pantanal Wetland. Braz. J. Biol. 2018, 78, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Chinder, G.B.; Hattas, D.; Massad, T.J. Growth and Functional Traits of Julbernardia Globiflora (Benth) Resprouts and Seedlings in Response to Fire Frequency and Herbivory in Miombo Woodlands. S. Afr. J. Bot. 2020, 135, 476–483. [Google Scholar] [CrossRef]
- Manrique-Pineda, D.A.; de Souza, E.B.; Paranhos Filho, A.C.; Cáceres Encina, C.C.; Damasceno-Junior, G.A. Fire, Flood and Monodominance of Tabebuia Aurea in Pantanal. For. Ecol. Manag. 2021, 479, 118599. [Google Scholar] [CrossRef]
- USGS. Available online: https://lpdaac.usgs.gov/products/mod13q1v006/ (accessed on 26 August 2022).
- Câmara, G.; Souza, R.C.M.; Freitas, U.M.; Garrido, J. Spring: Integrating Remote Sensing and Gis by Object-Oriented Data Modelling. Comput. Graph. 1996, 20, 395–403. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sens. Environ. 2017, 202, 18–27. Available online: https://earthengine.google.com/faq/ (accessed on 27 December 2022). [CrossRef]
- Gamarra, R.M.; Teixeira-Gamarra, M.C.; Carrijo, M.G.G.; Filho, A.C.P. Use of NDVI in Analysis of Structure Vegetation and Protection Effectiveness of Conservation Unit in the Cerrado. RA’E GA-O Espac. Geogr. Anal. 2016, 37, 307–332. [Google Scholar]
- Muma, M.H. Comparison of Three Methods for Estimating Solpugid (Arachnida) Populations. J. Arachnol. 1980, 8, 267–270. [Google Scholar]
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 20 November 2022).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Mangiafico, S. Package ‘Rcompanion’. Functions to Support Extension Education Program Evaluation. R package Version 2.4.18 2022; 136p. Available online: https://cran.r-project.org/package=rcompanion (accessed on 23 November 2022).
- Zar, J.H. Bioestatistical Analyses; Pearson Prentice Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- Bakeman, R. Recommended Effect Size Statistic. Behav. Res. Methods 2005, 37, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Kral, K.C.; Limb, R.F.; Harmon, J.P.; Hovick, T.J. Arthropods and Fire: Previous Research Shaping Future Conservation. Rangel. Ecol. Manag. 2017, 70, 589–598. [Google Scholar] [CrossRef]
- Antunes, S.C.; Curado, N.; Castro, B.B.; Gonçalves, F. Short-Term Recovery of Soil Functional Parameters and Edaphic Macro-Arthropod Community after a Forest Fire. J. Soils Sediments 2009, 9, 267–278. [Google Scholar] [CrossRef]
- Greenberg, C.H.; Forrest, T.G.; Waldrop, T. Short-Term Response of Ground-Dwelling Arthropods to Prescribed Hardwood Forest. For. Sci. 2010, 56, 112–121. [Google Scholar]
- Jansen, R.; Makaka, L.; Little, I.T.; Dippenaar-Schoeman, A. Response of Ground-Dwelling Spider Assemblages (Arachnida, Araneae) to Montane Grassland Management Practices in South Africa. Insect Conserv. Divers. 2013, 6, 572–589. [Google Scholar] [CrossRef]
- Parr, C.L.; Robertson, H.G.; Biggs, H.C.; Chown, S.L. Response of African Savanna Ants to Long-Term Fire Regimes. J. Appl. Ecol. 2004, 41, 630–642. [Google Scholar] [CrossRef]
- van Mantgem, E.F.; Keeley, J.E.; Witter, M. Faunal Responses to Fire in Chaparral and Sage Scrub in California, USA. Fire Ecol. 2015, 11, 128–148. [Google Scholar] [CrossRef]
- Podgaiski, L.R.; Joner, F.; Lavorel, S.; Moretti, M.; Ibanez, S.; Mendonça, M.d.S.; Pillar, V.D. Spider Trait Assembly Patterns and Resilience under Fire-Induced Vegetation Change in South Brazilian Grasslands. PLoS ONE 2013, 8, e60207. [Google Scholar] [CrossRef]
- Bengtsson, J. Disturbance and Resilience in Soil Animal Communities. Eur. J. Soil Biol. 2002, 38, 119–125. [Google Scholar] [CrossRef]
- Moretti, M.; Conedera, M.; Duelli, P.; Edwards, P.J. The Effects of Wildfire on Ground-Active Spiders in Deciduous Forests on the Swiss Southern Slope of the Alps. J. Appl. Ecol. 2002, 39, 321–336. [Google Scholar] [CrossRef]
- Uehara-Prado, M.; Bello, A.D.M.; Fernandes, J.D.O.; Santos, A.J.; Silva, I.A.; Cianciaruso, M.V. Abundance of Epigaeic Arthropods in a Brazilian Savanna under Different Fire Frequencies. Zoologia 2010, 27, 718–724. [Google Scholar] [CrossRef]
- Pressler, Y.; Moore, J.C.; Cotrufo, M.F. Belowground Community Responses to Fire: Meta-Analysis Reveals Contrasting Responses of Soil Microorganisms and Mesofauna. Oikos 2019, 128, 309–327. [Google Scholar] [CrossRef]
- Taiton, N.M.; Mentis, M.T. Fire in Grassland. In Ecological Effects of Fire in South African Ecosystems; Booysen, P.V., Taiton, N.M., Eds.; Springer: Berlin, Germany, 1984; pp. 115–147. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Malmström, A.; Zaitsev, A.S.; Shakhab, S.V.; Bengtsson, J.; Persson, T. Do Burned Areas Recover from inside? An Experiment with Soil Fauna in a Heterogeneous Landscape. Appl. Soil Ecol. 2012, 59, 73–86. [Google Scholar] [CrossRef]
- Howard, D.R.; Hill, P.S.M. The Effect of Fire on Spatial Distributions of Male Mating Aggregations in Gryllotalpa Major Saussure (Orthoptera: Gryllotalpidae) at the Nature Conservancy’s Tallgrass Prairie Preserve in Oklahoma: Evidence of a Fire-Dependent Species. J. Kansas Entomol. Soc. 2007, 80, 51–64. [Google Scholar] [CrossRef]
- Parmenter, R.R.; Kreutzian, M.; Moore, D.I.; Lightfoot, D.C. Short-Term Effects of a Summer Wildfire on a Desert Grassland Arthropod Community in New Mexico. Environ. Entomol. 2011, 40, 1051–1066. [Google Scholar] [CrossRef]
- Anjos, A.G.; Alvarado, S.T.; Sol, M. Patch and Landscape Features Drive Fire Regime in a Brazilian Flammable Ecosystem. J. Nat. Conserv. 2022, 69, 126261. [Google Scholar] [CrossRef]
- Wikars, L.O. Dependence on Fire in Wood-Living Insects: An Experiment with Burned and Unburned Spruce and Birch Logs. J. Insect Conserv. 2002, 6, 1–12. [Google Scholar] [CrossRef]
- Zanzarini, F.V.; Pissarra, T.C.T.; Brandão, F.J.C.; Teixeira, D.D.B. Correlação Espacial Do Índice de Vegetação (NDVI) de Imagem Landsat/ETM+ Com Atributos Do Solo. Rev. Bras. Eng. Agric. Ambient. 2013, 17, 608–614. [Google Scholar] [CrossRef]
- Ribeiro, M.N.; Sanchez, M.; Pedroni, F.; Peixoto, K.d.S. Fogo e Dinâmica Da Comunidade Lenhosa Em Cerrado Sentido Restrito, Barra Do Garças, Mato Grosso. Acta Bot. Bras. 2012, 26, 203–217. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Persson, T. Recovery of Soil Macrofauna after Wildfires in Boreal Forests. Soil Biol. Biochem. 2013, 57, 182–191. [Google Scholar] [CrossRef]
- Zaitsev, A.S.; Gongalsky, K.B.; Persson, T.; Bengtsson, J. Connectivity of Litter Islands Remaining after a Fire and Unburnt Forest Determines the Recovery of Soil Fauna. Appl. Soil Ecol. 2014, 83, 101–108. [Google Scholar] [CrossRef]
- Yekwayo, I.; Pryke, J.S.; Gaigher, R.; Samways, M.J. Wandering Spiders Recover More Slowly than Web-Building Spiders after Fire. Oecologia 2019, 191, 231–240. [Google Scholar] [CrossRef]
- Martínez, F.J.; Cheli, G.H.; Grismado, C.J.; Bisigato, A.J. Ground-Dwelling Arachnids and Fire Disturbance: A Case Study in Northeastern Patagonia (Argentina). Fire 2022, 5, 91. [Google Scholar] [CrossRef]
- Bell, J.R.; Philip Wheater, C.; Rod Cullen, W. The Implications of Grassland and Heathland Management for the Conservation of Spider Communities: A Review. J. Zool. 2001, 255, 377–387. [Google Scholar] [CrossRef]
- Yang, X.; Liu, R.T.; Shao, M.A.; Wei, X.R.; Li, T.C.; Chen, M.Y.; Li, Z.Y.; Dai, Y.C.; Gan, M. Short-Term Effects of Wildfire on Soil Arthropods in a Semi-Arid Grassland on the Loess Plateau. Front. Microbiol. 2022, 13, 989351. [Google Scholar] [CrossRef]
- Koltz, A.M.; Burkle, L.A.; Pressler, Y.; Dell, J.E.; Vidal, M.C.; Richards, L.A.; Murphy, S.M. Global Change and the Importance of Fire for the Ecology and Evolution of Insects. Curr. Opin. Insect Sci. 2018, 29, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Xavier, E.; Rocha, L.S. Autoecology and Description of Mummucia Mauryi (Solifugae, Mummuciidae), a New Solifugae from Brazilian Semi-Arid Caatinga. J. Arachnol. 2001, 29, 127–134. [Google Scholar] [CrossRef]
- Belozerov, V.N. Seasonal Aspects of the Life Cycle of Solifuges (Arachnida, Solifugae) as Compared with Pseudoscorpions (Arachnida, Pseudoscorpiones). Entomol. Rev. 2013, 93, 1050–1072. [Google Scholar] [CrossRef]
- Pausas, J.G. Generalized Fire Response Strategies in Plants and Animals. Oikos 2019, 128, 147–153. [Google Scholar] [CrossRef]
- Warren, S.D.; Scifres, C.J.; Teel, P.D. Response of Grassland Arthropods to Burning: A Review. Agric. Ecosyst. Environ. 1987, 19, 105–130. [Google Scholar] [CrossRef]
- De Araujo Lira, A.F.; Lorenzo, E.P.; Desouza, A.M. The First Record of Gaucha Mauryi (Solifugae: Mummucidae) in the State of Pernambuco, Brazil, and Notes on Brazilian Solifuge Species Distributiont. Entomol. News 2020, 129, 406–413. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).