Technological Aspects of Methane–Hydrogen Mixture Transportation through Operating Gas Pipelines Considering Industrial and Fire Safety
Abstract
:1. Introduction
2. Materials and Methods
2.1. Possibilities of Hydrogen Transportation through the Existing Gas Pipeline Network with due Regard to Fire Safety
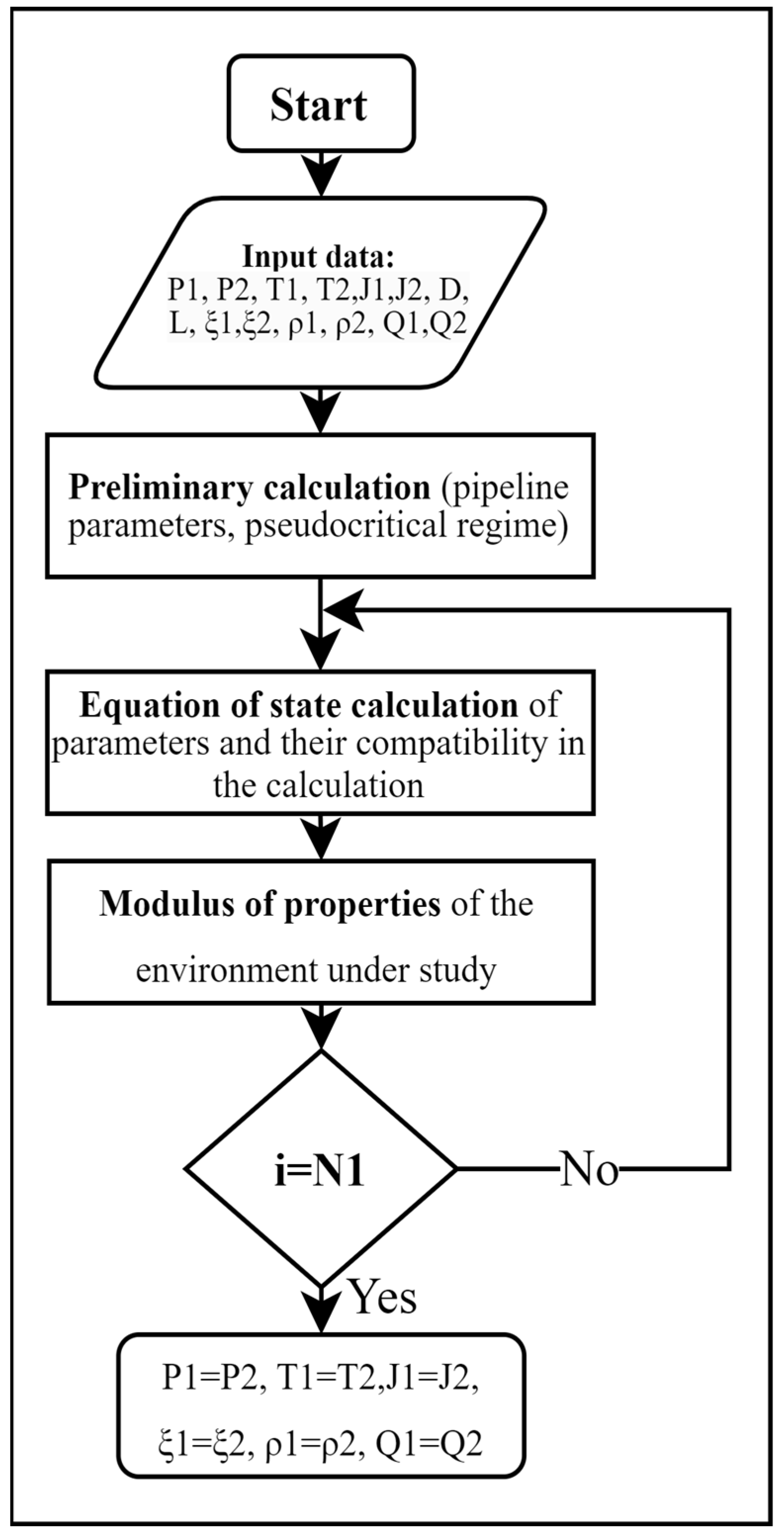
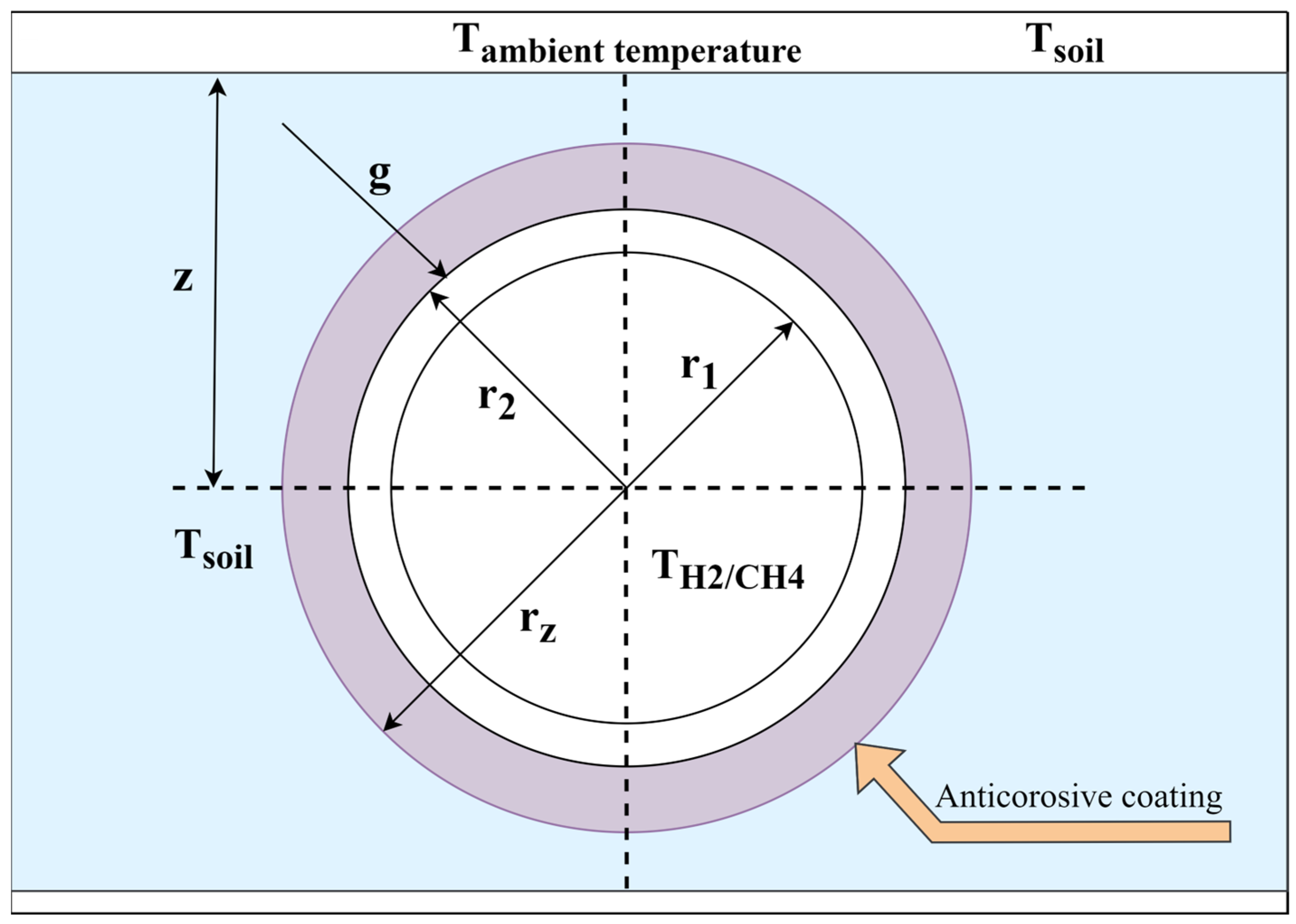
2.2. Model of Methane–Hydrogen Mixture Transportation
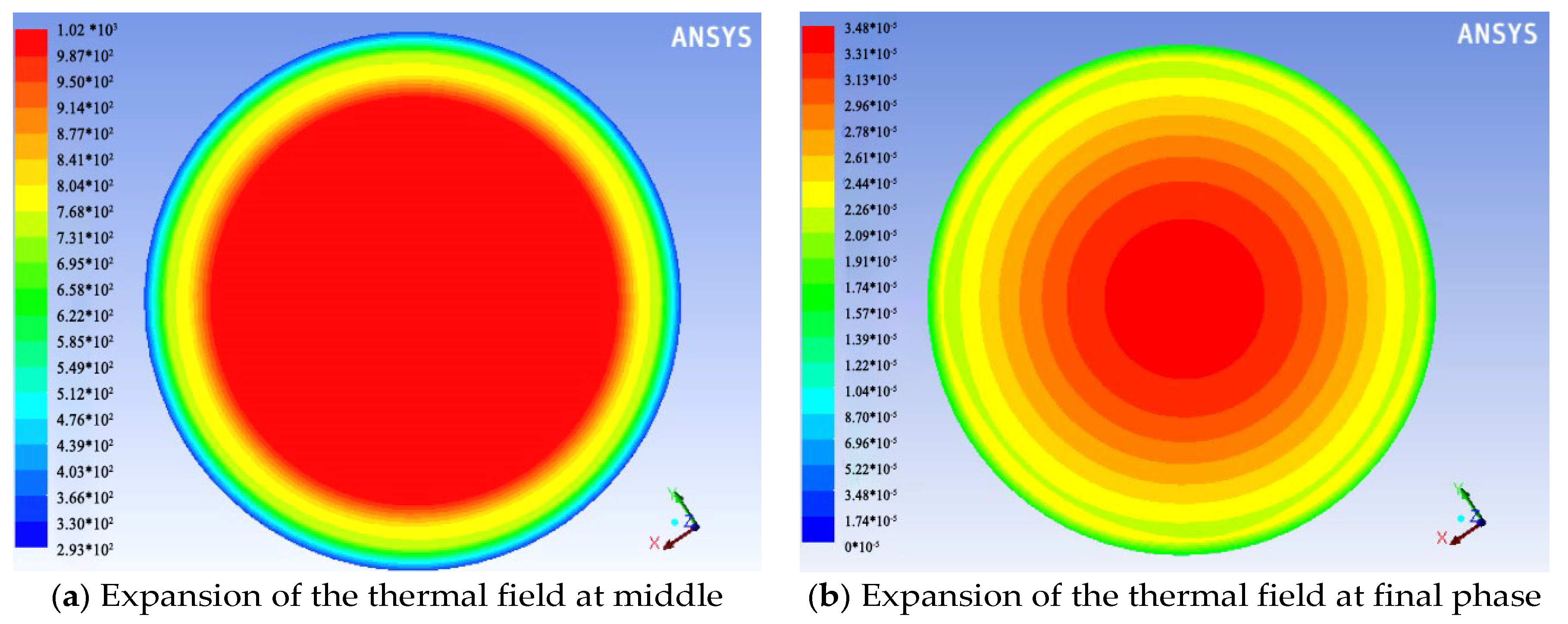
3. Results
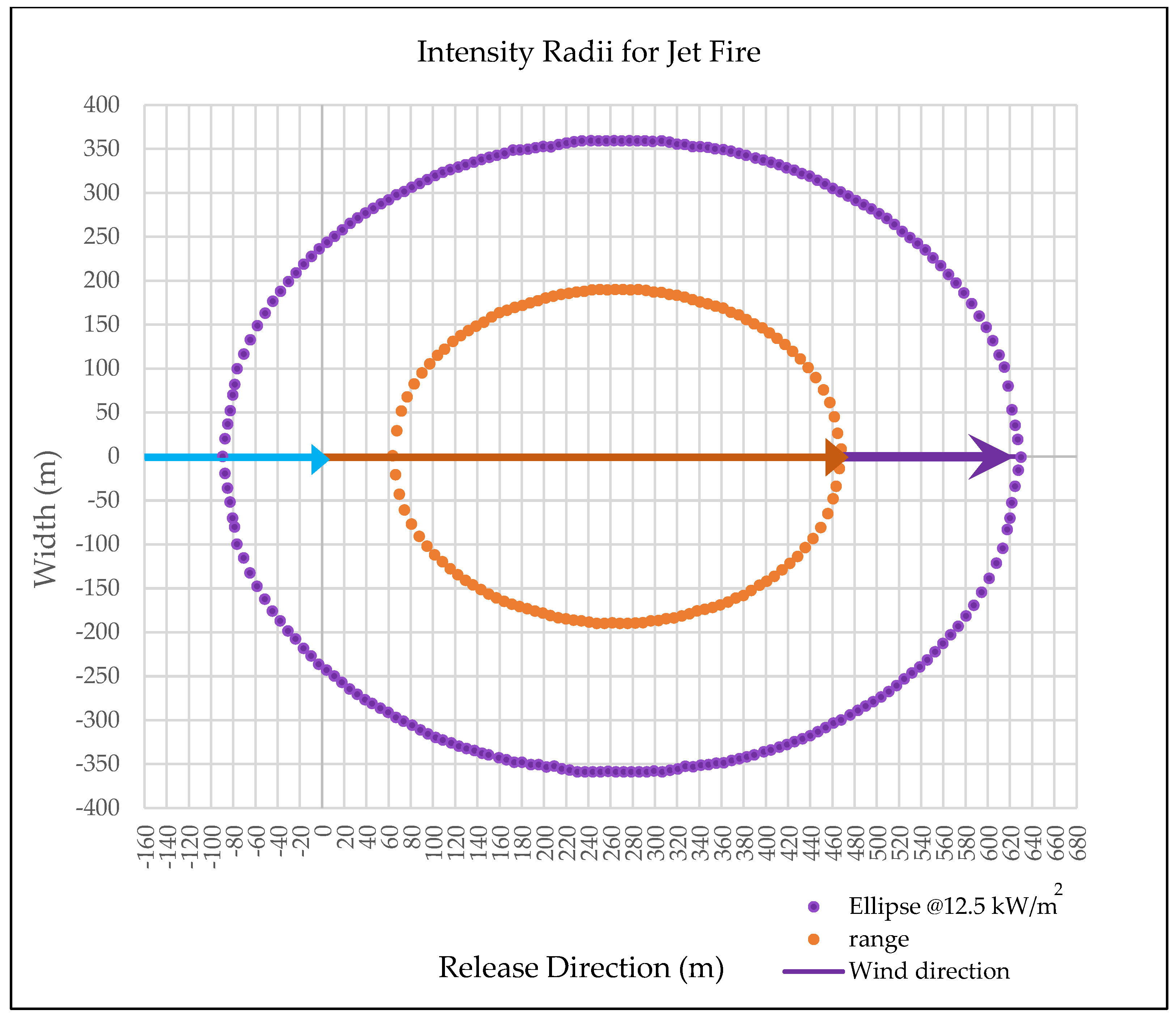
Fire Risk Assessment Considering Industrial Safety in Case of Hydrogen Leakage on the Pipeline
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deymi-Dashtebayaz, M.; Ebrahimi-Moghadam, A.; Pishbin, S.I.; Pourramezan, M. Investigating the effect of hydrogen injection on natural gas thermo-physical properties with various compositions. Energy 2019, 167, 235–245. [Google Scholar] [CrossRef]
- Ebrahimi-Moghadam, A.; Farzaneh-Gord, M.; Deymi-Dashtebayaz, M. Correlations for estimating natural gas leakage from above-ground and buried urban distribution pipelines. J. Nat. Gas Sci. Eng. 2016, 34, 185–196. [Google Scholar] [CrossRef]
- De Boer, H.S.; Grond, L.; Moll, H.; Benders, R. The application of power-to-gas, pumped hydro storage and compressed air energy storage in an electricity system at different wind power penetration levels. Energy 2014, 72, 360–370. [Google Scholar] [CrossRef]
- Farzaneh-Gord, M.; Arabkoohsar, A.; Dasht-bayaz, M.D.; Farzaneh-Kord, V. Feasibility of accompanying uncontrolled linear heater with solar system in natural gas pressure drop stations. Energy 2012, 41, 420–428. [Google Scholar] [CrossRef]
- Colbertaldo, P.; Guandalini, G.; Campanari, S. Modelling the integrated power and transport energy system: The role of power-to-gas and hydrogen in long-term scenarios for Italy. Energy 2018, 154, 592–601. [Google Scholar] [CrossRef]
- Pellegrino, S.; Lanzini, A.; Leone, P. Greening the gas network—The need for modelling the distributed injection of alternative fuels. Renew. Sustain. Energy Rev. 2017, 70, 266–286. [Google Scholar] [CrossRef]
- Guandalini, G.; Colbertaldo, P.; Campanari, S. Dynamic modeling of natural gas quality within transport pipelines in presence of hydrogen injections. Appl. Energy 2017, 185, 1712–1723. [Google Scholar] [CrossRef]
- Abeysekera, M.; Wu, J.; Jenkins, N.; Rees, M. Steady state analysis of gas networks with distributed injection of alternative gas. Appl. Energy 2016, 164, 991–1002. [Google Scholar] [CrossRef]
- Babyr, N.V.; Babyr, K.V. To improve the contact adaptability of mechanical roof support. E3S Web Conf. 2021, 266, 03015. [Google Scholar] [CrossRef]
- Korsakas, V.; Melaika, M.; Pukalskas, S.; Stravinskas, P. Hydrogen Addition Influence for the Efficient and Ecological Parameters of Heavy-Duty Natural Gas Si Engine. Procedia Eng. 2017, 187, 395–401. [Google Scholar] [CrossRef]
- Yue, J. Effect of hydrogen addition on performance of low heat value gas engine. Int. J. Hydrogen Energy 2016, 41, 15800–15804. [Google Scholar] [CrossRef]
- Marinina, O.; Nechitailo, A.; Stroykov, G.; Tsvetkova, A.; Reshneva, E.; Turovskaya, L. Technical and Economic Assessment of Energy Efficiency of Electrification of Hydrocarbon Production Facilities in Underdeveloped Areas. Sustainability 2023, 15, 9614. [Google Scholar] [CrossRef]
- Melaina, M.W.; Antonia, O.; Penem, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues. Technical Report NREL/TP-5600-51995. 2013. Available online: https://www.nrel.gov/docs/fy13osti/51995.pdf (accessed on 29 January 2023).
- Reynolds, R.A.; Slager, W.L. Pipeline Transportation of Hydrogen. In Hydrogen Energy; Veziroğlu, T.N., Ed.; Springer: Boston, MA, USA, 1975. [Google Scholar] [CrossRef]
- Liu, R.; Yuan, C.; Ma, W.; Liu, S.; Lu, S.; Zhang, H.; Gong, J. Simulation Study on Aircraft Fire Extinguishing Pipeline with Different Filling Conditions and Pipeline Characteristics. Fire 2022, 5, 86. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Pham, T.V.; Rogachev, M.K.; Korobov, G.Y.; Parfenov, D.V.; Zhurkevich, A.O.; Islamov, S.R. A comprehensive method for determining the dewaxing interval period in gas lift wells. J. Petrol. Explor. Prod. Technol. 2023, 13, 1163–1179. [Google Scholar] [CrossRef]
- Ni, X.; Chen, Y.; Huang, Q.; Zhao, C.; Li, S.; Huang, J.; Wang, J. An Experimental Study on the Transportation Characteristics of Perfluoro(2-methyl-3-pentanone) in a Straight Pipe. Fire 2023, 6, 156. [Google Scholar] [CrossRef]
- Litvinenko, V.S.; Tsvetkov, P.S.; Dvoynikov, M.V.; Buslaev, G.V. Barriers to implementation of hydrogen initiatives in the context of global energy sustainable development. J. Min. Inst. 2020, 244, 428–438. [Google Scholar] [CrossRef]
- Hydro-Pac, Inc. High Pressure Hydrogen Compressors. Available online: http://hydropac.com/hydrogen-compression.html (accessed on 29 August 2023).
- Lurie, M.V. Transportation of Hydrogen Batches by a Gas Pipeline in the Flow of Natural Gas. Territ. “NEFTEGAS” Oil Gas Territ. 2020, 11–12, 84–88. (In Russian) [Google Scholar]
- Golunov, N.N.; Lurie, M.V.; Musailov, I.T. Transportation of Hydrogen through Gas Pipeline in the Form of Methane-Hydrogen Mixture. Territ. “NEFTEGAS” Oil Gas Territ. 2021, 1–2, 74–82. (In Russian) [Google Scholar]
- Lurye, M.V.; Musailov, I.T. Peculiarities of Gas Transportation via the Turkish Stream Gas Pipeline Impellers. Territ. “NEFTEGAS” Oil Gas Territ. 2018, 3, 42–50. (In Russian) [Google Scholar]
- Zhang, Y.; Wu, Y.; Savenok, O.V.; Yin, J.; Geng, H.; Li, D. Experimental Investigation on Cracking Characteristics of Dry and Saturated Shales in Nitrogen Fracturing after Liquid Nitrogen (LN2) Injection. Geofluids 2023, 2023, 8861524. [Google Scholar] [CrossRef]
- Bolobov, V.I.; Latipov, I.U.; Zhukov, V.S.; Popov, G.G. Using the Magnetic Anisotropy Method to Determine Hydrogenated Sections of a Steel Pipeline. Energies 2023, 16, 5585. [Google Scholar] [CrossRef]
- Mingazhev, A.; Shchipachev, A.; Mingazheva, A. Oil spill response technology for ice-covered waters in the Arctic. E3S Web Conf. 2023, 378, 05007. [Google Scholar] [CrossRef]
- The HyUnder Project. Available online: http://hyunder.eu/ (accessed on 29 January 2023).
- Aspen Plus, Version 8.0, User Guide. 2010. Available online: https://courses.washington.edu/overney/Aspen/Aspen_Tutorial.pdf (accessed on 29 August 2023).
- Tananykhin, D.S.; Struchkov, I.A.; Khormali, A.; Roschinp, V. Investigation of the influences of asphaltene deposition on oilfield development using reservoir simulation. Pet. Explor. Dev. 2022, 49, 1138–1149. [Google Scholar] [CrossRef]
- Zemenkova, M.Y.; Chizhevskaya, E.L.; Zemenkov, Y.D. Intelligent monitoring of the condition of hydrocarbon pipeline transport facilities using neural network technologies. J. Min. Inst. 2022, 258, 933–944. [Google Scholar] [CrossRef]
- Ludtke, K.H. Process Centrifugal Compressors. Basics, Operation, Design, Application; Springer: Berlin, Germany, 2004. [Google Scholar] [CrossRef]
- Shammazov, I.; Dzhemilev, E.; Sidorkin, D. Improving the Method of Replacing the Defective Sections of Main Oil and Gas Pipelines Using Laser Scanning Data. Appl. Sci. 2023, 13, 48. [Google Scholar] [CrossRef]
- Shammazov, I.A.; Sidorkin, D.I.; Batyrov, A.M. Ensuring the stability of above-ground main pipelines in areas of continuous permafrost distribution. Georesources Eng. 2022, 333, 200–207. [Google Scholar] [CrossRef]
- Afanaseva, O.; Bezyukov, O.; Pervukhin, D.; Tukeev, D. Experimental Study Results Processing Method for the Marine Diesel Engines Vibration Activity Caused by the Cylinder-Piston Group Operations. Inventions 2023, 8, 71. [Google Scholar] [CrossRef]
- Ignatenko, A.; Afanaseva, O. Application of system analysis methods for the research of mining enterprise activity. In Proceedings of the 2023 Sixth International Conference of Women in Data Science at Prince Sultan University (WiDS PSU), Riyadh, Saudi Arabia, 14–15 March 2023; pp. 180–184. [Google Scholar] [CrossRef]
- Arefiev, I.B.; Afanaseva, O.V. Implementation of Control and Forecasting Problems of Human-Machine Complexes on the Basis of Logic-Reflexive Modeling. In System Analysis in Engineering and Control. SAEC 2021; Vasiliev, Y.S., Pankratova, N.D., Volkova, V.N., Shipunova, O.D., Lyabakh, N.N., Eds.; Lecture Notes in Networks and Systems; Springer: Cham, Switzerland, 2022; Volume 442. [Google Scholar] [CrossRef]
- Jungmeister, D.A.; Gasımov, E.E.; Isaev, A.I. Substantiation of the design and parameters of the device for regulating the air flow in down-the-hole hammers of roller-cone drilling rigs. Min. Informational Anal. Bull. 2022, 2, 251–267. [Google Scholar] [CrossRef]
- Yungmeyster, D.; Isaev, A.; Gasymov, E. Substantiation of dth air drill hammer parameters for penetration rate adjustment using air flow. Gorn. Zhurnal 2022, 7, 72–77. [Google Scholar] [CrossRef]
- Kukharova, T.V.; Asadulagi, M.-A.M. Investigation of the OA-300M Electrolysis Cell Temperature Field of Metallurgical Production. Energies 2022, 15, 9001. [Google Scholar] [CrossRef]
- Karyakina, E.D.; Shammazov, I.; Shalygin, A.V. Main aspects of liquefied natural gas process line thermal and hydraulic calculations. IOP Conf. Ser. Earth Environ. Sci. 2021, 677, 052056. [Google Scholar] [CrossRef]
- Martirosyan, A.V.; Ilyushin, Y.V. Modeling of the Natural Objects’ Temperature Field Distribution Using a Supercomputer. Informatics 2022, 9, 62. [Google Scholar] [CrossRef]
- Pershin, I.M.; Papush, E.G.; Kukharova, T.V.; Utkin, V.A. Modeling of Distributed Control System for Network of Mineral Water Wells. Water 2023, 15, 2289. [Google Scholar] [CrossRef]
- Plotnikov, A.V.; Trushnikov, V.E.; Pervukhin, D.A.; Shestopalov, M.Y. Mathematical Simulation of the Formation Pressure Monitoring System in the Water-Drive Gas Reservoir. In Proceedings of the 2023 XXVI International Conference on Soft Computing and Measurements (SCM), Saint Petersburg, Russia, 24–26 May 2023; pp. 77–81. [Google Scholar] [CrossRef]
- Shammazov, I.A.; Batyrov, A.M.; Sidorkin, D.I.; Van Nguyen, T. Study of the Effect of Cutting Frozen Soils on the Supports of Above-Ground Trunk Pipelines. Appl. Sci. 2023, 13, 3139. [Google Scholar] [CrossRef]
- Katysheva, E. Analysis of the Interconnected Development Potential of the Oil, Gas and Transport Industries in the Russian Arctic. Energies 2023, 16, 3124. [Google Scholar] [CrossRef]
- Asadulagi, M.M. Synthesis of Lumped and Distributed Controllers for Control System of Hydrodynamic Process. In Proceedings of the 2019 International Multi-Conference on Industrial Engineering and Modern Technologies, FarEastCon 2019, Vladivostok, Russia, 1–4 October 2019; p. 8933859. [Google Scholar] [CrossRef]
- Ede, A.J. The heat transfer coefficient for flow in a pipe. Int. J. Heat Mass Transf. 1961, 4, 105–110. [Google Scholar] [CrossRef]
- Jang, C.B.; Choi, S.-W.; Baek, J.-B. CFD modeling and fire damage analysis of jet fire on hydrogen pipeline in a pipe rack structure. Int. J. Hydrogen Energy 2015, 40, 15760–15772. [Google Scholar] [CrossRef]
- Wang, C.J.; Wen, J.X.; Chen, Z.B.; Dembele, S. Predicting radiative characteristics of hydrogen and hydrogen/methane jet fires using FireFOAM. Int. J. Hydrogen Energy 2014, 39, 20560–20569. [Google Scholar] [CrossRef]
- Schefer, R.W.; Houf, W.G.; Bourne, B.; Colton, J. Spatial and radiative properties of an open-flame hydrogen plume. Int. J. Hydrogen Energy 2006, 31, 1332–1340. [Google Scholar] [CrossRef]
- Mashhadimoslem, H.; Ghaemi, A.; Palacios, A.; Behroozi, A.H. A new method for comparison thermal radiation on large-scale hydrogen and propane jet fires based on experimental and computational studies. Fuel 2020, 282, 118864. [Google Scholar] [CrossRef]
- Nobili, M.; Caruso, G. Comparative CFD simulations of a hydrogen fire scenario. IOP Conf. Ser. J. Phys. Conf. Ser. 2017, 796, 012035. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Z.; Chen, G.; Zhang, Q.; Yang, Y. Study on leakage and explosion consequence for hydrogen blended natural gas in urban distribution networks. Int. J. Hydrogen Energy 2022, 47, 27096–27115. [Google Scholar] [CrossRef]
- Froeling, H.A.J.; Dröge, M.T.; Nane, G.F.; Van Wijk, A.J.M. Quantitative risk analysis of a hazardous jet fire event for hydrogen transport in natural gas transmission pipelines. Int. J. Hydrogen Energy 2021, 46, 10411–10422. [Google Scholar] [CrossRef]
- Jo, Y.-D.; Ahn, B.J. Analysis of hazard area associated with hydrogen gas transmission pipelines. Int. J. Hydrogen Energy 2006, 31, 2122–2130. [Google Scholar] [CrossRef]
- Li, Y.; Kuang, Z.; Fan, Z.; Shuai, J. Evaluation of the safe separation distances of hydrogen-blended natural gas pipelines in a jet fire scenario. Int. J. Hydrogen Energy 2023, 48, 18804–18815. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, X.; Liu, M.; Liu, J. A theoretical framework for calculating full-scale jet fires induced by high-pressure hydrogen/natural gas transient leakage. Int. J. Hydrogen Energy 2018, 43, 22765–22775. [Google Scholar] [CrossRef]
- Islamov, S.R.; Bondarenko, A.V.; Mardashov, D.V. A selection of emulsifiers for preparation of invert emulsion drilling fluids. Top. Issues Ration. Use Nat. Resour. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Zolotov, O.I.; Iliushina AN Novozhilov, I.M. Spatially Distributed System for Monitoring of Fields Technical Condition in Mineral Resources Sector. In Proceedings of the 2021 XXIV International Conference on Soft Computing and Measurements (SCM), St. Petersburg, Russia, 26–28 May 2021; pp. 93–95. [Google Scholar] [CrossRef]
- Vasiliev, G.G.; Dzhaljabov, A.A.; Leonovich, I.A. Analysis of the causes of engineering structures deformations at gas industry facilities in the permafrost zone. J. Min. Inst. 2021, 249, 377–385. [Google Scholar] [CrossRef]

| H2/CH4 % | M, kg/s | P1, MPa | kg/m3 | C1, m/s | VH2, m3/s | VCH4, m3/s | H2 kg/s | CH4 kg/s | P2 MPa | kg/m3 | C2 m/s | p MPa | T2 K | VΣ m3/s |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10/90 | 449 | 9.8 | 32 | 10.4 | 4 | 7.2 | 5 | 445 | 5,5 | 27 | 20 | 4.5 | 300 | 8 |
| 25/75 | 383 | 9.8 | 42 | 10.6 | 2 | 6 | 13 | 370 | 7,5 | 27 | 17 | 3.5 | 299 | 8 |
| 50/50 | 269 | 9.8 | 53 | 10.6 | 0.09 | 4 | 26 | 243 | 8 | 24 | 14 | 2.5 | 299 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fetisov, V.; Davardoost, H.; Mogylevets, V. Technological Aspects of Methane–Hydrogen Mixture Transportation through Operating Gas Pipelines Considering Industrial and Fire Safety. Fire 2023, 6, 409. https://doi.org/10.3390/fire6100409
Fetisov V, Davardoost H, Mogylevets V. Technological Aspects of Methane–Hydrogen Mixture Transportation through Operating Gas Pipelines Considering Industrial and Fire Safety. Fire. 2023; 6(10):409. https://doi.org/10.3390/fire6100409
Chicago/Turabian StyleFetisov, Vadim, Hadi Davardoost, and Veronika Mogylevets. 2023. "Technological Aspects of Methane–Hydrogen Mixture Transportation through Operating Gas Pipelines Considering Industrial and Fire Safety" Fire 6, no. 10: 409. https://doi.org/10.3390/fire6100409
APA StyleFetisov, V., Davardoost, H., & Mogylevets, V. (2023). Technological Aspects of Methane–Hydrogen Mixture Transportation through Operating Gas Pipelines Considering Industrial and Fire Safety. Fire, 6(10), 409. https://doi.org/10.3390/fire6100409








