Recent Advances in Cold Atmospheric Pressure Plasma for E. coli Decontamination in Food: A Review
Abstract
1. Introduction
- (I)
- (II)
- (III)
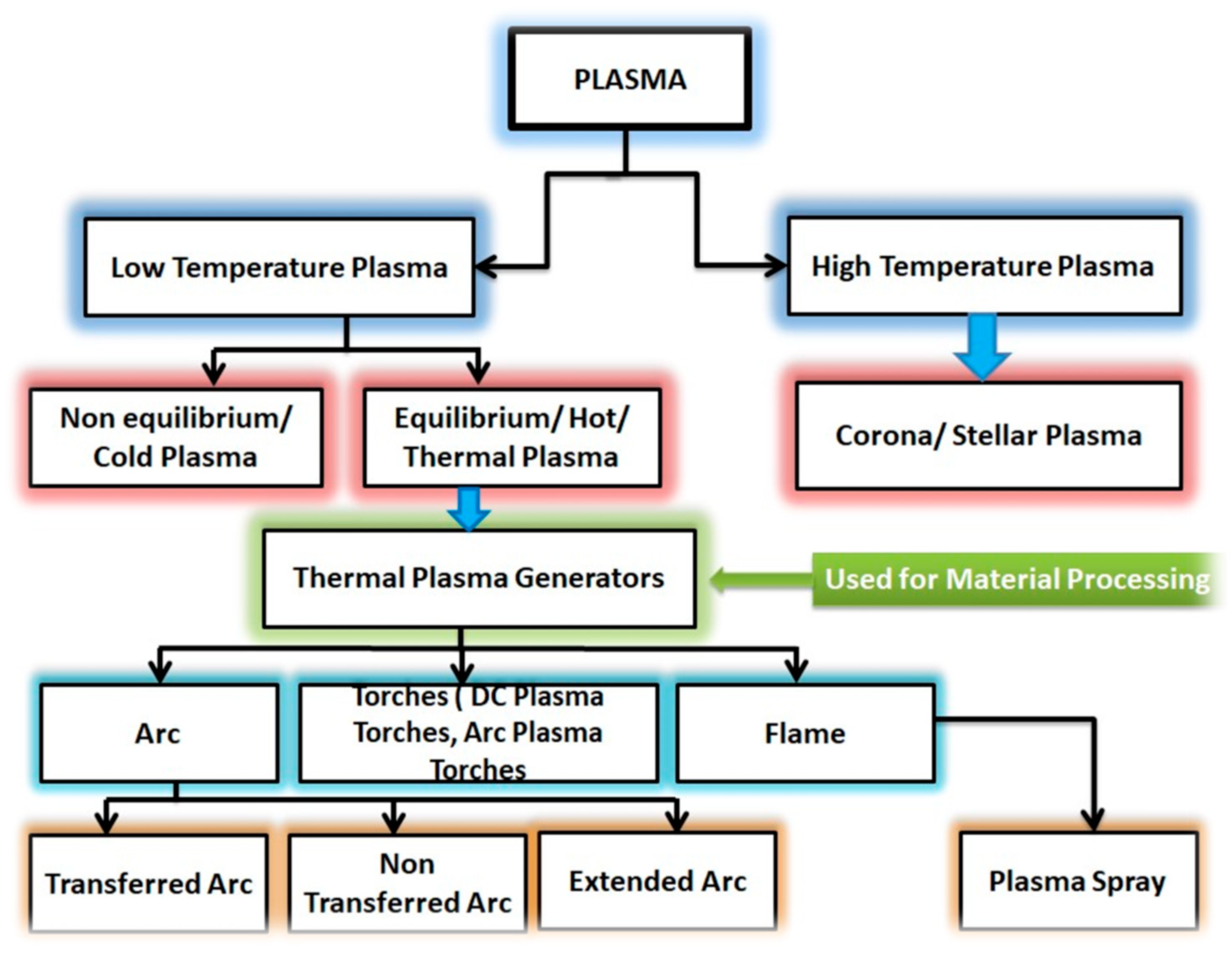
2. Mechanism of CAP in Microbial Inactivation
2.1. Basic Principles of Plasma Science
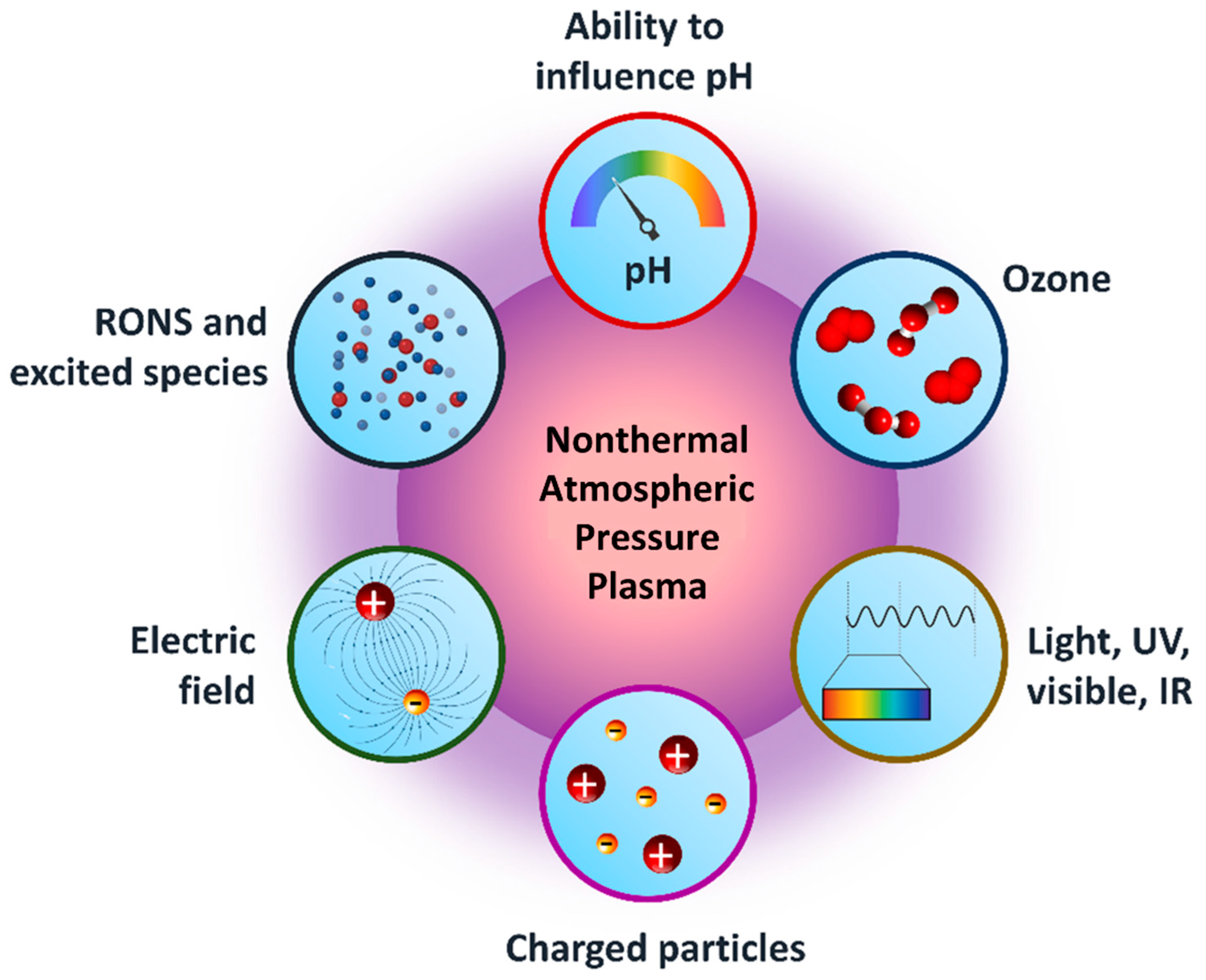
2.2. Reactive Species Generated in CAP
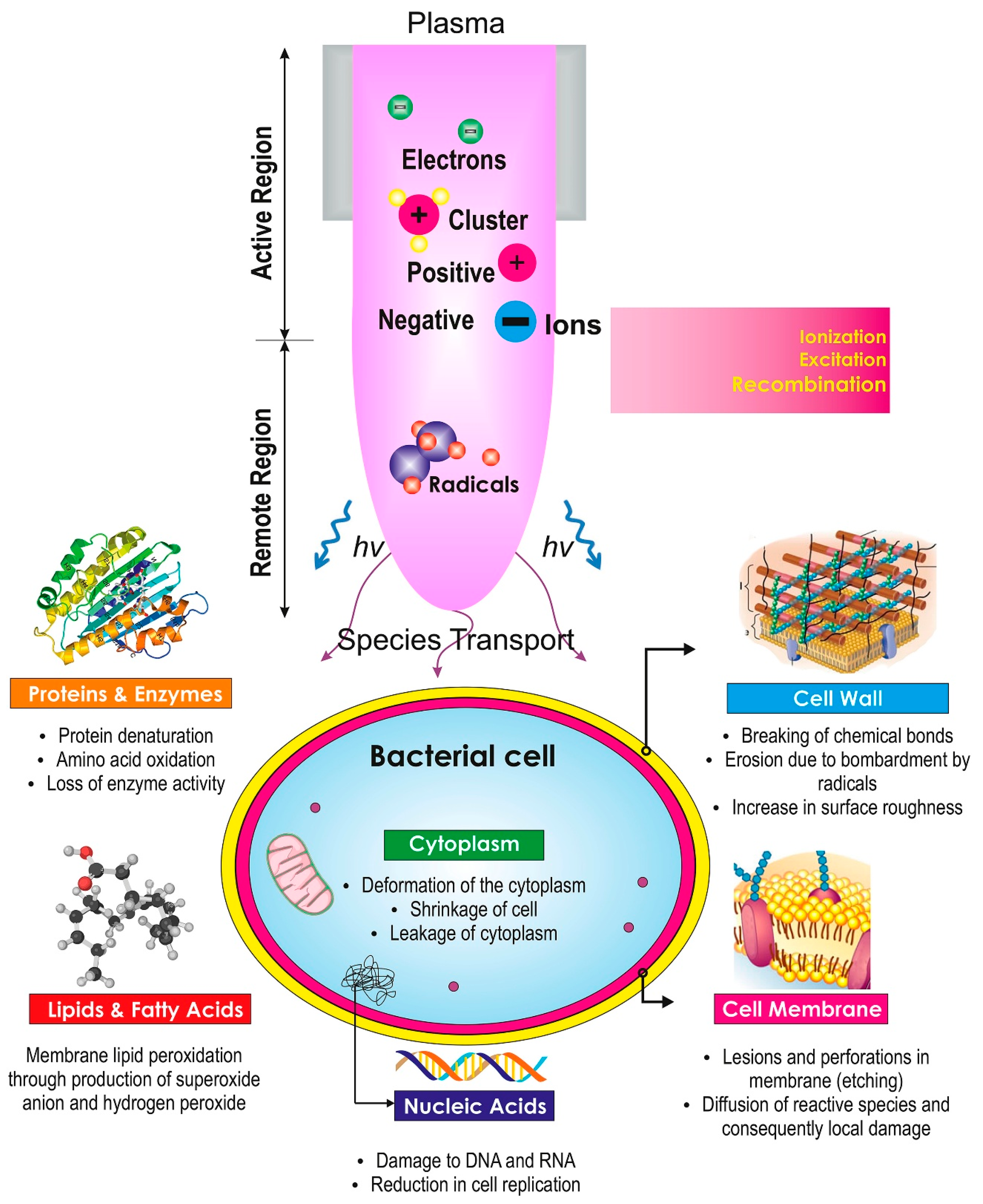
2.3. Mode of Action Against E. coli
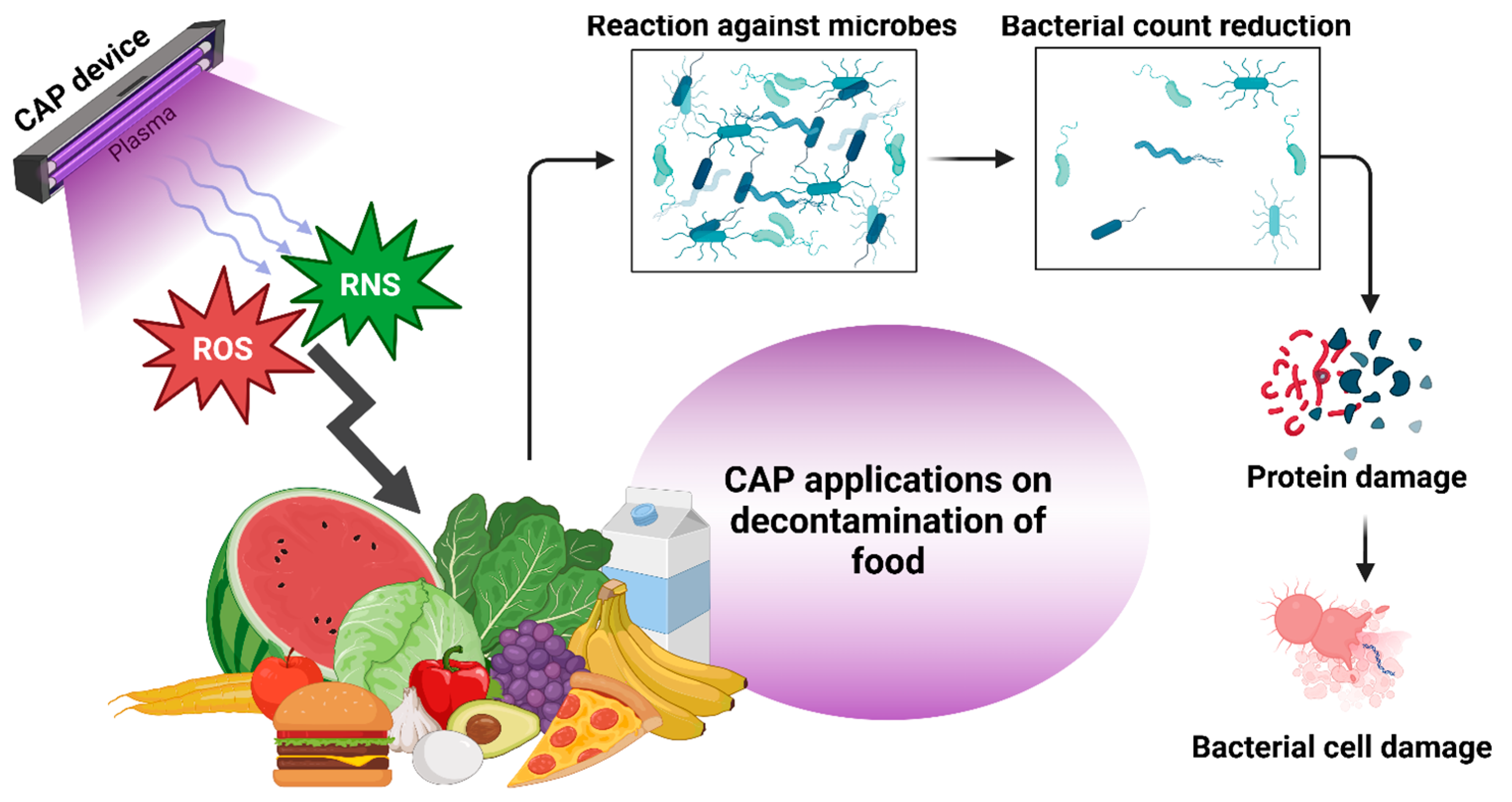
3. E. coli Decontamination Using CAP in Different Food Systems
3.1. Fresh Fruits and Vegetables
3.2. Meat and Poultry
3.3. Dairy Products
3.4. Seafood
3.5. Processed and Ready-to-Eat Foods
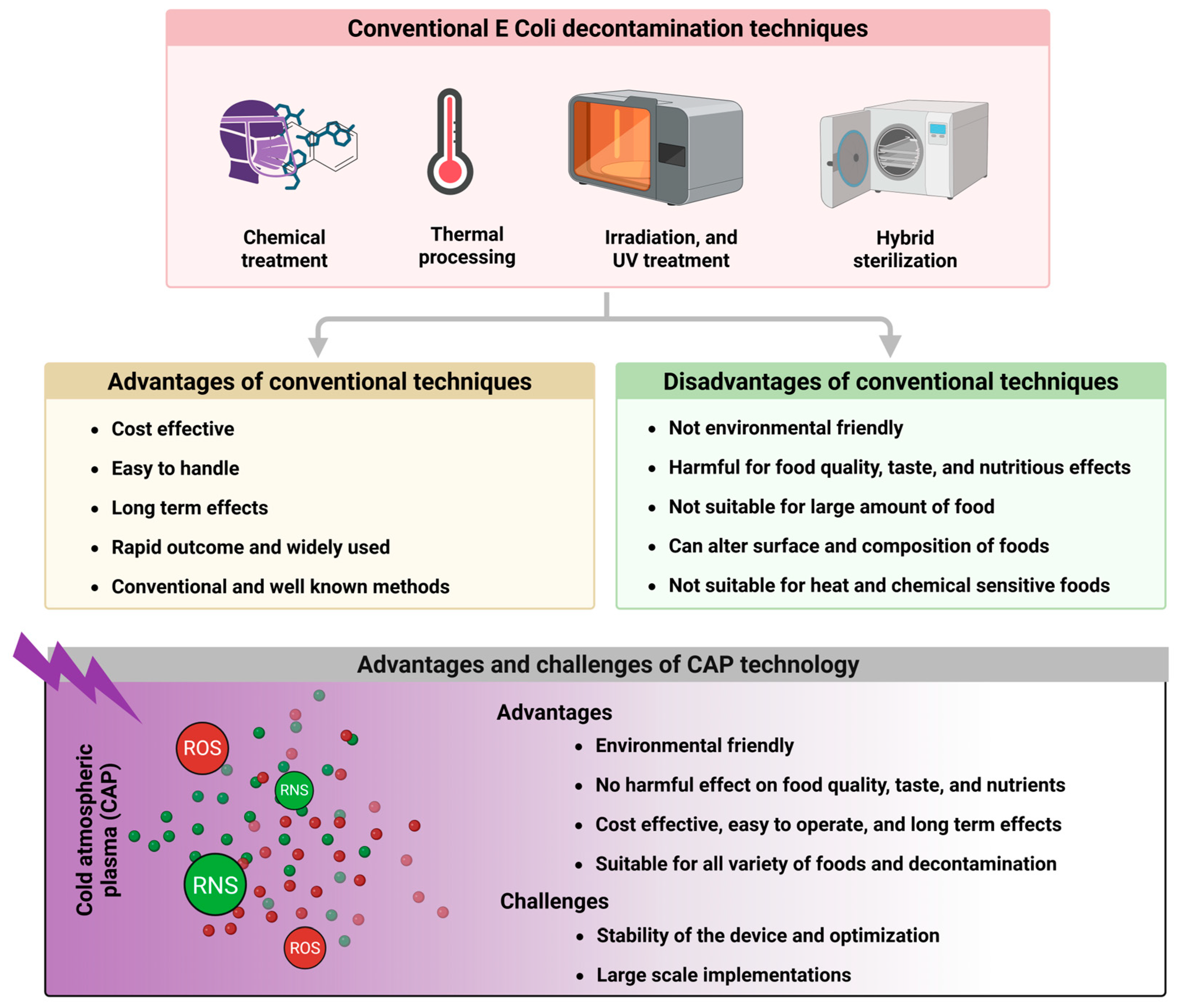
4. Comparison of CAP with Conventional E. coli Decontamination Methods
| No | Food Product | CAP Sources and Parameters | E. coli Inactivation | Ref. |
|---|---|---|---|---|
| 1 | Apple Cider | Atmospheric Cold Plasma (ACP) using simulated air (80% N2 + 20% O2) for 180 s | Significant reduction | [194] |
| 2 | Sour Cherry Juice | Dielectric Barrier Discharge (DBD) plasma with 1% oxygen in argon, 50 kV/cm field intensity, 9-min exposure | 6-log reduction | [195] |
| 3 | Orange Juice | Atmospheric Pressure Plasma Jet (APPJ) treatment | Significant reduction | [46] |
| 4 | Golden Delicious Apples | Atmospheric Cold Plasma using purified air as carrier gas, treatment time not specified | Significant reduction | [196] |
| 5 | Fresh Produce | High-Voltage Atmospheric Cold Plasma (HVACP) treatment | Significant reduction | [197] |
| 6 | Liquid Media | Dielectric Barrier Discharge Atmospheric Cold Plasma (DBD-ACP) generated inside a sealed package; 20 s direct exposure | Complete inactivation (7-log reduction) | [10] |
| 7 | Cherry Tomatoes, strawberries | Dielectric Barrier Discharge Atmospheric Cold Plasma (DBD-ACP) at 70 kV RMS for 120–300 s | Reduction to undetectable levels from initial 6.3 log10 CFU/sample | [198] |
| 8 | Chicken Fillets | Dielectric Barrier Discharge Atmospheric Cold Plasma (DBD-ACP) treatment voltage and time varied | Significant reduction | [199] |
| 9 | Fresh Produce | Atmospheric Cold Plasma (ACP) treatment parameters varied | Significant reduction | [200] |
| 10 | Meat and Meat Products | Dielectric Barrier Discharge Cold Atmospheric Plasma (DBD-CAP); parameters varied | Significant reduction | [201] |
| 11 | Grape Tomato, Spinach, and Cantaloupe | Cold Plasma-Activated Hydrogen Peroxide Aerosol; parameters varied | Significant reduction | [202] |
| 12 | Roma Tomatoes | X-Ray Radiation; parameters varied | Significant reduction | [203] |
| 13 | Fresh Produce | Atmospheric Cold Plasma (ACP) treatment parameters varied | Significant reduction | [204] |
| 14 | FFP3 Face Masks | Surface Micro-Discharge (SMD) plasma device; nitrogen mode at 12 kVpp, 5 kHz; 1-min exposure | 5-log reduction | [205] |
| 15 | Liquid Media | Dielectric Barrier Discharge Atmospheric Cold Plasma (DBD-ACP) inside a sealed package; 20 s direct exposure | Complete inactivation (7-log reduction) | [10] |
| 16 | Cherry Tomatoes | Dielectric Barrier Discharge Atmospheric Cold Plasma (DBD-ACP) at 70 kV RMS for 120 s | Reduction to undetectable levels from initial 6.3 log10 CFU/sample | [198] |
4.1. Chemical Treatments
- (i)
- After chemical treatment, some chemical residues remain in foods longer, causing serious health risks for consumers.
- (ii)
- Such chemical treatments are not long-lasting and have antibacterial effects for a limited time.
- (iii)
- Some food products, including raw food, ready-to-eat foods, and fruits, have limited penetration ability; therefore, these chemicals can only treat upper surfaces, leaving bactericidal effects inside foods.
4.2. Thermal Processing
- (i)
- Some foods are temperature-sensitive and can lose their nutritional quality, taste, effects of ingredients, and necessary vitamins at high temperatures. For example, boiling water can disturb water hardness, green vegetables may lose iron, and the taste of fruits may also change.
- (ii)
- Depending on the mass volume of foods, the heating process can not completely and permanently eliminate biofilms.
4.3. Irradiation and UV Treatment
- (i)
- Irradiations can alter food taste and biological composition, altering food flavor and producing undesirable effects, especially when preserved for a long time.
- (ii)
- Some radiations are restricted to use, like nuclear radiations, and even many radiations have consumers’ perceptions of radiated foods, limiting widespread adoption.
- (iii)
- Due to its lower penetration depth and intensity challenges than foods’ penetration depth, it is less effective against embedded bacteria in food matrices and multilayer biofilms.
4.4. Potential Synergies with CAP
5. Challenges and Future Prospects of CAP in Food Decontamination
5.1. Current Limitations of CAP
- (i)
- The transition of CAP research from laboratory studies to large-scale industrial applications faces significant challenges, particularly in optimizing key parameters such as inactivation efficiency, power consumption, processing costs, and reactor installation expenses. To facilitate this transition, further research is needed on the design, development, scalability, and throughput of optimized processing conditions, ensuring the successful implementation of CAP technology at an industrial level [39].
- (ii)
- CAP encompasses various types, including arc plasma, spark discharge, and corona discharge, each generating RONS, electric fields, UV radiation, electron energies, ion energies, and electron and ion densities at different intensities. These variations can influence food surfaces differently, sometimes altering color, texture, composition, and sensory attributes. Developing and optimizing CAP sources tailored to specific food categories based on properties such as heat sensitivity, texture stability, and nutritional constraints is essential to address these challenges. In summary, selecting an appropriate CAP source for a given application is crucial to ensuring effective and controlled food processing [223].
- (iii)
- In many cases, it is reported that CAP-generated RONS are short-lived, and there is a chance of biofilm regrowth if processed food is not adequately protected from environment-borne pathogens. Therefore, innovative multidisciplinary approaches and advanced control mechanisms are essential for effectively generating high-potency plasma species capable of permanently eliminating E. coli biofilms [224].
- (iv)
- The most challenging issue in the CAP treatment of foods is operational cost. Since CAP requires gases, reactor development, typical electrode assembly, and input energy, it is less economically friendly. Profound innovation and optimization of the reactors that consume low energy while processing foods using CAP are required [116].
5.2. Emerging Innovations
- (i)
- A hybrid plasma system could be the best approach for completely sterilizing biofilms. Combining CAP with ozone treatments and using the mists of chemicals like hydrogen peroxide can be more effective [225].
- (ii)
- CAP-induced nanoparticles can act as medical probes for sterilizing foods from biofilms by transferring CAP effects deep inside the foods. Such nanoparticles should be extracted from foods, plants, and other human consumer items to avoid harmful side effects [225].
- (iii)
- The challenging issue can be solved by developing automated and adaptive plasma devices, in which advanced robotics and artificial intelligence should be used to develop smart and portable plasma systems. Such techniques are convenient for transforming laboratory-scale work to the industrial level [226].
- (iv)
- Washing foods to overcome microbial effects like E. coli can be useful, and plasma-activated waters (PALs) can play a vital role in this purpose, especially for sterilizing ready-to-eat foods [227].
- (v)
- The design and development of continuous plasma-generating reactors capable of large-scale food processing are essential for enhancing the feasibility of CAP treatments in the food processing industry. These advancements would enable efficient and uninterrupted plasma-based food treatment, ensuring greater practicality and scalability in industrial applications [1,228].
5.3. Regulatory and Consumer Acceptance Issues
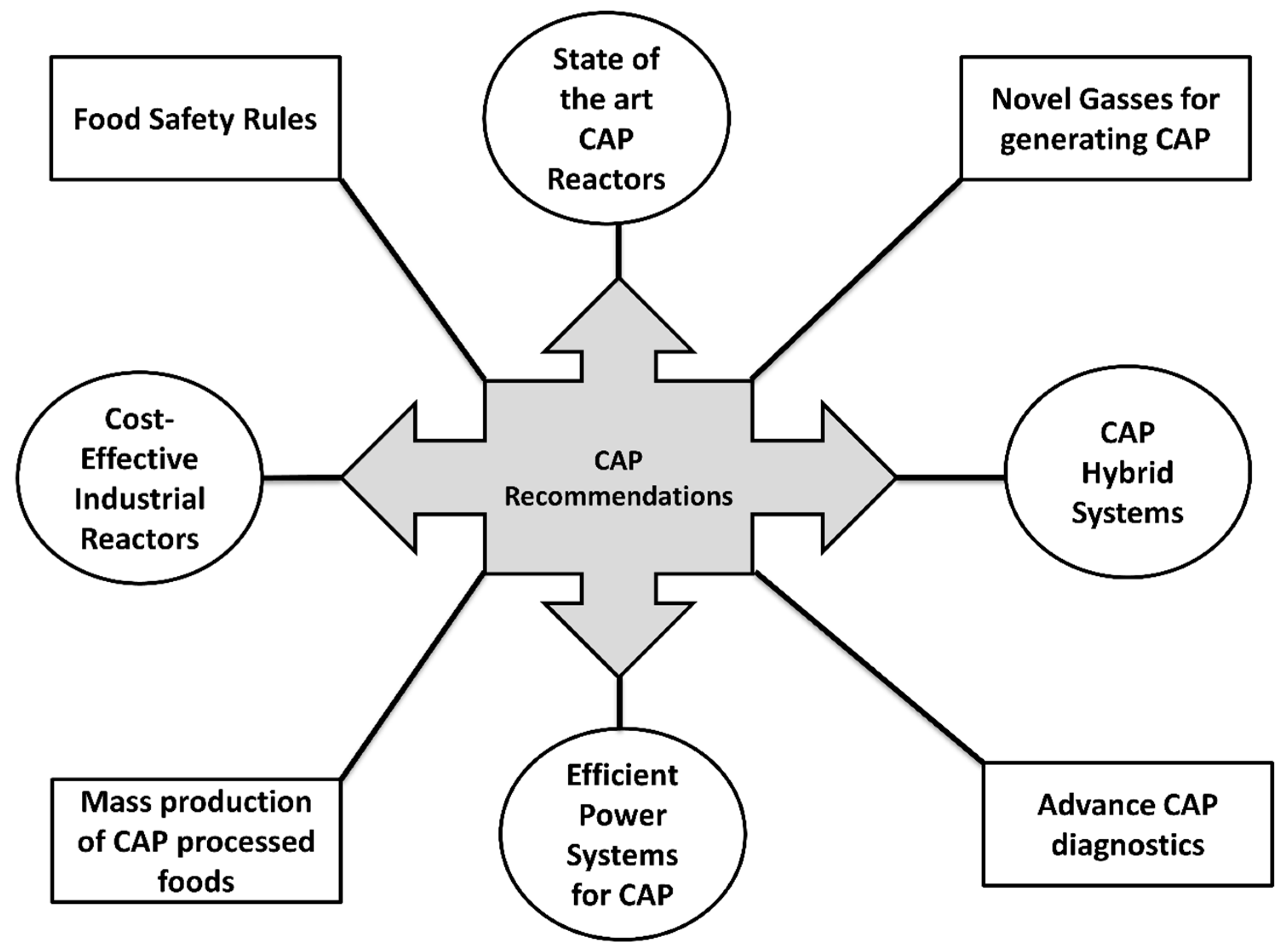
6. Future Recommendations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAP | Cold atmospheric pressure plasma |
| E. coli | Escherichia coli |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| DBD | Dielectric barrier discharge |
| UV | Ultraviolet |
| CFU | Colony-forming unit |
| EHEC | Enterohemorrhagic Escherichia coli |
| ETEC | Enterotoxigenic Escherichia coli |
| EPEC | Enteropathogenic Escherichia coli |
| EAEC | Enteroaggregative Escherichia coli |
| EIEC | Enteroinvasive Escherichia coli |
| STEC | Shiga toxin-producing Escherichia coli |
| FDA | Food and Drug Administration |
| PALs | Plasma-activated liquids |
| LTP | Low-temperature plasma |
| HTP | High-temperature plasma |
| HUS | Hemolytic uremic syndrome |
| RONS | Reactive oxygen and nitrogen species |
References
- Niemira, B.A. Cold plasma decontamination of foods. Annu. Rev. Food Sci. Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal Plasma Inactivation of Food-Borne Pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef]
- Likotrafiti, E.; Rhoades, J. Chapter 32—Probiotics, Prebiotics, Synbiotics, and Foodborne Illness. In Probiotics, Prebiotics, and Synbiotics; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 469–476. [Google Scholar] [CrossRef]
- Fung, F.; Wang, H.-S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S. Cold plasma technology for sustainable food production: Meeting the United Nations sustainable development goals. Sustain. Food Technol. 2025, 3, 32–53. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljević, V.; O’Donnell, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Venkitanarayanan, K.S.; Ezeike, G.O.; Hung, Y.C.; Doyle, M.P. Efficacy of electrolyzed oxidizing water for inactivating Escherichia coli O157:H7, Salmonella enteritidis, and Listeria monocytogenes. Appl. Environ. Microbiol. 1999, 65, 4276–4279. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Gibson, K.E. Irrigation water and contamination of fresh produce with bacterial foodborne pathogens. Curr. Opin. Food Sci. 2022, 47, 100889. [Google Scholar] [CrossRef]
- Niveditha, A.; Pandiselvam, R.; Prasath, V.A.; Singh, S.K.; Gul, K.; Kothakota, A. Application of cold plasma and ozone technology for decontamination of Escherichia coli in foods—A review. Food Control 2021, 130, 108338. [Google Scholar] [CrossRef]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.M.; Bourke, P. Atmospheric cold plasma inactivation of Escherichia coli in liquid media inside a sealed package. J. Appl. Microbiol. 2013, 114, 778–787. [Google Scholar] [CrossRef]
- Umair, M.; Xun, S.; Jabbar, S.; Abid, M.; Rajoka, M.S.R.; Zhendan, H. Recent breakthroughs of non-thermal cold plasma food processing: A review. Carbohydr. Polym. Technol. Appl. 2025, 9, 100673. [Google Scholar] [CrossRef]
- Luo, H.; Liang, D.; Liu, Q.; Niu, L.; Temirlan, K.; Li, W. Electron beam irradiation combined with cold plasma modification of chitosan to enhance physicochemical and functional properties. Carbohydr. Polym. 2025, 354, 123308. [Google Scholar] [CrossRef] [PubMed]
- Saedi, Z.; Panchal, D.; Lu, Q.; Kuddushi, M.; Pour, S.E.; Zhang, X. Integrating multiple cold plasma generators and Bernoulli-driven microbubble formation for large-volume water treatment. Sustain. Mater. Technol. 2025, 44, e01300. [Google Scholar] [CrossRef]
- Krewing, M.; Weisgerber, K.M.; Dirks, T.; Bobkov, I.; Schubert, B.; Bandow, J.E. Iron-sulfur cluster proteins present the weak spot in non-thermal plasma-treated Escherichia coli. Redox Biol. 2025, 81, 103562. [Google Scholar] [CrossRef]
- Tadesse, D.A.; Zhao, S.; Tong, E.; Ayers, S.; Singh, A.; Bartholomew, M.J.; McDermott, P.F. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950–2002. Emerg. Infect. Dis. 2012, 18, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Gregova, G.; Kmet, V. Antibiotic resistance and virulence of Escherichia coli strains isolated from animal rendering plant. Sci. Rep. 2020, 10, 17108. [Google Scholar] [CrossRef]
- Christodoulou, M.K. Comparison of Antimicrobial Resistance in Escherichia coli Strains Isolated From Swine, Poultry, and Farm Workers in the Respective Livestock Farming Units in Greece. Cureus 2023, 15, e51073. [Google Scholar] [CrossRef]
- Sevenich, R.; Bark, F.; Crews, C.; Anderson, W.; Pye, C.; Riddellova, K.; Hradecky, J.; Moravcova, E.; Reineke, K.; Knorr, D. Effect of high pressure thermal sterilization on the formation of food processing contaminants. Innov. Food Sci. Emerg. Technol. 2013, 20, 42–50. [Google Scholar] [CrossRef]
- Bharti, B.; Li, H.; Ren, Z.; Zhu, R.; Zhu, Z. Recent advances in sterilization and disinfection technology: A review. Chemosphere 2022, 308, 136404. [Google Scholar] [CrossRef]
- Li, X.; Farid, M. A review on recent development in non-conventional food sterilization technologies. J. Food Eng. 2016, 182, 33–45. [Google Scholar] [CrossRef]
- Shahi, S.; Khorvash, R.; Goli, M.; Ranjbaran, S.M.; Najarian, A.; Mohammadi Nafchi, A. Review of proposed different irradiation methods to inactivate food-processing viruses and microorganisms. Food Sci. Nutr. 2021, 9, 5883–5896. [Google Scholar] [CrossRef]
- Udachin, V.; Dahle, S.; Fink, R. Dielectric Barrier Discharge Air Plasma as a Comprehensive Disinfection Approach to E. Coli and S. Aureus Biofilm Management. Phys. Scr. 2025, 100, 25015. [Google Scholar] [CrossRef]
- Gökmen, V. Introduction: Potential safety risks associated with thermal processing of foods. In Acrylamide in Food; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Madani, A.; Esfandiari, Z.; Shoaei, P.; Ataei, B. Evaluation of Virulence Factors, Antibiotic Resistance, and Biofilm Formation of Escherichia coli Isolated from Milk and Dairy Products in Isfahan, Iran. Foods 2022, 11, 960. [Google Scholar] [CrossRef] [PubMed]
- Melini, F.; Melini, V.; Luziatelli, F.; Ruzzi, M. Raw and Heat-Treated Milk: From Public Health Risks to Nutritional Quality. Beverages 2017, 3, 54. [Google Scholar] [CrossRef]
- Du, R.; Yang, X.; Cheng, J.-H. Inhibition regulatory mechanism in Staphylococcus aureus under cold plasma oxidative stress. Food Biosci. 2025, 65, 106040. [Google Scholar] [CrossRef]
- Tumuluri, T.; Inanoglu, S.; Schaffner, D.W.; Karwe, M. V Effect of surface roughness on the efficacy of Plasma Activated Mist (PAM) for inactivation of Listeria innocua and Klebsiella michiganensis. Int. J. Food Microbiol. 2025, 431, 111070. [Google Scholar] [CrossRef]
- Yan, B.; Jian, L.; Qi-Chun, L.; Yanyan, H.; Shi-Lin, C.; Lang-Hong, W.; and Zeng, X.-A. From Laboratory to Industry: The Evolution and Impact of Pulsed Electric Field Technology in Food Processing. Food Rev. Int. 2025, 41, 373–398. [Google Scholar] [CrossRef]
- Rød, S.K.; Hansen, F.; Leipold, F.; Knøchel, S. Cold atmospheric pressure plasma treatment of ready-to-eat meat: Inactivation of Listeria innocua and changes in product quality. Food Microbiol. 2012, 30, 233–238. [Google Scholar] [CrossRef]
- Kim, B.; Yun, H.; Jung, S.; Jung, Y.; Jung, H.; Choe, W.; Jo, C. Effect of atmospheric pressure plasma on inactivation of pathogens inoculated onto bacon using two different gas compositions. Food Microbiol. 2011, 28, 9–13. [Google Scholar] [CrossRef]
- Fernández, A.; Noriega, E.; Thompson, A. Inactivation of Salmonella enterica serovar Typhimurium on fresh produce by cold atmospheric gas plasma technology. Food Microbiol. 2013, 33, 24–29. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Munir, M.T.; Federighi, M. Control of Foodborne Biological Hazards by Ionizing Radiations. Foods 2020, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Vyas, H.K.N.; Hoque, M.M.; Xia, B.; Alam, D.; Cullen, P.J.; Rice, S.A.; Mai-Prochnow, A. Transcriptional Signatures Associated with the Survival of Escherichia coli Biofilm During Treatment with Plasma-Activated Water. Biofilm 2025, 9, 100266. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef] [PubMed]
- Niemira, B.A. Cold plasma reduction of Salmonella and Escherichia coli O157:H7 on almonds using ambient pressure gases. J. Food Sci. 2012, 77, M171–M175. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Wan, Z.; Colonna, W.; Keener, K.M. Effect of high voltage atmospheric cold plasma on white grape juice quality. J. Sci. Food Agric. 2017, 97, 4016–4021. [Google Scholar] [CrossRef]
- Yawut, N.; Mekwilai, T.; Vichiansan, N.; Braspaiboon, S.; Leksakul, K.; Boonyawan, D. Cold plasma technology: Transforming food processing for safety and sustainability. J. Agric. Food Res. 2024, 18, 101383. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A Review on Non-thermal Atmospheric Plasma for Food Preservation: Mode of Action, Determinants of Effectiveness, and Applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef]
- Ding, C.; Sun, Z.; Wang, Y.; Zhou, H.; Huang, Q.; Song, W. Effect of Flexible Plasma Pad on HaCaT Cells and Bacteria. Plasma Process. Polym. 2025, e2400244. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef]
- Samukawa, S.; Hori, M.; Rauf, S.; Tachibana, K.; Bruggeman, P.; Kroesen, G.; Whitehead, J.C.; Murphy, A.B.; Gutsol, A.F.; Starikovskaia, S.; et al. The 2012 Plasma Roadmap. J. Phys. D Appl. Phys. 2012, 45, 253001. [Google Scholar] [CrossRef]
- Adamovich, I.; Agarwal, S.; Ahedo, E.; Alves, L.L.; Baalrud, S.; Babaeva, N.; Bogaerts, A.; Bourdon, A.; Bruggeman, P.J.; Canal, C.; et al. The 2022 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2022, 55, 373001. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S.; Han, I.; Choi, E.H. Unveiling the Therapeutic Potential of Soft Plasma Jet and Nitric-Oxide Enriched Plasma-Activated Water (NO-PAW) on Oral Cancer YD-10B Cells: A Comprehensive Investigation of Direct and Indirect Treatments. Plasma Chem. Plasma Process. 2025, 45, 725–752. [Google Scholar] [CrossRef]
- Dasan, B.; Boyacı, I. Effect of Cold Atmospheric Plasma on Inactivation of Escherichia coli and Physicochemical Properties of Apple, Orange, Tomato Juices, and Sour Cherry Nectar. Food Bioprocess Technol. 2018, 11, 334–343. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Q.; Yang, Y.; Zhang, J.; Han, R.; Wang, J. Research Progress and Future Trends of Low Temperature Plasma Application in Food Industry: A Review. Molecules 2023, 28, 4714. [Google Scholar] [CrossRef]
- Bogaerts, A.; Tu, X.; Whitehead, J.C.; Centi, G.; Lefferts, L.; Guaitella, O.; Azzolina-Jury, F.; Kim, H.-H.; Murphy, A.B.; Schneider, W.F.; et al. The 2020 plasma catalysis roadmap. J. Phys. D Appl. Phys. 2020, 53, 443001. [Google Scholar] [CrossRef]
- Shabbir, A.; Hassan, S.A.; Hanif, H.; Rauf, R.; Muntaha, S.T.; Jubbar, M.; Aadil, R.M. Applications of cold plasma technique to enhance the safety and quality of different food products. Meas. Food 2024, 15, 100183. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.E.; Chung, M.-S.; Min, S.C. Cold plasma treatment for the microbiological safety of cabbage, lettuce, and dried figs. Food Microbiol. 2015, 51, 74–80. [Google Scholar] [CrossRef]
- Noriega, E.; Shama, G.; Laca, A.; Díaz, M.; Kong, M.G. Cold atmospheric gas plasma disinfection of chicken meat and chicken skin contaminated with Listeria innocua. Food Microbiol. 2011, 28, 1293–1300. [Google Scholar] [CrossRef]
- Ravash, N.; Hesari, J.; Khorram, S.; Roopesh, M.S. Application of atmospheric cold plasma (ACP) for processing of raw bovine colostrum: Investigation of antimicrobial efficacy of different ACP treatments. Int. Dairy J. 2025, 161, 106140. [Google Scholar] [CrossRef]
- Ranjbar, S.; Shahmansouri, M.; Attri, P.; Bogaerts, A. Effect of plasma-induced oxidative stress on the glycolysis pathway of Escherichia coli. Comput. Biol. Med. 2020, 127, 104064. [Google Scholar] [CrossRef]
- Xie, M.; Koch, E.H.W.; van Walree, C.A.; Sobota, A.; Sonnen, A.F.P.; Killian, J.A.; Breukink, E.; Lorent, J.H. Synergistic effects of oxidative and acid stress on bacterial membranes of Escherichia coli and Staphylococcus simulans. Commun. Biol. 2024, 7, 1161. [Google Scholar] [CrossRef]
- Marcén, M.; Ruiz, V.; Serrano, M.J.; Condón, S.; Mañas, P. Oxidative stress in E. coli cells upon exposure to heat treatments. Int. J. Food Microbiol. 2017, 241, 198–205. [Google Scholar] [CrossRef]
- Gruber, C.C.; Babu, V.M.P.; Livingston, K.; Joisher, H.; Walker, G.C. Degradation of the Escherichia coli Essential Proteins DapB and Dxr Results in Oxidative Stress, which Contributes to Lethality through Incomplete Base Excision Repair. MBio 2022, 13, e03756-21. [Google Scholar] [CrossRef] [PubMed]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Taccogna, F.; Dilecce, G. Non-equilibrium in low-temperature plasmas. Eur. Phys. J. D 2016, 70, 251. [Google Scholar] [CrossRef]
- Olanbiwoninu, A.A.; Popoola, B.M. Biofilms and their impact on the food industry. Saudi J. Biol. Sci. 2023, 30, 103523. [Google Scholar] [CrossRef]
- Elafify, M.; Liao, X.; Feng, J.; Ahn, J.; Ding, T. Biofilm formation in food industries: Challenges and control strategies for food safety. Food Res. Int. 2024, 190, 114650. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Ababneh, A.M.; Al-Holy, M.; Al-Nabulsi, A.; Osaili, T.; Abughoush, M.; Ayyash, M.; Holley, R.A. A Review of Bacterial Biofilm Components and Formation, Detection Methods, and Their Prevention and Control on Food Contact Surfaces. Microbiol. Res. 2024, 15, 1973–1992. [Google Scholar] [CrossRef]
- Hosseini, H.; Mousavi, M. Chapter 7—Cold plasma technology: A novel technology for shrimp preservation. In Postharvest Technologies and Quality Control of Shrimp; Nirmal, N.P., Benjakul, S., G.B.T.-P.T., Bono, Q.C.O.S., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 203–219. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Segat, A.; Ishikawa, K. Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci. Technol. 2016, 55, 39–47. [Google Scholar] [CrossRef]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Keidar, M.; Shashurin, A.; Volotskova, O.; Stepp, M.A.; Srinivasan, P. Cold Atmospheric Plasma in Cancer Therapy Cold atmospheric plasma in cancer therapy. Phys. Plasmas 2013, 20, 057101. [Google Scholar] [CrossRef]
- Science, P.S.; Sunka, P.; Babicky, V.; Lukes, P.; Simek, M. Generation of chemically active species by electrical discharges in water. Plasma Sources Sci. Technol. 1999, 8, 258. [Google Scholar] [CrossRef]
- Čobanović, R.; Maletić, D.; Kocić-Tanackov, S.; Čabarkapa, I.; Kokić, B.; Kojić, P.; Milošević, S.; Stulić, V.; Pavičić, T.V.; Vukić, M. Comparison of the Bacterial Inactivation Efficiency of Water Activated by a Plasma Jet Source and a Pin-to-Pin Electrode Configuration Source. Processes 2023, 11, 3286. [Google Scholar] [CrossRef]
- Kutasi, K.; Krstulović, N.; Jurov, A.; Salamon, K.; Popović, D.; Milošević, S. Controlling: The composition of plasma-activated water by Cu ions. Plasma Sources Sci. Technol. 2021, 30, 45015. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S.; Choi, E.H.; Han, I. ROS production in response to high-power microwave pulses induces p53 activation and DNA damage in brain cells: Radiosensitivity and biological dosimetry evaluation. Front. Cell Dev. Biol. 2023, 11, 1067861. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.N.; Mumtaz, S.; Han, I.; Choi, E.H. Formation of reactive species via high power microwave induced DNA damage and promoted intrinsic pathway-mediated apoptosis in lung cancer cells: An in vitro investigation. Fundam. Res. 2024, 4, 1542–1556. [Google Scholar] [CrossRef]
- Mumtaz, S.; Javed, R.; Rana, J.N.; Iqbal, M.; Choi, E.H. Pulsed high power microwave seeds priming modulates germination, growth, redox homeostasis, and hormonal shifts in barley for improved seedling growth: Unleashing the molecular dynamics. Free Radic. Biol. Med. 2024, 222, 371–385. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S.; Han, I.; Choi, E.H. Harnessing the synergy of nanosecond high-power microwave pulses and cisplatin to increase the induction of apoptosis in cancer cells through the activation of ATR/ATM and intrinsic pathways. Free Radic. Biol. Med. 2024, 225, 221–235. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.-D. The kINPen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D. Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Lim, J.S.; Javed, R.; Choi, E.H.; Han, I. Effect of Plasma On-Time with a Fixed Duty Ratio on Reactive Species in Plasma-Treated Medium and Its Significance in Biological Applications. Int. J. Mol. Sci. 2023, 24, 5289. [Google Scholar] [CrossRef] [PubMed]
- Veerana, M.; Mumtaz, S.; Rana, J.N.; Javed, R.; Panngom, K.; Ahmed, B.; Akter, K.; Choi, E.H. Recent Advances in Non-Thermal Plasma for Seed Germination, Plant Growth, and Secondary Metabolite Synthesis: A Promising Frontier for Sustainable Agriculture. Plasma Chem. Plasma Process. 2024, 44, 2263–2302. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.D.; Von Woedtke, T. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Laroque, D.A.; Seó, S.T.; Valencia, G.A.; Laurindo, J.B.; Carciofi, B.A.M. Cold plasma in food processing: Design, mechanisms, and application. J. Food Eng. 2022, 312, 110748. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Wemlinger, E.; Pedrow, P.; Barbosa-Cánovas, G.; Garcia-Perez, M. Effect of atmospheric pressure cold plasma (APCP) on the inactivation of Escherichia coli in fresh produce. Food Control 2013, 34, 149–157. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C. Applications of cold plasma technology for microbiological safety in meat industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Wang, X.; Chen, M.; Lu, Y.; Yu, P.; Zhang, C.; Huang, C.; Yang, Z.; Chen, Y.; Zhou, J. Inactivation of Multidrug-Resistant Bacteria Using Cold Atmospheric-Pressure Plasma Technology. Front. Med. 2025, 12, 1522186. [Google Scholar] [CrossRef]
- Ranjan, R.; Gupta, A.K.; Pandiselvam, R.; Chauhan, A.K.; Akhtar, S.; Jha, A.K.; Pratiksha; Ghosh, T.; Purohit, S.R.; Rather, M.A.; et al. Plasma treatment: An alternative and sustainable green approach for decontamination of mycotoxin in dried food products. J. Agric. Food Res. 2023, 14, 100867. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. A comprehensive review of the application of cold plasma technology in lignocellulosic biomass pretreatment. Biofuels Bioprod. Biorefin. 2024, 19, 453–468. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Schoonjans, R.; Castenmiller, J.; Chaudhry, Q.; Cubadda, F.; Daskaleros, T.; Franz, R.; Gott, D.; Mast, J.; Mortensen, A.; Oomen, A.G.; et al. Regulatory safety assessment of nanoparticles for the food chain in Europe. Trends Food Sci. Technol. 2023, 134, 98–111. [Google Scholar] [CrossRef]
- Meijer, G.W.; Lähteenmäki, L.; Stadler, R.H.; Weiss, J. Issues surrounding consumer trust and acceptance of existing and emerging food processing technologies. Crit. Rev. Food Sci. Nutr. 2021, 61, 97–115. [Google Scholar] [CrossRef]
- Kantono, K.; Hamid, N.; Malavalli, M.M.; Liu, Y.; Liu, T.; Seyfoddin, A. Consumer Acceptance and Production of In Vitro Meat: A Review. Sustainability 2022, 14, 4910. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Qasim, I.; Riaz, S.; Ur-Rehman, N.; Bukhari, H. DNA Reduction of Waterborne E.coli by Underwater Capillary Discharge. Jordan J. Phys. 2022, 15, 207–214. [Google Scholar] [CrossRef]
- Dezest, M.; Bulteau, A.-L.; Quinton, D.; Chavatte, L.; Le Bechec, M.; Cambus, J.P.; Arbault, S.; Nègre-Salvayre, A.; Clément, F.; Cousty, S. Oxidative modification and electrochemical inactivation of Escherichia coli upon cold atmospheric pressure plasma exposure. PLoS ONE 2017, 12, e0173618. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef]
- Verma, C.; Sharma, A.; Singh, P.; Somani, M.; Singh, S.; Patra, S.; Mukhopadhyay, S.; Gupta, B. 15—Functional designing of textile surfaces for biomedical devices. In Fiber and Textile Engineering in Drug Delivery Systems; Sharma, N., Butola, B.S., Eds.; The Textile Institute Book Series; Woodhead Publishing: Sawston, UK, 2023; pp. 443–460. [Google Scholar] [CrossRef]
- Kulkarni, S. Chapter 3—Plasma Assisted Polymer Synthesis and Processing. In Non-Thermal Plasma Technology for Polymeric Materials; Thomas, S., Mozetič, M., Cvelbar, U., Špatenka, P., K.M., P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–93. [Google Scholar] [CrossRef]
- Mumtaz, S.; Khan, R.; Rana, J.N.; Javed, R.; Iqbal, M.; Choi, E.H.; Han, I. Review on the Biomedical and Environmental Applications of Nonthermal Plasma. Catalysts 2023, 13, 685. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, L.; Deng, W.; Wang, J.; Zhang, J. The molecular reactive pathway between lipoxygenase and lipase and reactive species generated in dielectric barrier discharge atmospheric cold plasma: An investigation using molecular docking. Food Chem. 2025, 465, 141973. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-L.; Di, J.-J.; Lian, H.-Y.; Yi, X.; Zhou, H.; Liu, Y. Cold plasma pretreatment of ramsdellite-MnO2 to enhance remediation of antibiotic rifampicin from water by the self-generated reactive oxygen species. Appl. Surf. Sci. 2025, 693, 162811. [Google Scholar] [CrossRef]
- Martinet, A.; Miebach, L.; Heisterberg, L.; Neugebauer, A.; Enderle, M.D.; Bekeschus, S. Reactive Species Production and Colon Cancer Cytotoxicity of an Electrosurgical Cold Argon Plasma Device. Plasma Process. Polym. 2025, 22, e2400240. [Google Scholar] [CrossRef]
- Lim, J.; Park, S.; Ryu, S.; Park, S.; Kim, M.S. Different Inactivation Mechanisms of Staphylococcus aureus and Escherichia coli in Water by Reactive Oxygen and Nitrogen Species Generated from an Argon Plasma Jet. Environ. Sci. Technol. 2025, 59, 3276–3285. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Choi, S.; Lyakhov, K.; Shaislamov, U.; Mongre, R.K.; Jeong, D.K.; Suresh, R.; Lee, H.J. High-frequency underwater plasma discharge application in antibacterial activity. Plasma Phys. Reports 2017, 43, 381–392. [Google Scholar] [CrossRef]
- Chaturvedi Misra, V.; Tiwari, N.; Bhale, D.R.; Ghorui, S. Frequency-Tunable Plasma: Insights Into Ozone and Reactive Species Dynamics for Biomedical Use. Plasma Process. Polym. 2025, 22, e70002. [Google Scholar] [CrossRef]
- Marotta, E.; Paradisi, C. The importance of mechanistic studies in the development of cold plasma-based degradation of persistent organic pollutants in water. Curr. Opin. Green Sustain. Chem. 2025, 52, 100999. [Google Scholar] [CrossRef]
- Gupta, K.K.; Routray, W. Cold plasma: A nonthermal pretreatment, extraction, and solvent activation technique for obtaining bioactive compounds from agro-food industrial biomass. Food Chem. 2025, 472, 142960. [Google Scholar] [CrossRef] [PubMed]
- Dilip, D.; Modupalli, N.; Rahman, M.M.; Kariyat, R. Atmospheric cold plasma alters plant traits and negatively affects the growth and development of fall armyworm in rice. Sci. Rep. 2025, 15, 3680. [Google Scholar] [CrossRef]
- Karthik, C.; Mavelil-Sam, R.; Thomas, S.; Thomas, V. Cold Plasma Technology Based Eco-Friendly Food Packaging Biomaterials. Polymers 2024, 16, 230. [Google Scholar] [CrossRef]
- Abbas, H.; Altamim, E.; Salama, M.; Fouad, M.; Zahran, H. Cold Plasma Technology: A Sustainable Approach to Milk Preservation by Reducing Pathogens and Enhancing Oxidative Stability. Sustainability 2024, 16, 8754. [Google Scholar] [CrossRef]
- Moutiq, R.; Misra, N.N.; Mendonça, A.; Keener, K. In-package decontamination of chicken breast using cold plasma technology: Microbial, quality and storage studies. Meat Sci. 2020, 159, 107942. [Google Scholar] [CrossRef]
- Mahmoud, Y.A.-G.; Elkaliny, N.E.; Darwish, O.A.; Ashraf, Y.; Ebrahim, R.A.; Das, S.P.; Yahya, G. Comprehensive review for aflatoxin detoxification with special attention to cold plasma treatment. Mycotoxin Res. 2025, 41, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Palmer, J.; Pedley, J.; Petcu, M.; Newson, H.L.; Keener, K.; Flint, S. The effect of variations in cold plasma conditions on the detoxification of Aflatoxin M1 and degradation products. Int. Dairy J. 2025, 160, 106103. [Google Scholar] [CrossRef]
- Eshtiaghi, M.N.; Nakthong, N.; Samani, B.H.; Taki, K.; Tuntithavornwat, S. Effective Inactivation of Fungi in Grain Using Atmospheric Pressure Cold Plasma. Heliyon 2025, 11, e43018. [Google Scholar] [CrossRef]
- Khalid, W.; Benmebarek, I.E.; Zargarchi, S.; Kumar, P.; Javed, M.; Moreno, A.; Sharma, A.; Nayik, G.A.; Esatbeyoglu, T. Optimization of the effect of cold plasma treatment on UAE-NADES green extraction of chickpea roots (Cicer arietinum) bioactive compounds. Ultrason. Sonochem. 2025, 114, 107276. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Jiang, L.; Wang, J.; Zhang, W. Investigating binding mechanism between coconut globulin and tannic acid mediated by atmospheric cold plasma: Protein structure and stability. Food Chem. 2025, 464, 141670. [Google Scholar] [CrossRef]
- Nicol, M.K.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef]
- Mao, L.; Mhaske, P.; Zing, X.; Kasapis, S.; Majzoobi, M.; Farahnaky, A. Cold plasma: Microbial inactivation and effects on quality attributes of fresh and minimally processed fruits and Ready-To-Eat vegetables. Trends Food Sci. Technol. 2021, 116, 146–175. [Google Scholar] [CrossRef]
- Chizoba Ekezie, F.-G.; Sun, D.-W.; Cheng, J.-H. A review on recent advances in cold plasma technology for the food industry: Current applications and future trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Farooq, S.; Dar, A.H.; Dash, K.K.; Srivastava, S.; Pandey, V.K.; Ayoub, W.S.; Pandiselvam, R.; Manzoor, S.; Kaur, M. Cold plasma treatment advancements in food processing and impact on the physiochemical characteristics of food products. Food Sci. Biotechnol. 2023, 32, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Bezerra, J.; Lamarão, C.V.; Sanches, E.A.; Rodrigues, S.; Fernandes, F.A.N.; Ramos, G.L.P.A.; Esmerino, E.A.; Cruz, A.G.; Campelo, P.H. Cold plasma as a pre-treatment for processing improvement in food: A review. Food Res. Int. 2023, 167, 112663. [Google Scholar] [CrossRef] [PubMed]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; von Woedtke, T.; Brandenburg, R.; von dem Hagen, T.; Weltmann, K.-D. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2010, 44, 13002. [Google Scholar] [CrossRef]
- Jha, N.; Ryu, J.J.; Choi, E.H.; Kaushik, N.K. Generation and Role of Reactive Oxygen and Nitrogen Species Induced by Plasma, Lasers, Chemical Agents, and Other Systems in Dentistry. Oxid. Med. Cell. Longev. 2017, 2017, 7542540. [Google Scholar] [CrossRef]
- Triantaphyllidou, I.-E.; Aggelopoulos, C.A. Insights on bacteria inactivation in water by cold plasma: Effect of water matrix and pulsed plasmas waveform on physicochemical water properties, species formation and inactivation efficiency of Escherichia coli. Environ. Res. 2025, 266, 120467. [Google Scholar] [CrossRef]
- Movasaghi, M.; Heydari, M.M.; Schwean-Lardner, K.; Kirychuk, S.; Thompson, B.; Zhang, L. Investigating cold plasma jet effectiveness for eggshell surface decontamination. Food Control 2025, 168, 110928. [Google Scholar] [CrossRef]
- Rao, W.; Li, Y.; Dhaliwal, H.; Feng, M.; Xiang, Q.; Roopesh, M.S.; Pan, D.; Du, L. The Application of Cold Plasma Technology in Low-Moisture Foods. Food Eng. Rev. 2023, 15, 86–112. [Google Scholar] [CrossRef]
- Ke, Z.; Bai, Y.; Yi, Y.; Ding, Y.; Wang, W.; Liu, S.; Zhou, X.; Ding, Y. Why plasma-activated water treatment reduced the malonaldehyde content in muscle foods. Food Chem. 2023, 403, 134387. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; López-Delacalle, M.; Ródenas, R.; Martínez, V.; Rubio, F.; Rivero, R.M. Critical responses to nutrient deprivation: A comprehensive review on the role of ROS and RNS. Environ. Exp. Bot. 2019, 161, 74–85. [Google Scholar] [CrossRef]
- Surowsky, B.; Bußler, S.; Schlüter, O.K. Chapter 7—Cold Plasma Interactions With Food Constituents in Liquid and Solid Food Matrices. In Cold Plasma in Food and Agriculture; Misra, N.N., Schlüter, O., Cullen, P.J.B.T.-C.P., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 179–203. [Google Scholar] [CrossRef]
- Bayati, M.; Lund, M.N.; Tiwari, B.K.; Poojary, M.M. Chemical and physical changes induced by cold plasma treatment of foods: A critical review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13376. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Francis, K.; Zhang, X. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A novel Non-Thermal Technology for Food Processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Kumar, N.; Panghal, A.; Garg, M.K. Thermal Food Engineering Operations; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Mitharwal, S.; Rani, P.; Shanker, M.A.; Kumar, A.; Aslam, R.; Barut, Y.T.; Kothakota, A.; Rustagi, S.; Bhati, D.; et al. The influence of non-thermal technologies on color pigments of food materials: An updated review. Curr. Res. food Sci. 2023, 6, 100529. [Google Scholar] [CrossRef] [PubMed]
- Harikrishna, S.; Pawase, P.; Dash, K. Cold plasma as an emerging nonthermal technology for food processing: A comprehensive review. J. Agric. Food Res. 2023, 14, 100747. [Google Scholar] [CrossRef]
- Yudhistira, B.; Punthi, F.; Gavahian, M.; Chang, C.-K.; Hazeena, S.H.; Hou, C.-Y.; Hsieh, C.-W. Nonthermal technologies to maintain food quality and carbon footprint minimization in food processing: A review. Trends Food Sci. Technol. 2023, 141, 104205. [Google Scholar] [CrossRef]
- Knorr, D.; Ade-Omowaye, B.I.O.; Heinz, V. Nutritional improvement of plant foods by non-thermal processing. Proc. Nutr. Soc. 2002, 61, 311–318. [Google Scholar] [CrossRef]
- Wang, X.; Hou, M.; Liu, T.; Ren, J.; Li, H.; Yang, H.; Hu, Z.; Gao, Z. Continuous cold plasma reactor for the processing of NFC apple juice: Effect on quality control and preservation stability. Innov. Food Sci. Emerg. Technol. 2025, 100, 103905. [Google Scholar] [CrossRef]
- Chaplot, S.; Yadav, B.; Jeon, B.; Roopesh, M.S. Atmospheric Cold Plasma and Peracetic Acid–Based Hurdle Intervention To Reduce Salmonella on Raw Poultry Meat. J. Food Prot. 2019, 82, 878–888. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.-H.; Jo, C. Prospective Applications of Cold Plasma for Processing Poultry Products: Benefits, Effects on Quality Attributes, and Limitations. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1292–1309. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.S.; Ebaid, E.M.S.M.; Khalel, K.H.M.; Imre, K.; Morar, A.; Herman, V.; EL-Nawawi, F.A.M. Decontamination of chicken meat using dielectric barrier discharge cold plasma technology: The effect on microbial quality, physicochemical properties, topographical structure, and sensory attributes. LWT 2022, 165, 113739. [Google Scholar] [CrossRef]
- Souza, V.R.; Illera, A.E.; Keener, K.M. High voltage atmospheric cold plasma technology as a food safety intervention for decontamination of cutting tools during ready-to-eat poultry meat slicing. Innov. Food Sci. Emerg. Technol. 2022, 80, 103065. [Google Scholar] [CrossRef]
- Akhtar, J.; Abrha, M.G.; Teklehaimanot, K.; Gebrekirstos, G. Cold plasma technology: Fundamentals and effect on quality of meat and its products. Food Agric. Immunol. 2022, 33, 451–478. [Google Scholar] [CrossRef]
- Lin, L.; Liao, X.; Cui, H. Cold plasma treated thyme essential oil/silk fibroin nanofibers against Salmonella Typhimurium in poultry meat. Food Packag. Shelf Life 2019, 21, 100337. [Google Scholar] [CrossRef]
- Gao, Y.; Yeh, H.-Y.; Bowker, B.; Zhuang, H. Effects of different antioxidants on quality of meat patties treated with in-package cold plasma. Innov. Food Sci. Emerg. Technol. 2021, 70, 102690. [Google Scholar] [CrossRef]
- Gu, Y.; Lu, H.; Shao, Y.; Fu, D.; Wu, J.; Hu, J.; Tu, J.; Song, X.; Qi, K. Acetoacetyl-CoA transferase ydiF regulates the biofilm formation of avian pathogenic Escherichia coli. Res. Vet. Sci. 2022, 153, 144–152. [Google Scholar] [CrossRef]
- Sinnott-Stutzman, V.; Sykes, J.E. 53—Gram-Negative Bacterial Infections. In Greene’s Infectious Diseases of the Dog and Cat, 5th ed.; Sykes, J.E., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2021; pp. 643–654. [Google Scholar] [CrossRef]
- Feng, A.; Akter, S.; Leigh, S.A.; Wang, H.; Pharr, G.T.; Evans, J.; Branton, S.L.; Landinez, M.P.; Pace, L.; Wan, X.-F. Genomic diversity, pathogenicity and antimicrobial resistance of Escherichia coli isolated from poultry in the southern United States. BMC Microbiol. 2023, 23, 15. [Google Scholar] [CrossRef]
- Johnson, J.R.; Sannes, M.R.; Croy, C.; Johnston, B.; Clabots, C.; Kuskowski, M.A.; Bender, J.; Smith, K.E.; Winokur, P.L.; Belongia, E.A. Antimicrobial drug-resistant Escherichia coli from humans and poultry products, Minnesota and Wisconsin, 2002–2004. Emerg. Infect. Dis. 2007, 13, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Rothrock, M.J.; Lawrence, K.C.; Gamble, G.R.; Bowker, B.C. Effects of in-package cold plasma treatment on poultry breast meat packaged in high CO2 atmosphere. Poult. Sci. 2024, 103, 104085. [Google Scholar] [CrossRef]
- Yeh, H.-Y.; Line, J.E.; Hinton, A., Jr.; Gao, Y.; Zhuang, H. The effect of rosemary Extract and cold plasma treatments on bacterial community diversity in poultry ground meats. Heliyon 2019, 5, e02719. [Google Scholar] [CrossRef]
- Reinstein, S.; Fox, J.T.; Shi, X.; Alam, M.J.; Renter, D.G.; Nagaraja, T.G. Prevalence of Escherichia coli O157:H7 in organically and naturally raised beef cattle. Appl. Environ. Microbiol. 2009, 75, 5421–5423. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Moraes, J.; Ferreira, M.V.S.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; et al. Cold plasma processing of milk and dairy products. Trends Food Sci. Technol. 2018, 74, 56–68. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, R.K. Cold plasma treatment of dairy proteins in relation to functionality enhancement. Trends Food Sci. Technol. 2020, 102, 30–36. [Google Scholar] [CrossRef]
- Nikmaram, N.; Keener, K.M. The effects of cold plasma technology on physical, nutritional, and sensory properties of milk and milk products. LWT 2022, 154, 112729. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kahar, S.P.; Ranveer, R.C.; Annapure, U.S. Cold plasma an emerging nonthermal technology for milk and milk products: A review. Int. J. Dairy Technol. 2021, 74, 615–626. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Fernandes, L.M.; Moraes, J.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; Borges, F.O.; et al. Processing chocolate milk drink by low-pressure cold plasma technology. Food Chem. 2019, 278, 276–283. [Google Scholar] [CrossRef]
- Kanca, N.; Avşar, Y.K. Cold Plasma Technology and Its Effects on Some Properties of Milk and Dairy Products TT—Soğuk Plazma Teknolojisinin Süt ve Süt Ürünlerinin Bazı Özellikleri Üzerine Etkileri. Atatürk Üniversitesi Ziraat Fakültesi Derg. 2023, 54, 89–94. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Pimentel, T.C.; Freitas, M.Q.; Moraes, J.; Fernandes, L.M.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; Borges, F.O.; et al. Chocolate milk drink processed by cold plasma technology: Physical characteristics, thermal behavior and microstructure. LWT 2019, 102, 324–329. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhang, Y.; Lü, X.; Zhao, L.; Song, Y.; Zhang, L.; Jiang, H.; Zhang, J.; Ge, W. Processing sheep milk by cold plasma technology: Impacts on the microbial inactivation, physicochemical characteristics, and protein structure. LWT 2022, 153, 112573. [Google Scholar] [CrossRef]
- Hunt, J.M. Shiga toxin-producing Escherichia coli (STEC). Clin. Lab. Med. 2010, 30, 21–45. [Google Scholar] [CrossRef]
- Onyeaka, H.N.; Nwabor, O.F. Chapter 3—Microbial food contamination and foodborne diseases. In Food Preservation and Safety of Natural Products; Onyeaka, H.N., Nwabor, O.F., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 19–37. [Google Scholar] [CrossRef]
- Zarei, O.; Shokoohizadeh, L.; Hossainpour, H.; Alikhani, M.Y. The Prevalence of Shiga Toxin-Producing Escherichia coli and Enteropathogenic Escherichia coli Isolated from Raw Chicken Meat Samples. Int. J. Microbiol. 2021, 2021, 3333240. [Google Scholar] [CrossRef] [PubMed]
- Farrokh, C.; Jordan, K.; Auvray, F.; Glass, K.; Oppegaard, H.; Raynaud, S.; Thevenot, D.; Condron, R.; De Reu, K.; Govaris, A.; et al. Review of Shiga-toxin-producing Escherichia coli (STEC) and their significance in dairy production. Int. J. Food Microbiol. 2013, 162, 190–212. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Khan, S.U.H.; Khan, M.J.; Khattak, B.; Fozia, F.; Ahmad, I.; Wadaan, M.A.; Khan, M.F.; Baabbad, A.; Goyal, S.M. Multiple-Drug Resistant Shiga Toxin-Producing E. coli in Raw Milk of Dairy Bovine. Trop. Med. Infect. Dis. 2024, 9, 64. [Google Scholar] [CrossRef]
- Kwenda, A.; Nyahada, M.; Musengi, A.; Mudyiwa, M.; Muredzi, P. An Investigation on the Causes of Escherichia coli and Coliform Contamination of Cheddar Cheese and How to Reduce the Problem (A Case Study at a Cheese Manufacturing Firm in Harare, Zimbabwe). Int. J. Nutr. Food Sci. 2014, 3, 6–14. [Google Scholar] [CrossRef]
- Machado, M.A.M.; Castro, V.S.; da Cunha-Neto, A.; Vallim, D.C.; Pereira, R.D.C.L.; Dos Reis, J.O.; de Almeida, P.V.; Galvan, D.; Conte-Junior, C.A.; Figueiredo, E.E.D.S. Heat-resistant and biofilm-forming Escherichia coli in pasteurized milk from Brazil. Brazilian J. Microbiol. 2023, 54, 1035–1046. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Shiekh, K.A.; Benjakul, S. Pros and cons of cold plasma technology as an alternative non-thermal processing technology in seafood industry. Trends Food Sci. Technol. 2021, 111, 617–627. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kulawik, P.; Ozogul, Y.; Ozogul, F.; Bekhit, A.E.-D.A. Recent developments in non-thermal processing for seafood and seafood products: Cold plasma, pulsed electric field and high hydrostatic pressure. Int. J. Food Sci. Technol. 2022, 57, 774–790. [Google Scholar] [CrossRef]
- Hu, J.; Xie, K.; Zhu, H.; Giusti, A.; Li, M.; Zheng, Y.; Chen, J.; Armani, A.; Ying, X.; Deng, S. Effects of atmospheric cold plasma treatment mode on muscle quality and bacterial community of red shrimp during cold storage. Food Res. Int. 2025, 207, 116051. [Google Scholar] [CrossRef]
- Costa, R.A. Escherichia coli in seafood: A brief overview. Adv. Biosci. Biotechnol. 2013, 2013, 450–454. [Google Scholar] [CrossRef]
- Loest, D.; Uhland, F.C.; Young, K.M.; Li, X.-Z.; Mulvey, M.R.; Reid-Smith, R.; Sherk, L.M.; Carson, C.A. Carbapenem-resistant Escherichia coli from shrimp and salmon available for purchase by consumers in Canada: A risk profile using the Codex framework. Epidemiol. Infect. 2022, 150, e148. [Google Scholar] [CrossRef]
- Vásquez-García, A.; de Oliveira, A.P.S.C.; Mejia-Ballesteros, J.E.; de Godoy, S.H.S.; Barbieri, E.; de Sousa, R.L.M.; Fernandes, A.M. Escherichia coli detection and identification in shellfish from southeastern Brazil. Aquaculture 2019, 504, 158–163. [Google Scholar] [CrossRef]
- Beraldo, L.G.; Borges, C.A.; Maluta, R.P.; Cardozo, M.V.; Rigobelo, E.C.; de Ávila, F.A. Detection of Shiga toxigenic (STEC) and enteropathogenic (EPEC) Escherichia coli in dairy buffalo. Vet. Microbiol. 2014, 170, 162–166. [Google Scholar] [CrossRef]
- Tatarczak, M.; Wieczorek, K.; Possē, B.; Osek, J. Identification of putative adhesin genes in shigatoxigenic Escherichia coli isolated from different sources. Vet. Microbiol. 2005, 110, 77–85. [Google Scholar] [CrossRef]
- Ibrahim, C.; Hammami, S.; Mejri, S.; Mehri, I.; Pothier, P.; Hassen, A. Detection of Aichi virus genotype B in two lines of wastewater treatment processes. Microb. Pathog. 2017, 109, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.-Y.I.; Habib, I. Pathogenic E. coli in the Food Chain across the Arab Countries: A Descriptive Review. Foods 2023, 12, 3726. [Google Scholar] [CrossRef]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef] [PubMed]
- Nataro, J.P.; Martinez, J. Diagnosisand Investigation of Diarrheagenic Escherichia coli. Methods Mol. Med. 1998, 15, 387–406. [Google Scholar] [CrossRef]
- Ekonomou, S.I.; Boziaris, I.S. Non-Thermal Methods for Ensuring the Microbiological Quality and Safety of Seafood. Appl. Sci. 2021, 11, 833. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Pan, W.; Han, Q.; Wei, Y.; Li, Y.; Hu, Y.; Ying, X.; Armani, A.; Guidi, A.; et al. Antibacterial mechanism of atmospheric cold plasma against Pseudomonas fluorescens and Pseudomonas putida and its preservation application on in-packaged red shrimp paste. Food Chem. 2025, 464, 141590. [Google Scholar] [CrossRef]
- Üçok, D.; Akan, T.; Kartal, S.; Tosun, Ş.Y.; Mol, S.; Coşansu, S.; Doğruyol, H.; Ulusoy, Ş.; Bostan, K. An opportunity for post-harvest seafood safety: Atmospheric pressure air or helium cold plasma to control Salmonella Enteritidis in sea bass. Int. J. Food Sci. Technol. 2024, 59, 3773–3780. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ou, Y.; Li, F.; Jiao, Y.; Shi, H. Pathogens inactivation and preservation of Pacific white shrimp by atmospheric cold plasma treatment. Innov. Food Sci. Emerg. Technol. 2024, 93, 103638. [Google Scholar] [CrossRef]
- Matra, K.; Zonklin, B.; Hamkratok, S.; Pachekrepapol, U. Effect of dielectric barrier discharge cold plasma combined with green tea extract on quality and safety of ready-to-eat fish patties during storage. Discov. Food 2025, 5, 46. [Google Scholar] [CrossRef]
- Denoya, G.I.; Szerman, N.; Vaudagna, S.R. Application of Direct and Indirect Non-thermal Plasma in the Development of Ready-to-Eat Foods. Curr. Food Sci. Technol. Reports 2024, 2, 45–54. [Google Scholar] [CrossRef]
- Huang, M.; Adhikari, B.; Lv, W.; Xu, J. Application of novel-assisted radio frequency technology to improve ready-to-eat foods quality: A critical review. Food Biosci. 2024, 59, 104182. [Google Scholar] [CrossRef]
- Lee, H.W.; Oh, Y.J.; Min, S.C. Microbial inhibition in mixed vegetables packaged in plastic containers using combined treatment with hydrogen peroxide and cold plasma. Food Control 2024, 161, 110107. [Google Scholar] [CrossRef]
- Yadav, B.; Spinelli, A.C.; Govindan, B.N.; Tsui, Y.Y.; McMullen, L.M.; Roopesh, M.S. Cold plasma treatment of ready-to-eat ham: Influence of process conditions and storage on inactivation of Listeria innocua. Food Res. Int. 2019, 123, 276–285. [Google Scholar] [CrossRef]
- Yadav, B.; Spinelli, A.C.; Misra, N.N.; Tsui, Y.Y.; McMullen, L.M.; Roopesh, M.S. Effect of in-package atmospheric cold plasma discharge on microbial safety and quality of ready-to-eat ham in modified atmospheric packaging during storage. J. Food Sci. 2020, 85, 1203–1212. [Google Scholar] [CrossRef]
- Zeraatpisheh, F.; Tabatabaei Yazdi, F.; Shahidi, F. Investigation of effect of cold plasma on microbial load and physicochemical properties of ready-to-eat sliced chicken sausage. J. Food Sci. Technol. 2022, 59, 3928–3937. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Sysolyatina, E.; Mukhachev, A.; Yurova, M.; Grushin, M.; Karalnik, V.; Petryakov, A.; Trushkin, N.; Ermolaeva, S.; Akishev, Y. Role of the Charged Particles in Bacteria Inactivation by Plasma of a Positive and Negative Corona in Ambient Air. Plasma Process. Polym. 2014, 11, 315–334. [Google Scholar] [CrossRef]
- Lu, X.; Ye, T.; Cao, Y.; Sun, Z.; Xiong, Q.; Tang, Z.; Xiong, Z.; Hu, J.; Jiang, Z.; Pan, Y. The roles of the various plasma agents in the inactivation of bacteria. J. Appl. Phys. 2008, 104, 53309. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.-P.; Canal, C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: A brief review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.-D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from Physical Plasmas: Redox Chemistry for Biomedical Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef] [PubMed]
- Ozen, E.; Kumar, G.D.; Mishra, A.; Singh, R.K. Inactivation of Escherichia coli in apple cider using atmospheric cold plasma. Int. J. Food Microbiol. 2022, 382, 109913. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Rostami, S.; Hosseinzadeh Samani, B.; Lorigooini, Z. The effect of atmospheric pressure cold plasma on the inactivation of Escherichia coli in sour cherry juice and its qualitative properties. Food Sci. Nutr. 2020, 8, 870–883. [Google Scholar] [CrossRef]
- Kilonzo-Nthenge, A.; Liu, S.; Yannam, S.; Patras, A. Atmospheric Cold Plasma Inactivation of Salmonella and Escherichia coli on the Surface of Golden Delicious Apples. Front. Nutr. 2018, 5, 120. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of Inactivation by High-Voltage Atmospheric Cold Plasma Differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.M.; Bourke, P. Atmospheric cold plasma inactivation of Escherichia coli, Salmonella enterica serovar Typhimurium and Listeria monocytogenes inoculated on fresh produce. Food Microbiol. 2014, 42, 109–116. [Google Scholar] [CrossRef]
- Wang, J.M.; Zhuang, H.; Lawrence, K.; Zhang, J.H. Disinfection of chicken fillets in packages with atmospheric cold plasma: Effects of treatment voltage and time. J. Appl. Microbiol. 2018, 124, 1212–1219. [Google Scholar] [CrossRef]
- Feizollahi, E.; Misra, N.N.; Roopesh, M.S. Factors influencing the antimicrobial efficacy of Dielectric Barrier Discharge (DBD) Atmospheric Cold Plasma (ACP) in food processing applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 666–689. [Google Scholar] [CrossRef]
- Nasiru, M.M.; Frimpong, E.B.; Muhammad, U.; Qian, J.; Mustapha, A.T.; Yan, W.; Zhuang, H.; Zhang, J. Dielectric barrier discharge cold atmospheric plasma: Influence of processing parameters on microbial inactivation in meat and meat products. Compr. Rev. food Sci. food Saf. 2021, 20, 2626–2659. [Google Scholar] [CrossRef]
- Jiang, Y.; Sokorai, K.; Pyrgiotakis, G.; Demokritou, P.; Li, X.; Mukhopadhyay, S.; Jin, T.; Fan, X. Cold plasma-activated hydrogen peroxide aerosol inactivates Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria innocua and maintains quality of grape tomato, spinach and cantaloupe. Int. J. Food Microbiol. 2017, 249, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, B.S.M. The effects of X-ray radiation on Escherichia coli O157:H7, Listeria monocytogenes, Salmonella enterica and Shigella flexneri inoculated on whole Roma tomatoes. Food Microbiol. 2010, 27, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Yepez, X.; Illera, A.E.; Baykara, H.; Keener, K. Recent Advances and Potential Applications of Atmospheric Pressure Cold Plasma Technology for Sustainable Food Processing. Foods 2022, 11, 1833. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Tschang, C.Y.T.; Sann, J.; Thoma, M.H. Cold Atmospheric Plasma Decontamination of FFP3 Face Masks and Long-Term Material Effects. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 493–502. [Google Scholar] [CrossRef]
- Alonso, V.P.P.; Furtado, M.M.; Iwase, C.H.T.; Brondi-Mendes, J.Z.; Nascimento, M. da S. Microbial resistance to sanitizers in the food industry: Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 654–669. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Ramezan, Y.; Hematabadi, H.; Ramezan, M.; Khani, M.R.; Kamkari, A.; Najafi Tabrizi, A. Effect of cold atmospheric plasma torch distance on the microbial inactivation and sensorial properties of ready-to-eat olivier salad. Food Sci. Technol. Int. 2022, 29, 710–717. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, C.; Zou, F.; Sun, Y.; Shang, N.; Wu, W. Impacts of Cold Plasma Technology on Sensory, Nutritional and Safety Quality of Food: A Review. Foods 2022, 11, 2818. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Keener, K.M. Effects of Cold Plasma on Food Quality: A Review. Foods 2018, 7, 4. [Google Scholar] [CrossRef]
- Ho, K.K.H.Y.; Redan, B.W. Impact of thermal processing on the nutrients, phytochemicals, and metal contaminants in edible algae. Crit. Rev. Food Sci. Nutr. 2022, 62, 508–526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, L.; Zhang, J.; Shi, J.; Wang, Y.; Wang, S. Adverse Effects of Thermal Food Processing on the Structural, Nutritional, and Biological Properties of Proteins. Annu. Rev. Food Sci. Technol. 2021, 12, 259–286. [Google Scholar] [CrossRef]
- Khalaf, A.T.; Abdalla, A.N.; Ren, K.; Liu, X. Cold atmospheric plasma (CAP): A revolutionary approach in dermatology and skincare. Eur. J. Med. Res. 2024, 29, 487. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Zhao, Y.; Sun, Y.; Duan, M.; Wang, H.; Dai, R.; Liu, Y.; Li, X.; Jia, F. Bactericidal efficacy difference between air and nitrogen cold atmospheric plasma on Bacillus cereus: Inactivation mechanism of Gram-positive bacteria at the cellular and molecular level. Food Res. Int. 2023, 173, 113204. [Google Scholar] [CrossRef] [PubMed]
- Stańczyk, B.; Wiśniewski, M. The Promising Potential of Cold Atmospheric Plasma Therapies. Plasma 2024, 7, 465–497. [Google Scholar] [CrossRef]
- Roberts, P. Food irradiation is safe: Half a century of studies. Radiat. Phys. Chem. 2014, 105, 78–82. [Google Scholar] [CrossRef]
- Koutchma, T. UV Light for Processing Foods. Ozone Sci. Eng. 2008, 30, 93–98. [Google Scholar] [CrossRef]
- Indiarto, R.; Irawan, A.N.; Subroto, E. Meat Irradiation: A Comprehensive Review of Its Impact on Food Quality and Safety. Foods 2023, 12, 1845. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Gururani, P.; Bisht, B.; Kumar, V.; Kumar, N.; Joshi, R.; Vlaskin, M.S. Impact of irradiation on physico-chemical and nutritional properties of fruits and vegetables: A mini review. Heliyon 2022, 8, e10918. [Google Scholar] [CrossRef]
- Choi, E.H.; Uhm, H.S.; Kaushik, N.K. Plasma bioscience and its application to medicine. AAPPS Bull. 2021, 31, 10. [Google Scholar] [CrossRef]
- Rossi, F.; Kylián, O.; Hasiwa, N. Decontamination of Surfaces by Low Pressure Plasma Discharges. Plasma Process. Polym. 2006, 3, 431–442. [Google Scholar] [CrossRef]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of production and application in dentistry and oncology. Med. Gas Res. 2013, 3, 21. [Google Scholar] [CrossRef]
- Das, S.; Gajula, V.P.; Mohapatra, S.; Singh, G.; Kar, S. Role of cold atmospheric plasma in microbial inactivation and the factors affecting its efficacy. Health Sci. Rev. 2022, 4, 100037. [Google Scholar] [CrossRef]
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm Formation and Control of Foodborne Pathogenic Bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef] [PubMed]
- Sohbatzadeh, F.; Haqpanah, H.; Shabannejad, A.; Yazdanshenas, H. Eradication of exotoxin A and its producer in freshwater by means of cold-vaporized hydrogen peroxide-enhanced SDBD: A sustainable processing. Aquaculture 2022, 559, 738380. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R. Substantial capabilities of robotics in enhancing industry 4.0 implementation. Cogn. Robot. 2021, 1, 58–75. [Google Scholar] [CrossRef]
- Perinban, S.; Orsat, V.; Lyew, D.; Raghavan, V. Effect of plasma activated water on Escherichia coli disinfection and quality of kale and spinach. Food Chem. 2022, 397, 133793. [Google Scholar] [CrossRef] [PubMed]
- Konchekov, E.M.; Gusein-zade, N.; Burmistrov, D.E.; Kolik, L.V.; Dorokhov, A.S.; Izmailov, A.Y.; Shokri, B.; Gudkov, S. V Advancements in Plasma Agriculture: A Review of Recent Studies. Int. J. Mol. Sci. 2023, 24, 15093. [Google Scholar] [CrossRef]
- Niemira, B.A. Chapter 13—Regulatory status of cold plasma in food applications. In Advances in Cold Plasma Applications for Food Safety and Preservation; Bermudez-Aguirre, D., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 341–349. [Google Scholar] [CrossRef]
- Keener, K.M.; Misra, N.N. Chapter 14—Future of Cold Plasma in Food Processing. In Cold Plasma in Food and Agriculture; Misra, N.N., Schlüter, O., Cullen, P.J., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 343–360. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The Potential of Cold Plasma for Safe and Sustainable Food Production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Onyeaka, H.; Miri, T.; Obileke, K.; Anumudu, C.; Hart, A. A Cold Plasma Technology for Ensuring the Microbiological Safety and Quality of Foods. Food Eng. Rev. 2022, 14, 535–554. [Google Scholar] [CrossRef]

| No | E. coli Type | Antibiotic-Resistant Profile | Ref. |
|---|---|---|---|
| 1 | Enterohemorrhagic E. coli (EHEC) | Notably, strains like O157:H7 have resisted multiple antibiotics, including ampicillin and tetracycline. Multidrug resistance (resistance to ≥3 antimicrobial classes) in E. coli has increased from 7.2% in the 1950s to 63.6% in the 2000s. Commonly found in foods like undercooked ground beef, unpasteurized milk and dairy, raw vegetables, fruits, and unpasteurized juices. | [15] |
| 2 | Enterotoxigenic E. coli (ETEC) | ETEC strains have resisted antibiotics such as ampicillin, trimethoprim–sulfamethoxazole, and ciprofloxacin. Commonly found in contaminated water, raw vegetables, unpasteurized milk, and undercooked seafood. | [16] |
| 3 | Enteropathogenic E. coli (EPEC) | EPEC strains have demonstrated resistance to multiple antibiotics, including ampicillin, tetracycline, and co-trimoxazole. The presence of multidrug-resistant EPEC strains has been reported in various studies. It exists in Contaminated water, raw or undercooked meats, unpasteurized milk, and dairy. | [16] |
| 4 | Enteroaggregative E. coli (EAEC) | EAEC strains have shown resistance to a range of antibiotics, including ampicillin, tetracycline, and ciprofloxacin. Multidrug-resistant EAEC strains have been identified, complicating treatment options. It is found in Contaminated water, fresh produce, unpasteurized juices, and raw meats. | [16] |
| 5 | Enteroinvasive E. coli (EIEC) | EIEC strains have been found to be resistant to antibiotics such as ampicillin and trimethoprim–sulfamethoxazole. The occurrence of multidrug-resistant EIEC strains has been documented in various regions. This type of E. coli is commonly found in Contaminated water, raw vegetables, soft cheeses, and undercooked meats. | [16] |
| 6 | Shiga toxin-producing E. coli (STEC) | STEC strains, including O157:H7, have exhibited resistance to multiple antibiotics, such as ampicillin and tetracycline. The rise in multidrug-resistant STEC strains is a growing public health concern. This type of E. coli exists in Undercooked beef, raw milk, unpasteurized juices, raw sprouts, contaminated water, and soft cheeses. | [15] |
| 7 | Uropathogenic E. coli (UPEC) | UPEC strains have shown resistance to various antibiotics, including ampicillin, ciprofloxacin, and trimethoprim–sulfamethoxazole. The prevalence of multidrug-resistant UPEC strains has increased, leading to challenges in treating urinary tract infections. This type is not commonly linked to food but may be transmitted through contaminated water or poor hygiene. | [16] |
| 8 | Avian Pathogenic E. coli (APEC) | APEC strains have demonstrated resistance to antibiotics such as tetracycline, streptomycin, and sulfonamides. The emergence of multidrug-resistant APEC strains affects both animal health and poses potential risks to human health. It exists mainly in Poultry products. | [17] |
| 9 | Neonatal Meningitis-causing E. coli (NMEC) | NMEC strains have been found to be resistant to antibiotics, including ampicillin and gentamicin. The presence of multidrug-resistant NMEC strains complicates the management of neonatal meningitis. This type of E. coli primarily infects newborns, possibly transmitted through contaminated water, dairy products, or maternal transmission. | [16] |
| 10 | Adherent-Invasive E. coli (AIEC) | AIEC strains have shown resistance to multiple antibiotics, including ampicillin and ciprofloxacin. Detecting multidrug-resistant AIEC strains is concerning, especially given their association with inflammatory bowel diseases. This type of E. coli is not directly foodborne; rather, it is possibly linked to contaminated water, dairy products, and poor hygiene. | [16] |
| Low-Temperature Plasma | High-Temperature Plasma |
|---|---|
e.g., Low-pressure plasma (glow discharge) | (e. g., Fusion plasmas) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.W.; Gul, K.; Mumtaz, S. Recent Advances in Cold Atmospheric Pressure Plasma for E. coli Decontamination in Food: A Review. Plasma 2025, 8, 18. https://doi.org/10.3390/plasma8020018
Ahmed MW, Gul K, Mumtaz S. Recent Advances in Cold Atmospheric Pressure Plasma for E. coli Decontamination in Food: A Review. Plasma. 2025; 8(2):18. https://doi.org/10.3390/plasma8020018
Chicago/Turabian StyleAhmed, Muhammad Waqar, Kainat Gul, and Sohail Mumtaz. 2025. "Recent Advances in Cold Atmospheric Pressure Plasma for E. coli Decontamination in Food: A Review" Plasma 8, no. 2: 18. https://doi.org/10.3390/plasma8020018
APA StyleAhmed, M. W., Gul, K., & Mumtaz, S. (2025). Recent Advances in Cold Atmospheric Pressure Plasma for E. coli Decontamination in Food: A Review. Plasma, 8(2), 18. https://doi.org/10.3390/plasma8020018









