Plasma Treatment of Polystyrene Films—Effect on Wettability and Surface Interactions with Au Nanoparticles
Abstract
1. Introduction
2. Experimental Details
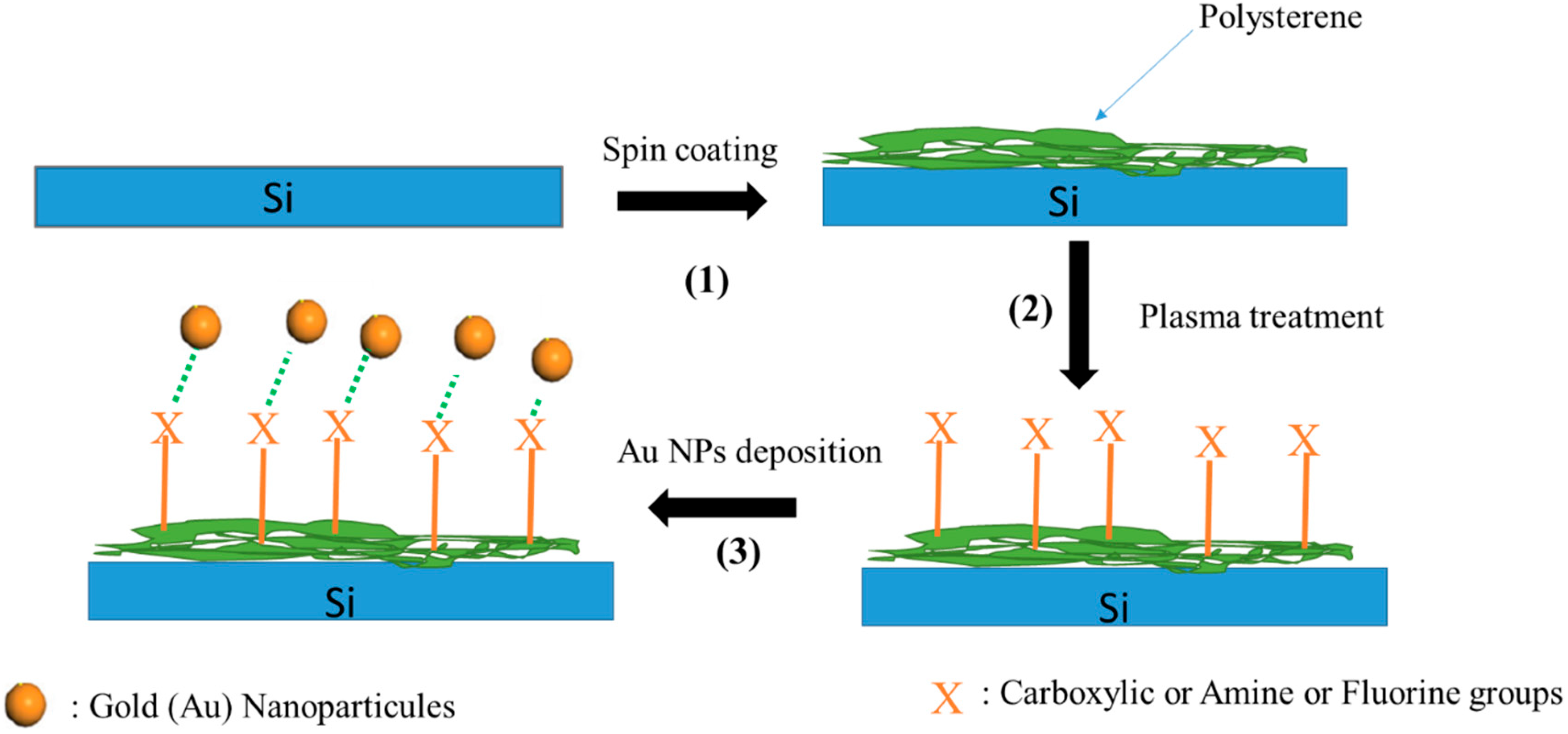
2.1. Spin Coating of Polystyrene Films
2.2. Plasma Treatment
2.3. Au NPs Deposition
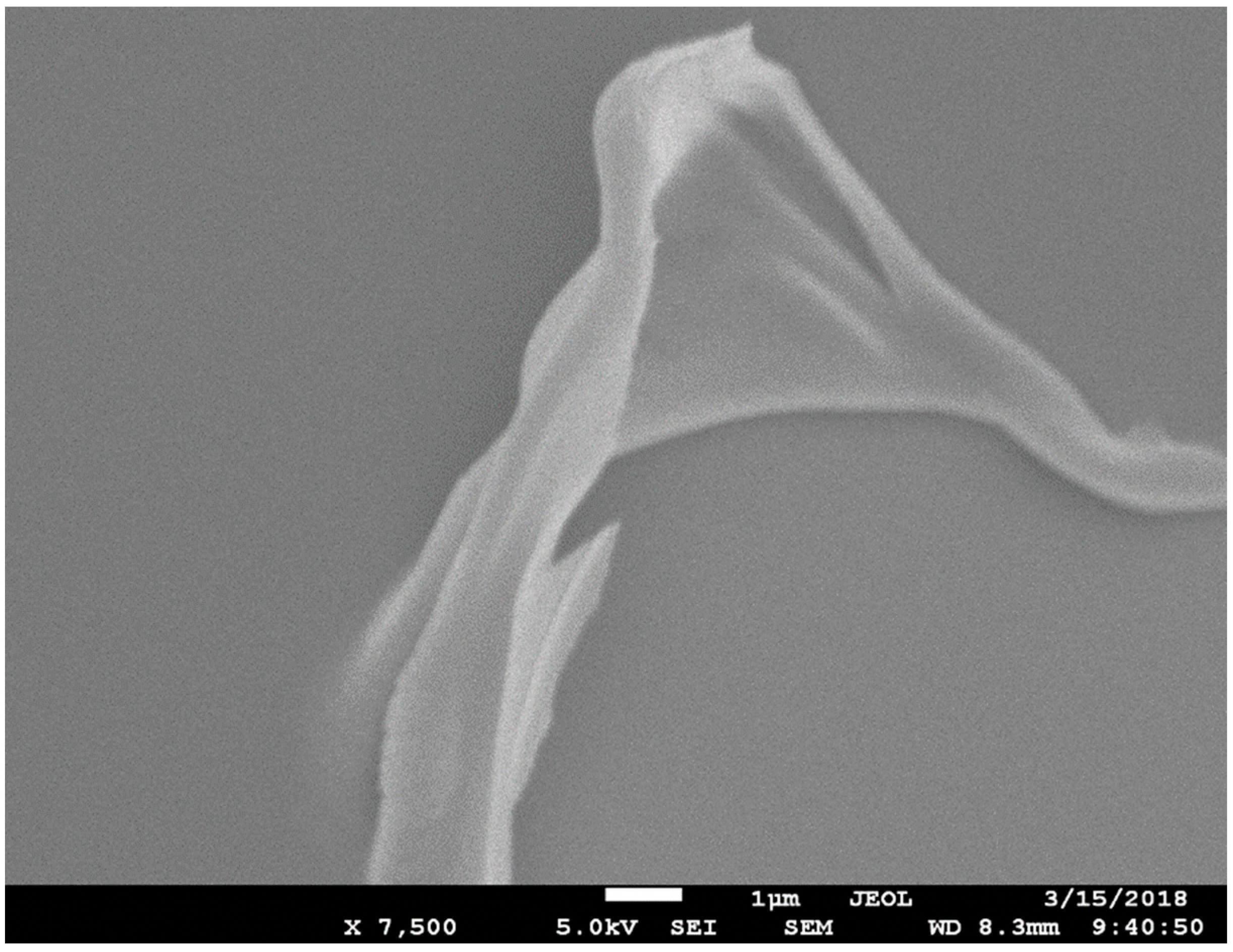
2.4. Materials Characterization
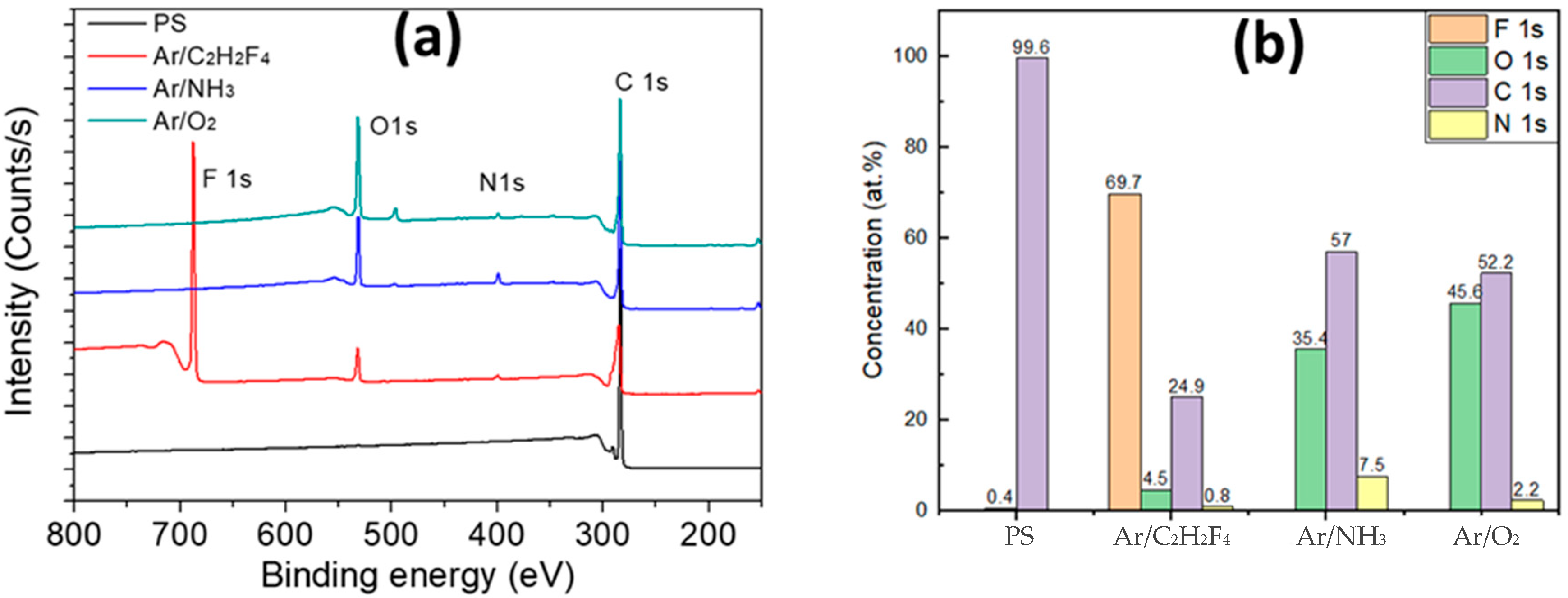
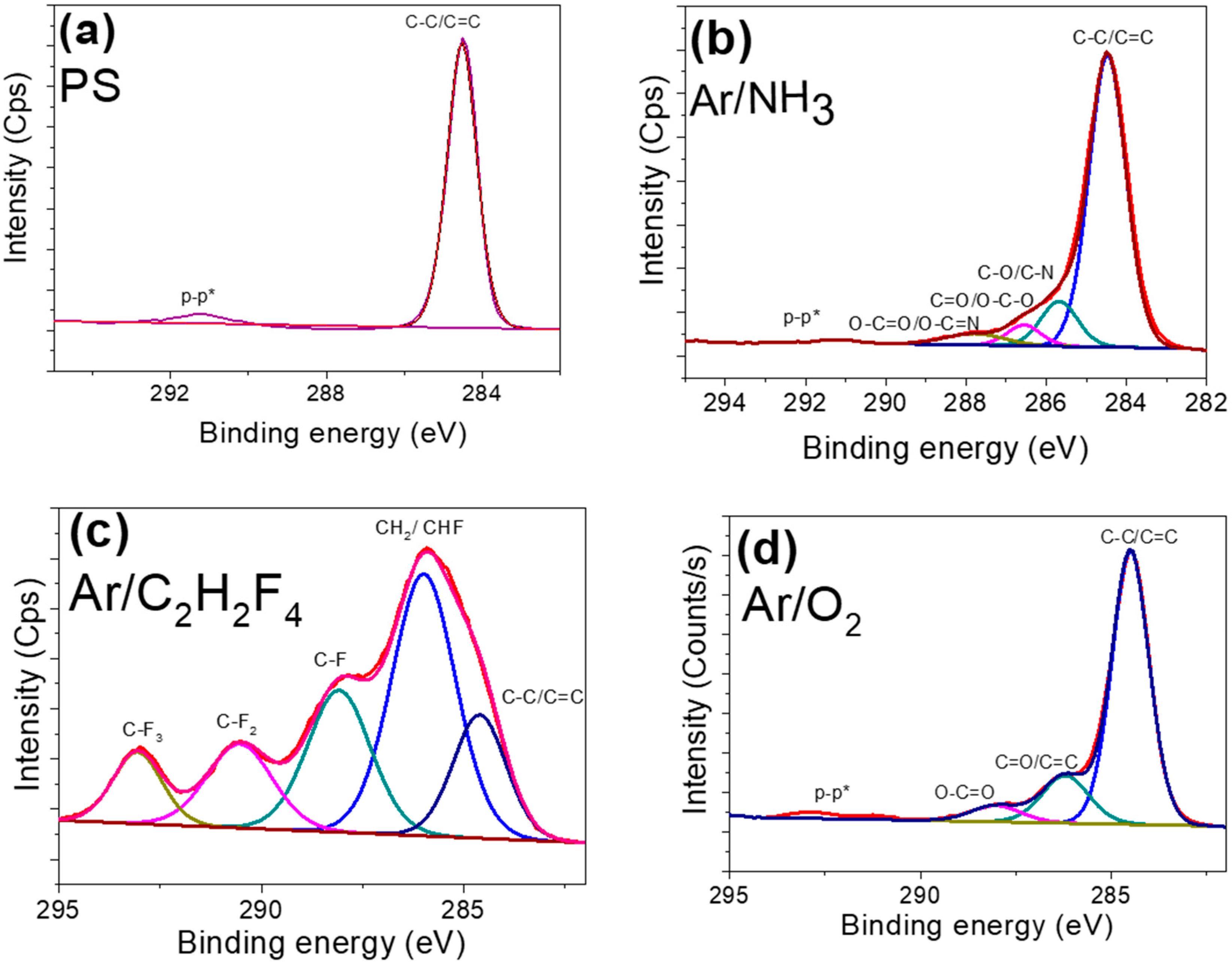
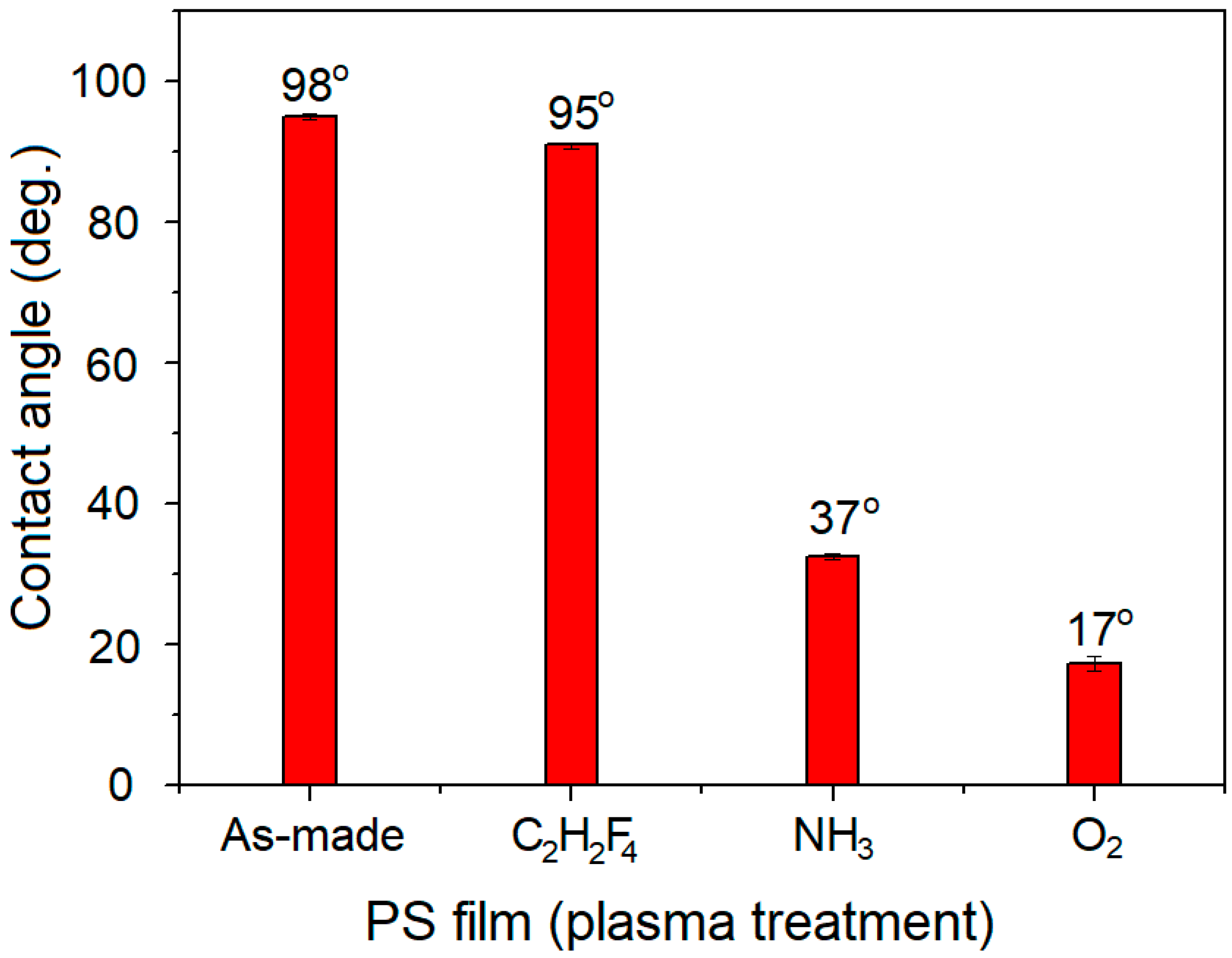
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gurman, J.L.; Baier, L.; Levin, B.C. Polystyrenes: A Review of the Literature on the Products of Thermal Decomposition and Toxicity. NIST Interagency/Internal Rep. 1986. Available online: https://www.nist.gov/publications/polystyrenes-review-literature-products-thermal-decomposition-and-toxicity (accessed on 29 November 2022).
- Cooper, I. Plastics and chemical migration into food. Chem. Migr. Food Contact Mater. 2007, 1, 228–250. [Google Scholar] [CrossRef]
- Wünsch, J.R. Polystyrene: Synthesis, Production and Applications; Rapra Technology Ltd.: Shawbury, UK, 2000; Volume 10. [Google Scholar]
- Haider, S.; Kausar, A.; Muhammad, B. Overview on Polystyrene/Nanoclay Composite: Physical Properties and Application. Polym. Technol. Eng. 2017, 56, 917–931. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, B.; Cremaschi, L. Review of moisture behavior and thermal performance of polystyrene insulation in building applications. Build. Environ. 2017, 123, 50–65. [Google Scholar] [CrossRef]
- Liston, E.M.; Martinu, L.; Wertheimer, M.R. Plasma surface modification of polymers for improved adhesion: A critical review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Yasuda, H. Plasma treatment for improved bonding: A review. J. Macromol. Sci. Part A Chem. 1976, 30, 199–218. [Google Scholar]
- Matouk, Z.; Torriss, B.; Rincón, R.; Dorris, A.; Beck, S.; Berry, R.M.; Chaker, M. Functionalization of cellulose nanocrystal films using Non-Thermal atmospheric–Pressure plasmas. Appl. Surf. Sci. 2020, 511, 145566. [Google Scholar] [CrossRef]
- Beaulieu, I.; Geissler, M.; Mauzeroll, J. Oxygen Plasma Treatment of Polystyrene and Zeonor: Substrates for Adhesion of Patterned Cells. Langmuir 2009, 25, 7169–7176. [Google Scholar] [CrossRef]
- Tian, X.; Yu, Q.; Kong, X.; Zhang, M. Preparation of Plasmonic Ag@PS Composite via Seed-Mediated In Situ Growth Method and Application in SERS. Front. Chem. 2022, 10, 142. [Google Scholar] [CrossRef]
- Calis, B.; Yilmaz, M. Fabrication of gold nanostructure decorated polystyrene hybrid nanosystems via poly(L-DOPA) and their applications in surface-enhanced Raman Spectroscopy (SERS), and catalytic activity. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 622, 126654. [Google Scholar] [CrossRef]
- Eisen, C.; Ge, L.; Santini, E.; Gia, M.C.; Reithofer, M.R. Hyper crosslinked polymer supported NHC stabilized gold nanoparticles with excellent catalytic performance in flow processes. Nanoscale Adv. 2023, 5, 1095–1101. [Google Scholar] [CrossRef]
- Li, H.; Li, A.; Zhao, Z.; Li, M.; Song, Y. Heterogeneous Wettability Surfaces: Principle, Construction, and Applications. Small Struct. 2020, 1, 2000028. [Google Scholar] [CrossRef]
- Hendrich, K.; Peng, W.; Vana, P. Controlled Arrangement of Gold Nanoparticles on Planar Surfaces via Constrained Dewetting of Surface-Grafted RAFT Polymer. Polymers 2020, 12, 1214. [Google Scholar] [CrossRef]
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D.K. Review on gold nanoparticles and their applications. Toxicol. Environ. Health Sci. 2012, 3, 193–205. [Google Scholar] [CrossRef]
- Al-Hamdani, A.H.; A Madlool, R.; Abdulazeez, N.Z. Effect of gold nanoparticle size on the linear and nonlinear optical properties. AIP Conf. Proc. 2020, 2290, 050029. [Google Scholar]
- Trudel, S. Unexpected magnetism in gold nanostructures: Making gold even more attractive. Gold Bull. 2011, 44, 3–13. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, L.; Wu, X.; Tian, Y.; Zhou, X.; Xu, S.; Xie, Z.; Ma, Y. Size and Shape Effect of Gold Nanoparticles in “Far-Field” Surface Plasmon Resonance. Part. Part. Syst. Charact. 2019, 36, 1800077. [Google Scholar] [CrossRef]
- Lin, C.; Tao, K.; Hua, D.; Ma, Z.; Zhou, S. Size Effect of Gold Nanoparticles in Catalytic Reduction of p-Nitrophenol with NaBH4. Molecules 2013, 18, 12609–12620. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef]
- Solaymani, S.; Yadav, R.P.; Ţălu, Ş.; Achour, A.; Rezaee, S.; Nezafat, N.B. Averaged power spectrum density, fractal and multifractal spectra of Au nano-particles deposited onto annealed TiO2 thin films. Opt. Quantum Electron. 2020, 52, 1–16. [Google Scholar] [CrossRef]
- García, T.; López, J.M.; Solsona, B.; Sanchis, R.; Willock, D.J.; Davies, T.E.; Lu, L.; He, Q.; Kiely, C.J.; Taylor, S.H. The Key Role of Nanocasting in Gold-based Fe2O3 Nanocasted Catalysts for Oxygen Activation at the Metal-support Interface. Chemcatchem 2019, 11, 1915–1927. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Wang, Q.; Zhou, X.; Li, J.; Song, S.; Zhang, H. CeO2 supported low-loading Au as an enhanced catalyst for low temperature oxidation of carbon monoxide. Crystengcomm 2019, 21, 7108–7113. [Google Scholar] [CrossRef]
- Achour, A.; Islam, M.; Solaymani, S.; Vizireanu, S.; Saeed, K.; Dinescu, G. Influence of plasma functionalization treatment and gold nanoparticles on surface chemistry and wettability of reactive-sputtered TiO2 thin films. Appl. Surf. Sci. 2018, 458, 678–685. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying thiol–gold interactions towards the efficient strength control. Nat. Commun. 2014, 5, 4348. [Google Scholar] [CrossRef] [PubMed]
- Achour, A.; Solaymani, S.; Vizireanu, S.; Baraket, A.; Vesel, A.; Zine, N.; Errachid, A.; Dinescu, G.; Pireaux, J. Effect of nitrogen configuration on carbon nanowall surface: Towards the improvement of electrochemical transduction properties and the stabilization of gold nanoparticles. Mater. Chem. Phys. 2019, 228, 110–117. [Google Scholar] [CrossRef]
- Lee, G.; Lee, H.; Nam, K.; Han, J.-H.; Yang, J.; Lee, S.W.; Yoon, D.S.; Eom, K.; Kwon, T. Nanomechanical characterization of chemical interaction between gold nanoparticles and chemical functional groups. Nanoscale Res. Lett. 2012, 7, 608. [Google Scholar] [CrossRef]
- Ponraj, S.B.; Chen, Z.; Li, L.H.; Shankaranarayanan, J.S.; Rajmohan, G.D.; du Plessis, J.; Sinclair, A.J.; Chen, Y.; Wang, X.; Kanwar, J.R.; et al. Fabrication of Boron Nitride Nanotube–Gold Nanoparticle Hybrids Using Pulsed Plasma in Liquid. Langmuir 2014, 30, 10712–10720. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Lin, F. The Basic Properties of Gold Nanoparticles and their Applications in Tumor Diagnosis and Treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef]
- Balfourier, A.; Luciani, N.; Wang, G.; Lelong, G.; Ersen, O.; Khelfa, A.; Alloyeau, D.; Gazeau, F.; Carn, F. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 103–113. [Google Scholar] [CrossRef]
- Sändig, N.; Zerbetto, F. Molecules on gold. Chem. Commun. 2010, 46, 667–676. [Google Scholar] [CrossRef]
- Carnerero, J.M.; Jimenez-Ruiz, A.; Castillo, P.M.; Prado-Gotor, R. Covalent and non-covalent DNA-gold-nanoparticle interactions: New avenues of research. Chemphyschem 2017, 18, 17–33. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Kim, J.; Tran, V.T.; Suzuki, T.; Neethirajan, S.; Lee, J.; Park, E.Y. In situ self-assembly of gold nanoparticles on hydrophilic and hydrophobic substrates for influenza virus-sensing platform. Sci. Rep. 2017, 7, srep44495. [Google Scholar] [CrossRef]
- Prasad, M.D.; Krishna, M. Au nanoparticle embedded Poly(methyl-methacrylate) and Poly(styrene) free-standing films for wettability and surface enhanced Raman scattering applications. Mater. Sci. Eng. B 2021, 272, 115324. [Google Scholar] [CrossRef]
- Arshad, M.; Karamti, H.; Awrejcewicz, J.; Grzelczyk, D.; Galal, A.M. Thermal Transmission Comparison of Nanofluids over Stretching Surface under the Influence of Magnetic Field. Micromachines 2022, 13, 1296. [Google Scholar] [CrossRef]
- Arshad, M.; Hussain, A.; Elfasakhany, A.; Gouadria, S.; Awrejcewicz, J.; Pawłowski, W.; Elkotb, M.A.; Alharbi, F.M. Magneto-Hydrodynamic Flow above Exponentially Stretchable Surface with Chemical Reaction. Symmetry 2022, 14, 1688. [Google Scholar] [CrossRef]
- Ghasemi, M.; Minier, M.; Tatoulian, M.; Arefi-Khonsari, F. Determination of Amine and Aldehyde Surface Densities: Application to the Study of Aged Plasma Treated Polyethylene Films. Langmuir 2007, 23, 11554–11561. [Google Scholar]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Morgan, D.J. Comments on the XPS Analysis of Carbon Materials. C 2021, 7, 51. [Google Scholar] [CrossRef]
- López-Santos, C.; Yubero, F.; Cotrino, J.; Contreras, L.; Barranco, A.; González-Elipe, A.R. Formation of Nitrogen Functional Groups on Plasma Treated DLC. Plasma Process. Polym. 2009, 6, 555–565. [Google Scholar] [CrossRef]
- Vesel, A.; Junkar, I.; Cvelbar, U.; Kovac, J.; Mozetic, M. Surface modification of polyester by oxygen- and nitrogen-plasma treatment. Surf. Interface Anal. 2008, 40, 1444–1453. [Google Scholar] [CrossRef]
- Fluorovo, H.P. Hydrophobization of polymer polystyrene in fluorine plasma. Mater. Tehnol. 2011. Available online: http://mit.imt.si/izvodi/mit113/vesel.pdf (accessed on 29 November 2022).
- Sakai, Y.; Norimatsu, H.; Saito, Y.; Inomata, H.; Mizuno, T. Silica coating on plastics by liquid phase deposition (LPD) method. Thin Solid Films 2001, 392, 294–298. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: The role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Boccia, A.; Zanoni, R.; Arduini, A.; Pescatori, L.; Secchi, A. Structural electronic study via XPS and TEM of subnanometric gold particles protected by calixarenes for silicon surface anchoring. Surf. Interface Anal. 2012, 44, 1086–1090. [Google Scholar] [CrossRef]
- Xie, X.; Long, J.; Xu, J.; Chen, L.; Wang, Y.; Zhang, Z.; Wang, X. Nitrogen-doped graphene stabilized gold nanoparticles for aerobic selective oxidation of benzylic alcohols. RSC Adv. 2012, 2, 12438–12446. [Google Scholar] [CrossRef]
- Suarez-Martinez, I.; Bittencourt, C.; Ke, X.; Felten, A.; Pireaux, J.; Ghijsen, J.; Drube, W.; Van Tendeloo, G.; Ewels, C. Probing the interaction between gold nanoparticles and oxygen functionalized carbon nanotubes. Carbon 2009, 47, 1549–1554. [Google Scholar] [CrossRef]




| Sample ID | Plasma Treatment: 20 W, 5 min | Gold Nanoparticles | |
|---|---|---|---|
| Pressure [mbar] | Reactive Species in Gas Mixture Ar:X 10:25 [sccm] | ||
| As made | - | - | 9 W, 10 s, 30 sccm Ar flux, 5 × 10−3 mbar |
| Ar/O2 | 0.35 | O2 | |
| Ar/NH3 | 0.90 | NH3 | |
| Ar/C2H2F4 | 0.35 | C2H2F4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.; Matouk, Z.; Ouldhamadouche, N.; Pireaux, J.-J.; Achour, A. Plasma Treatment of Polystyrene Films—Effect on Wettability and Surface Interactions with Au Nanoparticles. Plasma 2023, 6, 322-333. https://doi.org/10.3390/plasma6020022
Islam M, Matouk Z, Ouldhamadouche N, Pireaux J-J, Achour A. Plasma Treatment of Polystyrene Films—Effect on Wettability and Surface Interactions with Au Nanoparticles. Plasma. 2023; 6(2):322-333. https://doi.org/10.3390/plasma6020022
Chicago/Turabian StyleIslam, Mohammad, Zineb Matouk, Nadir Ouldhamadouche, Jean-Jacques Pireaux, and Amine Achour. 2023. "Plasma Treatment of Polystyrene Films—Effect on Wettability and Surface Interactions with Au Nanoparticles" Plasma 6, no. 2: 322-333. https://doi.org/10.3390/plasma6020022
APA StyleIslam, M., Matouk, Z., Ouldhamadouche, N., Pireaux, J.-J., & Achour, A. (2023). Plasma Treatment of Polystyrene Films—Effect on Wettability and Surface Interactions with Au Nanoparticles. Plasma, 6(2), 322-333. https://doi.org/10.3390/plasma6020022









