Modeling Study of OH Radical-Dominated H-Abstraction Reaction for Understanding Nucleotides Oxidation Induced by Cold Atmospheric Plasmas
Abstract
1. Introduction
2. Simulation Setup
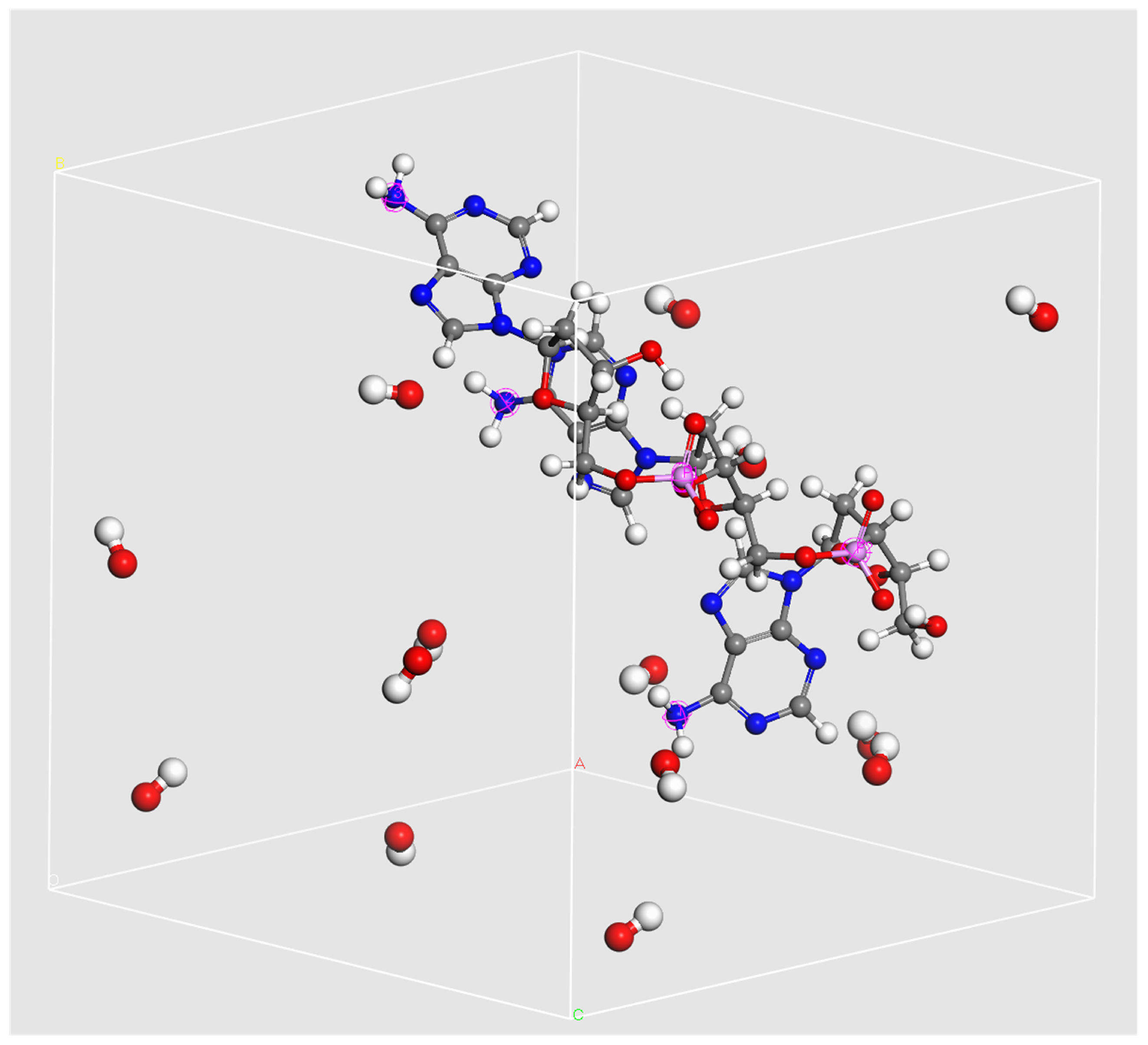
2.1. Molecular Structure of the Nucleotide
2.2. Generation of CAP and Reactive Species
2.3. Simulation Details
3. Results and Discussion
3.1. The Impact of OH Radicals on Nucleosides
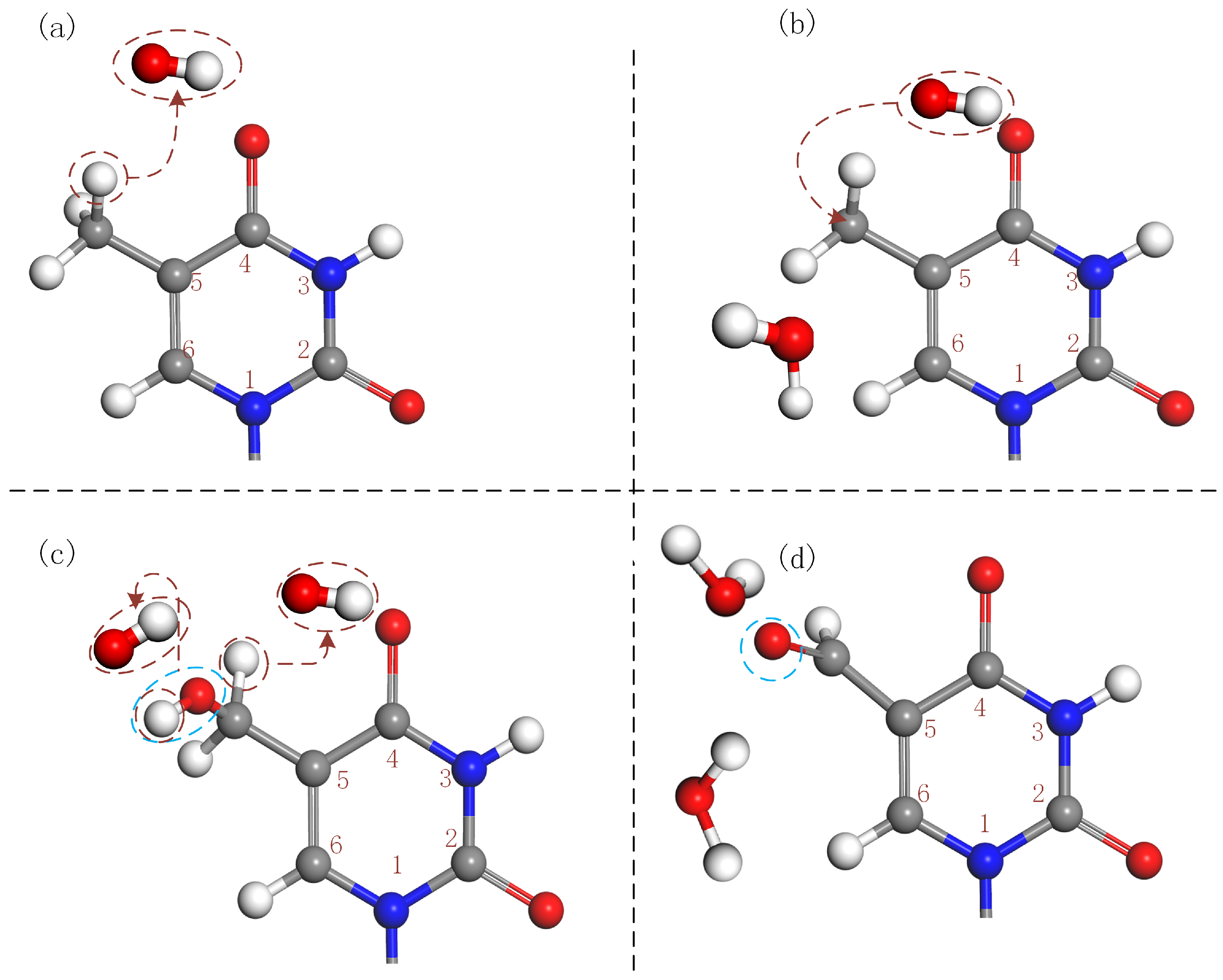
3.1.1. Oxidation of Methyl Group at Thymine
3.1.2. H-Abstraction from Amino Group at Guanine
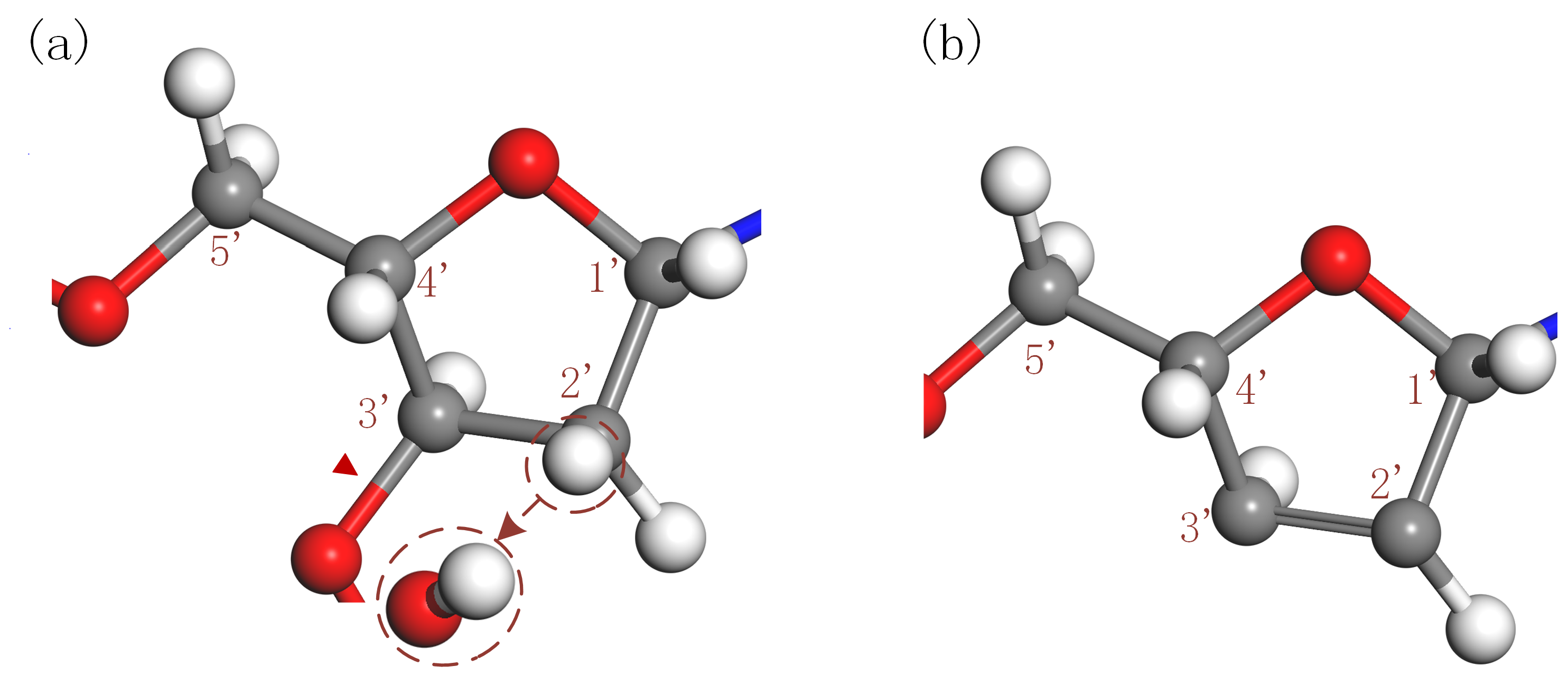
3.1.3. Strand Breakage Arising from H-Abstraction at C2′ Site of 2-deoxyribose
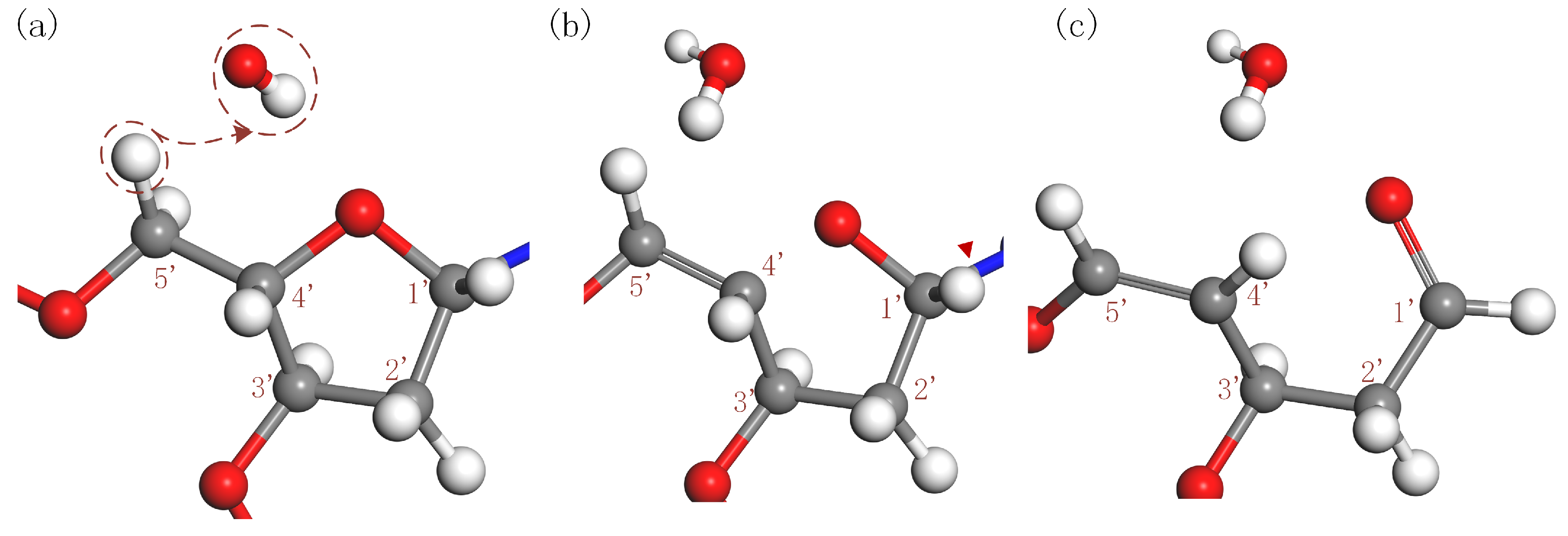
3.1.4. Base Release Resulting from H-Abstraction at C5′ Site of 2-deoxyribose
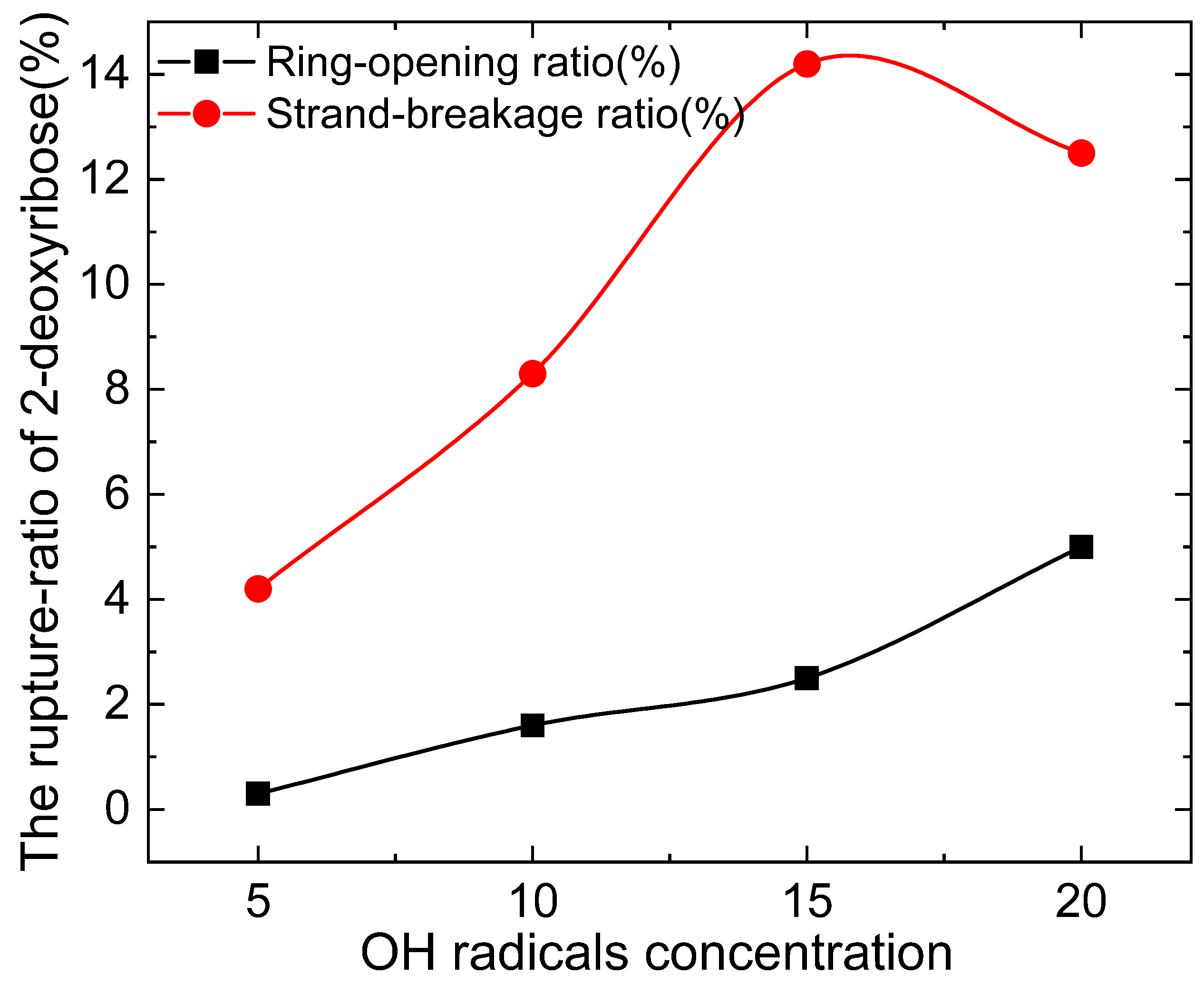
3.2. Dose Effects of OH Radical on Nucleobases and Sugar Moiety
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Efthimion, P.; Kaganovich, I.; Raitses, Y.; Keidar, M.; Lee, H.C.; Shneider, M.; Car, R. Critical Need for a National Initiative in Low Temperature Plasma Research. arXiv 2020, arXiv:2007.09199. [Google Scholar]
- Laroussi, M. Cold plasma in medicine and healthcare: The new frontier in low temperature plasma applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, L.; Zheng, Q.; Sherman, J.H.; Canady, J.; Trink, B.; Keidar, M. Micro-sized cold atmospheric plasma source for brain and breast cancer treatment. Plasma Med. 2018, 8, 2. [Google Scholar] [CrossRef]
- Reuter, S.; Von Woedtke, T.; Weltmann, K.D. The kINPen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.D.; von Woedtke, T. The plasma jet kINPen—A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Weltmann, K.; Von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2016, 59, 014031. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma medicine: A field of applied redox biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef]
- Ryan, T.P.; Stalder, K.R. Overview of current applications in plasma medicine. In Proceedings of the Energy-based Treatment of Tissue and Assessment IX. International Society for Optics and Photonics, San Francisco, CA, USA, 29–30 January 2017; Volume 10066, p. 1006606. [Google Scholar]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef]
- Reuter, S.; Tresp, H.; Wende, K.; Hammer, M.U.; Winter, J.; Masur, K.; Schmidt-Bleker, A.; Weltmann, K.D. From RONS to ROS: Tailoring plasma jet treatment of skin cells. IEEE Trans. Plasma Sci. 2012, 40, 2986–2993. [Google Scholar] [CrossRef]
- Ji, W.O.; Lee, M.H.; Kim, G.H.; Kim, E.H. Quantitation of the ROS production in plasma and radiation treatments of biotargets. Sci. Rep. 2019, 9, 19837. [Google Scholar] [CrossRef]
- Liu, D.; He, T.; Liu, Z.; Wang, S.; Liu, Z.; Rong, M.; Kong, M.G. Spatial-temporal distributions of ROS in model tissues treated by a He+ O2 plasma jet. Plasma Process. Polym. 2018, 15, 1800057. [Google Scholar] [CrossRef]
- Kelly-Wintenberg, K.; Hodge, A.; Montie, T.; Deleanu, L.; Sherman, D.; Reece Roth, J.; Tsai, P.; Wadsworth, L. Use of a one atmosphere uniform glow discharge plasma to kill a broad spectrum of microorganisms. J. Vac. Sci. Technol. A Vac. Surf. Film. 1999, 17, 1539–1544. [Google Scholar] [CrossRef]
- Montie, T.C.; Kelly-Wintenberg, K.; Roth, J.R. An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans. Plasma Sci. 2000, 28, 41–50. [Google Scholar] [CrossRef]
- Guo, J.S.; Tian, S.Q.; Zhang, Y.T. Reactive molecular dynamics simulations on interaction mechanisms of cold atmospheric plasmas and peptides. Phys. Plasmas 2023, 30, 043512. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Ghasemitarei, M.; Ghorbi, T.; Yusupov, M.; Zhang, Y.; Zhao, T.; Shali, P.; Bogaerts, A. Effects of Nitro-Oxidative Stress on Biomolecules: Part 1—Non-Reactive Molecular Dynamics Simulations. Biomolecules 2023, 13, 1371. [Google Scholar] [CrossRef]
- Yusupov, M.; Neyts, E.C.; Verlackt, C.C.; Khalilov, U.; Van Duin, A.C.; Bogaerts, A. Inactivation of the endotoxic biomolecule lipid a by oxygen plasma species: A reactive molecular dynamics study. Plasma Process. Polym. 2015, 12, 162–171. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, T.; Zou, L.; Wang, X.; Zhang, Y. ReaxFF-based molecular dynamics simulation of DNA molecules destruction in cancer cells by plasma ROS. Phys. Plasmas 2019, 26, 083504. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, T.; Wang, X.; Wang, D.; Zhang, Y. ReaxFF-based molecular dynamics simulation of the interactions between OH radicals in plasma and succinate dehydrogenase in cancer cell mitochondria. Plasma Process. Polym. 2022, 19, 2200083. [Google Scholar] [CrossRef]
- Li, Y.; Tan, S.; Liu, D.; Zhang, Y. Molecular dynamics simulation research on the interaction between plasma and living organisms: A comprehensive review. Plasma Process. Polym. 2024, 21, 2300119. [Google Scholar] [CrossRef]
- Abolfath, R.M.; Biswas, P.; Rajnarayanam, R.; Brabec, T.; Kodym, R.; Papiez, L. Multiscale QM/MM molecular dynamics study on the first steps of guanine damage by free hydroxyl radicals in solution. J. Phys. Chem. A 2012, 116, 3940–3945. [Google Scholar] [CrossRef]
- Khosravian, N.; Kamaraj, B.; Neyts, E.; Bogaerts, A. Structural modification of P-glycoprotein induced by OH radicals: Insights from atomistic simulations. Sci. Rep. 2016, 6, 19466. [Google Scholar] [CrossRef]
- Razzokov, J.; Yusupov, M.; Cordeiro, R.M.; Bogaerts, A. Atomic scale understanding of the permeation of plasma species across native and oxidized membranes. J. Phys. D Appl. Phys. 2018, 51, 365203. [Google Scholar] [CrossRef]
- Bogaerts, A.; Yusupov, M.; Razzokov, J.; Van der Paal, J. Plasma for cancer treatment: How can RONS penetrate through the cell membrane? Answers from computer modeling. Front. Chem. Sci. Eng. 2019, 13, 253–263. [Google Scholar] [CrossRef]
- Yao, X.; Guo, J.S.; Zhang, Y.T. Unveiling pathways of oxytetracycline degradation induced by cold atmospheric plasma. AIP Adv. 2022, 12, 035046. [Google Scholar] [CrossRef]
- Chai, Z.N.; Wang, X.C.; Yusupov, M.; Zhang, Y.T. Unveiling the interaction mechanisms of cold atmospheric plasma and amino acids by machine learning. Plasma Process. Polym. 2024, e2300230. [Google Scholar] [CrossRef]
- Bogaerts, A.; Khosravian, N.; Van der Paal, J.; Verlackt, C.C.; Yusupov, M.; Kamaraj, B.; Neyts, E.C. Multi-level molecular modelling for plasma medicine. J. Phys. D Appl. Phys. 2015, 49, 054002. [Google Scholar] [CrossRef]
- Van der Paal, J.; Aernouts, S.; Van Duin, A.C.; Neyts, E.C.; Bogaerts, A. Interaction of O and OH radicals with a simple model system for lipids in the skin barrier: A reactive molecular dynamics investigation for plasma medicine. J. Phys. D Appl. Phys. 2013, 46, 395201. [Google Scholar] [CrossRef]
- Yusupov, M.; Bogaerts, A.; Huygh, S.; Snoeckx, R.; Van Duin, A.C.; Neyts, E.C. Plasma-induced destruction of bacterial cell wall components: A reactive molecular dynamics simulation. J. Phys. Chem. C 2013, 117, 5993–5998. [Google Scholar] [CrossRef]
- Tian, S.Q.; Wang, X.L.; Zhang, Y.T. Numerical study on interactions of atmospheric plasmas and vegetable oils by reactive molecular dynamic simulations. Plasma Process. Polym. 2021, 18, 2100124. [Google Scholar] [CrossRef]
- Verlackt, C.; Neyts, E.; Jacob, T.; Fantauzzi, D.; Golkaram, M.; Shin, Y.; Van Duin, A.; Bogaerts, A. Atomic-scale insight into the interactions between hydroxyl radicals and DNA in solution using the ReaxFF reactive force field. New J. Phys. 2015, 17, 103005. [Google Scholar] [CrossRef]
- Branỳ, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold atmospheric plasma: A powerful tool for modern medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef]
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P.K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Verma, S.K.; Suar, M. Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Mater. Today Bio 2022, 13, 100200. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Chi, Y.Y.; He, J. Numerical Simulation on the Production of Reactive Oxygen Species in Atmospheric Pulse-Modulated RF Discharges with He/O2 Mixtures. Plasma Process. Polym. 2014, 11, 639–646. [Google Scholar] [CrossRef]
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y. Generation of reactive oxygen species in helium–oxygen radio-frequency discharges at atmospheric pressure. IEEE Trans. Plasma Sci. 2013, 41, 2979–2986. [Google Scholar] [CrossRef]
- Zhang, Y.T.; He, J. Frequency effects on the production of reactive oxygen species in atmospheric radio frequency helium-oxygen discharges. Phys. Plasmas 2013, 20, 013502. [Google Scholar] [CrossRef]
- Chenoweth, K.; Van Duin, A.C.; Goddard, W.A. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef]
- Kuznetsova, A.A.; Knorre, D.G.; Fedorova, O.S. Oxidation of DNA and its components with reactive oxygen species. Russ. Chem. Rev. 2009, 78, 659. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O− in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Pelle, E.; Mammone, T.; Marenus, K.; Maes, D.; Huang, X.; Frenkel, K. Ultraviolet-B-induced oxidative DNA base damage in primary normal human epidermal keratinocytes and inhibition by a hydroxyl radical scavenger. J. Investig. Dermatol. 2003, 121, 177–183. [Google Scholar] [CrossRef]
- Hayon, E.; Simic, M. Addition of hydroxyl radicals to pyrimidine bases and electron transfer reactions of intermediates to quinones. J. Am. Chem. Soc. 1973, 95, 1029–1035. [Google Scholar] [CrossRef]
- HAZRA, D.; STSTEENKEN. Pattern of OH Radical-Addition to Cytosine and 1-Substituted, 3-Substituted, 5-Substituted and 6-Substituted cytosines-electron-transfer and dehydration reactions of the OH adducts. J. Am. Chem. Soc. 1983, 105, 4380–4386. [Google Scholar] [CrossRef]
- Myers, L.S., Jr.; Hollis, M.L.; Theard, L.M.; Peterson, F.C.; Warnick, A. Pulse radiolysis of nucleic acid constituents and related compounds. III. Optical spectra and reactivity of organic free radicals formed by reaction of hydroxyl free radicals with pyrimidine bases. J. Am. Chem. Soc. 1970, 92, 2875–2882. [Google Scholar] [CrossRef]
- Monari, A.; Dumont, E. Understanding DNA under oxidative stress and sensitization: The role of molecular modeling. Front. Chem. 2015, 3, 43. [Google Scholar]
- Rodríguez-Muñiz, G.M.; Marin, M.L.; Lhiaubet-Vallet, V.; Miranda, M.A. Reactivity of Nucleosides with a Hydroxyl Radical in Non-aqueous Medium. Chem. Eur. J. 2012, 18, 8024–8027. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.L. Oxidatively generated base damage to cellular DNA. Free Radic. Biol. Med. 2010, 49, 9–21. [Google Scholar] [CrossRef]
- Pogozelski, W.K.; Tullius, T.D. Oxidative strand scission of nucleic acids: Routes initiated by hydrogen abstraction from the sugar moiety. Chem. Rev. 1998, 98, 1089–1108. [Google Scholar] [CrossRef]
- Dedon, P.C. The chemical toxicology of 2-deoxyribose oxidation in DNA. Chem. Res. Toxicol. 2008, 21, 206–219. [Google Scholar] [CrossRef]
- Ji, Y.; Xia, Y.; Zhao, M.; Li, F.; Huang, B. Reactions of ·OH with thymine studied using density functional theory. Int. J. Quantum Chem. 2005, 101, 211–218. [Google Scholar] [CrossRef]
- Wu, Y.; Mundy, C.J.; Colvin, M.E.; Car, R. On the mechanisms of OH radical induced DNA-base damage: A comparative quantum chemical and Car- Parrinello molecular dynamics study. J. Phys. Chem. A 2004, 108, 2922–2929. [Google Scholar] [CrossRef]
- Burrows, C.J. Oxidative nucleobase modifications leading to strand scission. Chem. Rev. 1998, 98, 1109–1151. [Google Scholar] [CrossRef]
- Cadet, J.; Delatour, T.; Douki, T.; Gasparutto, D.; Pouget, J.P.; Ravanat, J.L.; Sauvaigo, S. Hydroxyl radicals and DNA base damage. Mutat. Res. Mol. Mech. Mutagen. 1999, 424, 9–21. [Google Scholar] [CrossRef]
- Wagner, J.R.; Van Lier, J.; Berger, M.; Cadet, J. Thymidine hydroperoxides: Structural assignment, conformational features, and thermal decomposition in water. J. Am. Chem. Soc. 1994, 116, 2235–2242. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Simic, M.G. Mechanism of OH radical reactions with thymine and uracil derivatives. J. Am. Chem. Soc. 1986, 108, 5968–5972. [Google Scholar] [CrossRef]
- Hong, J.; Kim, D.G.; Yoo, J.S.; Cheong, C. Damaged products of thymine in hydroxyl radical solution under UV irradiation. Microchem. J. 1999, 63, 109–118. [Google Scholar] [CrossRef]
- Douki, T.; Dealtour, T.; Bianchini, F.; Cadet, J. Observation and prevention of an artefactual formation of oxidized DNA bases and nucleosides in the GC-EMS method. Carcinogenesis 1996, 17, 347–353. [Google Scholar] [CrossRef]
- Frelon, S.; Douki, T.; Ravanat, J.L.; Pouget, J.P.; Tornabene, C.; Cadet, J. High-performance liquid chromatography- tandem mass spectrometry measurement of radiation-induced base damage to isolated and cellular DNA. Chem. Res. Toxicol. 2000, 13, 1002–1010. [Google Scholar] [CrossRef]
- Ravanat, J.L.; Douki, T. UV and ionizing radiations induced DNA damage, differences and similarities. Radiat. Phys. Chem. 2016, 128, 92–102. [Google Scholar] [CrossRef]
- Breen, A.P.; Murphy, J.A. Reactions of oxyl radicals with DNA. Free Radic. Biol. Med. 1995, 18, 1033–1077. [Google Scholar] [CrossRef]
- Jena, N.; Mishra, P. Formation of ring-opened and rearranged products of guanine: Mechanisms and biological significance. Free Radic. Biol. Med. 2012, 53, 81–94. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; D’Angelantonio, M.; Guerra, M.; Kaloudis, P.; Mulazzani, Q.G. A reevaluation of the ambident reactivity of the guanine moiety towards hydroxyl radicals. Angew. Chem. Int. Ed. 2009, 48, 2214–2217. [Google Scholar] [CrossRef]
- Matter, B.; Seiler, C.L.; Murphy, K.; Ming, X.; Zhao, J.; Lindgren, B.; Jones, R.; Tretyakova, N. Mapping three guanine oxidation products along DNA following exposure to three types of reactive oxygen species. Free Radic. Biol. Med. 2018, 121, 180–189. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Von Sonntag, C.; Schulte-Frohlinde, D. Strand breaks and sugar release by .gamma.-irradiation of DNA in aqueous solution. J. Am. Chem. Soc. 1975, 97, 2277–2278. [Google Scholar] [CrossRef]
- Richmond, R.C.; Zimbrick, J.D. In vivo radiation-induced thymine residue release from E. coli DNA. Biochem. Biophys. Res. Commun. 1975, 64, 391–398. [Google Scholar] [CrossRef]
- Richmond, R.; Zimbrick, J. Base residue release from 3 H-thymine labeled DNA in irradiated E. coli under conditions of enzyme inhibition. In Oxygen and Oxy-Radicals in Chemistry and Biology; Rodgers, M.A.J., Powers, E.L., Eds.; Academic Press; Washington, DC, USA, 1981.
- Yao, W.; Ma, X.; Li, S.; Gao, Y.; Nian, F.; Zhou, L. Theoretical study of mechanism and kinetics for the reaction of hydroxyl radical with 2′-deoxycytidine. Struct. Chem. 2018, 29, 1359–1366. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.-X.; Chen, Y.; Zhang, Y.-T. Modeling Study of OH Radical-Dominated H-Abstraction Reaction for Understanding Nucleotides Oxidation Induced by Cold Atmospheric Plasmas. Plasma 2024, 7, 498-509. https://doi.org/10.3390/plasma7020026
Jiang Y-X, Chen Y, Zhang Y-T. Modeling Study of OH Radical-Dominated H-Abstraction Reaction for Understanding Nucleotides Oxidation Induced by Cold Atmospheric Plasmas. Plasma. 2024; 7(2):498-509. https://doi.org/10.3390/plasma7020026
Chicago/Turabian StyleJiang, Yu-Xuan, Yang Chen, and Yuan-Tao Zhang. 2024. "Modeling Study of OH Radical-Dominated H-Abstraction Reaction for Understanding Nucleotides Oxidation Induced by Cold Atmospheric Plasmas" Plasma 7, no. 2: 498-509. https://doi.org/10.3390/plasma7020026
APA StyleJiang, Y.-X., Chen, Y., & Zhang, Y.-T. (2024). Modeling Study of OH Radical-Dominated H-Abstraction Reaction for Understanding Nucleotides Oxidation Induced by Cold Atmospheric Plasmas. Plasma, 7(2), 498-509. https://doi.org/10.3390/plasma7020026





