Long-Term Response of Fuel to Mechanical Mastication in South-Eastern Australia
Abstract
:1. Introduction
- How does fuel load and structure change overtime following mastication?
- Do these fuel trajectories vary as a function of landscape aridity?
2. Materials and Methods
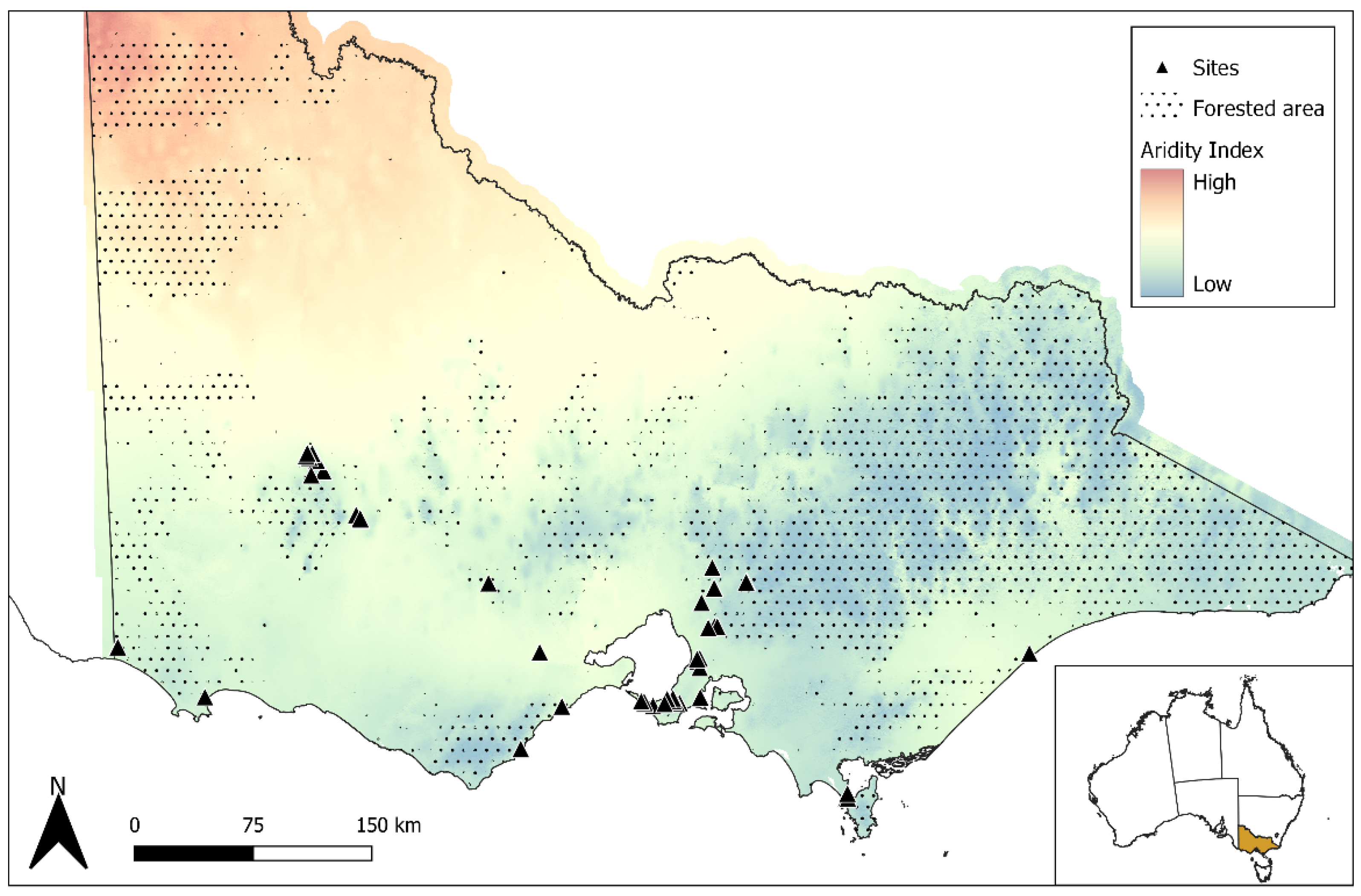
2.1. Study Sites
2.2. Field Measurements
2.3. Data Analysis
3. Results
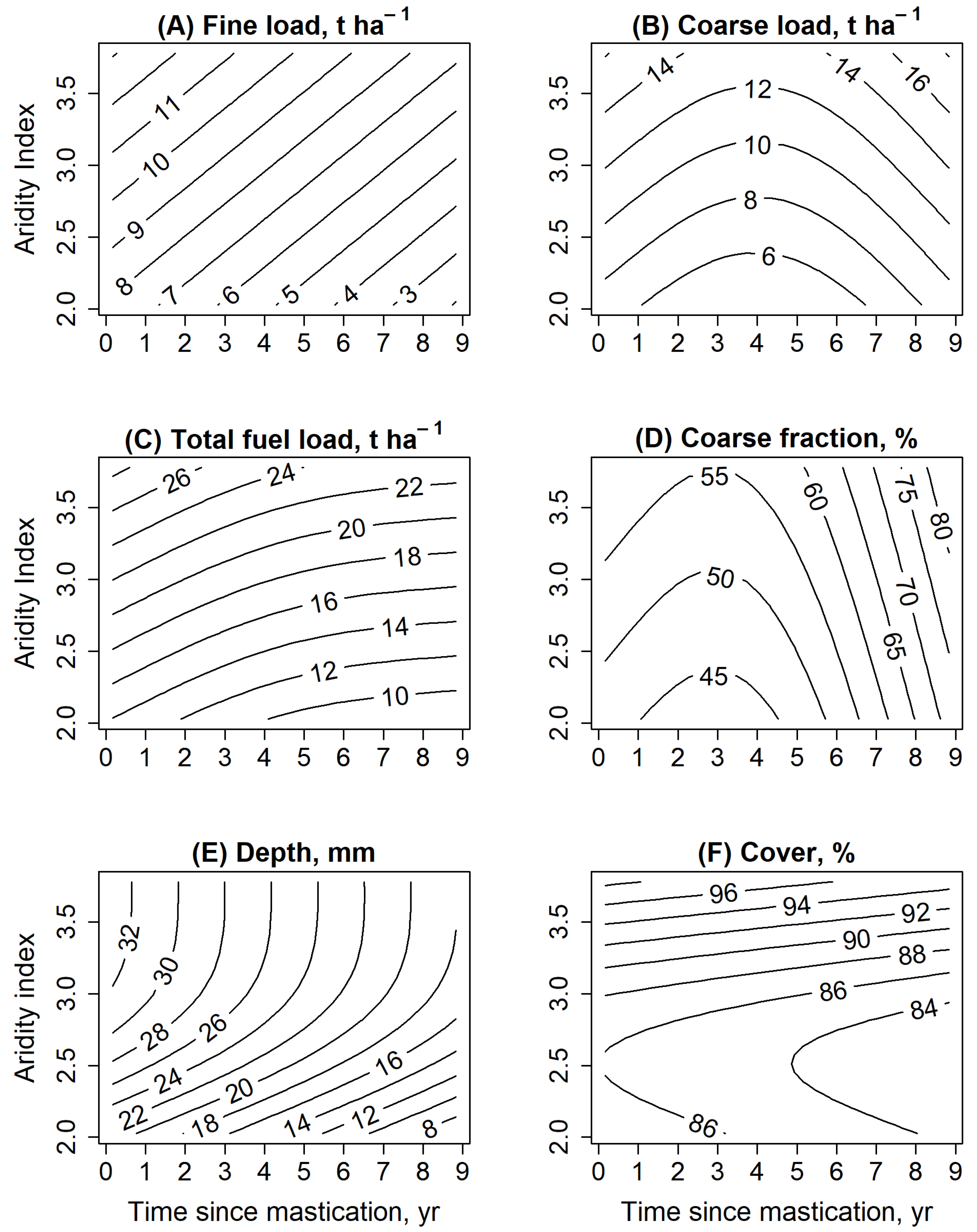
3.1. Changes in Surface Fuels
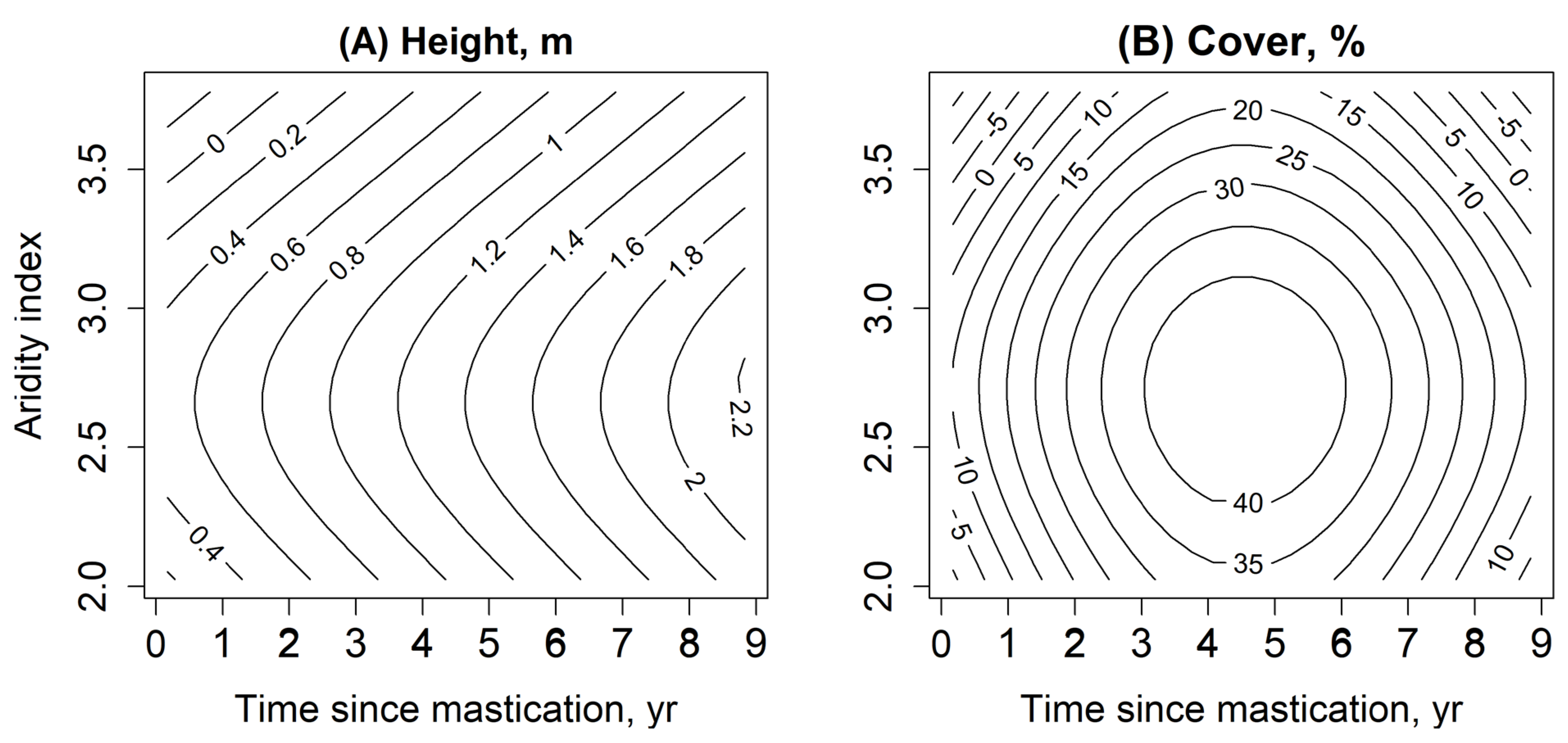
3.2. Changes in Standing Fuel
4. Discussion
4.1. Changes in Surface Fuels
4.2. Changes in Standng Fuel
4.3. Management Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salis, M.; Laconi, M.; Ager, A.A.; Alcasena, F.J.; Arca, B.; Lozano, O.; de Oliveira, A.F.; Spano, D. Evaluating alternative fuel treatment strategies to reduce wildfire losses in a Mediterranean area. For. Ecol. Manag. 2016, 368, 207–221. [Google Scholar] [CrossRef]
- Penman, T.D.; Collins, L.; Duff, T.; Price, O.F. Scientific Evidence Regarding Effectiveness of Prescribed Burning, in Prescribed Burning in Australasia: The Science, Practice and Politics of Burning the Bush; Bushfire and Natural Hazards CRC, Ed.; AFAC: Melbourne, Australia, 2020; pp. 99–111. [Google Scholar]
- Stephens, S.L.; Husari, S.; Thomas Nichols, H.; Sugihara, N.G. Fire and Fuel Management. In Fire in California's Ecosystems; van Wagtendonkm, J.W., Ed.; University of California Press: Berkeley, CA, USA, 2018; pp. 411–428. [Google Scholar]
- Duff, T.J.; Cawson, J.G.; Penman, T.D. Prescribed Burning. In Encyclopedia of Wildfires and Wildland–Urban Interface (WUI) Fires; Manzello, S.L., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–11. [Google Scholar]
- Black, A.E.; Hayes, P.; Strickland, R. Organizational learning from prescribed fire escapes: A review of developments over the last 10 years in the USA and Australia. Curr. For. Rep. 2020, 6, 41. [Google Scholar] [CrossRef]
- Reisen, F.; Meyer, C.M.; McCaw, L.; Powell, J.C.; Tolhurst, K.; Keywood, M.D.; Gras, J.L. Impact of smoke from biomass burning on air quality in rural communities in southern Australia. Atmos. Environ. 2011, 45, 3944–3953. [Google Scholar] [CrossRef]
- Quinn-Davidson, L.N.; Varner, J.M. Impediments to prescribed fire across agency, landscape and manager: An example from northern California. Int. J. Wildland Fire 2012, 21, 210–218. [Google Scholar] [CrossRef]
- Keane, R.E.; Sikkink, P.G.; Jain, T.B. Physical and Chemical Characteristics of Surface Fuels in Masticated Mixed-Conifer Stands of the U.S. Rocky Mountains; Rocky Mountain Research Station, Department of Agriculture, Forest Service: Fort Collins, CO, USA, 2018; p. 56. Available online: https://www.fs.usda.gov/treesearch/pubs/55649 (accessed on 27 March 2022).
- Kreye, J.K.; Kobziar, L.N. The effect of mastication on surface fire behaviour, fuels consumption and tree mortality in pine flatwoods of Florida, USA. Int. J. Wildland Fire 2015, 24, 573–579. [Google Scholar] [CrossRef]
- Battaglia, M.A.; Rocca, M.E.; Rhoades, C.C.; Ryan, M.G. Surface fuel loadings within mulching treatments in Colorado coniferous forests. For. Ecol. Manag. 2010, 260, 1557–1566. [Google Scholar] [CrossRef]
- Hudak, A.T.; Rickert, I.; Morgan, P.; Strand, E.; Lewis, S.A.; Robichaud, P.R.; Hoffman, C.; Holden, Z.A. Review of fuel treatment effectiveness in forests and rangelands and a case study from the 2007 megafires in Central Idaho USA. USDA For. Serv. Gen. Tech. Rep. RMRS-GTR 2011, 60, 252. [Google Scholar]
- Grant, M.A.; Duff, T.J.; Penman, T.D.; Pickering, B.J.; Cawson, J.G. Mechanical mastication reduces fuel structure and modelled fire behaviour in Australian shrub encroached ecosystems. Forests 2021, 12, 812. [Google Scholar] [CrossRef]
- Kane, J.M.; Varner, J.M.; Knapp, E.E. Novel fuelbed characteristics associated with mechanical mastication treatments in northern California and south-western Oregon, USA. Int. J. Wildland Fire 2009, 18, 686–697. [Google Scholar] [CrossRef] [Green Version]
- Kreye, J.K.; Brewer, N.W.; Morgan, P.; Varner, J.M.; Smith, A.M.; Hoffman, C.M.; Ottmar, R.D. Fire behavior in masticated fuels: A review. For. Ecol. Manag. 2014, 314, 193–207. [Google Scholar] [CrossRef]
- Cawson, J.G.; Pickering, B.; Penman, T.D.; Filkov, A. Quantifying the effect of mastication on flaming and smouldering durations in eucalypt forests and woodlands under laboratory conditions. Int. J. Wildland Fire 2021, 30, 611–624. [Google Scholar] [CrossRef]
- Lyon, Z.D.; Morgan, P.; Stevens-Rumann, C.S.; Sparks, A.M.; Keefe, R.F.; Smith, A.M. Fire behaviour in masticated forest fuels: Lab and prescribed fire experiments. Int. J. Wildland Fire Aust. 2018, 4, 280. [Google Scholar] [CrossRef]
- Reed, W.P.; Varner, J.M.; Knapp, E.E.; Kreye, J.K. Long-term changes in masticated woody fuelbeds in northern California and southern Oregon, USA. Int. Assoc. Wildland Fire 2020, 29, 807–819. [Google Scholar] [CrossRef]
- Brennan, T.J.; Keeley, J.E. Effect of mastication and other mechanical treatments on fuel structure in chaparral. Int. J. Wildland Fire 2015, 24, 949–963. [Google Scholar] [CrossRef]
- Wozniak, S.S.; Strand, E.K.; Johnson, T.R.; Hulet, A.; Roundy, B.A.; Young, K. Treatment longevity and changes in surface fuel loads after pinyon–juniper mastication. Ecosphere 2020, 11, e03226. [Google Scholar] [CrossRef]
- Pandey, R.R.; Sharma, G.; Tripathi, S.K.; Singh, A.K. Litterfall, litter decomposition and nutrient dynamics in a subtropical natural oak forest and managed plantation in northeastern India. For. Ecol. Manag. 2007, 240, 96–104. [Google Scholar] [CrossRef]
- Stevens-Rumann, C.S.; Hudak, A.T.; Morgan, P.; Arnold, A.; Strand, E.K. Fuel dynamics following wildfire in US Northern Rockies forests. Front. For. Glob. Chang. 2020, 3, 51. [Google Scholar] [CrossRef]
- Thomas, P.B.; Watson, P.J.; Bradstock, R.A.; Penman, T.D.; Price, O.F. Modelling surface fine fuel dynamics across climate gradients in eucalypt forests of south-eastern Australia. Ecography 2014, 37, 827–837. [Google Scholar] [CrossRef]
- Coop, J.D.; Grant, T.A., III; Magee, P.A.; Moore, E.A. Mastication treatment effects on vegetation and fuels in pinon-juniper woodlands of central Colorado, USA. For. Ecol. Manag. 2017, 396, 68. [Google Scholar] [CrossRef]
- Kane, J.M.; Varner, J.M.; Knapp, E.E.; Powers, R.F. Understory vegetation response to mechanical mastication and other fuels treatments in a ponderosa pine forest. Appl. Veg. Sci. 2010, 13, 207–220. [Google Scholar] [CrossRef]
- Battaglia, M.A.; Fornwalt, P.J.; Rhoades, C.C. Mastication Effects on Fuels, Plants, and Soils in Four Western U.S. Ecosystems: Trends with Time-since-Treatment; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2015; p. 37.
- Potts, J.B.; Marino, E.; Stephens, S.L. Chaparral shrub recovery after fuel reduction: A comparison of prescribed fire and mastication techniques. Plant Ecol. 2010, 210, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Martorano, C.A.; Kane, J.M.; Engber, E.A.; Gibson, J. Long-term fuel and understorey vegetation response to fuel treatments in oak and chaparral stands of northern California. Appl. Veg. Sci. 2021, 24, e12551. [Google Scholar] [CrossRef]
- Lindner, A.; Stein, K.; Freiberg, M. Abundance and vigor of three selected understory species along environmental gradients in south-eastern Brazil. Ecotropica 2010, 16, 101–112. [Google Scholar]
- Brenes-Arguedas, T.; Roddy, A.B.; Coley, P.D.; Kursar, T.A. Do differences in understory light contribute to species distributions along a tropical rainfall gradient? Oecologia 2011, 166, 443–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyman, P.; Sherwin, C.B.; Langhans, C.; Lane, P.N.; Sheridan, G.J. Downscaling regional climate data to calculate the radiative index of dryness in complex terrain. Aust. Meteorol. Oceanogr. J. 2014, 64, 109–122. [Google Scholar] [CrossRef]
- Petrů, M.; Tielbörger, K.; Belkin, R.; Sternberg, M.; Jeltsch, F. Life history variation in an annual plant under two opposing environmental constraints along an aridity gradient. Ecography 2006, 29, 66–74. [Google Scholar] [CrossRef]
- Ding, J.; Eldridge, D.J. Community-level responses to increasing dryness vary with plant growth form across an extensive aridity gradient. J. Biogeogr. 2021, 48, 1788. [Google Scholar] [CrossRef]
- Alessandro, O.; Nyman, P. Aridity indices predict organic matter decomposition and comminution processes at landscape scale. Ecol. Indic. 2017, 78, 531–540. [Google Scholar] [CrossRef]
- Pueyo, Y.; Alados, C.L. Effects of fragmentation, abiotic factors and land use on vegetation recovery in a semi-arid Mediterranean area. Basic Appl. Ecol. 2007, 8, 158–170. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Moritz, M.A.; Batllori, E.; Bradstock, R.A.; Gill, A.M.; Handmer, J.; Hessburg, P.F.; Leonard, J.; McCaffrey, S.; Odion, D.C.; Schoennagel, T.; et al. Learning to coexist with wildfire. Nature 2014, 515, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Knapp, E.E.; Varner, J.M.; Busse, M.D.; Skinner, C.N.; Shestak, C.J. Behaviour and effects of prescribed fire in masticated fuelbeds. Int. J. Wildland Fire 2011, 20, 932–945. [Google Scholar] [CrossRef]
- Kreye, J.K.; Varner, J.M.; Kane, J.M.; Knapp, E.E.; Reed, W.P. The impact of aging on laboratory fire behaviour in masticated shrub fuelbeds of California and Oregon, USA. Int. J. Wildland Fire 2016, 25, 1002–1008. [Google Scholar] [CrossRef]
- Gallant, J.; Wilson, N.; Dowling, T.; Read, A.; Inskeep, C. SRTM-derived 1 second Digital Elevation Models version 1.0. Record 1; 2011. Available online: http://pid.geoscience.gov.au/dataset/ga/72759 (accessed on 3 January 2020).
- Bureau of Meteorology. Climate classification of Australia. 2005. Available online: http://www.bom.gov.au/climate/how/newproducts/images/zones.shtml (accessed on 5 March 2021).
- Department of Environment Land Water and Planning. Bioregions and EVC Benchmarks. 2021. Available online: https://www.environment.vic.gov.au/biodiversity/bioregions-and-evc-benchmarks (accessed on 13 September 2021).
- Zenni, R.D.; Wilson, J.R.U.; Le Roux, J.J.; Richardson, D.M. Evaluating the invasiveness of Acacia paradoxa in South Africa. South Afr. J. Bot. 2009, 75, 485–496. [Google Scholar] [CrossRef]
- Lucidcentral. Environmental Weeds of Australia. 2021. Available online: https://keyserver.lucidcentral.org/weeds/data/media/Html/index.htm (accessed on 15 February 2022).
- Charles Sturt University. South West Slopes Revegetation Guide; Kunzea Ericoides. 2022. Available online: https://science-health.csu.edu.au/herbarium/sws/database/kunzea/kunzea-ericoides#sws (accessed on 15 February 2022).
- Specht, R.L. Foliage Projective Cover and Standing Biomass. In Vegetation Classification in Australia; Gillison, A.N., Anderson, D.J., Eds.; Commonwealth Scientific and Industrial Research Organization in Association with Australian National University Press: Canberra, Australia, 1981; p. 18. [Google Scholar]
- Hines, F.; Tolhurst, K.G.; Wilson, A.A.G.; McCarthy, G.J. Overall Fuel Hazard Assessment Guide, 4th ed.; Fire Management Branch, Dept of Natural Resources and Environment: East Melbourne, Australia, 2010. [Google Scholar]
- Elzinga, C.L. Measuring and Monitoring Plant Populations; U.S. Dept. of the Interior, Bureau of Land Management, National Applied Resource Sciences Center: Denver, Colorado, 1998; pp. 112–113, 182. Available online: http://msuinvasiveplants.org/documents/archives_cism/BLM_Measuring_and_monitoring.pdf (accessed on 27 March 2022).
- Wood, S.N.; Augustin, N.H. GAMs with integrated model selection using penalized regression splines and applications to environmental modelling. Ecol. Model. 2002, 157, 157–177. [Google Scholar] [CrossRef] [Green Version]
- Harrell, F.E.J. Hmisc: Harrell Miscellaneous, R package version 4.3-0. 2019. Available online: https://cran.r-project.org/web/packages/Hmisc/index.html (accessed on 31 March 2022).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Shakespear, A.W. Fuel Response to mechanical mastication of Pinyon-Juniper woodlands in Utah. Master’s Thesis, Department of Plant and Wildlife Sciences, Brigham Young University, BYU Scholars Archive, Provo, UT, USA, 2014. [Google Scholar]
- Fasth, B.G.; Harmon, M.E.; Sexton, J.; White, P. Decomposition of fine woody debris in a deciduous forest in North Carolina. J. Torrey Bot. Soc. 2011, 138, 192–206. [Google Scholar] [CrossRef]
- Burrows, N.; McCaw, L. Prescribed burning in southwestern Australian forests. Front. Ecol. Environ. 2013, 11, e25–e34. [Google Scholar] [CrossRef]
- Kane, J.M.; Knapp, E.; Varner, J.M. Variability in loading of mechanically masticated fuel beds in Northern California and Southwestern Oregon. In Fuels Management–How to Measure Success: Conference Proceedings; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006. [Google Scholar]
- Reed, W.P. Long-Term Fuel and Vegetation Responses to Mechanical Mastication in Northern California and Southern Oregon; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2016. [Google Scholar]
- Pausas, J.G.; Keeley, J.E. Evolutionary ecology of resprouting and seeding in fire-prone ecosystems. New Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Murphy, B.P.; Russell-Smith, J.; Nano, C.E.; Bradstock, R.; Enright, N.J.; Fontaine, J.B.; Gosper, C.R.; Radford, I.; et al. A synthesis of postfire recovery traits of woody plants in Australian ecosystems. Sci. Total Environ. 2015, 534, 31–42. [Google Scholar] [CrossRef]
- Vilà, M.; Sardans, J. Plant competition in mediterranean-type vegetation. J. Veg. Sci. 1999, 10, 281–294. [Google Scholar] [CrossRef]
- Zylstra, P.; Bradstock, R.A.; Bedward, M.; Penman, T.D.; Doherty, M.D.; Weber, R.O.; Gill, A.M.; Cary, G.J. Biophysical mechanistic modelling quantifies the effects of plant traits on fire severity: Species, not surface fuel loads, determine flame dimensions in Eucalypt forests. PLoS ONE 2016, 11, e0160715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, C.; Vega, J.A.; Fonturbel, T. Does shrub recovery differ after prescribed burning, clearing and mastication in a Spanish heathland? Plant Ecol. 2015, 216, 429–437. [Google Scholar] [CrossRef]
- Brennan, T.J.; Keeley, J.E. Impacts of mastication fuel treatments on California, USA, Chaparral vegetation structure and composition. Fire Ecol. 2017, 13, 120–138. [Google Scholar] [CrossRef] [Green Version]
- Wilkin, K.M.; Ponisio, L.C.; Fry, D.L.; Tubbesing, C.L.; Potts, J.B.; Stephens, S.L. Decade-long plant community responses to shrubland fuel hazard reduction. Fire Ecol. 2017, 13, 105–136. [Google Scholar] [CrossRef] [Green Version]
- Pickering, B.J.; Duff, T.J.; Baillie, C.; Cawson, J.G. Darker, cooler, wetter: Forest understories influence surface fuel moisture. Agric. For. Meteorol. 2021, 300, 108311. [Google Scholar] [CrossRef]
- Rothermel, R. How to Predict the Spread and Intensity of Forest and Range Fires The Bark Beetles, Fuels, and Fire Bibliography. 1983. Available online: https://www.fs.usda.gov/treesearch/pubs/24635 (accessed on 27 March 2022).
- Busse, M.D.; Hubbert, K.R.; Fiddler, G.O.; Shestak, C.J.; Powers, R.F. Lethal soil temperatures during burning of masticated forest residues. Int. J. Wildland Fire 2005, 14, 267–276. [Google Scholar] [CrossRef]
- Halbrook, J.; Han, H.S.; Graham, R.T.; Jain, T.B.; Denner, R. Mastication: A fuel reduction and site preperation alternative. In Proceedings of the 29th Council on Forest Engineering Conference, Coeur d’Alene, ID, USA, 31 July–2 August 2006. [Google Scholar]
- Fernandes, P.M.; Botelho, H.S. A Review of Prescribed Burning Effectiveness in Fire Hazard Reduction. Int. J. Wildland Fire 2003, 12, 117–128. [Google Scholar] [CrossRef] [Green Version]


| Fuel Property | Definition |
|---|---|
| 25 grid point measurements | |
| Surface fuel cover, % | Percentage cover of surface fuels determined as a proportion of the total 25 measurements where surface fuels were present (number of points present/25 × 100) [47]. |
| Surface fuel depth, mm | Mean depth of surface fuel (leaves, bark, twigs and other masticated debris on the forest floor) where it was present. Points with no surface fuel were not included in this calculation [46]. |
| Cover of shrub fuel, % | Percentage cover of shrub fuel determined from the proportion of the total 25 points where it was present (number of points present/25 × 100) [47]. Shrubs include vegetation exceeding 2 m in height (above the height pole) but exclude tree canopy. |
| Height of shrub fuel, cm | Mean height of shrub fuel. Sites where there were less than five measuments made were excluded. Shrubs include vegetation exceeding 2 m in height (above the height pole) but exclude tree canopy. |
| Cover of standing fuel for each 10 cm height increment (up to 2 m), % | Percentage cover of standing fine and coarse fuel as a function of height. Determined from the proportion of the total 25 points where this vegetation was present within each height increment (number hits/25 × 100) [47]. |
| Plot corner measurements | |
| Surface fine fuel load, t ha−1 | Oven-dry mass per unit area of the surface fine fuel (<6 mm); mean for the plot and converted to tonnes per hectare. |
| Surface coarse fuel load, t ha−1 | Oven-dry mass per unit area of the surface coarse fuel (6–25 mm); mean for the plot and converted to tonnes per hectare. |
| Total surface fuel load, t ha−1 | Oven-dry mass per unit area of surface fine and coarse fuel; mean for the plot and converted to tonnes per hectare. |
| Coarse fraction, % | Percentage of total surface fuel load that is coarse fuel (6–25 mm) |
| Model | R2 Adjusted | Deviance Explained, % | Smoothed Terms | e.d.f | p-Value |
|---|---|---|---|---|---|
| Fine fuel load, t ha−1 | 0.12 | 15.1 | Time since mastication | 1 | 0.03 * |
| Aridity | 1 | 0.046 * | |||
| Coarse fuel load, t ha−1 | 0.14 | 18.0 | Time since mastication | 1.7 | 0.26 |
| Aridity | 1 | <0.01 ** | |||
| Total fuel load, t ha−1 | 0.12 | 15.3 | Time since mastication | 1.3 | 0.32 |
| Aridity | 1 | <0.01 ** | |||
| Coarse fraction, % | 0.15 | 19.3 | Time since mastication | 1.9 | 0.01 * |
| Aridity | 1 | 0.09 | |||
| Depth, mm | 0.19 | 22.1 | Time since mastication | 1 | 0.01 * |
| Aridity | 1.5 | 0.02 * | |||
| Cover, % | 0.001 | 4.52 | Time since mastication | 1 | 0.70 |
| Aridity | 1.5 | 0.48 |
| Model | R2 Adjusted | Deviance Explained, % | Smoothed Terms | e.d.f | p-Value |
|---|---|---|---|---|---|
| Cover, % | 0.22 | 27.4 | Time since mastication | 3.5 | <0.01 ** |
| Aridity | 2.2 | 0.26 | |||
| Height, m | 0.32 | 37.2 | Time since mastication | 1 | <0.01 ** |
| Elevation | 1.7 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pickering, B.J.; Burton, J.E.; Penman, T.D.; Grant, M.A.; Cawson, J.G. Long-Term Response of Fuel to Mechanical Mastication in South-Eastern Australia. Fire 2022, 5, 76. https://doi.org/10.3390/fire5030076
Pickering BJ, Burton JE, Penman TD, Grant MA, Cawson JG. Long-Term Response of Fuel to Mechanical Mastication in South-Eastern Australia. Fire. 2022; 5(3):76. https://doi.org/10.3390/fire5030076
Chicago/Turabian StylePickering, Bianca J., Jamie E. Burton, Trent D. Penman, Madeleine A. Grant, and Jane G. Cawson. 2022. "Long-Term Response of Fuel to Mechanical Mastication in South-Eastern Australia" Fire 5, no. 3: 76. https://doi.org/10.3390/fire5030076
APA StylePickering, B. J., Burton, J. E., Penman, T. D., Grant, M. A., & Cawson, J. G. (2022). Long-Term Response of Fuel to Mechanical Mastication in South-Eastern Australia. Fire, 5(3), 76. https://doi.org/10.3390/fire5030076






