Non-Thermal Plasma Treatment of Dye-Contaminated Wastewater: A Sustainable Approach for Pollutant Degradation and Enhanced Plant Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
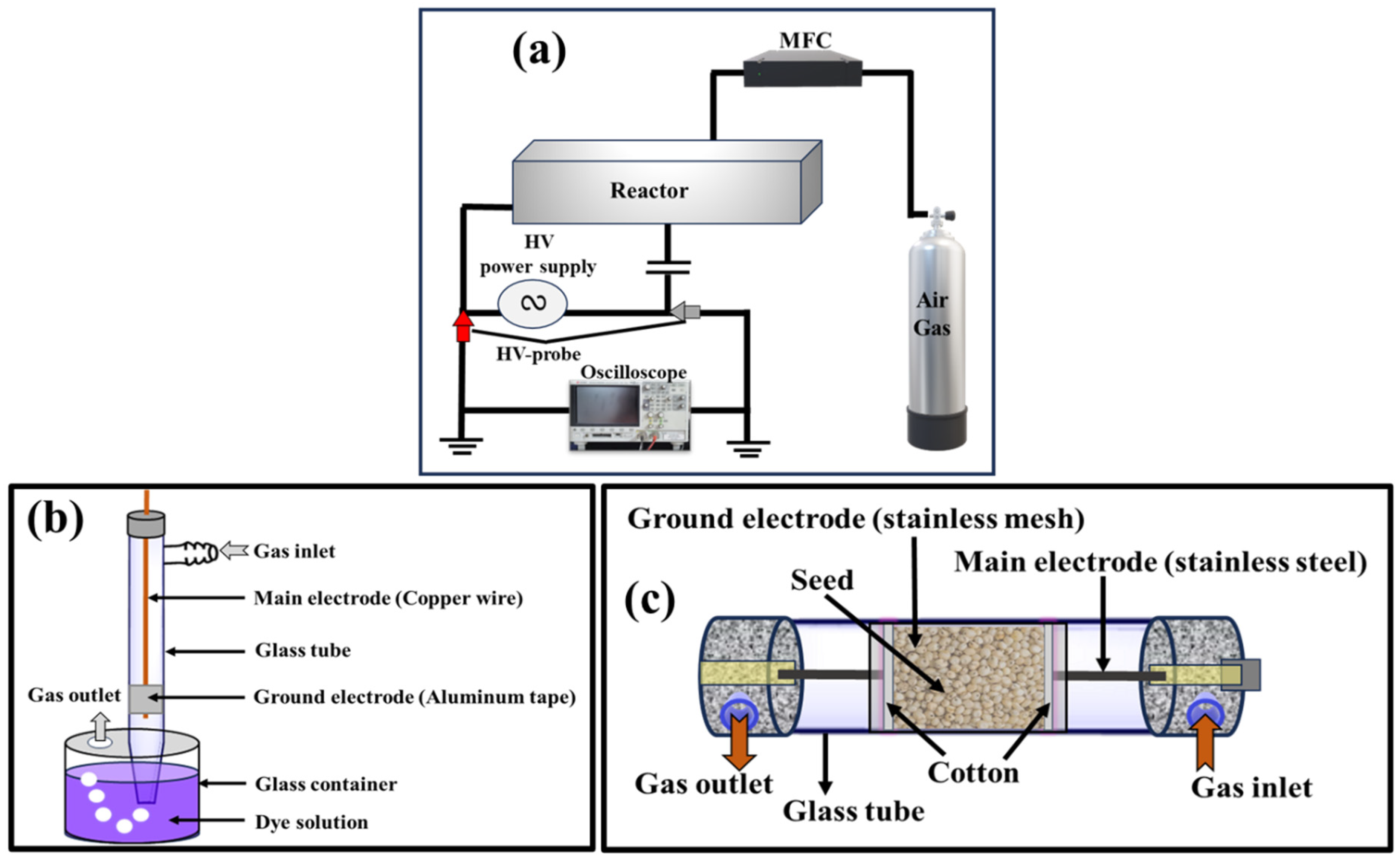
2.2. Non-Thermal Plasma Reactors
2.2.1. Water Activation/Treatment Reactor
2.2.2. Seed Treatment Reactor
2.2.3. Plasma Discharge Power Measurement
2.3. Seed and Water Treatment Procedure
2.4. Analytical Devices
3. Results and Discussion
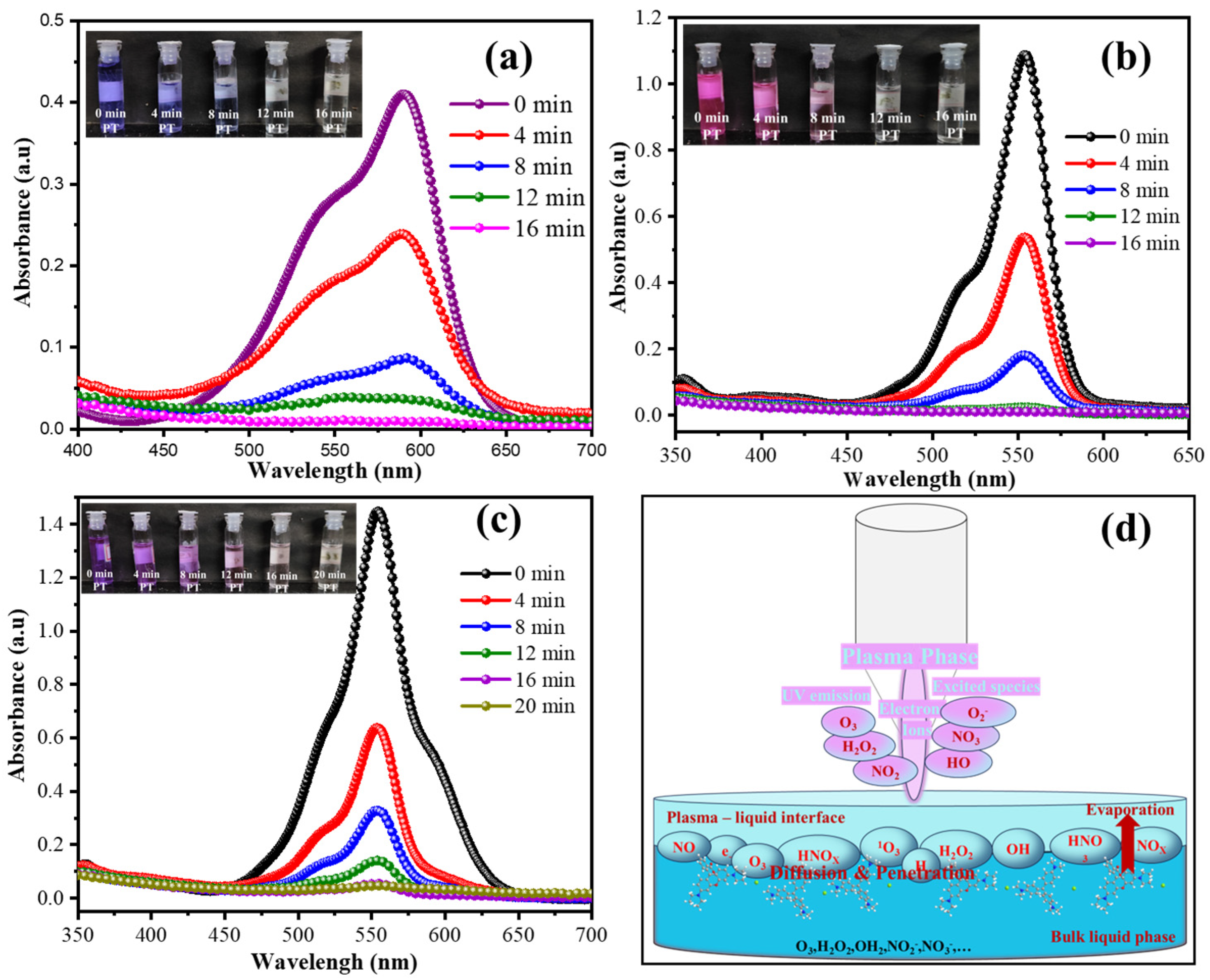
3.1. Crystal Violet (CV) and/or Rhodamine B (RhB) Degradation
3.2. Dye Mineralization and By-Product Identification
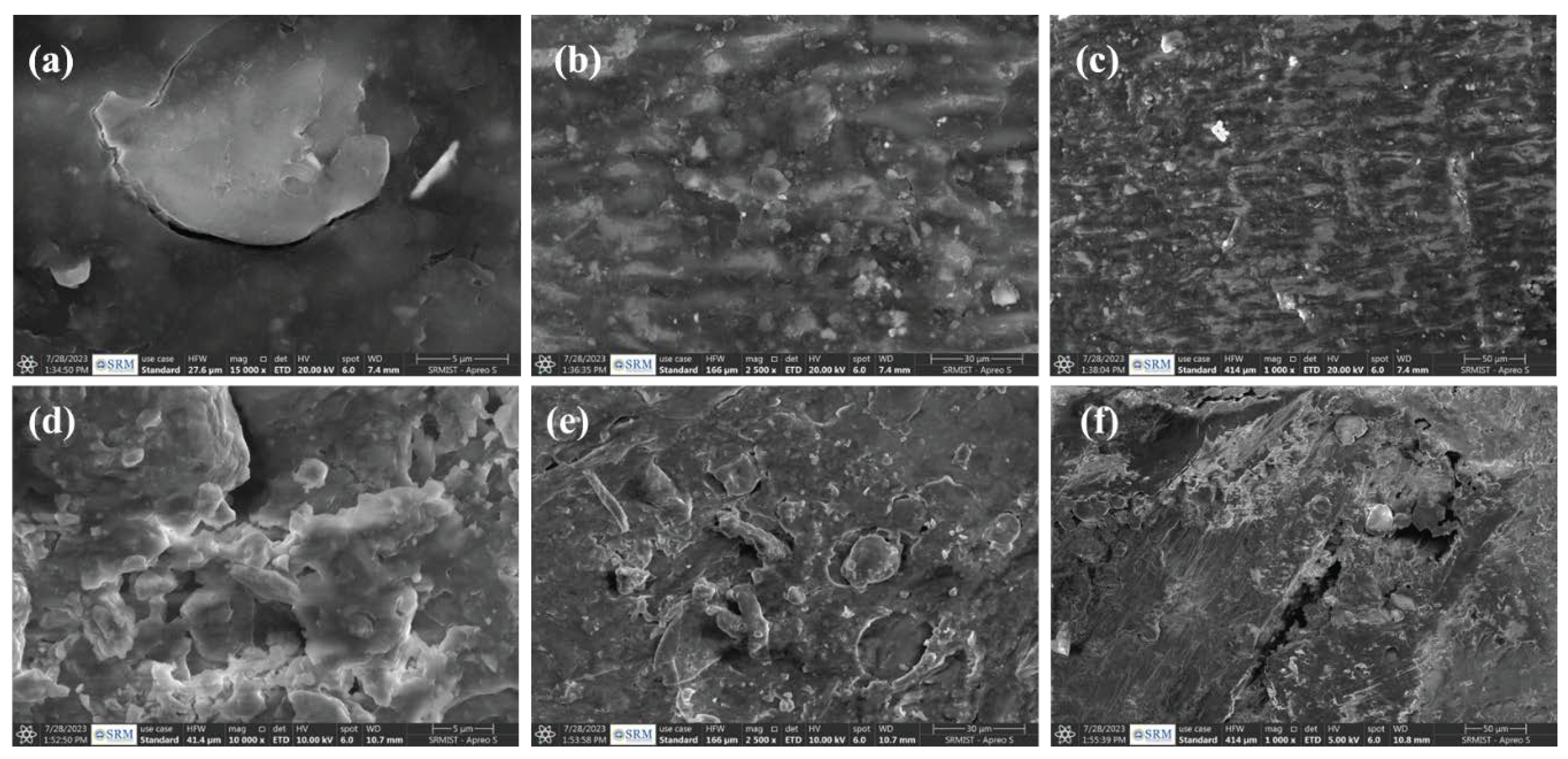
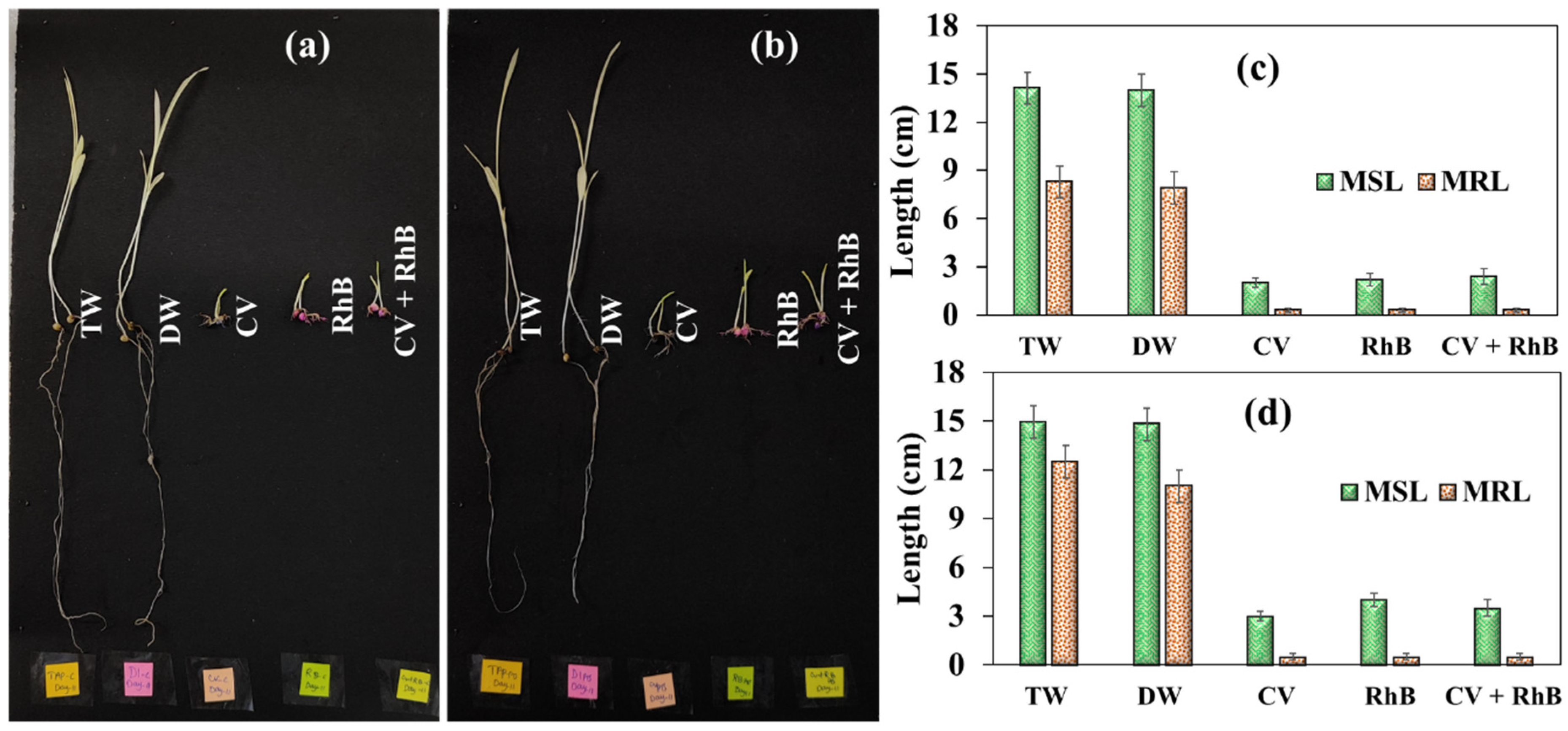
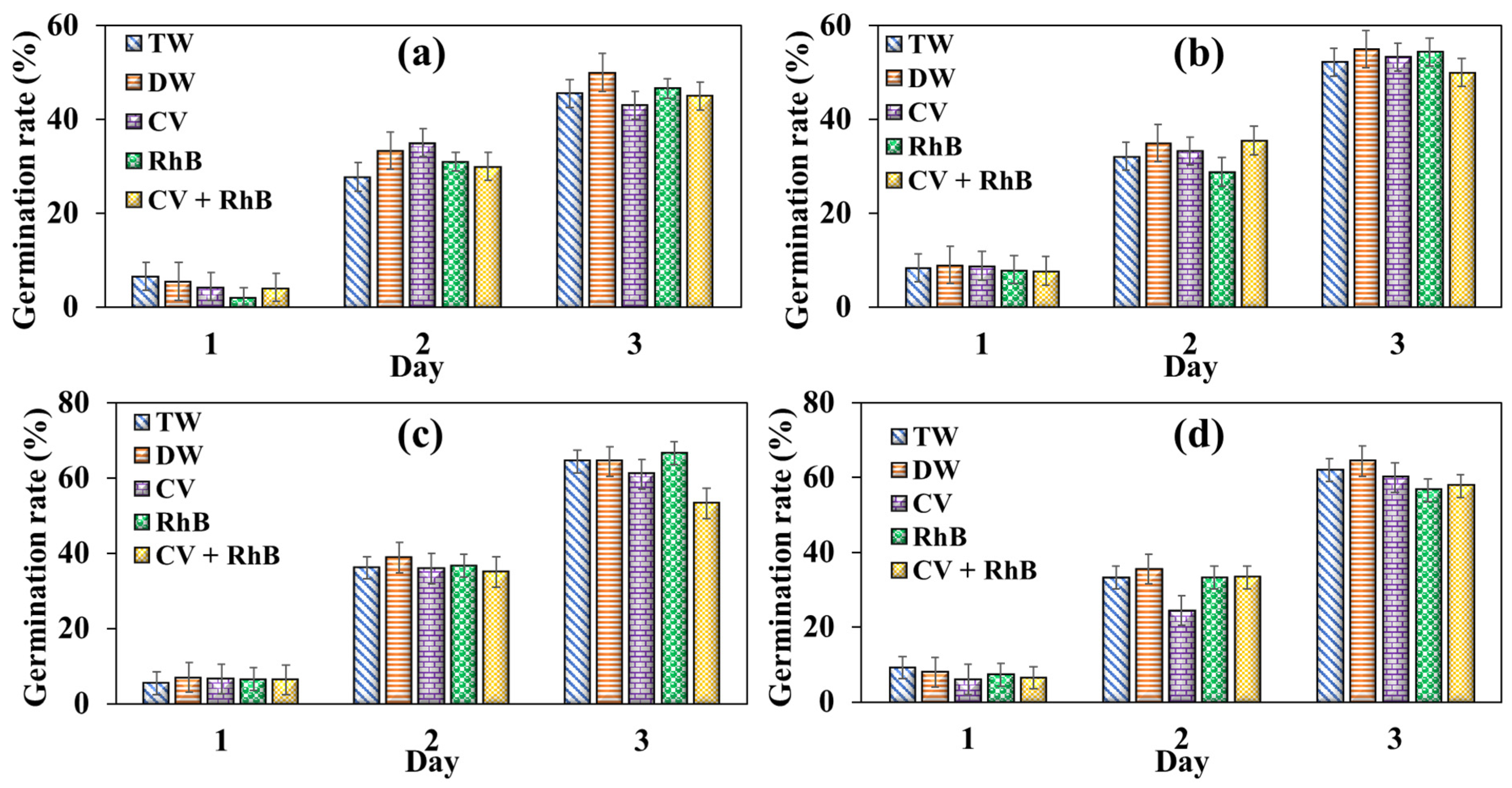
3.3. Effect of Dye-Contaminated Water on Seed Germination
3.4. Effects of Dye-Contaminated Water on Post-Germination Plant Growth
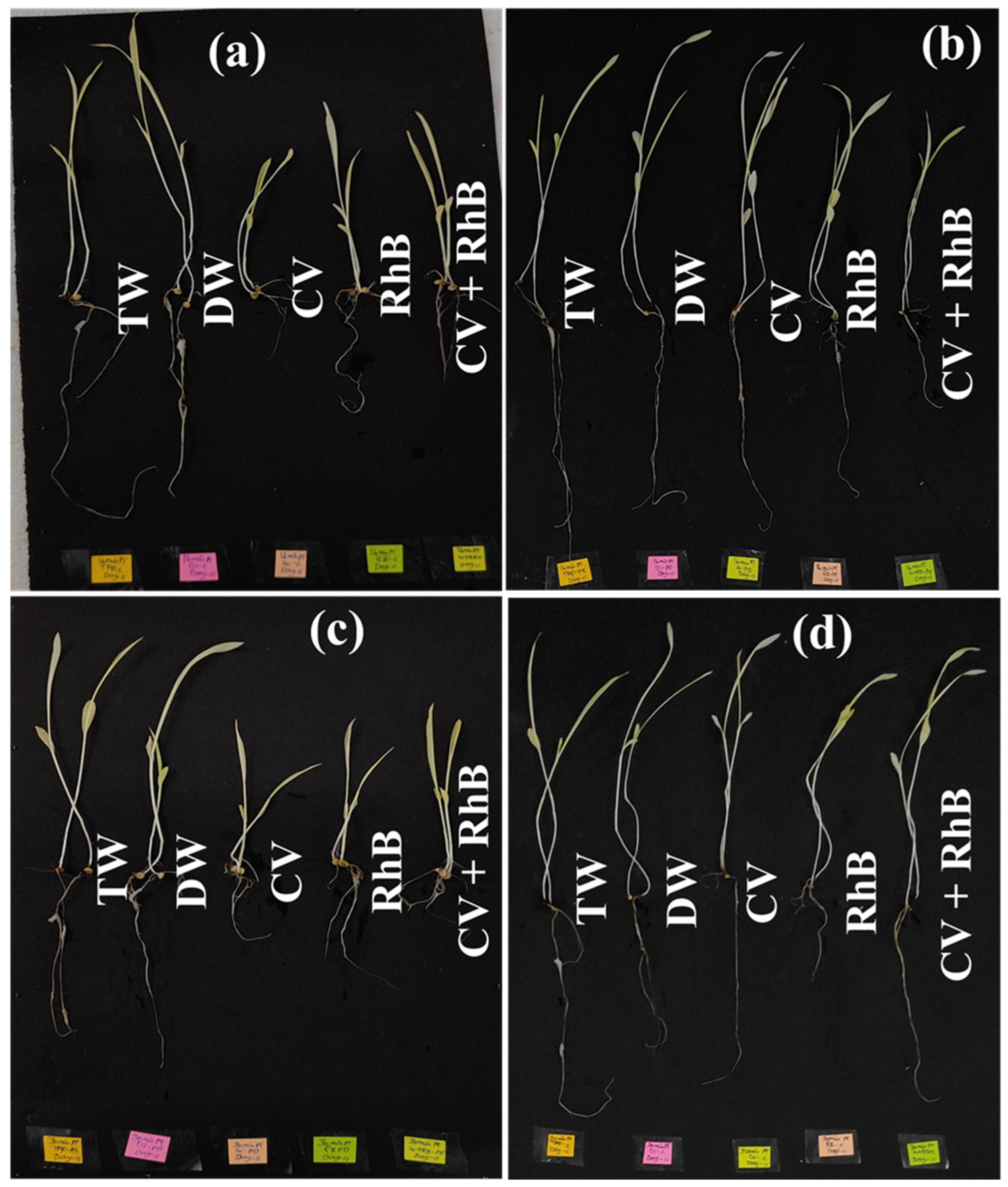
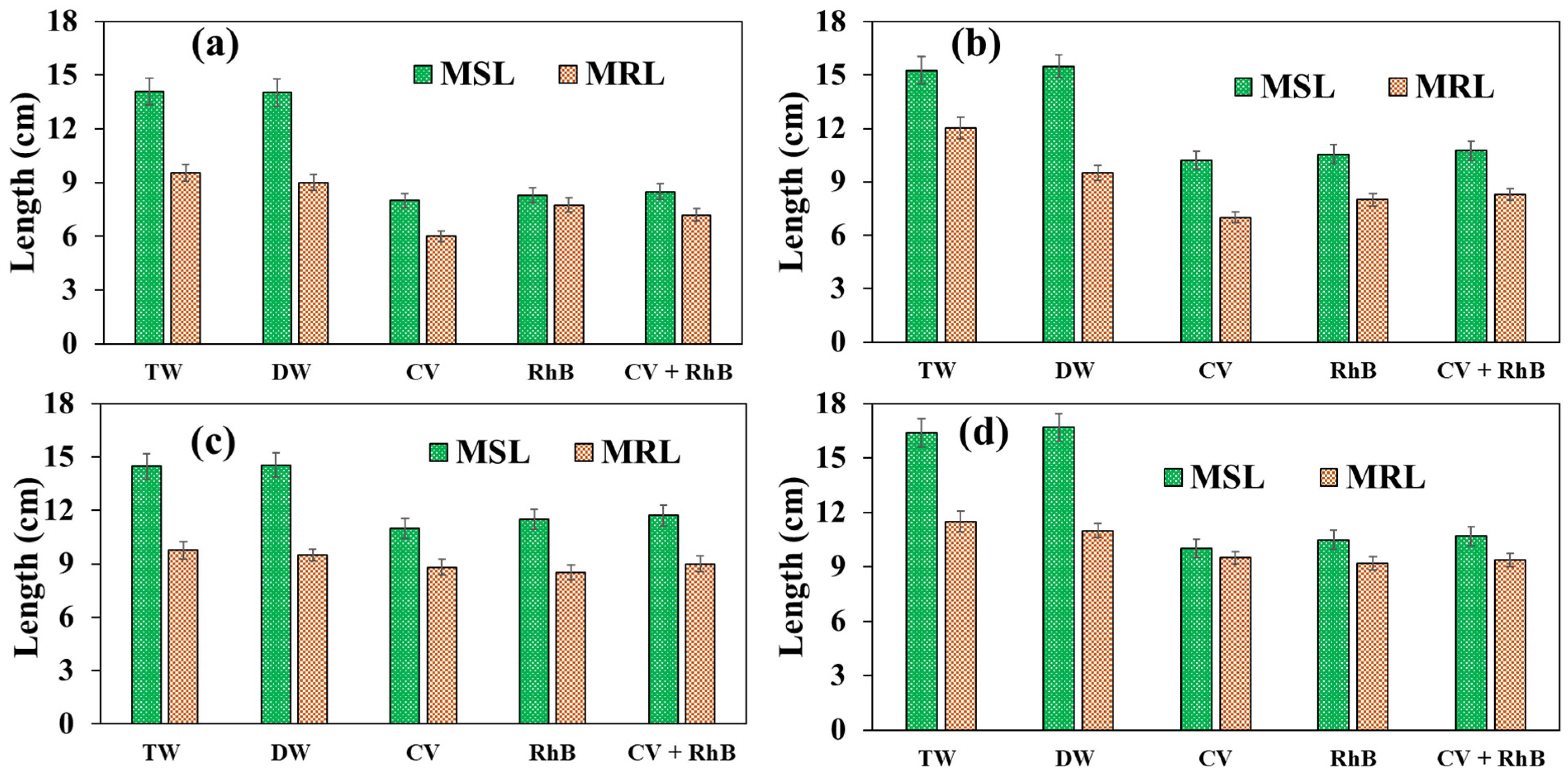
3.5. Seed Germination Using Plasma-Treated Water Contaminated with CV and RhB
3.6. Effect of Plasma-Treated Water Contaminated with CV and RhB on Post-Germination Plant Growth
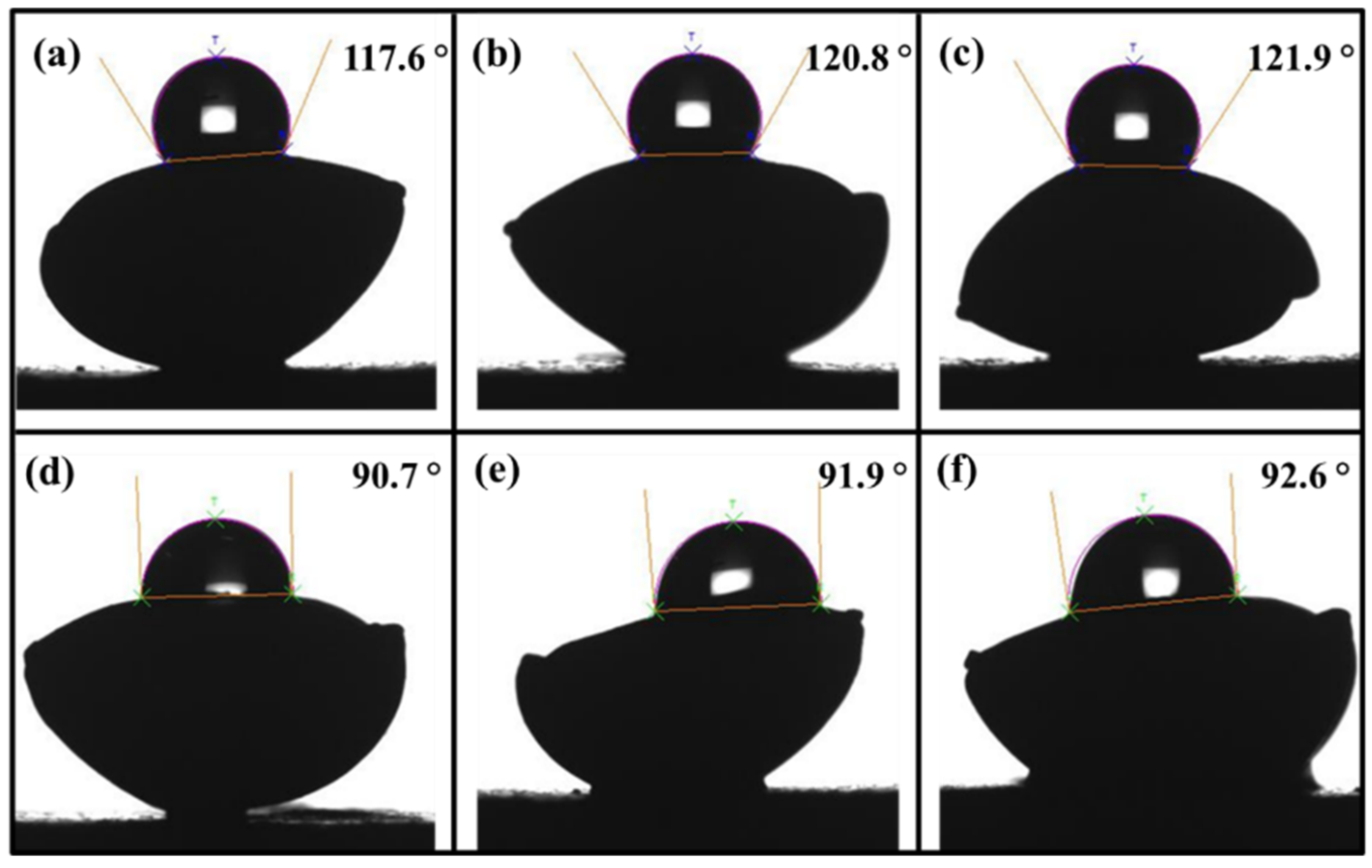
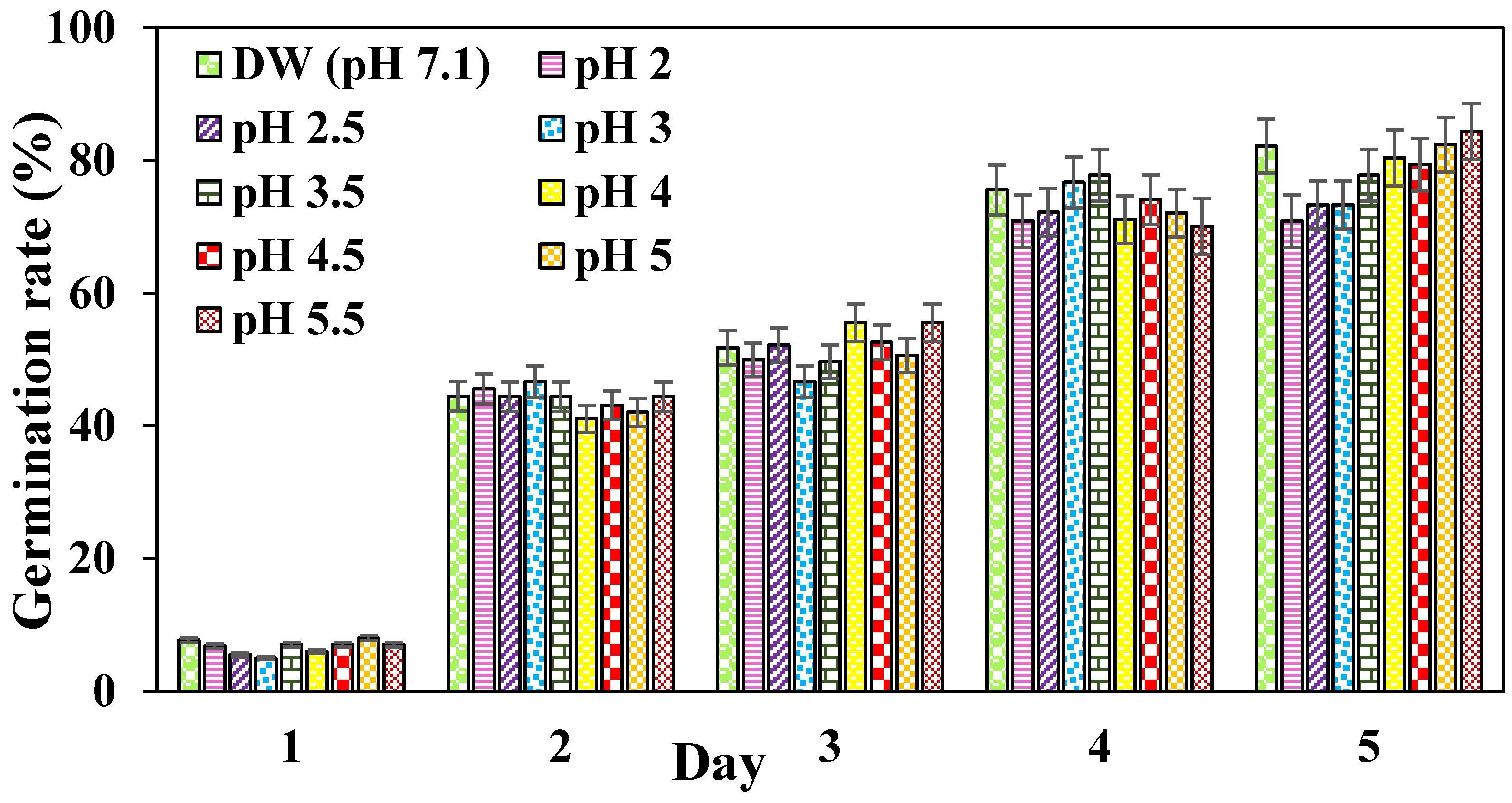
3.7. Effect of pH on Seed Germination and Plant Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhry, F.N.; Malik, M.F. Factors Affecting Water Pollution: A Review. J. Ecosyst. Ecography 2017, 7, 6–8. [Google Scholar] [CrossRef]
- Bilea, F.; Bradu, C.; Cicirma, M.; Medvedovici, A.V.; Magureanu, M. Plasma treatment of sulfamethoxazole contaminated water: Intermediate products, toxicity assessment and potential agricultural reuse. Sci. Total Environ. 2024, 909, 168524. [Google Scholar] [CrossRef]
- Pearce, C.I.; Lloyd, J.R.; Guthrie, J.T. The removal of colour from textile wastewater using whole bacterial cells: A review. Dye. Pigment. 2003, 58, 179–196. [Google Scholar] [CrossRef]
- Affat, S.S. Classifications, Advantages, Disadvantages, Toxicity Effects of Natural and Synthetic Dyes: A review. Univ. Thi-Qar J. Sci. 2021, 8, 130–135. [Google Scholar]
- Slama, H.B.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef]
- Ahmad, A.; Jawaid, M.; Ibrahim, M.N.M.; Yaqoob, A.A.; Alshammari, M.B. (Eds.) Nanohybrid Materials for Treatment of Textiles Dyes; Springer Nature: Singapore, 2023. [Google Scholar] [CrossRef]
- Arora, N.K. Microorganisms for Sustainability Volume 26 Series Editor. Available online: https://link.springer.com/book/10.1007/978-981-15-7455-9 (accessed on 18 January 2021).
- Nawaz, N.; Ali, S.; Shabir, G.; Rizwan, M.; Shakoor, M.B.; Shahid, M.J.; Afzal, M.; Arslan, M.; Hashem, A.; Abd, E.F.; et al. Bacterial augmented floating treatmentwetlands for efficient treatment of synthetic textile dye wastewater. Sustainability 2020, 12, 3731. [Google Scholar] [CrossRef]
- Adams, D. Decolourisation of Selected Dyes by Lettuce and Mung Bean Seedlings: A Potential Phytoremediation Strategy. Master’s Thesis, University of Canterbury, Christchurch, New Zealand, 2019. [Google Scholar]
- Torbati, S. Toxicological risks of Acid Bordeaux B on duckweed and the plant potential for effective remediation of dye-polluted waters. Environ. Sci. Pollut. Res. 2019, 26, 27699–27711. [Google Scholar] [CrossRef]
- Tandon, S.A.; Shaikh, S.; Kumar, R. Development and use of soil bacterial consortia for bioremediation of dye polluted soil and municipal wastewater. Glob. J. Biosci. Biotechnol. 2014, 3, 284–291. [Google Scholar]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A Review on Photocatalysis Used for Wastewater Treatment: Dye Degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Karthika, T.S.; Mansiya, C.; Alagar, M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Vacchi, F.I.; Albuquerque, A.F.; Vendemiatti, J.A.; Morales, D.A.; Ormond, A.B.; Freeman, H.S.; Zocolo, G.J.; Zanoni, M.V.B.; Umbuzeiro, G. Chlorine disinfection of dye wastewater: Implications for a commercial azo dye mixture. Sci. Total Environ. 2013, 442, 302–309. [Google Scholar] [CrossRef]
- Miruka, A.C.; Gao, X.; Cai, L.; Zhang, Y.; Luo, P.; Otieno, G.; Zhang, H.; Song, Z.; Liu, Y. Effects of solution chemistry on dielectric barrier atmospheric non-thermal plasma for operative degradation of antiretroviral drug nevirapine. Sci. Total Environ. 2024, 923, 171369. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.M.K.; Raju, B.R.; Karuppiah, J.; Reddy, E.L.; Subrahmanyam, C. Degradation and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem. Eng. J. 2013, 217, 41–47. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Pepe, G.; Campiglia, P.; Palma, V. Degradation of acid orange 7 azo dye in aqueous solution by a catalytic-assisted. non-thermal plasma process. Catalysts 2020, 10, 888. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.; Liu, Q.; Wu, M. Degradation of azo dye using non-thermal plasma advanced oxidation process in a circulatory airtight reactor system. Chem. Eng. J. 2012, 204–205, 32–39. [Google Scholar] [CrossRef]
- El-Tayeb, A.; El-Shazly, A.H.; Elkady, M.F. Investigation the influence of different salts on the degradation of organic dyes using non-thermal plasma. Energies 2016, 9, 874. [Google Scholar] [CrossRef]
- Joshi, D.; Prakash, G.V.; Ahammad, S.Z.; Kar, S.; Sreekrishnan, T.R. Development of low power non-thermal plasma jet and optimization of operational parameters for treating dyes and emerging contaminants. Plasma Sci. Technol. 2022, 24, 105501. [Google Scholar] [CrossRef]
- Chen, B.; Wang, Y.; Li, S.; Xu, N.; Fu, Y. Environment pollutants removal with non-thermal plasma technology. Int. J. Low-Carbon Technol. 2022, 17, 446–455. [Google Scholar] [CrossRef]
- Deb, U.K.; Bantilan, M.C.S.; Roy, A.D.; Rao, P.P. Global sorghum production scenario. In Sorghum Genetic Enhancement: Research Process, Dissemination and Impacts; International Crops Research Institute for the Semi-Arid Tropics (ICRISAT): Patancheru, India, 2004; Volume 2, pp. 21–38. [Google Scholar]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Mohandoss, S.; Mohan, H.; Balasubramaniyan, N.; Assadi, A.A.; Loganathan, S. Pearl Millet Seed Surface Modification and Improved Germination by Non-thermal Plasma Discharge: Understanding the Role of Reactive Species. Plasma Chem. Plasma Process. 2024, 44, 1031–1051. [Google Scholar] [CrossRef]
- Šerá, B.; Scholtz, V.; Jirešová, J.; Khun, J.; Julák, J.; Šerý, M. Effects of non-thermal plasma treatment on seed germination and early growth of leguminous plants—A review. Plants 2021, 10, 1616. [Google Scholar] [CrossRef]
- Han, B.; Yu, N.N.; Zheng, W.; Zhang, L.N.; Liu, Y.; Yu, J.B.; Zhang, Y.Q.; Park, G.; Sun, H.N.; Kwon, T. Effect of non-thermal plasma (NTP) on common sunflower (Helianthus annus L.) seed growth via upregulation of antioxidant activity and energy metabolism-related gene expression. Plant Growth Regul. 2021, 95, 271–281. [Google Scholar] [CrossRef]
- Meropoulis, S.; Aggelopoulos, C.A. Advancing Nanopulsed Plasma Bubbles for the Degradation of Organic Pollutants in Water: From Lab to Pilot Scale. Technologies 2024, 12, 189. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Privat-Maldonado, A.; Bogaerts, A. Analysis of Short-Lived Reactive Species in Plasma-Air-Water Systems: The Dos and the Do Nots. Anal. Chem. 2018, 90, 13151–13158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.N.; Mohapatro, S.; Dash, R.R. Removal of dyes from aqueous solutions using non-thermal plasma: A review. Int. J. Environ. Sci. Technol. 2024, 21, 7819–7836. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Tijani, J.O.; Badmus, K.O.; Pereao, O.; Babajide, O.; Fatoba, O.O.; Zhang, C.; Shao, T.; Sosnin, E.; Tarasenko, V.; et al. A critical review on ozone and co-species, generation and reaction mechanisms in plasma induced by dielectric barrier discharge technologies for wastewater remediation. J. Environ. Chem. Eng. 2021, 9, 105758. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, T.; Zhou, R.; Zhou, R.; (Ken) Ostikov, K.; Rezaeimotlagh, A.; Cullen, P.J. Plasma bubbles: A route to sustainable chemistry. AAPPS Bull. 2021, 31, 26. [Google Scholar] [CrossRef]
- Fan, H.J.; Huang, S.T.; Chung, W.H.; Jan, J.L.; Lin, W.Y.; Chen, C.C. Degradation pathways of crystal violet by Fenton and Fenton-like systems: Condition optimization and intermediate separation and identification. J. Hazard. Mater. 2009, 171, 1032–1044. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Sun, C.; Wang, L.; Wang, Q. Aqueous photofate of crystal violet under simulated and natural solar irradiation: Kinetics. products, and pathways. Water Res. 2016, 88, 173–183. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Holc, M.; Mozetič, M.; Recek, N.; Primc, G.; Vesel, A.; Zaplotnik, R.; Gselman, P. Wettability increase in plasma-treated agricultural seeds and its relation to germination improvement. Agronomy 2021, 11, 1467. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and Physiological Plant Processes Affected by Seed Treatment with Non-Thermal Plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of Plasma-Seed Treatments as a Potential Seed Processing Technology. Front. Phys. 2021, 9, 617345. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Parshetti, S.G.; Telke, A.A.; Kalyani, D.C.; Doong, R.A.; Govindwar, S.P. Biodegradation of Crystal Violet by Agrobacterium radiobacter. J. Environ. Sci. 2011, 23, 1384–1393. [Google Scholar] [CrossRef]
- Mani, S.; Bharagava, R.N. Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2016; pp. 71–104. [Google Scholar] [CrossRef]
- Sudarshan, S.; Bharti, V.S.; Harikrishnan, S.; Shukla, S.P.; RathiBhuvaneswari, G. Eco-toxicological effect of a commercial dye Rhodamine B on freshwater microalgae Chlorella vulgaris. Arch. Microbiol. 2022, 204, 658. [Google Scholar] [CrossRef]
- Sharma, J.; Singh, H.; Sharma, S.; Kumar, D.; Bhatt, U.; Soni, V. Rhodamine B induced alteration in antioxidant enzymes and photosynthetic performance of Eichhornia crassipes. Plant Physiol. Rep. 2022, 27, 603–617. [Google Scholar] [CrossRef]
- Brisset, J.; Lelievre, J.; Doubla, A.; Amouroux, J.; Brisset, J.L.; Lelièvre, J. Interactions with aqueous solutions of the air corona products Interactions with aqueous solutions of the air corona products (*). Rev. Phys. Appl. 1990, 25, 535–543. [Google Scholar] [CrossRef]
- Kusumandari, K.; Saraswati, T.E.; Prakoso, A.D. The in situ DBD plasma for Remazol dyes-based textile wastewater remediation. Int. J. Environ. Sci. Technol. 2023, 20, 4447–4458. [Google Scholar] [CrossRef]
- Miloh, N.; Kengne, V.K.; Acayanka, E.; Kouotou, P.M.; Kamgang, G.Y. Plasma-assisted Synthesis of Supported Superparamagnetic Oxides for Enhanced Fenton Reactions. Water Air Soil Pollut. 2024, 235, 631. [Google Scholar] [CrossRef]
- Herianto, S.; Arcega, R.D.; Hou, C.Y.; Chao, H.R.; Lee, C.C.; Lin, C.M.; Mahmudiono, T.; Chen, H.L. Chemical decontamination of foods using non-thermal plasma-activated water. Sci. Total Environ. 2023, 874, 162235. [Google Scholar] [CrossRef]
- Zhan, J.; Zhang, A.; Héroux, P.; Li, X.; Li, Z.; Zhao, J.; Guo, Y.; Liu, Y. Gasoline degradation and nitrogen fixation in soil by pulsed corona discharge plasma. Sci. Total Environ. 2019, 661, 266–275. [Google Scholar] [CrossRef]
- Priatama, R.A.; Pervitasari, A.N.; Park, S.; Park, S.J.; Lee, Y.K. Current Advancements in the Molecular Mechanism of Plasma Treatment for Seed Germination and Plant Growth. Int. J. Mol. Sci. 2022, 23, 4609. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sajib, S.A.; Rahi, M.S.; Tahura, S.; Roy, N.C.; Parvez, S.; Reza, M.A.; Talukder, M.R.; Kabir, A.H. Mechanisms and Signaling Associated with LPDBD Plasma Mediated Growth Improvement in Wheat. Sci. Rep. 2018, 8, 10498. [Google Scholar] [CrossRef]
- Hao, T.; Zhu, Q.; Zeng, M.; Shen, J.; Shi, X.; Liu, X.; Zhang, F.; de Vries, W. Quantification of the contribution of nitrogen fertilization and crop harvesting to soil acidification in a wheat-maize double cropping system. Plant Soil 2019, 434, 167–184. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The Roles of Plant Growth Promoting Microbes in Enhancing Plant Tolerance to Acidity and Alkalinity Stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Pham, H.T.T.; Nguyen, A.T.; Do, A.T.N.; Hens, L. Impacts of simulated acid rain on the growth and the yield of soybean (Glycine max (L.) merr.) in the mountains of northern vietnam. Sustainability 2021, 13, 4980. [Google Scholar] [CrossRef]
- Turner, G.D.; Lau, R.R.; Young, D.R. Effect of Acidity on Germination and Seedling Growth of Paulownia tomentosa. J. Appl. Ecol. 1988, 25, 561. [Google Scholar] [CrossRef]


| pH | H+ Ion
(mol/L) | Conductivity (μS/cm) | Total Dissolved Solid (TDS) (ppm) | Salinity (ppm) | |
|---|---|---|---|---|---|
| TW | 7.4 ± 0.2 | 3.9 × 10−8 | 1032 ± 6 | 745 ± 5.5 | 522.6 ± 6.1 |
| TW (16 min PT) | 6.8 ± 0.2 | 1.5 × 10−7 | 1324 ± 7 | 893 ± 7.6 | 644 ± 5.5 |
| TW (30 min PT) | 6.5 ± 0.2 | 3.1 × 10−7 | 1459 ± 4 | 998 ± 4.9 | 711 ± 6.1 |
| DW | 7.1 ± 0.2 | 7.9 × 10−8 | 25 ± 2 | 15.9 ± 2.3 | 22.2 ± 2.3 |
| DW (16 min PT) | 3.3 ± 0.2 | 5.0 × 10−4 | 58 ± 3 | 45.2 ± 2.9 | 33.4 ± 2.4 |
| DW (30 min PT) | 2.6 ± 0.2 | 2.5 × 10−3 | 114 ± 3 | 89.4 ± 4.8 | 75.4 ± 2.0 |
| CV | 5.7 ± 0.2 | 1.9 × 10−6 | 19 ± 3 | 14.6 ± 1.2 | 16.6 ± 1.8 |
| CV (16 min PT) | 3.6 ± 0.2 | 2.4 × 10−4 | 78 ± 6 | 52.5 ± 5.5 | 40 ± 1.7 |
| CV (30 min PT) | 2.8 ± 0.2 | 1.5 × 10−3 | 104 ± 5 | 62.7 ± 4.6 | 54.5 ± 2.7 |
| RhB | 5.7 ± 0.2 | 1.9 × 10−6 | 10 ± 1 | 6.8 ± 1.2 | 16.3 ± 1.6 |
| RhB (16 min PT) | 4.1 ± 0.2 | 7.9 × 10−5 | 57 ± 4 | 40.4 ± 4.7 | 35.1 ± 3.7 |
| RhB (30 min PT) | 3.4 ± 0.2 | 3.9 × 10−4 | 105 ± 5 | 69 ± 5.8 | 50.4 ± 5.3 |
| RhB + CV | 5.4 ± 0.2 | 3.9 × 10−6 | 18 ± 2 | 13.3 ± 1.7 | 16.9 ± 1.6 |
| RhB + CV (16 min PT) | 3.6 ± 0.2 | 2.5 × 10−4 | 70 ± 5 | 51.6 ± 4.4 | 44 ± 3.6 |
| RhB + CV (30 min PT) | 2.8 ± 0.2 | 1.5 × 10−3 | 100 ± 6 | 72.7 ± 4.5 | 54.4 ± 3.1 |
| Plasma Treatment Time | NO2− (mol/L) | NO3− (mol/L) | H2O2 (mol/L) |
|---|---|---|---|
| TW | BDL * | BDL * | BDL * |
| TW (16 min PT) | 6.08 × 10−7 | 2.4 × 10−5 | 9.9 × 10−5 |
| TW (30 min PT) | 3.4 × 10−6 | 3.8 × 10−5 | 1.2 × 10−4 |
| DW | BDL * | BDL * | BDL * |
| DW (16 min PT) | 1.6 × 10−6 | 2.4 × 10−5 | 9.9 × 10−5 |
| DW (30 min PT) | 1.7 × 10−6 | 4.1 × 10−5 | 1.2 × 10−4 |
| CV | BDL * | BDL * | BDL * |
| CV (16 min PT) | 1.6 × 10−6 | 4.1 × 10−5 | 1.23 × 10−4 |
| CV (30 min PT) | 4.0 × 10−6 | 6.6 × 10−5 | 1.9 × 10−4 |
| RhB | BDL * | BDL * | BDL * |
| RhB (16 min PT) | 1.6 × 10−6 | 4.1 × 10−5 | 1.23 × 10−4 |
| RhB (30 min PT) | 2.8 × 10−6 | 4.8 × 10−5 | 1.7 × 10−4 |
| RhB + CV | BDL * | BDL * | BDL * |
| RhB + CV (16 min PT) | 2.0 × 10−6 | 3.7 × 10−5 | 1.76 × 10−4 |
| RhB + CV (30 min PT) | 4.9 × 10−6 | 7.2 × 10−5 | 1.7 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohandoss, S.; Mohan, H.; Balasubramaniyan, N.; Loganathan, S. Non-Thermal Plasma Treatment of Dye-Contaminated Wastewater: A Sustainable Approach for Pollutant Degradation and Enhanced Plant Growth. Plasma 2025, 8, 40. https://doi.org/10.3390/plasma8040040
Mohandoss S, Mohan H, Balasubramaniyan N, Loganathan S. Non-Thermal Plasma Treatment of Dye-Contaminated Wastewater: A Sustainable Approach for Pollutant Degradation and Enhanced Plant Growth. Plasma. 2025; 8(4):40. https://doi.org/10.3390/plasma8040040
Chicago/Turabian StyleMohandoss, Subash, Harshini Mohan, Natarajan Balasubramaniyan, and Sivachandiran Loganathan. 2025. "Non-Thermal Plasma Treatment of Dye-Contaminated Wastewater: A Sustainable Approach for Pollutant Degradation and Enhanced Plant Growth" Plasma 8, no. 4: 40. https://doi.org/10.3390/plasma8040040
APA StyleMohandoss, S., Mohan, H., Balasubramaniyan, N., & Loganathan, S. (2025). Non-Thermal Plasma Treatment of Dye-Contaminated Wastewater: A Sustainable Approach for Pollutant Degradation and Enhanced Plant Growth. Plasma, 8(4), 40. https://doi.org/10.3390/plasma8040040








