1. Introduction
One of the most pressing global challenges facing mankind is the development and implementation of new types of fuels that could replace the oil derivatives commonly used in engines of various kinds and in power plants. This is necessary for combating global climate change by reducing CO2 emissions and achieving carbon neutrality, as well as for minimizing the emissions of other harmful substances such as soot, polycyclic aromatic hydrocarbons, and nitrogen oxides. Additionally, the depletion of oil fields is a growing concern.
Among fossil fuels, natural gas is the most environmentally friendly. However, methane, the main component of natural gas, due to the stability of its molecular structure, exhibits several properties that hinder its use as a fuel—a long ignition delay, low flame velocity, and narrow ignition limits. These properties make it challenging to use methane in internal combustion engines. To address these difficulties, introducing additives of more reactive compounds into methane is proposed—first of all, hydrogen, as well as diesel fuels, various ethers, etc. [
1,
2,
3,
4,
5].
For a considerable period, efforts have been made to introduce biofuels—combustible species produced from the various plant crops. During the growth process, these crops capture CO
2 from the air, ensuring carbon neutrality. Due to intense competition for fertile soils with food crops, which has attracted public criticism; thus, only so-called second- and third-generation biofuels are currently under consideration (using areas unsuitable for food agriculture and utilizing the aquatic environment, respectively). The specific chemical composition of biofuels in this case can vary widely depending on the process used; they can include various alcohols, ethers, and other compounds. Biofuels could be utilized both as independent fuels and as components of fuel blends [
6,
7].
An even more progressive approach to achieving carbon neutrality is the development of renewable energy sources and/or nuclear power or, in the distant future, thermonuclear power. However, the generation of environmentally friendly and cost-effective electrical energy does not immediately fulfill all the requirements of industry and transportation, as the energy densities achievable with electric accumulators in the foreseeable future are incomparable with the specific calorific values of fossil fuels. Consequently, projects are under consideration for utilizing “green” energy in the synthesis of chemical fuels with convenient properties, for subsequent use in various engines and power plants. Hydrogen is potentially one of the cleanest fuels, as it can be synthesized from water through the electrolysis process and subsequently burned without generating harmful emissions. Nevertheless, the use of pure hydrogen as a fuel encounters numerous technical challenges, including the insufficient development of safe transportation and storage means as well as its higher combustion temperature and flame propagation velocity, which contribute to a critical increase in thermal loads on engine design and an elevated concentration of nitrogen oxides in the combustion products. The transition to pure hydrogen combustion requires significant engine design modernization. In this context, the use of methane–hydrogen mixtures is considered a transitional stage. Studies have shown that adding hydrogen to methane already results in a substantial reduction in CO
2 and soot emissions [
8,
9]. It was suggested [
10] that the use of methane–hydrogen mixtures is safer not only for using hydrogen, but also for methane itself, as mixtures combine the positive qualities of hydrogen (high diffusion coefficient) and methane (lower flame propagation velocity and narrower ignition limits). Modeling has shown that the explosive property of methane–hydrogen is not much higher than that of methane, and that for both of these fuels, the explosion pressure is significantly lower than that of hydrogen. Another promising area for the use of methane–hydrogen mixtures, which has attracted great interest in recent years, is the safer transportation of hydrogen as an additive to methane, with the subsequent separation of hydrogen and its use as the main fuel in engines and power plants [
11].
However, the very possibility of a transition to purely hydrogen energy production is still disputed by many experts, and numerous safety-related issues have remained unresolved for an extended period [
12,
13,
14]. Therefore, the exact development trajectory of future energetic technologies remains unclear, and other compounds that could form the basis of promising fuel mixtures are still a subject of interest. Thus, the development of engines and power plants capable of utilizing fuel blends of various compositions during the transitional stages of global energy production changes is of particular importance as “omnivore” designs begin to achieve dramatic advantages, avoiding locking into wrong choices. Among the various options for such multicomponent combustible mixtures, the possibilities associated with combinations of methane–hydrogen mixtures with various promising biofuels are of particular interest. Some of the most well-known and promising examples of these biofuels are methanol and dimethyl ether (DME).
1.1. Methanol
Methyl alcohol CH
3OH (methanol) can be considered as a biofuel; as the simplest alcohol, it can be synthesized from a wide variety of biomasses and any carbonaceous stock, particularly by gasifying waste and using thermochemical (rather than biological) processes. It can also be a product of carbon capture technologies [
15], although synthesis from natural gas is currently the most widely used method for economic reasons. Methanol can actually be considered a synthetic fuel and a hydrogen carrier, as it contains 40% more hydrogen per volume than liquid hydrogen, without the issues of required energy input for storage, which are severe for molecular hydrogen. Methanol is already one of the most traded chemicals in the world. It possesses many desirable attributes, which make it a very promising spark-ignition engine fuel [
16]. Specifically, due to its lower adiabatic flame temperature, oxygenated molecule, and the absence of carbon–carbon bonds, methanol can reduce both NOx production and soot emissions [
17] and has exceptional knock resistance. It can also be used in the form of diesel/methanol dual-fuel combustion [
18].
1.2. DME
Dimethyl ether CH
3OCH
3 (DME) can also be made using various raw materials including biomasses, and is considered both as an independent fuel for diesel engines and gas turbines, and as a component of combustible mixtures [
19]. A potentially major use of dimethyl ether is also as a substitute for propane in LPG, widely used as fuel in households, transport, and industry [
20,
21]. Only moderate modifications are needed to convert a diesel engine to burn dimethyl ether. The simplicity of this short carbon chain compound with high oxygen content of about 35 wt. % leads to low emissions of particulate matter during combustion. It has a cetane number of 55–60 that is higher than that of diesel fuel. It is noteworthy that DME is a notable example of a two-stage ignition fuel demonstrating negative temperature dependencies of ignition delay [
22]. While all previous studies have shown that both H
2 and DME addition can greatly promote the ignition of CH
4, it is important to note that the DME oxidation mechanism is quite different from that of H
2, and one can expect that the details of ignition enhancement by adding DME to CH
4 would be different from that by adding H
2 to CH
4.
It is important to emphasize that the practical application of such complex multicomponent combustible mixtures is impossible without the creation and experimental verification of appropriate kinetic mechanisms that make it possible to describe their ignition and combustion under various conditions. The majority of contemporary mechanisms have been validated and optimized based on experimental datasets related to the combustion of fundamental fuels; thus, direct experimental investigation of multicomponent mixtures is valuable.
Thus, the primary goal of the present research was the experimental study of shock-induced ignition delays in methane–hydrogen mixtures partially substituted with promising biofuels, namely methanol and dimethyl ether, and subsequent validation of modern kinetic mechanisms using the obtained data.
The second goal of this research was to study the effect of methanol and dimethyl ether additives on flame velocity and temperature at elevated pressures and temperatures, simulating typical compressed engine conditions based on numerical modeling using verified modern kinetic mechanisms.
2. Materials and Methods
2.1. Experiments
Experiments to measure the ignition delay times of combustible mixtures were carried out in a stainless steel shock tube of a standard design with an inner diameter of 50 mm. The lengths of the high-pressure and low-pressure chambers were 1.5 and 3.0 m, respectively. The tube was equipped with two calibrated piezoelectric pressure gauges
PCB113B26 (G
1 and G
2) positioned at distances of 13 and 107 mm from the end plate, respectively, allowing measurements of the velocity of the incident shock wave (ISW), and with a pair of CaF
2 windows 13 mm from the end plate to allow for optical diagnostics. The parameters of the shock-heated gas media behind the reflected shock wave (RSW) were derived from initial conditions and incident shock wave velocity data according to common shock tube theory using the software package
SDToolBox, which enables the solution of standard problems for gas-phase explosions and shock wave propagation using realistic thermochemistry [
23]. Helium was used as a driver gas, and aluminum diaphragms 70 µm in thickness were used to obtain temperatures
TRSW in the range of 1050–1900 K at pressures
PRSW in the range of 3.5–5.5 bar behind the reflected wave. The scheme of the investigated section of the shock tube is presented in
Figure 1a.
The experimentally studied combustible mixtures were prepared manometrically and stored in a stainless steel mixing tank no less than 12 h before use to ensure uniform mixing. The mixtures were diluted with argon to ensure good shock wave front structure. All of the mixtures had the same 7 mol. % fraction of oxygen and were stoichiometric. Mixtures were designated as
[X], where α is the fraction of methane substituted by hydrogen and β is the fraction of the resulting methane–hydrogen fuel substituted by biofuel with designation X (MET for methanol, or DME). Note that substitution was carried out according to corresponding stoichiometric ratios; thus, for example, mixture M
20, where 20% of the original fuel (methane, stoichiometric ratio 1:2) was substituted by hydrogen (stoichiometric ratio 2:1), had equal resulting molar fractions of hydrogen and methane. All of the experimentally investigated mixtures are presented in
Table 1.
Experimental conditions were chosen to provide autoignition delay times less than 1 ms, considered as the working time of the shock tube, during which the temperature and pressure near the end plate remain constant. Ignition delay times were determined by registering the radiation of excited radicals OH*, which are the characteristic species indicating the development of combustion of hydrocarbon–oxygen mixtures. Radiation was registered by a photomultiplier (PM)
Hamamatsu H6780-04, equipped with an interference filter 310 ± 5 nm. In combustible mixtures, the energy release upon ignition is quite abrupt, and leads to a dramatic increase in the OH concentration in both the ground and excited states. Thus, a rapid rise in OH* chemiluminescence was considered as the end of induction time. The exact ignition moment was determined as the intersection of the inflectional tangent line of the OH* radiation intensity plot with the time axis. The increase in pressure was simultaneously recorded by a pressure gauge G
1 positioned at the same section of shock tube. The subsequent changes in OH* radiation intensity reflected the complex combination of kinetic and the intense gas dynamic processes in the post-ignition zone, and were not analyzed. An example of typical oscillograms obtained in the experiments is presented in
Figure 1b.
2.2. Modeling
Numerical kinetic modeling was performed using the freely distributed open-source
Cantera 3.0 software package [
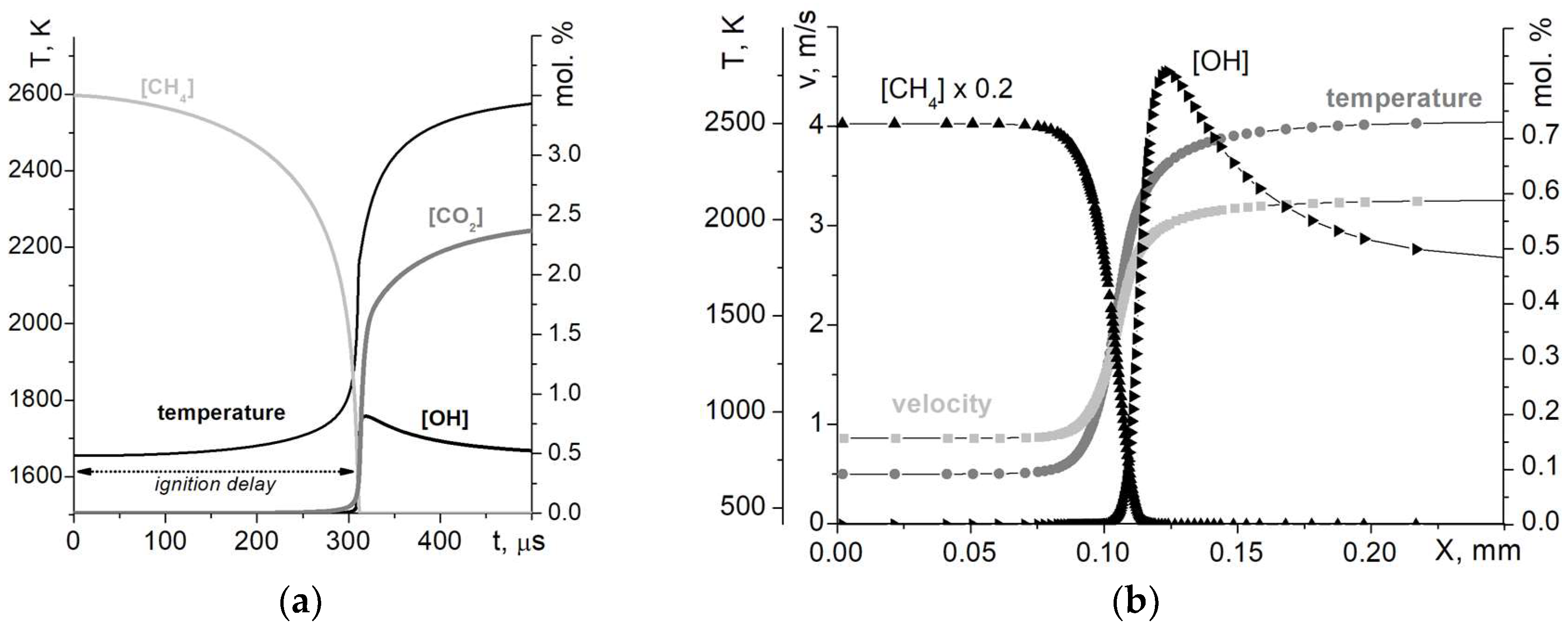
24], implemented as a Python library. Ignition delays were simulated in a zero-dimensional batch reactor of constant volume. The input parameters were mixture composition, pressure, and temperature, and the results were the time profiles of species concentrations. Similar to the approach used in the experiments, the moment of ignition was determined as the point of maximum slope of OH concentration. Typical modeling results for the zero-dimensional (0D) reactor are presented in
Figure 2a. As the pressure behind the reflected wave changes only slightly with temperature in the shock tube experiment series, calculations were carried out at a fixed initial pressure corresponding to the mean of the experimental series, while the initial temperature was varied. The obtained results represent the temperature dependencies of ignition delay time.
After verifying the kinetic mechanism used, a one-dimensional (1D) modeling of a flat premixed flame was performed to obtain dependencies of the normal flame velocity and adiabatic temperature on mixture composition. Calculations were carried out using a mixture-averaged transport model in a domain of a fixed width. The grid refinement criteria were the normalized slopes of species concentrations greater than 0.08 and normalized curvatures (i.e., change in slope) greater than 0.16. The results of calculations included spatial profiles of velocity, temperature, and species concentration. The velocity on the inlet boundary represents the flame velocity, and the temperature on the outlet boundary represents the adiabatic flame temperature of interest. Typical modeling results for a 1D reactor are presented in
Figure 2b.
The detailed kinetic mechanism of the
CRECK modeling group, describing the combustion and pyrolysis of primary fuels, alcohols, and ethers [
25,
26], was used as the main mechanism in this research. This hierarchically organized mechanism is a result of long-term efforts to model the combustion and pyrolysis of a large number of C
1–C
16-based hydrocarbon and oxygenated fuels under a wide range of experimental conditions. It has gained popularity, as it implements a modern approach to describing polycyclic hydrocarbons formation, and can also be supplemented with NO
x and soot formation reaction submodules. The performance of the mechanisms on methane–hydrogen mixtures autoignition was also compared with other classical kinetic schemes—
GRI-Mech 3.0, which has been the industry standard for the last two decades [
27];
FFCM-1, resulting from modern efforts of global constrained optimization within the uncertainties of reaction rate parameters [
28]; and
Aramco 2.0 [
29].
3. Experimental Results
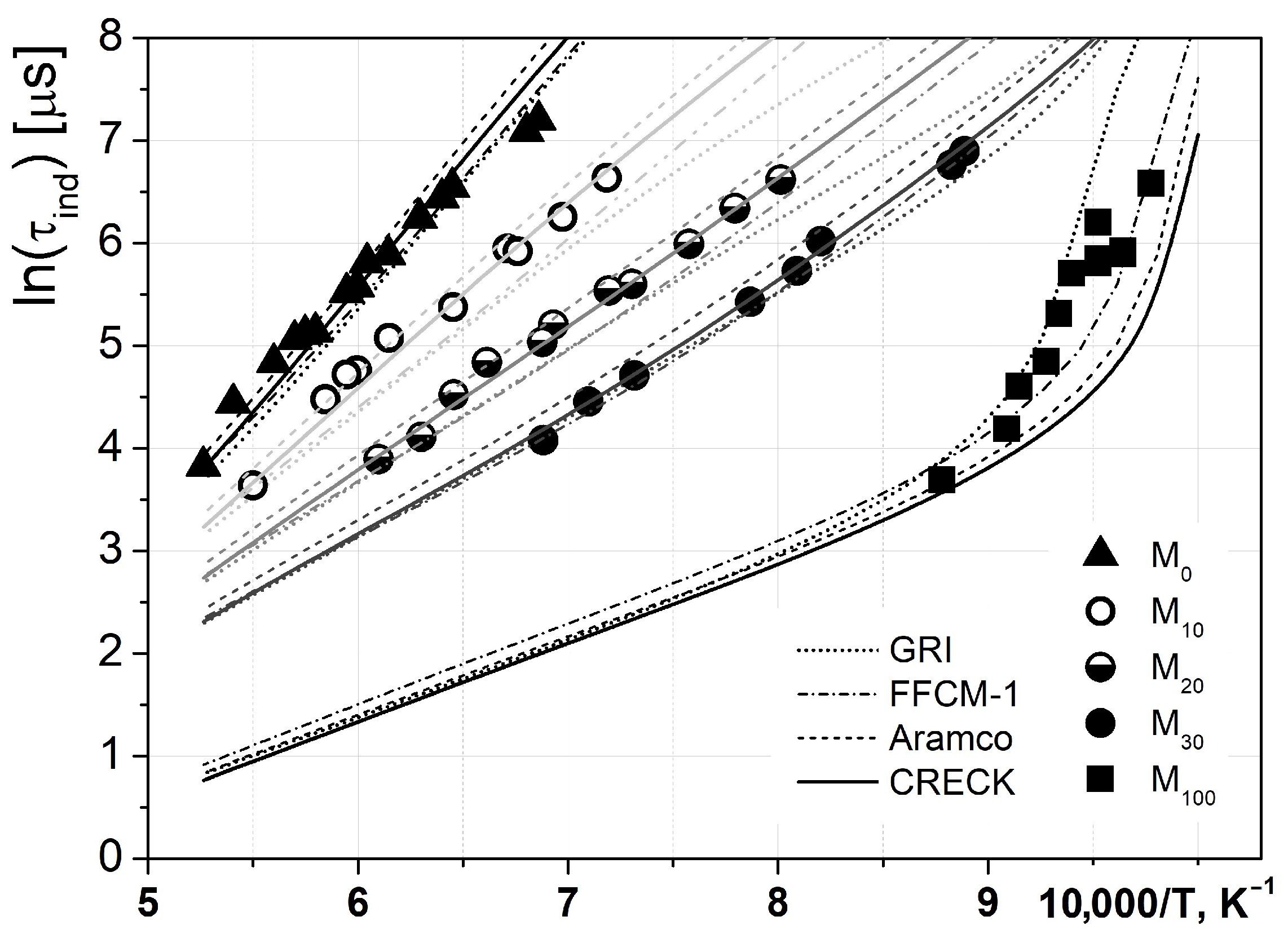
As the first stage of the present study, the ignition delay times in well-investigated mixtures of methane with hydrogen were measured to validate both experimental and numerical methods. In
Figure 3, the temperature dependencies of the experimentally measured (dots) and modeled (using different kinetic mechanisms (lines)) ignition delay times for methane–hydrogen mixtures are presented. All of the considered mechanisms provided very similar predictions of ignition delay times for well-studied methane–hydrogen combustion. The differences barely exceeded the precision of shock tube measurements, except for low-temperature hydrogen ignition, which has been a major challenge for combustion studies for decades [
30]. The observed good agreement between the experimental and modeled data confirms the reliability of the methods used.
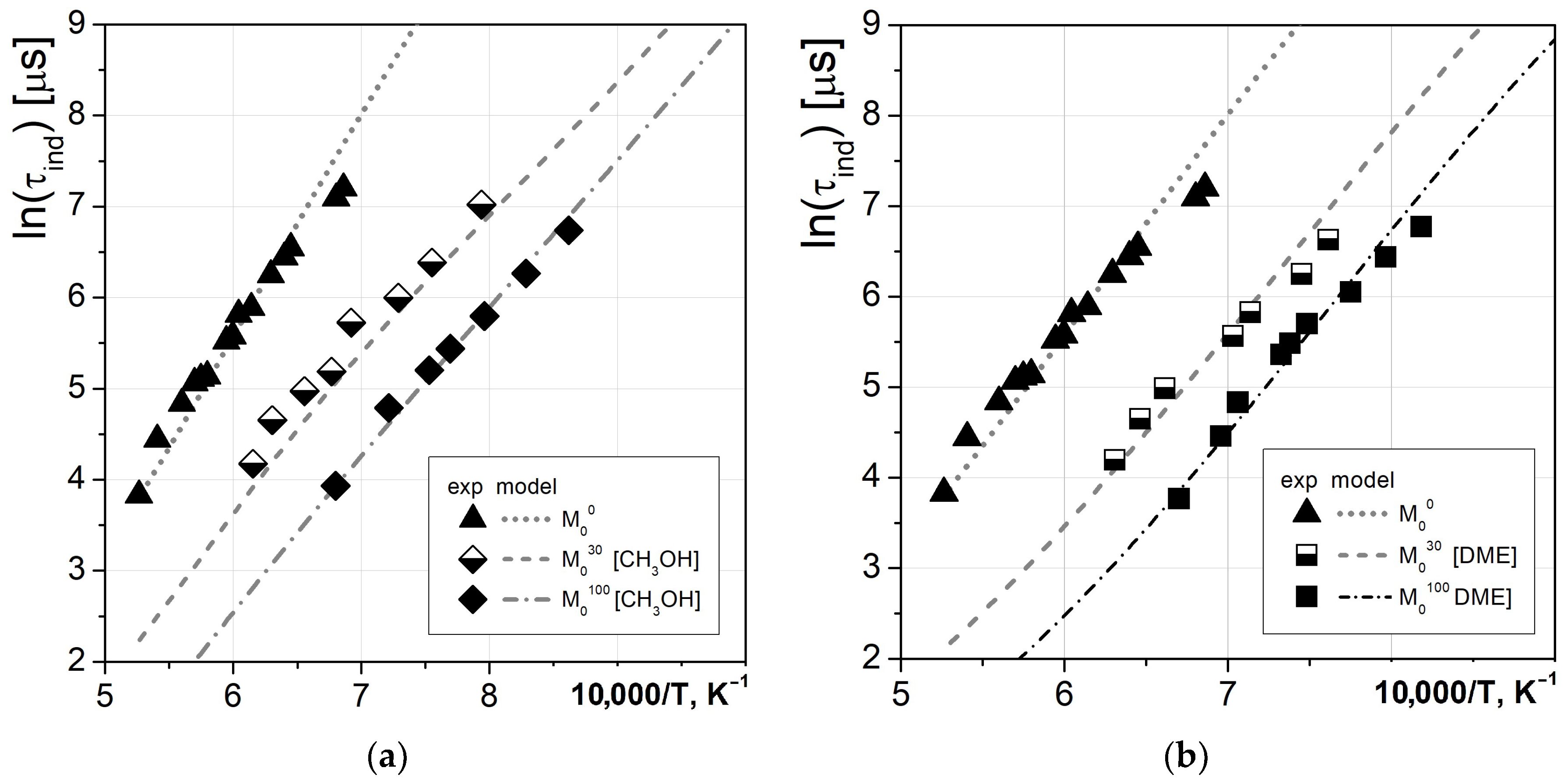
Next, the ignition properties of pure biofuels and their blends with methane were investigated, and the obtained data are presented in
Figure 4. Again, good agreement was observed, allowing us to conclude that the combustion kinetics of both types of blends is satisfactorily described by the used
CRECK mechanism.
Finally, the three-component fuels were investigated. The considered mixtures were based on an M
20 methane–hydrogen mixture which was substituted by 20% and 50% of methanol or DME. The obtained experimental values of the ignition delays and modeled temperature dependencies are presented in
Figure 5.
It can be seen that the substitution of 20% M20 mixture with methanol has no influence on the ignition delay time at lower temperatures, and only slightly shortens it at higher temperatures, which was observed in both the experiments and modeling. However, a notable decrease in ignition delay time was predicted by the modeling based on the CRECK mechanism for the 50% substitution, but was not observed experimentally.
Contrary to methanol, partial substitution of the M20 mixture with dimethyl ether resulted in a change in the slope of temperature dependence and ambiguous influence, namely accelerating ignition at T > 1330 K and decelerating it at lower temperatures. All of the features are in quite good agreement between the experimental and modeling data, indicating the high reliability of the considered kinetic mechanism for DME-related combustion studies.
The general conclusion that can be drawn from the performed measurements is that substitution of part of the methane–hydrogen mixture with methanol or DME at temperatures above 1400 K leads to acceleration of ignition processes, and this effect is most pronounced when DME is added.
4. Discussion
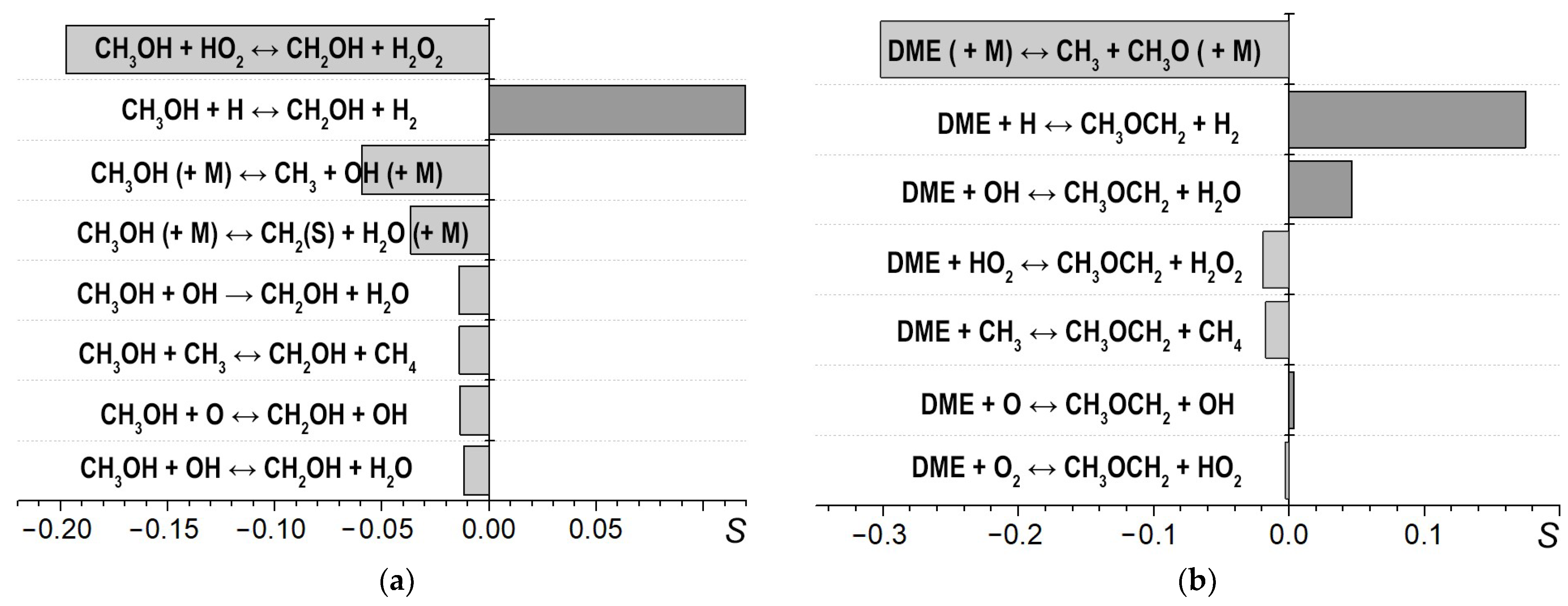
Numerical analysis was performed to determine the sensitivity of the ignition delay time to the rate constants of individual reactions with the direct participation of the biofuel additives under study. The calculations were carried out for the mixtures
[MET] and
[DME] at T = 1400 K and P = 4.5 bar, with ignition delay times of several hundred microseconds. The sensitivity value was determined as
, where
k0 is the undisturbed rate constant,
dk is its variation, and τ
0 and τ are ignition delay times for the original mechanism and the mechanism with the rate of the current reaction of interest changed, respectively. The sensitivity values for the most important reactions are presented in
Figure 6. Note that the negative values correspond to shortening ignition delay times, i.e., to the promotion of combustion.
It is noteworthy that maximal sensitivity among the reactions involving methanol is obtained by the exchange reaction CH
3OH + HO
2 ↔ CH
3OH + H
2O
2, while for DME, the most important is the reaction of its thermal decomposition CH
3OCH
3 (+M) ↔ CH
3 + CH
3O (+M), in which the CH
3 radical is formed. This reasonably corresponds to the change in the slope of temperature dependence of the ignition delay time in the presence of DME, and the lack of such a change in the presence of methanol (see
Figure 5).
From a practical standpoint, along with ignition delays, the flame propagation velocity is one of the key parameters for estimating the combustion properties of a given mixture and its potential compatibility as a fuel with existing engines.
To analyze the effect of biofuel additives on flame propagation velocity and temperature, modeling was performed for stoichiometric fuel–air mixtures at elevated temperatures and pressures. The values of the inlet pressure and temperature were determined for each mixture individually, and were assumed to correspond to adiabatic compression from normal conditions.
Calculations for two-component methane–hydrogen mixtures and mixtures partially substituted with methanol were performed assuming a V0/V = 10 compression ratio, which could be considered typical for internal combustion engines and gas turbines; the corresponding pressures and temperature ranges were P = 22.5–24.7 bar and T = 670–730 K.
In mixtures with a partial substitution of the methane–hydrogen mixture with DME, the high reactivity of DME posed a “cold boundary” problem for the calculation, as some kinetic rates became non-negligible; therefore, the reactant mixture composition changes ahead of the flame, and a steady-state solution does not exist for the inlet flow. Thus, to achieve a stable solution with the same calculation precision settings, a lower compression ratio of V
0/V = 7 was used for modeling in mixtures substituted with DME, resulting in P = 13.9–14.5 bar and T = 590–615 K, with minor changes in the flame parameters for non-substituted methane–hydrogen mixtures. All of the obtained modeling results are presented in
Figure 7.
The most notable feature of the obtained dependencies of flame velocity on the fraction of biofuel substitute is their minor slope. Contrary to the substitution of methane with hydrogen that results in sharp increase in flame velocity up to ten times, the substitution of the methane–hydrogen mixture with both methanol and dimethyl ether changes it only slightly. In mixtures containing a small amount of hydrogen (less than 20%), there is a slight increase in flame velocity as the biofuel content increases. At the same time, in mixtures containing 20–30% hydrogen, when replacing part of the mixture by both biofuels, a decrease in flame velocity is still observed, reaching 30–40%.
The influence of bio-additives on the flame temperature is also small. It is characteristic that the addition of methanol slightly reduces the flame temperature, and substituting the methane–hydrogen mixture with 50% DME has virtually no effect on the flame temperature.
Thus, mixing methane–hydrogen mixtures with biofuels results in a very modest change, and in some cases, even a decrease in flame temperature, which can slightly reduce the heat load on potential engine designs.
5. Conclusions
The shock-induced ignition of three-component methane+hydrogen+biofuel (methanol or DME) mixtures, considered as promising fuel blends, under argon-diluted conditions, was studied experimentally. Temperature dependencies of the ignition delay times of the mixtures in the temperature range of 1250–1650 K were obtained. These experimental data were analyzed through numerical simulations based on state-of-the-art kinetic mechanisms. The analysis showed good agreement between the numerical and experimental results. This made it possible to calculate the influence of biofuel additives on the combustion parameters of methane–hydrogen mixtures. The results show that under the investigated conditions, the substitution of methane–hydrogen mixtures with the considered biofuels results in an insignificant change in laminar flame velocity, adiabatic flame temperature, and ignition delay time. These results provide promising possibilities for the development of engines using these three-component fuel mixtures of varying compositions.
Author Contributions
Conceptualization, A.D. and A.E.; methodology, A.E.; software, A.D.; validation, A.D.; formal analysis, A.D.; investigation, A.D.; resources, A.E.; data curation, A.E.; writing—original draft preparation, A.D.; writing—review and editing, A.E.; visualization, A.D.; supervision, A.E.; project administration, A.E.; funding acquisition, A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science and the Higher Education of the Russian Federation (Agreement No. 075-15-2020-806 dated 29 September 2020).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tripathi, G.; Dhar, A. Performance, emissions, and combustion characteristics of methane-diesel dual-fuel engines: A review. Front. Therm. Eng. 2022, 2, 870077. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V.; Salgansky, E.A.; Arutyunov, A.V.; Arutyunov, V.S. A Comprehensive Review on the Prospects of Using Hydrogen–Methane Blends: Challenges and Opportunities. Energies 2022, 15, 2265. [Google Scholar] [CrossRef]
- Komatsu, K.; Asanuma, M.; Iijima, A.; Yoshida, K.; Shoji, H. A Study of an HCCI Engine Operating on a Blended Fuel of DME and Methane. SAE Int. J. Engines 2012, 5, 9–16. [Google Scholar] [CrossRef]
- Yang, H. Ethers and esters as alternative fuels for internal combustion engine: A review. Int. J. Engine Res. 2021, 24, 146808742110464. [Google Scholar] [CrossRef]
- Essen, M.; Gersen, S.; Dijk, G.; Emde, M. Literature Review on CNG/H2 Mixtures for Heavy-Duty CNG Vehicles; Concawe: Brussels, Belgium, 2021. [Google Scholar]
- Chen, Z.; Lim, K.; Liu, J.; Wang, X.; Jiang, S.; Zhang, C. Optimal design of glucose solution emulsified diesel and its effects on the performance and emissions of a diesel engine. Fuel 2015, 157, 9–15. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Wei, Z.; Wang, Y.; Deng, J. Effect of components on the emulsification characteristic of glucose solution emulsified heavy fuel oil. Energy 2022, 244, 123147. [Google Scholar] [CrossRef]
- Khatir, N.; Abdelkrim, L. Effect of hydrogen addition to methane on ICE emissions and performances: A brief review. J. Sci. Res. Rev. 2013, 2, 75–80. [Google Scholar]
- Karczewski, M.; Chojnowski, J.; Szamrej, G. A Review of Low-CO2 Emission Fuels for a Dual-Fuel RCCI Engine. Energies 2021, 14, 5067. [Google Scholar] [CrossRef]
- Middha, P.; Engel, D.; Hansen, O.R. Can the addition of hydrogen to natural gas reduce the explosion risk? Int. J. Hydrogen Energy 2011, 36, 2628–2636. [Google Scholar] [CrossRef]
- Lurie, M.V. Transportation of Hydrogen Batches by a Gas Pipeline in the Flow of Natural Gas. Territ. “NEFTEGAS” Oil Gas Territ. 2020, 11–12, 84–88. [Google Scholar]
- Fischer, M. Safety aspects of hydrogen combustion in hydrogen energy systems. Int. J. Hydrogen Energy 1986, 11, 593–601. [Google Scholar] [CrossRef]
- Najjar, Y.S.H. Hydrogen safety: The road toward green technology. Int. J. Hydrogen Energy 2013, 38, 10716–10728. [Google Scholar] [CrossRef]
- Arutyunov, V.S. Problems and challenges of hydrogen energy. Combust. Plasma Chem. 2021, 19, 245–255. [Google Scholar]
- Ugwu, A.; Osman, M.; Zaabout, A.; Amini, S. Carbon Capture Utilization and Storage in Methanol Production Using a Dry Reforming-Based Chemical Looping Technology. Energy Fuels 2022, 36, 9719–9735. [Google Scholar] [CrossRef]
- Verhelst, S.; Turner, J.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, Y.; Zhen, X.; Liu, Z. The effect of methanol production and application in internal combustion engines on emissions in the context of carbon neutrality: A review. Fuel 2022, 320, 123902. [Google Scholar] [CrossRef]
- Wang, X.; Gao, J.; Chen, H.; Chen, Z.; Zhang, P.; Chen, Z. Diesel/methanol dual-fuel combustion: An assessment of soot nanostructure and oxidation reactivity. Fuel Process. Technol. 2022, 237, 107464. [Google Scholar] [CrossRef]
- Putrasari, Y.; Lim, O. Dimethyl Ether as the Next Generation Fuel to Control Nitrogen Oxides and Particulate Matter Emissions from Internal Combustion Engines: A Review. ACS Omega 2022, 7, 32–37. [Google Scholar] [CrossRef]
- Stepanenko, D.; Kneba, Z. DME as alternative fuel for compression ignition engines—A review. Combust. Engines 2019, 177, 172–179. [Google Scholar] [CrossRef]
- Soltic, P.; Hilfiker, T.; Wright, Y.; Hardy, G.; Fröhlich, B.; Klein, D. The potential of dimethyl ether (DME) to meet current and future emissions standards in heavy-duty compression-ignition engines. Fuel 2024, 355, 129357. [Google Scholar] [CrossRef]
- Niu, K.; Yao, B.; Xu, Y.; Zhang, H.; Shi, Z.; Wang, Y. Study on Chemical Kinetics Mechanism of Ignition Characteristics of Dimethyl Ether Blended with Small Molecular Alkanes. Energies 2022, 15, 4652. [Google Scholar] [CrossRef]
- Browne, S.; Ziegler, J.; Bitter, N.; Schmidt, B.; Lawson, J.; Shepherd, J.E. SDToolbox—Numerical Tools for Shock and Detonation Wave Modeling. Explosion Dynamics Laboratory, GALCIT Technical Report FM2018.001 Revised April 2023. 2023. Available online: https://shepherd.caltech.edu/EDL/PublicResources/sdt/doc/ShockDetonation/ShockDetonation.pdf (accessed on 18 September 2023).
- Goodwin, D.G.; Moffat, H.K.; Schoegl, I.; Speth, R.L.; Weber, B.W. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes (2.6.0); Cantera Developers: Warrenville, IL, USA, 2022. [Google Scholar]
- Metcalfe, W.K.; Burke, S.M.; Ahmed, S.S.; Curran, H.J. A Hierarchical and Comparative Kinetic Modeling Study of C1 − C2 Hydrocarbon and Oxygenated Fuels. Int. J. Chem. Kinet. 2013, 45, 638–675. [Google Scholar] [CrossRef]
- Burke, U.; Somers, K.; O’Toole, P.; Zinner, C.; Marquet, N.; Bourque, G.; Petersen, E.; Metcalfe, W.; Serinyel, Z.; Curran, H. An ignition delay and kinetic modeling study of methane, dimethyl ether, and their mixtures at high pressures. Comb. Flame 2015, 162, 315–330. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C., Jr.; et al. GRI-Mech 3.0. Available online: http://www.me.berkeley.edu/gri_mech/ (accessed on 18 September 2023).
- Smith, G.P.; Tao, Y.; Wang, H. Foundational Fuel Chemistry Model Version 1.0 (FFCM-1). 2016. Available online: http://nanoenergy.stanford.edu/ffcm1 (accessed on 18 September 2023).
- NUI Galway Combustion Chemistry Centre. AramcoMech 2.0. 2017. Available online: http://www.nuigalway.ie/combustionchemistrycentre/mechanismdownloads/aramcomech20/ (accessed on 18 September 2023).
- Drakon, A.V.; Emelianov, A.V.; Eremin, A.V.; Gurentsov, E.V.; Petrushevich, Y.V.; Starostin, A.N.; Taran, M.D.; Fortov, V.E. Quantum Phenomena in Ignition and Detonation at Elevated Density. Phys. Rev. Lett. 2012, 109, 183201. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).