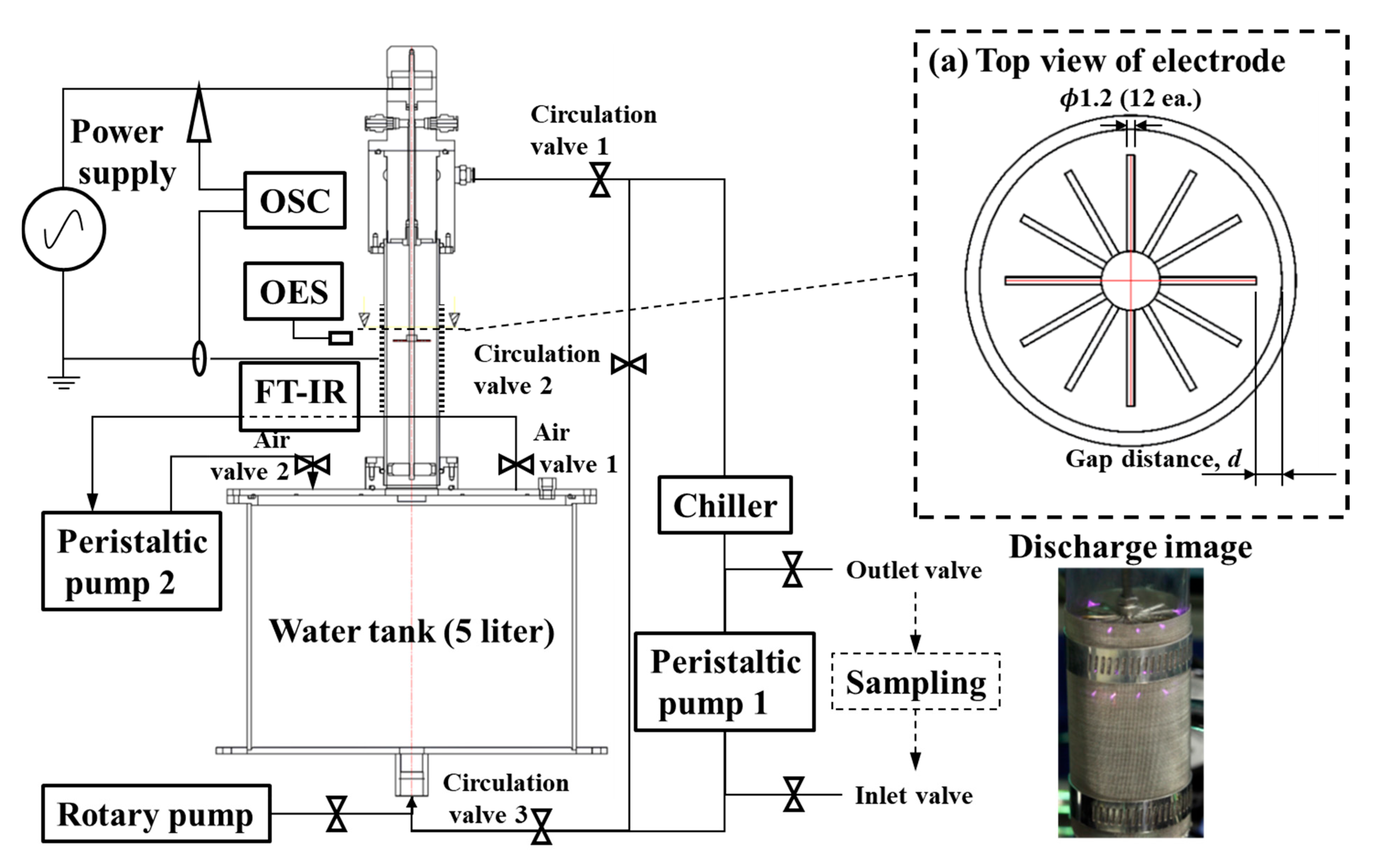
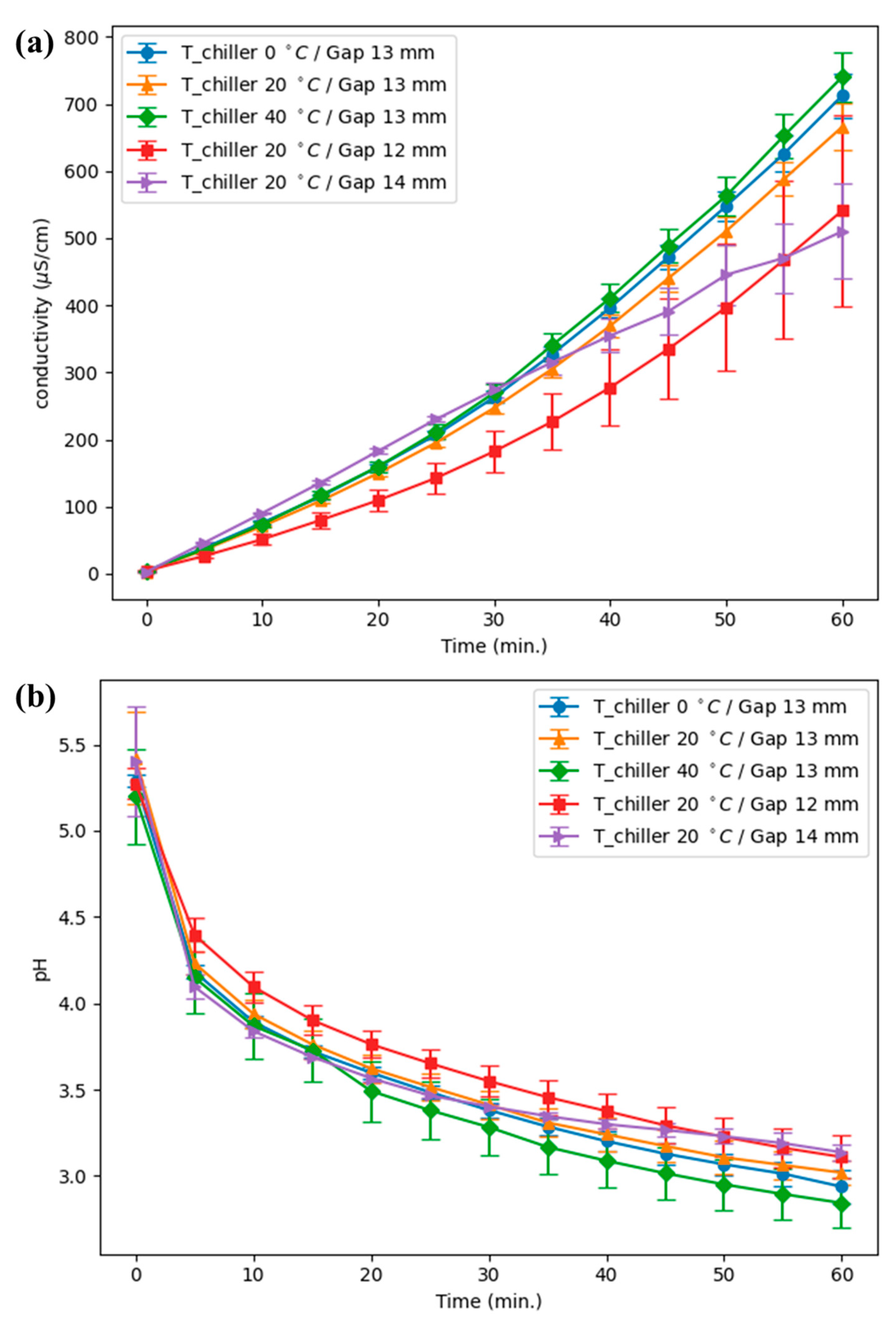
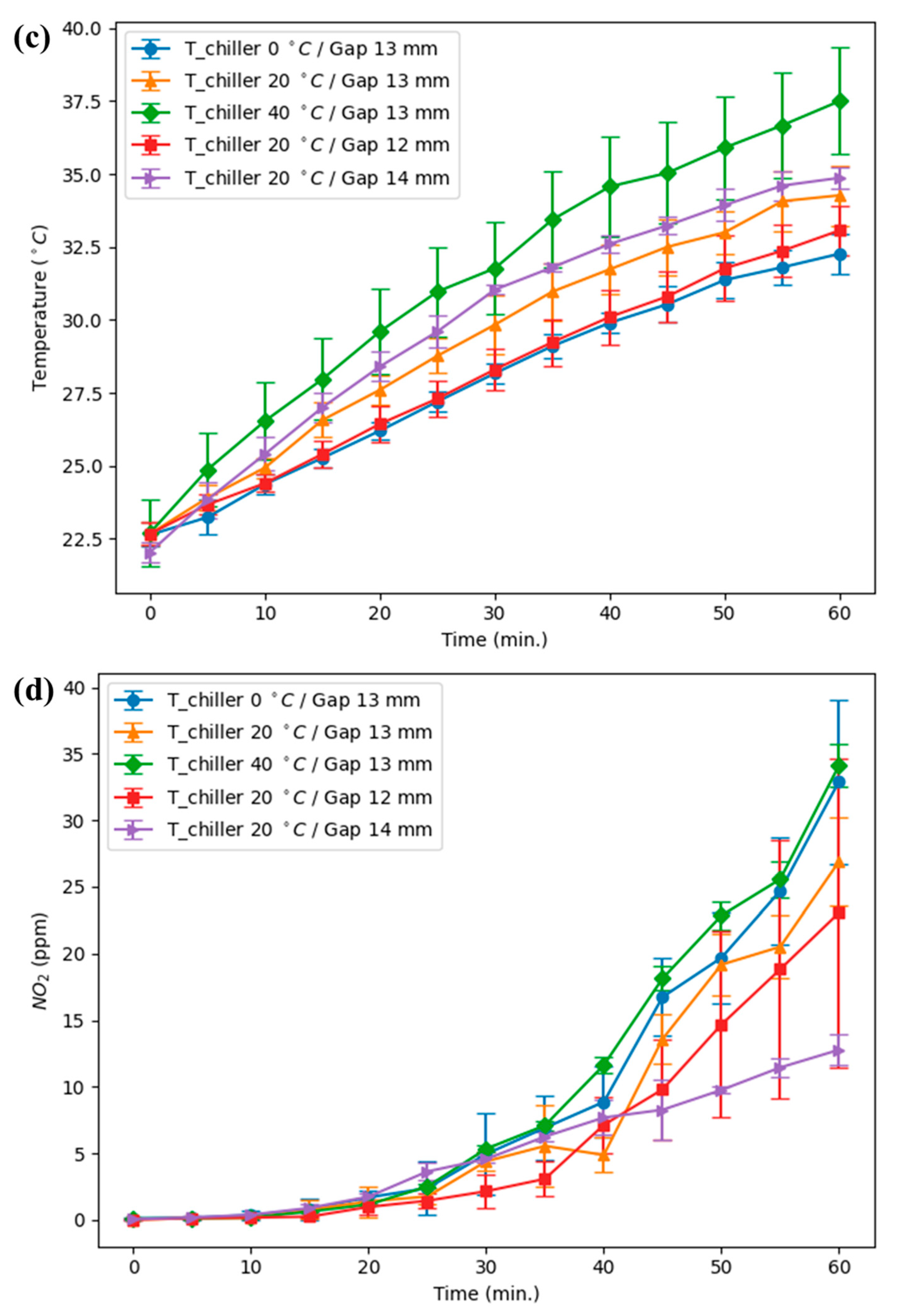
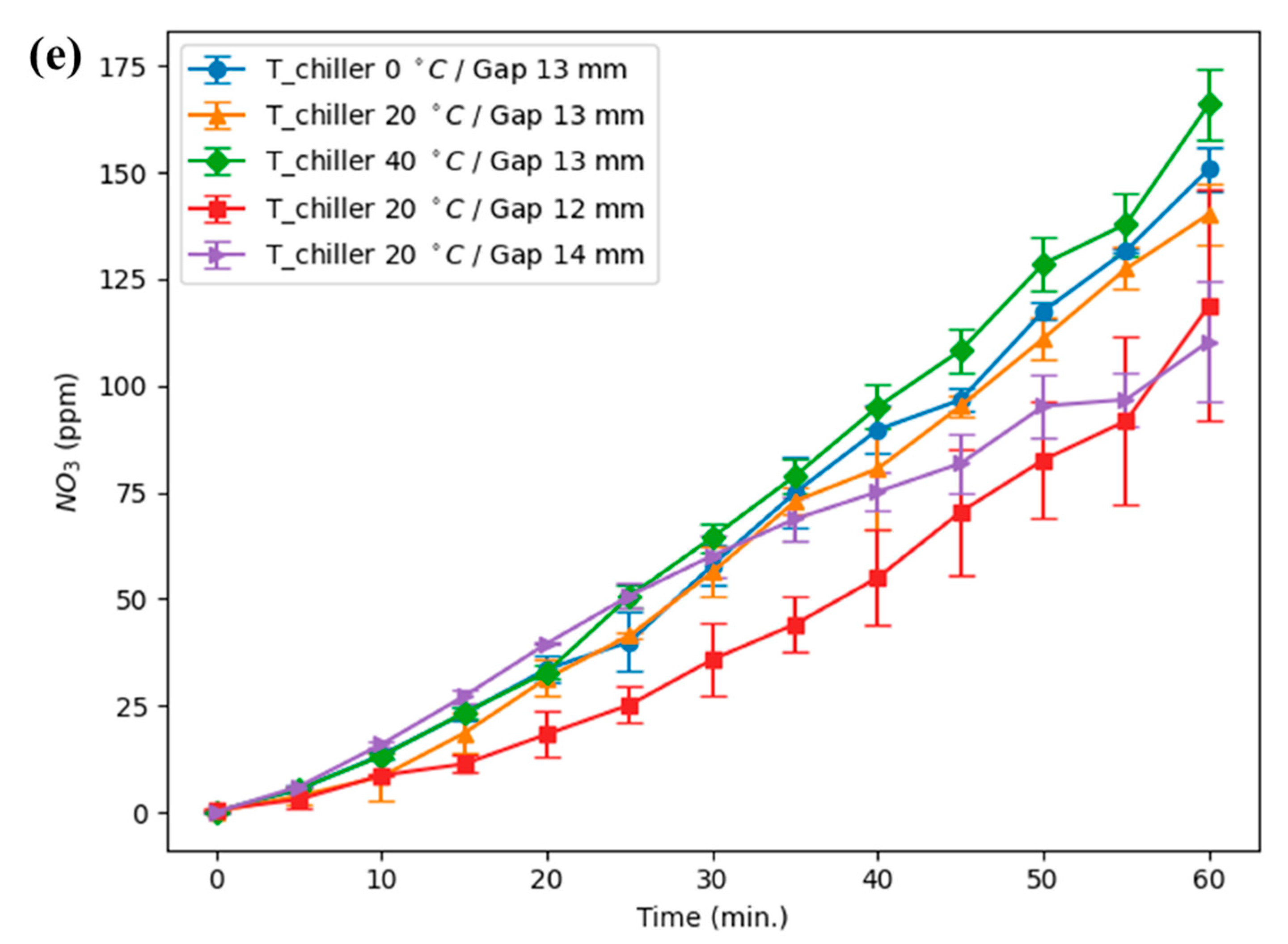
The experiments were carried out on a pin-to-water (PTW) discharge operating at 14.3 kHz and 0.2 kW, with electrode to quartz wall distance of 12, 13, and 14 mm and chiller setpoints of 0, 20, and 40 °C; the full apparatus is shown in
Figure 1. Across all tested conditions, the key, reproducible observation is that H
2O
2 concentration in the treated water does not increase monotonically with treatment time. Instead, the concentration rises rapidly during an initial period and then falls after reaching a clear maximum. Representative time traces in
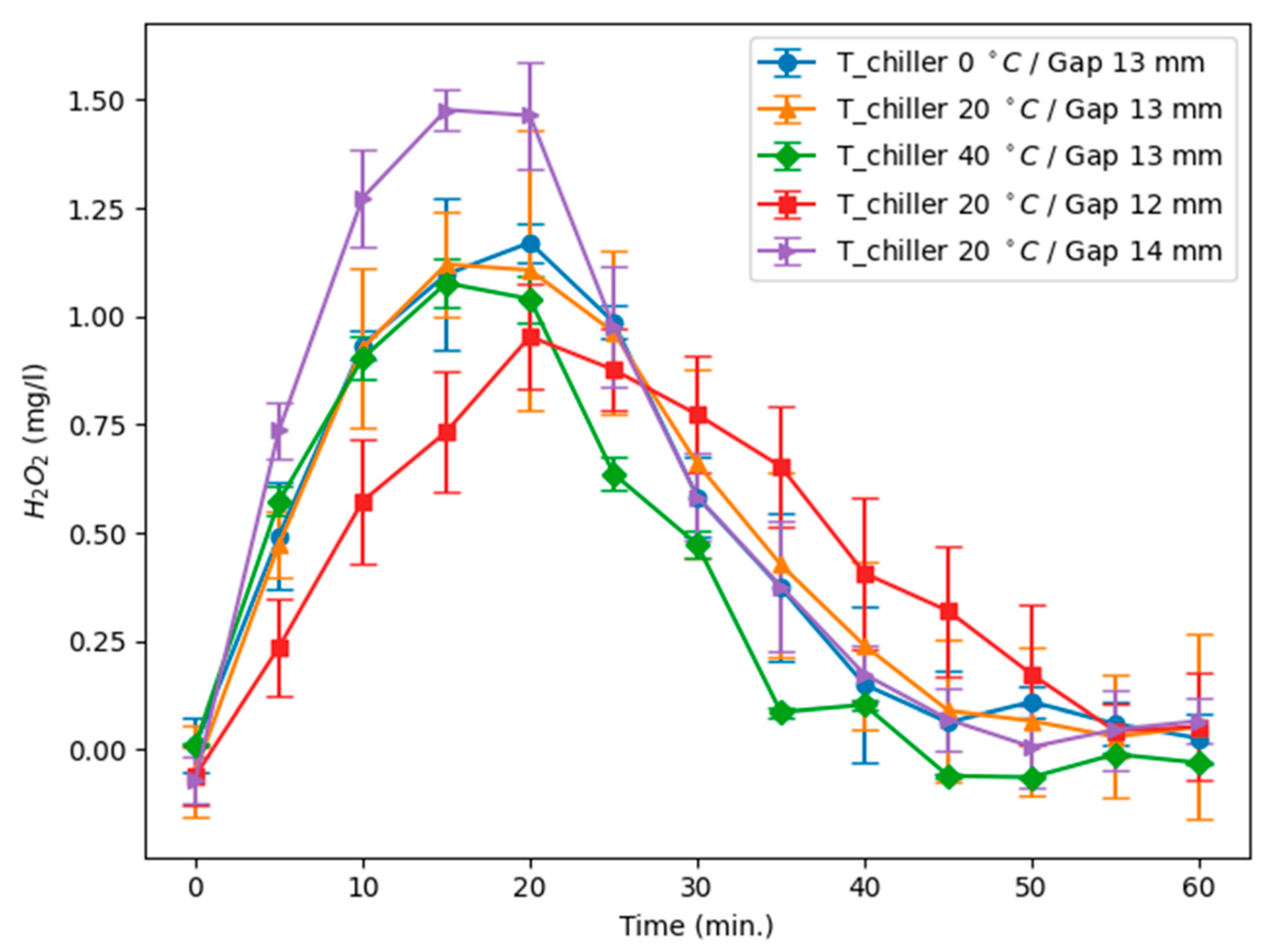
Figure 2 show that H
2O
2 typically attains its peak within ~15–25 min and declines over a longer interval (roughly 35–45 min under our conditions). This non-monotonic behavior was observed for every tested geometry and thermal setpoint and was reproduced in triplicate for each condition, establishing it as a systematic feature of these PTW discharges.
3.1. Time-Resolved Gas Phase Characteristics from Optical Diagnostics
To determine whether the drop in liquid H
2O
2 reflects changes in gas-phase production or a post-transfer liquid loss, we acquired time-resolved FTIR spectra of the discharge effluent.
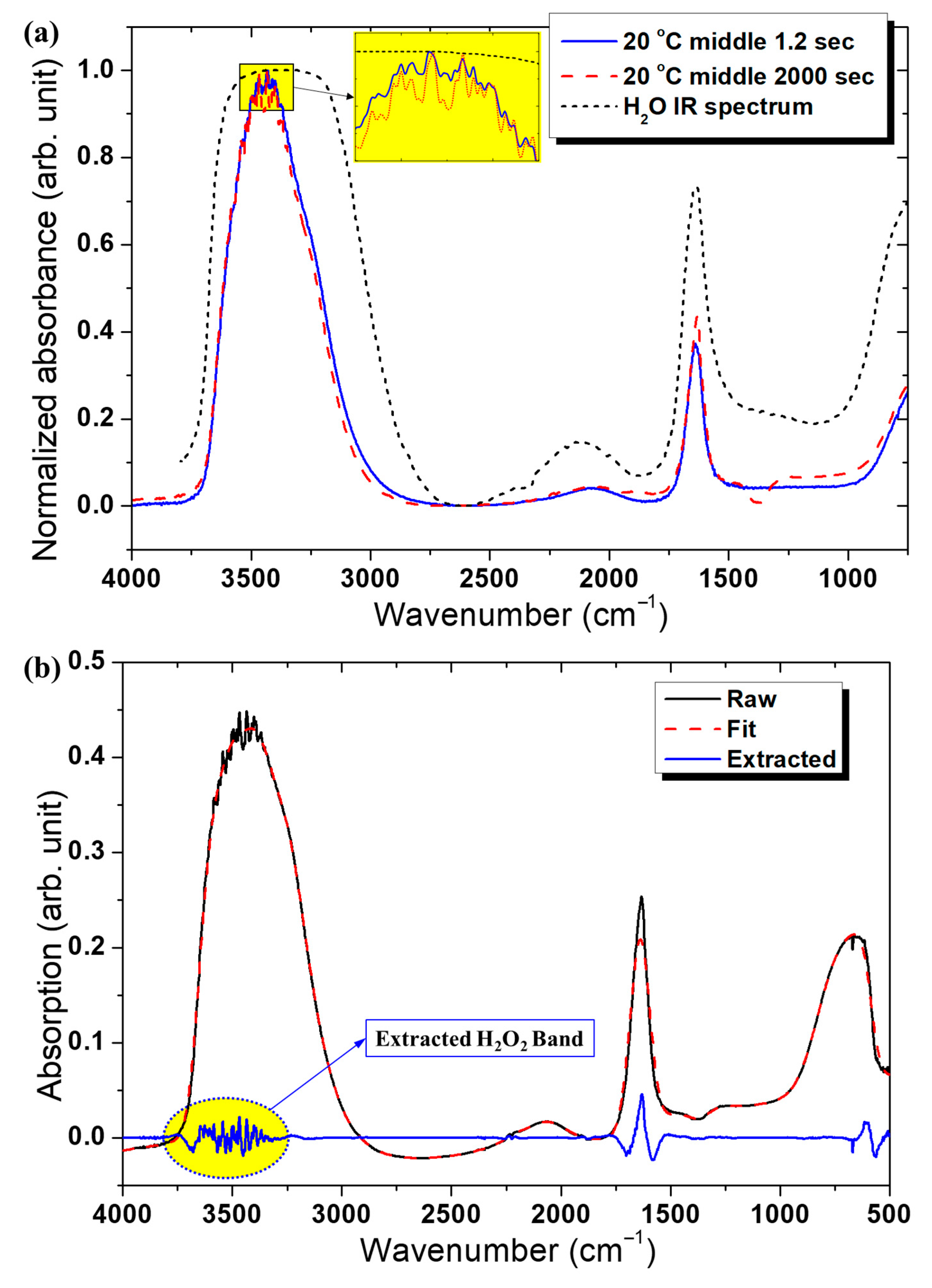
Figure 3 illustrates the spectral evolution. Gas-phase H
2O
2 was quantified from FTIR absorbance near 3500 cm
−1 after subtraction of the dominant H
2O vibrational envelope. Baseline correction employed a third-order polynomial fit across adjacent continuum windows; calibration was accomplished using prepared H
2O
2 reference gas mixtures. The reported FTIR intensities represent integrated absorbance within the 3490–3510 cm
−1 band, and the limit of detection under our optical path was estimated at 0.01 mg L
−1 (determined as 3σ of the baseline noise). After subtraction of the dominant H
2O background, a weak but identifiable H
2O
2 absorption band around 3500 cm
−1 [
17] becomes apparent and increases in intensity during the early operation period. Crucially, the FTIR H
2O
2 band shows an inflection and subsequent decrease at roughly the same time that the liquid-phase H
2O
2 peaks (~30 min), demonstrating that the reduction in aqueous H
2O
2 is mirrored by a decline in gas-phase H
2O
2 production rather than by a purely aqueous decomposition process. In
Figure 3c,d, the main absorption bands corresponding to H
2O
2 and unfiltered H
2O have been identified for clarity. The discontinuities observed at approximately 25 min, 31–35 min, and 54 min originate from periodic recalibration of the FT-IR spectrometer during long-term measurements, which slightly perturbs the baseline but does not affect the overall spectral trends.
Optical emission spectroscopy (OES) provides the link between plasma state and reactive-species production.
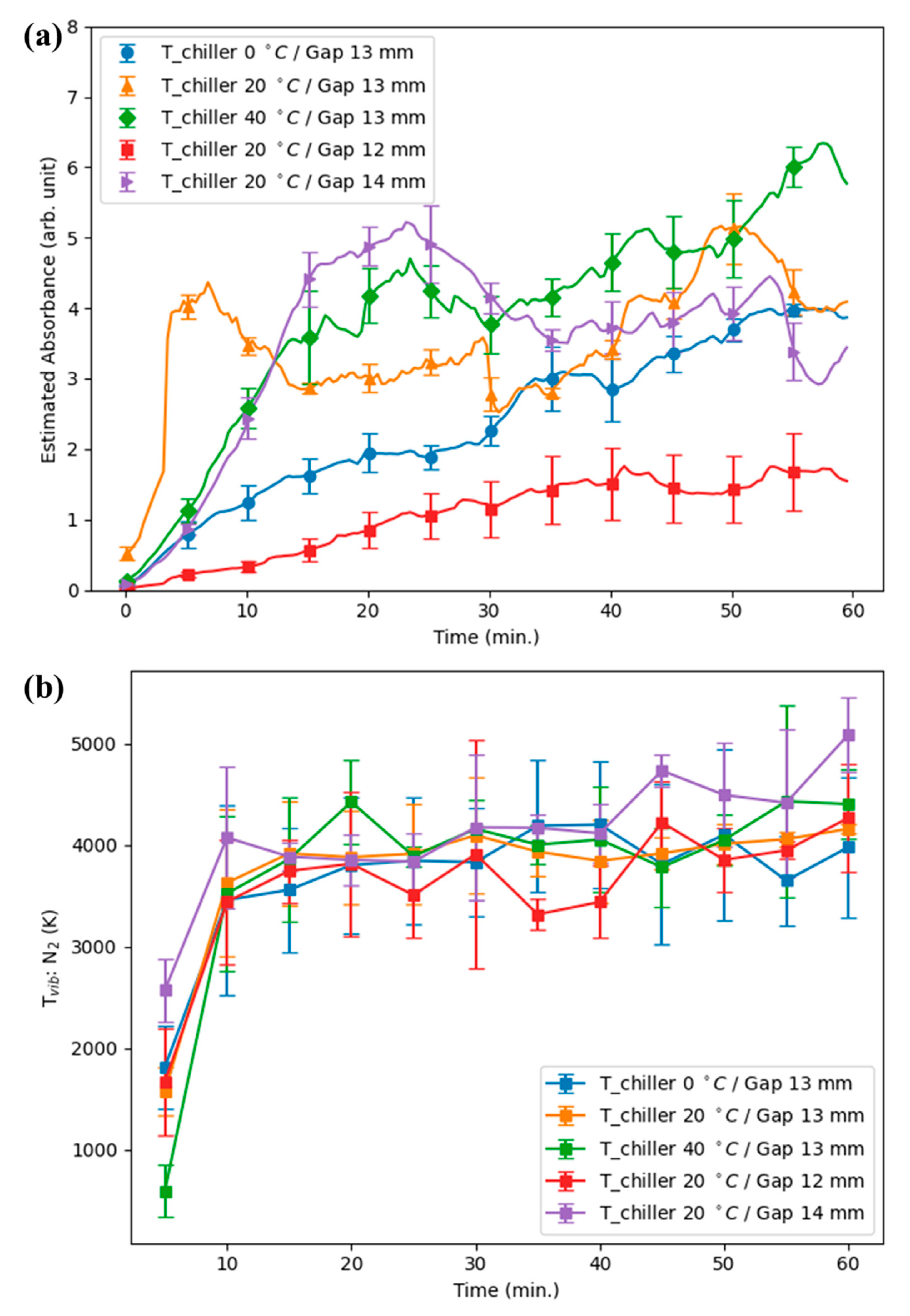
Figure 4a shows the time evolution of N
2 vibrational temperature (T
vib) extracted from OES [
18]: T
vib increases gradually from near 3000 K at ignition to beyond 5500 K with prolonged operation. When H
2O
2 production begins to decline, T
vib is consistently near ~4900 K (see the overlay and condition panels in
Figure 4b,c). Around T
vib~4900 K-where high-ν N
2 (ν ≥ 12) becomes significant; the reaction channel between excited N
2 and atomic O competes effectively with oxygen-radical recombination, diverting O and OH away from O
3 and H
2O
2 formation and into NO formation, in line with prior reports on mode transitions in humid air discharges [
19]. Shorter electrode gaps—i.e., higher local power density—accelerate the T
vib rise and thus bring forward the time of H
2O
2 suppression. The coincidence of the H
2O
2 inflection and a characteristic T
vib value across multiple conditions indicates that vibrational excitation of N
2 is a robust marker for the plasma-state change that reduces H
2O
2 yield; this observation is consistent with previous reports linking high N
2 vibrational excitation to mode transitions and altered ROS/RNS balance in humid air plasmas [
12,
13,
14].
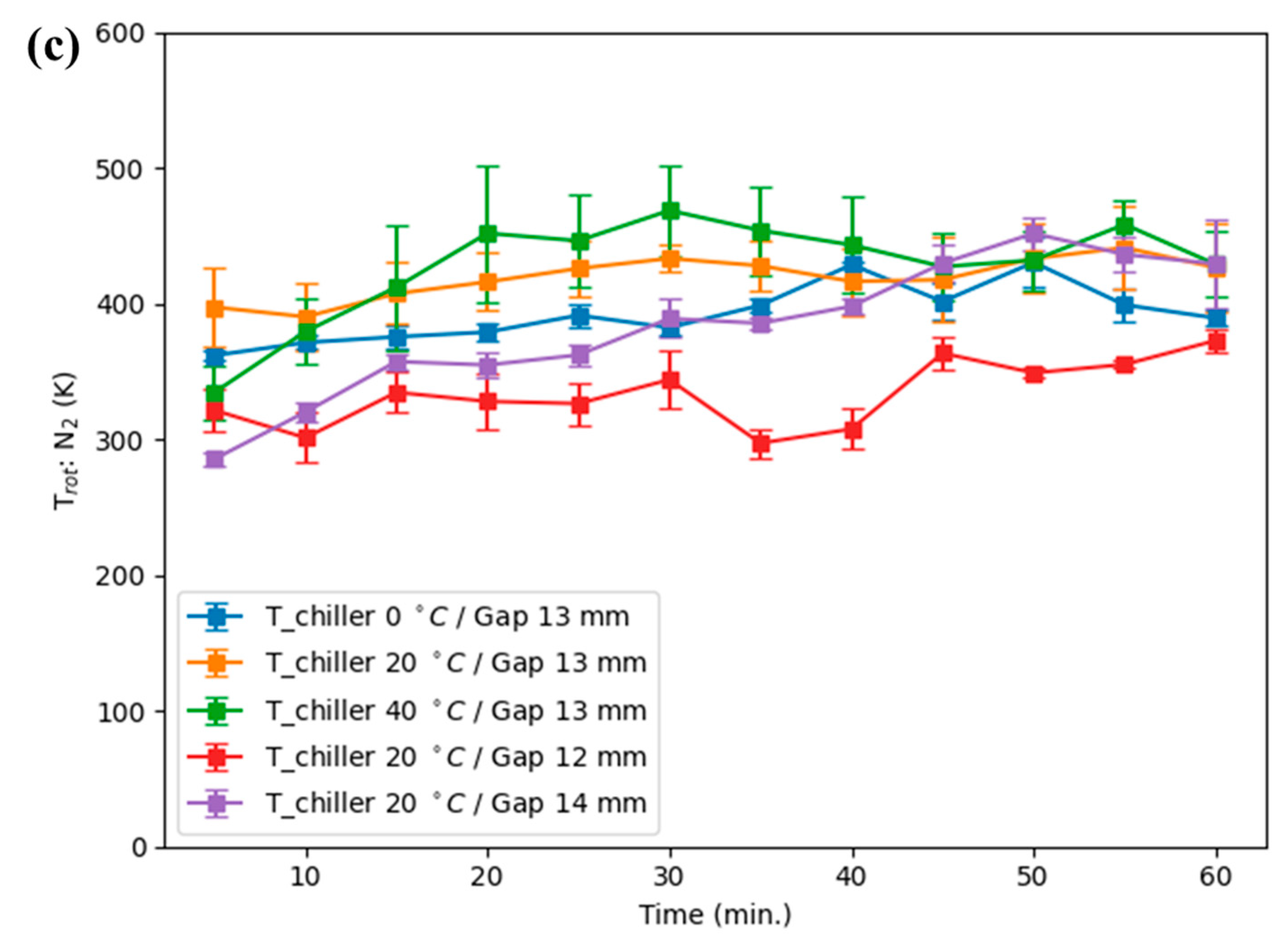
The vibrational temperature obtained from OES fitting spanned a range of 3000–5000 K (
Figure 4). The value of ~4900 K has been inferred from global modeling and from theoretical analysis reported previously [
14], and therefore represents an upper bound rather than a directly validated threshold in the present experiments. While this model-based estimate suggests a possible condition for significant changes in plasma–liquid chemistry, our current measurements only confirm the broader 3000–5000 K window.
Table 1 reports Pearson correlation coefficients (r) and two-tailed
p-values between the nearest-time matched H
2O
2 absorbance and the spectroscopically fitted temperatures (T
vib and T
rot) for each experimental condition (n = 12 matched points per condition). The primary numerical outcomes are reproduced below:
0 °C, 13 mm: r(Tvib, H2O2) = 0.643 (p = 0.024); r(Trot, H2O2) = 0.721 (p = 0.008).
20 °C, 13 mm: r(Tvib, H2O2) = −0.134 (p = 0.679); r(Trot, H2O2) = 0.189 (p = 0.555).
40 °C, 13 mm: r(Tvib, H2O2) = 0.821 (p = 0.001); r(Trot, H2O2) = 0.763 (p = 0.004).
20 °C, 12 mm: r(Tvib, H2O2) = 0.632 (p = 0.027); r(Trot, H2O2) = 0.453 (p = 0.139).
20 °C, 14 mm: r(Tvib, H2O2) = 0.462 (p = 0.131); r(Trot, H2O2) = 0.452 (p = 0.140).
These results show that Tvib exhibits statistically significant positive correlation with H2O2 in several operating conditions (notably 40 °C, 13 mm, 20 °C, 12 mm, and 0 °C, 13 mm), while Trot is statistically significant in some cases (notably 0 °C, 13 mm and 40 °C, 13 mm). The relative importance of Tvib vs. Trot is condition-dependent.
Note that the lowest T
vib values observed in this study (~1000 K) should be interpreted as detection-limited estimates obtained under conditions of weak N
2 emission, rather than as representative of the vibrational excitation under typical discharge operation. Similar low-end values have also been reported in the literature: Richard et al. observed T
vib as low as ~1000 K in pin-to-liquid plasma configurations, where plasma–liquid interactions and reduced excitation conditions limit vibrational energy transfer [
20], and Shimizu et al. demonstrated vibrational temperatures spanning 1000–4700 K in atmospheric pressure surface micro-discharges, noting that the lower-end values corresponded to regimes of reduced vibrational excitation [
14]. Taken together, these findings indicate that ~1000 K values can appear as plausible lower-bound estimates under constrained power density or signal-to-noise conditions, while the operationally relevant regime of our device consistently produces multi-thousand K vibrational excitation, consistent with the broader literature.
The combined quantitative evidence indicates that the decrease in H2O2 observed under prolonged operation is not primarily due to direct thermal decomposition, as Trot values remained in the hundreds of K (well below temperatures for rapid thermal H2O2 breakdown). Instead, rising Tvib–frequently preceding H2O2 decline–appears to mark a plasma-state transition that favors reaction channels (e.g., N2(v) + O → NOx) which divert reactive O and OH away from H2O2 formation. Trot plays a supporting role by modestly increasing collisional rates and transport, but it does not alone account for the observed suppression. Therefore, Tvib is a robust operational marker for plasma-mode change affecting H2O2 yield, while Trot provides complementary information relevant to thermal and transport effects.
3.3. Numerical Analysis
A control experiment explicitly ruled out bulk thermal decomposition as the dominant cause of H2O2 decline: an aqueous H2O2 solution held at 60 °C without plasma exposure showed negligible loss over the same time window. Therefore, the post-peak H2O2 decrease during plasma treatment is attributable to changes in plasma chemistry and transport rather than to direct thermal decomposition in the reservoir.
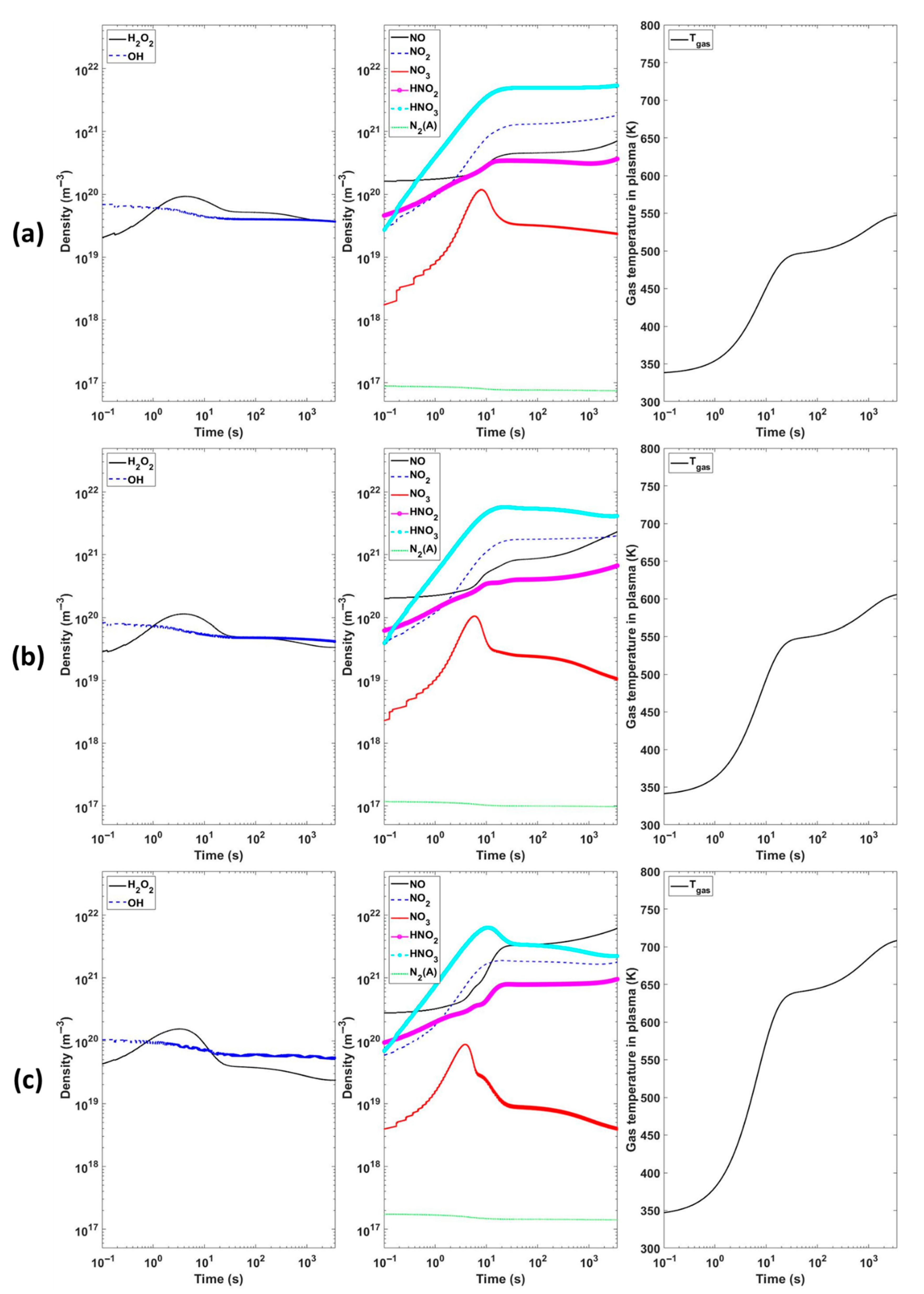
To mechanistically connect the measured trends and to explore parameter sensitivity, we coupled a global plasma chemistry model to 3D fluid dynamics simulations. The model, implemented as described in [
16], incorporates a comprehensive reaction set that has been previously validated against FTIR-measured RONS concentrations in humid-air discharges. Using this framework, we reproduce the characteristic non-monotonic H
2O
2 evolution: H
2O
2 initially accumulates and then declines while NO and NO
2 rise monotonically as effective gas temperature and vibrational excitation increase (
Figure 6). The agreement between simulation and our present measurements supports the interpretation that increasing T
vib shifts OH radical pathways away from H
2O
2 formation and toward NO
X chemistry.
During 1 h of plasma discharge from the global model, among the various chemical species, H
2O
2 and relevant major species for generation and loss are presented. The power density was assumed to be the value obtained by dividing the measured plasma power by plasma volume. In this work, we deduce the power density by dividing the input power by plasma volume. The plasma volume was deduced from the electrode radius and the distance between the electrode end and the water surface. The thickness of the water layer is assumed to be 1 mm. The gas temperature was not saturated but kept increasing. The gas temperature in the model indicates the value inside the plasma column. As the power density increased from 1.5 × 10
8 to 7.1 × 10
8 W/m
3, the maximum gas temperature increased from 525 K to 710 K. The calculated results clearly show the non-linear trends of H
2O
2 density. The H
2O
2 density increased after the beginning of discharge but gradually decreased without saturating at the maximum value. Note that the excited N
2(A) density decreases with the gas temperature. Because the N
2(A) consumes the ROS, which is needed to generate the O
3 and H
2O
2, the NO and NO
2 densities increased. The global model needs gas velocity to calculate the species densities that drift into and out of the plasma zone. We used complementary Fluent
® simulations to deduce the gas velocity from the water flow at the wall.
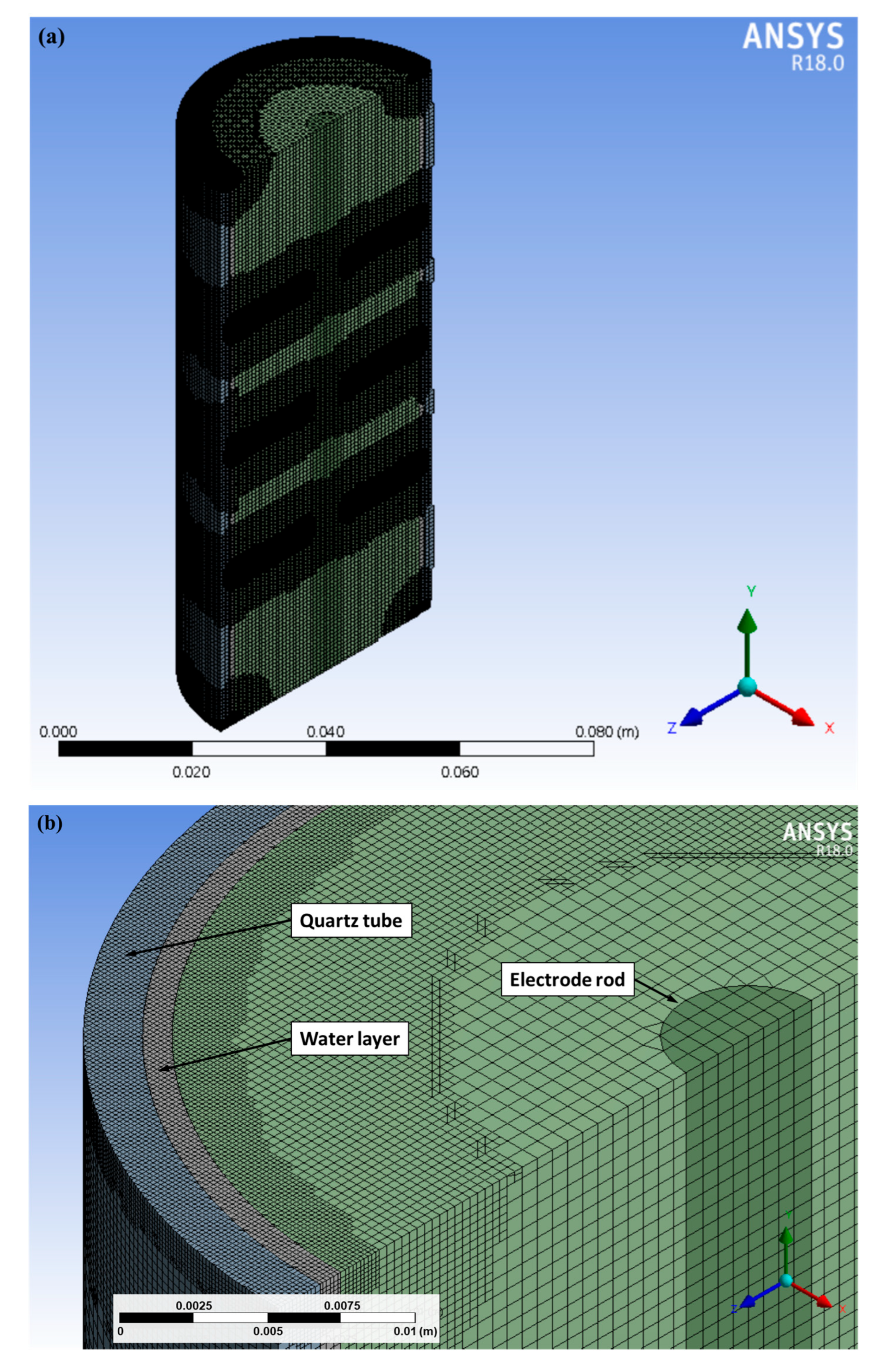
Figure 7 shows the domain of the simulation, and
Figure 8 shows the result. Fluent simulations were conducted in three dimensions, and
Figure 7 illustrates the computational domain and a representative cross-sectional mesh. The shaded regions indicate the positions of the three thin electrode layers, around which a finer mesh was applied to capture thermal gradients. Panel (a) shows the overall cross-sectional view, while panels (b) and (c) provide magnified views of the inlet region and the electrode/plasma zone, respectively. In the model, the plasma zone is represented as a localized heat source. The liquid layer was implemented as a tangentially injected water film flowing along the inner quartz wall, consistent with the experimental configuration, thereby approximating the observed stable water dynamics. In this calculation, we used the plasma zone as a heat source to consider the temperature effects on the gas flow.
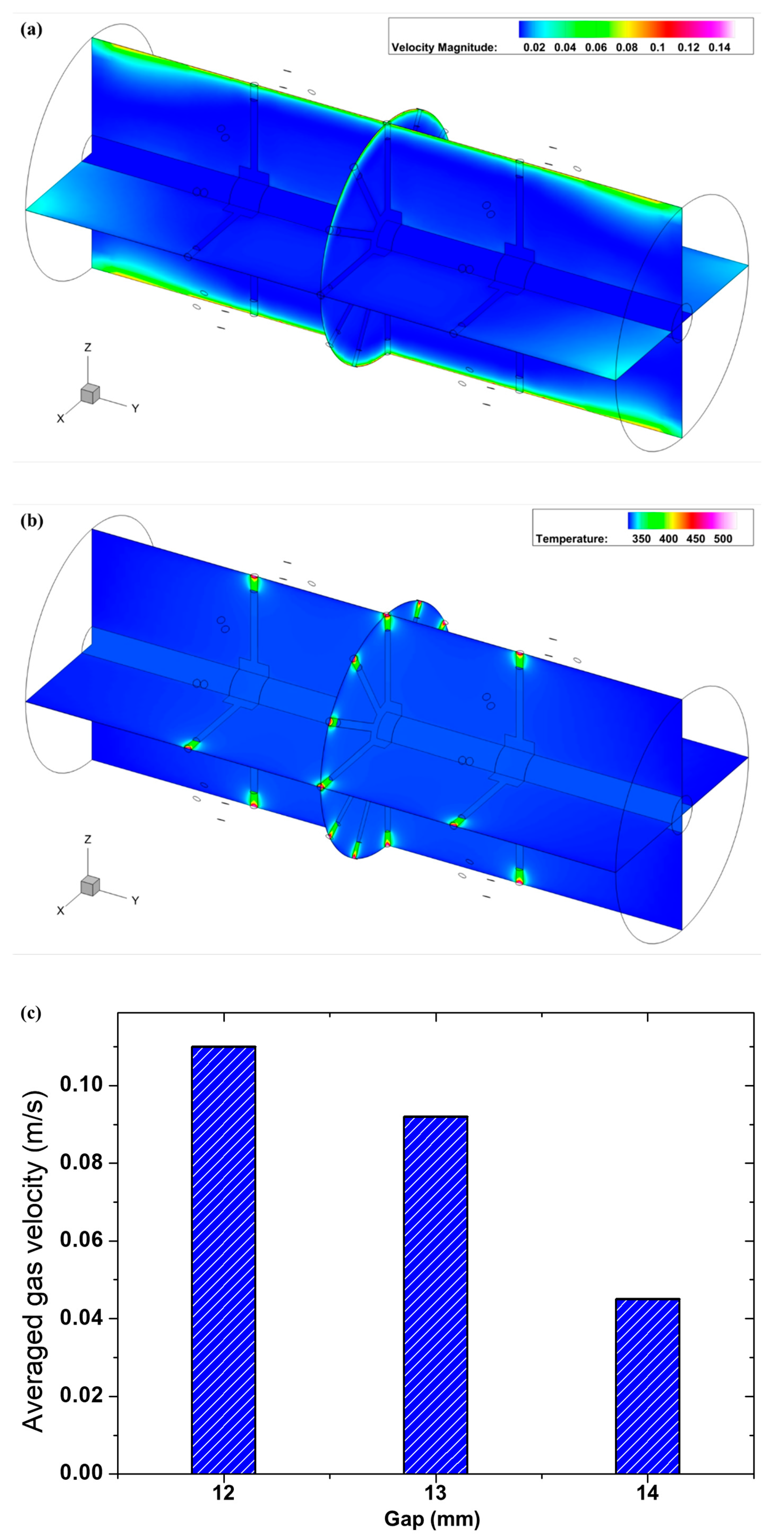
Figure 8a,b are velocity magnitude and temperature distribution in the tube, respectively. The averaged gas velocity in the plasma zone is presented in
Figure 8c. It reveals strong localized heating and convective patterns around the pin: regions of reduced convection near the liquid surface enhance local residence of reactive species and heat, while orthogonal flows remove ROS from the interface. The combined model results thus show how vibrational kinetics (captured by T
vib) and transport (flow and convective removal) jointly determine the time window over which H
2O
2 is favorably produced.
The mechanistic link between vibrational excitation and H
2O
2 production can be clarified by considering the role of hydroxyl radicals. It has been well established that H
2O
2 in humid plasmas is primarily formed through the recombination of OH radicals (OH + OH → H
2O
2), which originate from electron impact dissociation of water molecules [
21]. In this context, T
vib does not directly form H
2O
2 but acts as an indicator of enhanced electron–molecule vibrational excitation that promotes OH radical generation. Meanwhile, Trot provides complementary information on the thermal environment, which influences the lifetime and recombination probability of OH [
21]. Similar interpretations have been reported in plasma–liquid interaction studies, where correlations between vibrational excitation, OH radical density, and H
2O
2 yield have been observed [
22]. Accordingly, the observed coincidence of T
vib ~ 4900 K with the onset of H
2O
2 suppression should be understood as an operational marker of a plasma-state transition where OH pathways are diverted or consumed in competing reactions, rather than as a direct causal mechanism.
3.4. H2O2 Generation Control Knob
Taken together, the experimental and modeling evidence support the following mechanistic picture. In the initial regime (low T
vib), plasma processes produce abundant O, OH, and HO
2 that lead to net H
2O
2 formation in the gas phase and subsequent transfer to the liquid. As the operation proceeds, accumulated ions and changing surface conditions (increasing conductivity, surface deformation toward the pin) increase local current density and heating; T
vib rises accordingly. When T
vib approaches 4900 K, channels favoring NO formation (via vibrationally assisted N
2 + O pathways and related chemistry) become kinetically competitive [
13,
23,
24]. The enhanced NO/NO
2 chemistry produces soluble RNS that scavenge H
2O
2 (directly or via secondary aqueous reactions), acidify the liquid, and thereby suppress net H
2O
2 concentration despite continued plasma energy input. This T
vib-mediated ROS to RNS shift explains why simply increasing treatment time or power does not monotonically increase H
2O
2 yield and why geometry and flow control are essential levers.
These mechanistic conclusions directly inform device-level design rules for on-demand H
2O
2 generation.
Figure 9 summarizes the practical guidelines that emerge from our data and simulations. First, the electrode gap functions as an effective power-density control: selecting a longer gap reduces the rate of T
vib rise, thereby extending the H
2O
2-productive window. Second, real-time monitoring of T
vib by OES provides a practical process endpoint: terminate the discharge when T
vib approaches ~4900 K to harvest maximal H
2O
2 and avoid NOx domination. Third, flow geometry should be designed to minimize convective loss of ROS-orthogonal gas flows that sweep reactive oxygen away from the liquid interface, shorten the useful window, and reduce yield. Fourth, thermal management (chiller control) stabilizes bulk temperature but does not, by itself, prevent the vibrationally driven transition; therefore, chiller design is a secondary but necessary element of an integrated system. Finally, water quality (low conductivity DI water) and corrosion-resistant wetted materials are prerequisites for any practical implementation, particularly when downstream purity is required for industrial use. Although discharge current was not directly measured in the present work, the current characteristics of this PTW configuration were investigated in our previous study [
13]. It was shown that ions generated by the plasma increase the conductivity of the liquid, which provides positive feedback to the discharge and induces a transition from glow to abnormal glow mode. This mode transition is accompanied by changes in both plasma density and gas temperature, which are known to strongly influence H
2O
2 generation. The present results should therefore be interpreted in the context of these earlier findings, as the mode-dependent discharge behavior represents an additional factor that can modulate reactive species production.
We acknowledge the limitations of the present study that frame near-term work. First, the Tvib threshold reported here is an operationally useful marker within our experimental gas composition and geometry; cross-validation with alternative spectroscopic diagnostics and with varying gas mixtures (e.g., controlled O2 enrichment or reduced N2 fraction) would better constrain its universality. Second, although the global model captures the qualitative trends and parameter sensitivities, fully spatially resolved plasma-kinetic simulations that couple vibrational population dynamics with detailed surface chemistry are a logical next step for improved quantitative design. Third, for semiconductor applications in particular, additional qualification (TOC, ICP-MS for trace metal leaching, and energy efficiency metrics in mg H2O2 kW/h) is required before claiming process integration; the present work establishes the mechanistic and operational basis that such qualification should build upon.
Under our tested condition (0.2 kW, 60 min treatment of 600 mL, final H
2O
2 ≈ 1 mg·L
−1), the total H
2O
2 produced was ~0.6 mg, corresponding to an energy yield of ≈0.003 g·kWh
−1. This is substantially lower than values reported in parametric studies, which provide useful context for interpreting our fixed-condition results. For example, pulsed discharges in oxygen bubbles demonstrated that increasing pulse frequency enhances H
2O
2 generation while energy yield remains nearly constant; under optimized low-voltage long-pulse conditions, the maximum efficiency reached 61.8 mg·h
−1 and 2.1 g·kWh
−1 at 2.5 kV, 1 μs, 4 kHz [
25]. Power-scan studies with noble gas pin-to-water discharges showed that higher discharge power increases H
2O
2 concentration but decreases energy yield, with helium performing slightly better than argon [
26]. Atmospheric pressure plasma jets further revealed that feed gas humidity and power strongly affect H
2O
2 delivery, and that low-frequency pulsing can improve efficiency from ~0.2 g·kWh
−1 in continuous mode up to ~0.75 g·kWh
−1 [
27]. Compared to these reports, our results are modest because the operating frequency (14.3 kHz) and generator power (0.2 kW) were kept constant in this study. Nevertheless, the present findings establish a baseline for linking vibrational temperature to H
2O
2 production and can inform future optimization of frequency, power, and gas composition.
In summary, our combined experimental and modeling study shows that H2O2 generation in air PTW discharges is bounded by an operational window defined by N2 vibrational excitation. By using Tvib as an in-process diagnostic and by controlling geometry and flow to moderate its rise, PTW devices can be operated to maximize H2O2 yield while avoiding NOx-dominated suppression. These findings provide a pathway to rationally design compact, on-demand H2O2 generators for applications ranging from sterilization and agriculture to potential integration with downstream purification for high-purity industrial processes.