Plasma Deposition to Improve Barrier Performance of Biodegradable and Recyclable Substrates Intended for Food Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plasma-Deposition System
2.3. Coating Characterisation
2.4. Materials
3. Results and Discussion
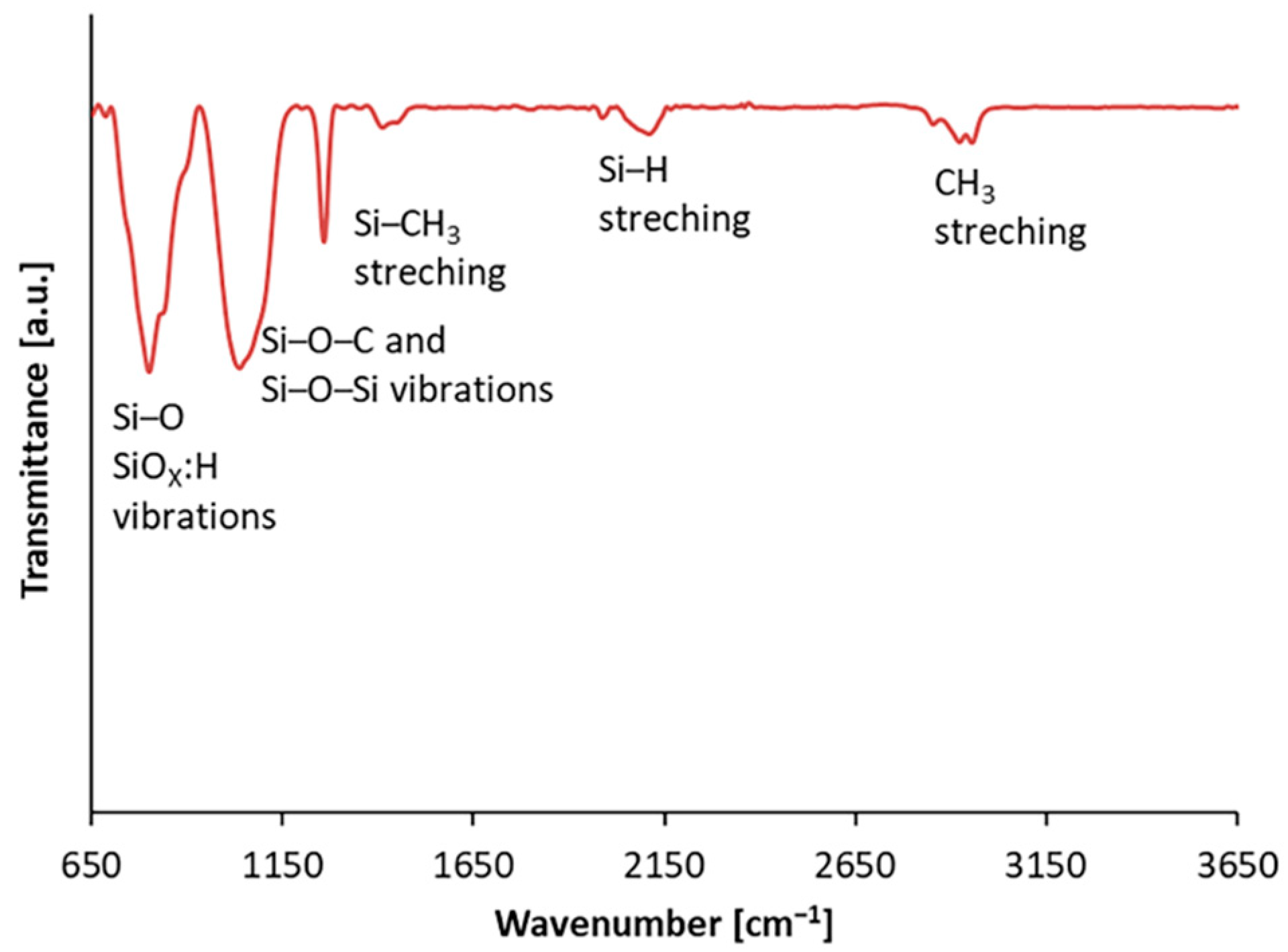
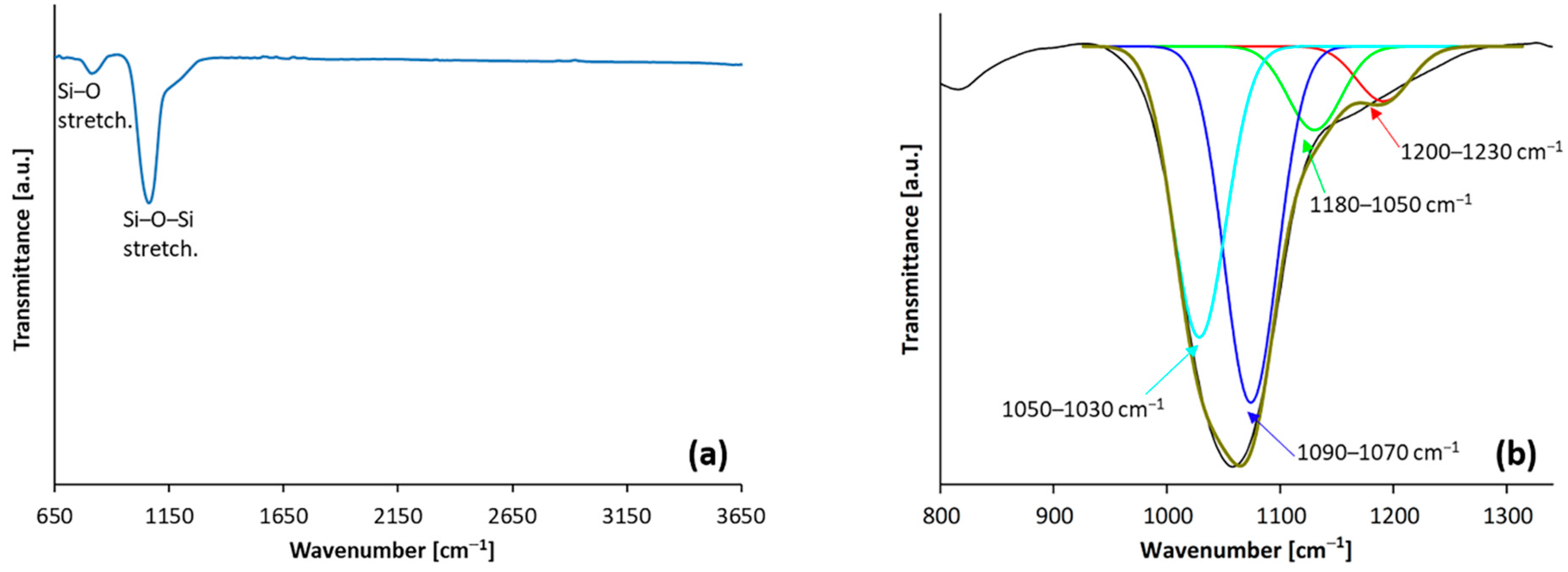
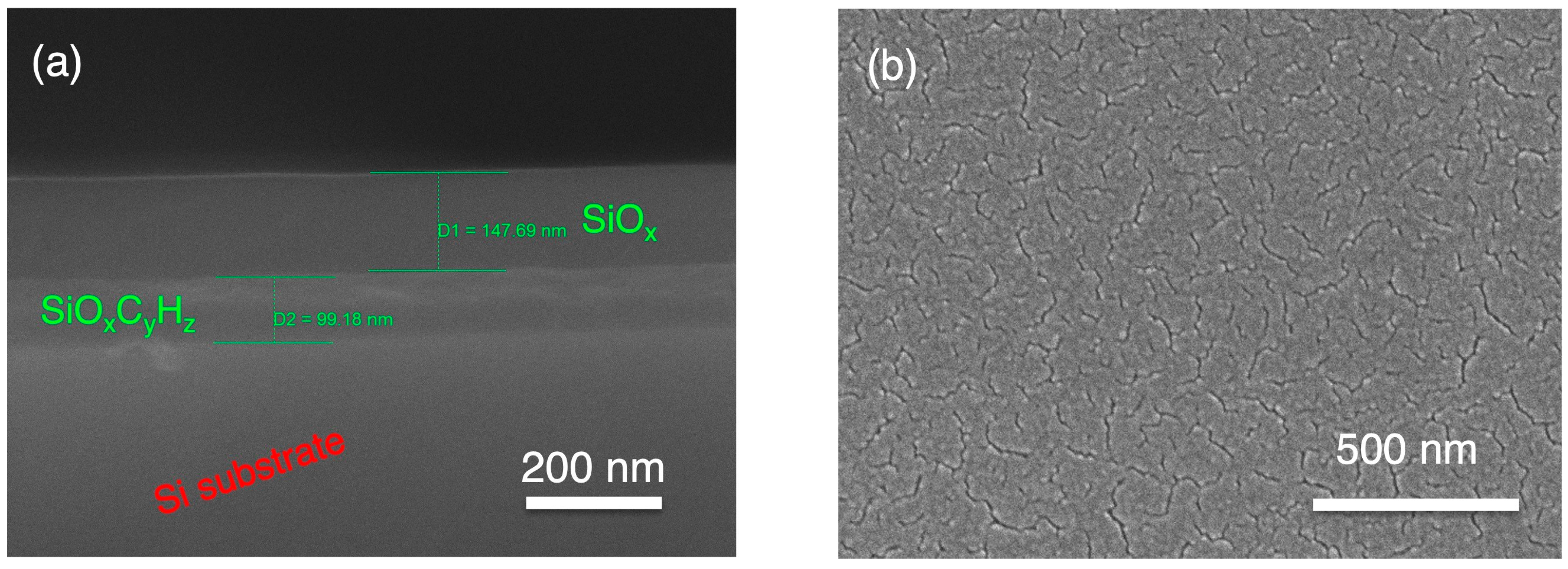
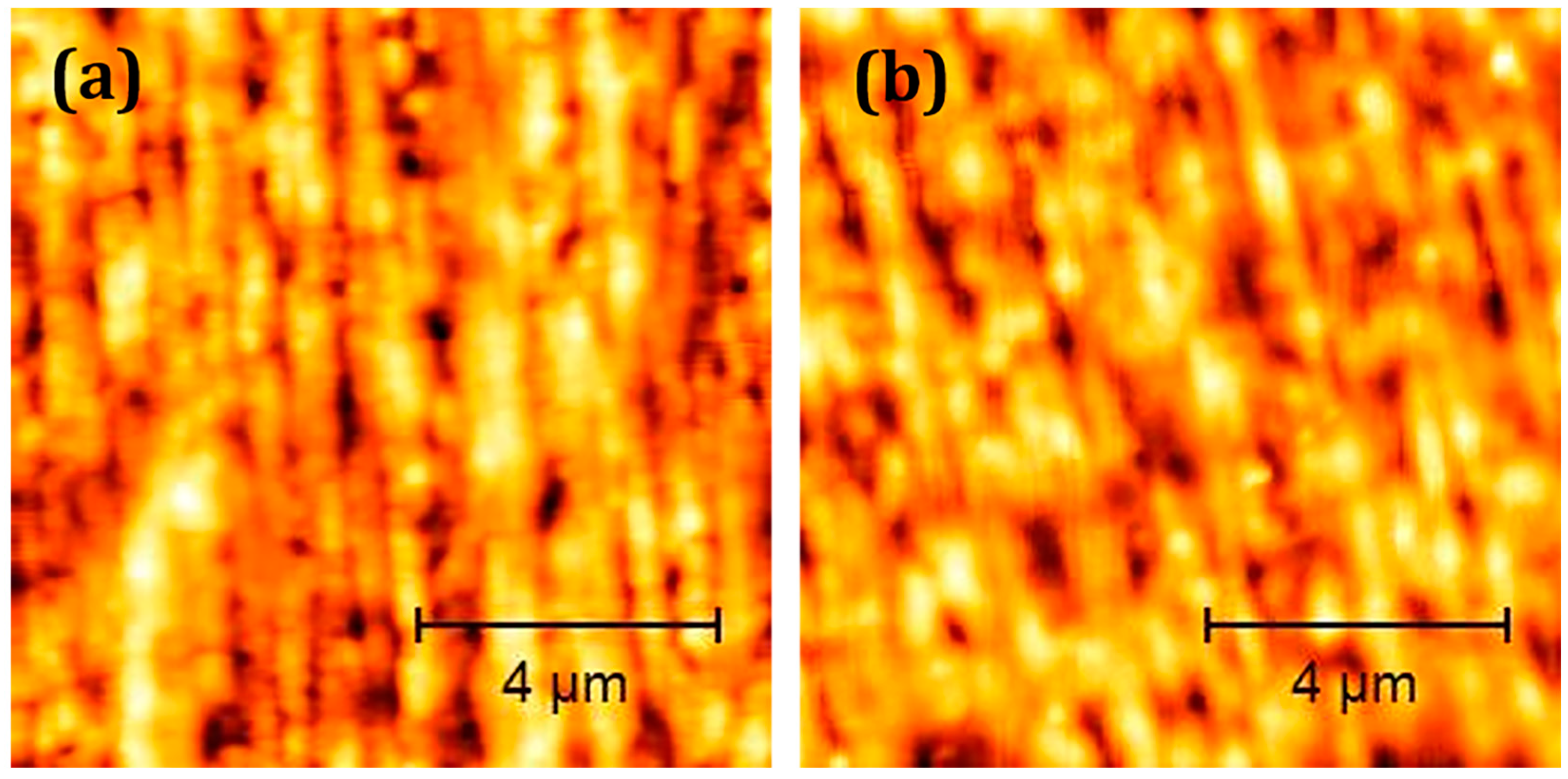
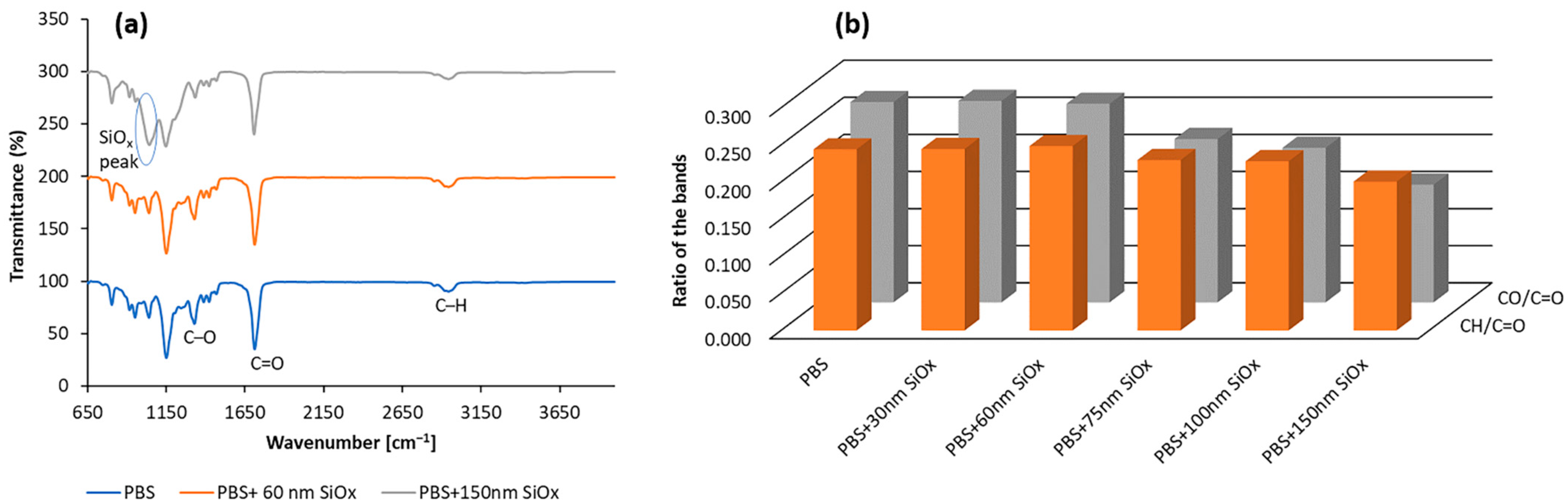
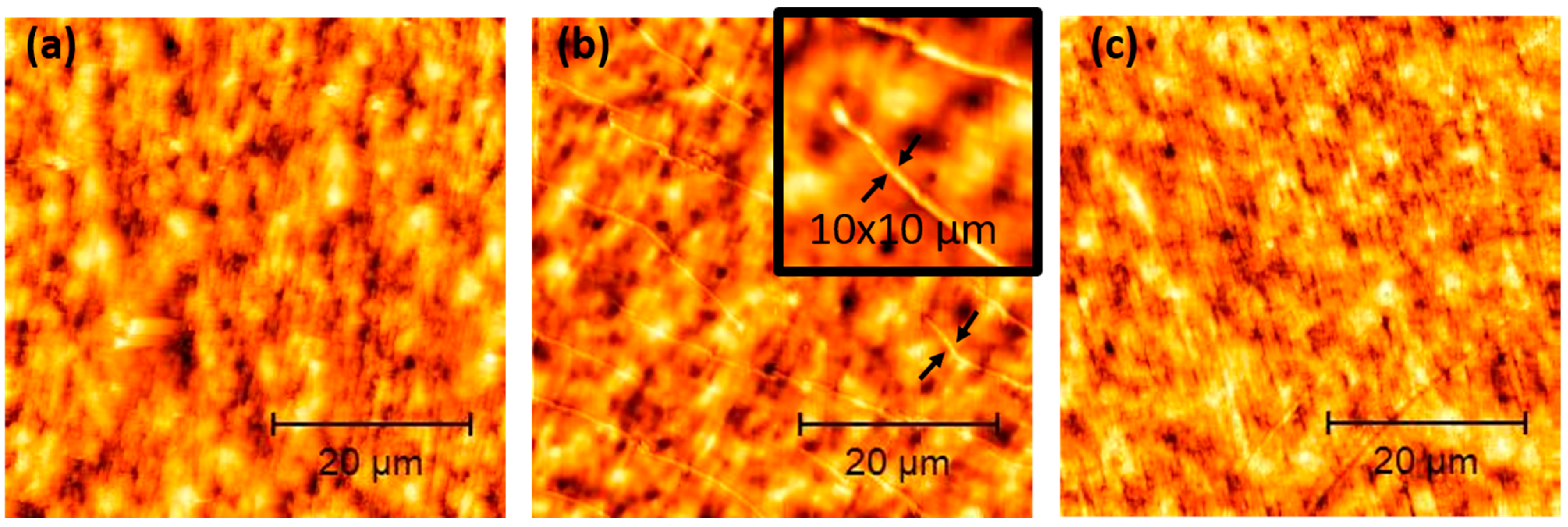
3.1. Characterisation of Coatings
3.2. Strategy to Eliminate the Stress in SiOx Barrier Coatings
3.3. Oxygen Transmission Rate Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kääriäinen, T.O.; Maydannik, P.; Cameron, D.C.; Lahtinen, K.; Johansson, P.; Kuusipalo, J. Atomic layer deposition on polymer based flexible packaging materials: Growth characteristics and diffusion barrier properties. Thin Solid Film. 2011, 519, 3146–3154. [Google Scholar] [CrossRef]
- Rügner, K.; Reuter, R.; Ellerweg, D.; de los Arcos, T.; von Keudell, A.; Benedikt, J. Insight into the Reaction Scheme of SiO2 Film Deposition at Atmospheric Pressure. Plasma Process. Polym. 2013, 10, 1061–1073. [Google Scholar] [CrossRef]
- Saito, K.; Uchiyama, Y.; Abe, K. Preparation of SiO2 thin films using the Cat-CVD method. Thin Solid Film. 2003, 430, 287–291. [Google Scholar] [CrossRef]
- Starostin, S.A.; Creatore, M.; Bouwstra, J.B.; van de Sanden, M.C.M.; de Vries, H.W. Towards Roll-to-Roll Deposition of High Quality Moisture Barrier Films on Polymers by Atmospheric Pressure Plasma Assisted Process. Plasma Process. Polym. 2015, 12, 545–554. [Google Scholar] [CrossRef]
- Starostin, S.A.; Keuning, W.; Schalken, J.-P.; Creatore, M.; Kessels, W.M.M.; Bouwstra, J.B.; van de Sanden, M.C.M.; de Vries, H.W. Synergy between Plasma-Assisted ALD and Roll-to-Roll Atmospheric Pressure PE-CVD Processing of Moisture Barrier Films on Polymers. Plasma Process. Polym. 2016, 13, 311–315. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Amar; Mohanty, K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Leterrier, Y. Durability of nanosized oxygen-barrier coatings on polymers. Prog. Mater. Sci. 2003, 48, 1–55. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Zhang, Y.; Liu, F.; Liu, Z. The power source effect on SiOx coating deposition by plasma enhanced chemical vapor deposition. Thin Solid Film. 2009, 517, 3850–3853. [Google Scholar] [CrossRef]
- Fahlteich, J.; Fahland, M.; Schönberger, W.; Schiller, N. Permeation barrier properties of thin oxide films on flexible polymer substrates. Thin Solid Film. 2009, 517, 3075–3080. [Google Scholar] [CrossRef]
- Fanelli, F.; Lovascio, S.; d’Agostino, R.; Arefi-Khonsari, F.; Fracassi, F. Ar/HMDSO/O2 Fed Atmospheric Pressure DBDs: Thin Film Deposition and GC-MS Investigation of By-Products. Plasma Process. Polym. 2010, 7, 535–543. [Google Scholar] [CrossRef]
- Hegemann, D.; Vohrer, U.; Oehr, C.; Riedel, R. Deposition of SiOx films from O2/HMDSO plasmas. Surf. Coat. Technol. 1999, 116–119, 1033–1036. [Google Scholar] [CrossRef]
- Lamendola, R.; d’Agostino, R.; Fracassi, F. Thin film deposition from hexamethyldisiloxane fed glow discharges. Plasma Polym 1997, 2, 147–164. [Google Scholar] [CrossRef]
- Teshima, K.; Inoue, Y.; Sugimura, H.; Takai, O. Gas barrier properties of silicon oxide films prepared by plasma-enhanced CVD using tetramethoxysilane. Vacuum 2002, 66, 353–357. [Google Scholar] [CrossRef]
- Su, B.J.; Youn, J.K.; Choi, Y.S.; Choi, I.S.; Han, J.G. Characterization of silicon oxide gas barrier films with controlling to the ion current density (ion flux) by plasma enhanced chemical vapor deposition. Thin Solid Film. 2010, 518, 6385–6389. [Google Scholar] [CrossRef]
- Sobrinho, A.S.d.; Latrèche, M.; Czeremuszkin, G.; Klemberg-Sapieha, J.E.; Wertheimer, M.R. Transparent barrier coatings on polyethylene terephthalate by single- and dual-frequency plasma-enhanced chemical vapor deposition. J. Vac. Sci. Technol. A 1998, 16, 3190. [Google Scholar] [CrossRef]
- Welle, F.; Franz, R. SiOx layer as functional barrier in polyethylene terephthalate (PET) bottles against potential contaminants from post-consumer recycled PET. Food Addit. Contam. Part A 2008, 25, 788–794. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doi, Y.; Kasuya, K.; Abe, H.; Koyama, N.; Shin-ichi, I.; Koichi, T.; Yoshida, Y. Evaluation of biodegradabilities of biosynthetic and chemosynthetic polyesters in river water. Polym. Degrad. Stab. 1996, 51, 281–286. [Google Scholar] [CrossRef]
- Agres, L.; Segui, Y.; Delsol, R.; Raynaud, P. 1996 Oxygen barrier efficiency of hexamethyldisiloxane/oxygen plasma deposited coating. J. Appl. Polym. Sci. 2015, 61, 2015–2022. [Google Scholar] [CrossRef]
- Roberts, A.P.; Henry, B.M.; Sutton, A.P.; Grovenor, C.R.M.; Briggs, G.A.D.; Miyamoto, T.; Kano, M.; Tsukaharab, Y.; Yanakab, M. Gas permeation in silicon-oxide/polymer (SiOx/PET) barrier films: Role of the oxide lattice, nano-defects and macro-defects. J. Membr. Sci. 2002, 208, 75–88. [Google Scholar] [CrossRef]
- Bensaoula, A.; Wolfe, J.C.; Ignatiev, A.; Fong, F.O.; Leung, T.S. Direct-current magnetron deposition of molybdenum and tungsten with rf-substrate bias. J. Vac. Sci. Technol. 1984, A2, 389. [Google Scholar] [CrossRef]
- Milella, A.; Creatore, M.; Blauw, M.A.; van de Sanden, M.C.M. Remote Plasma Deposited Silicon Dioxide-Like Film Densification by Means of RF SubstrateBiasing: Film Chemistry and Morphology. Plasma Process. Polym. 2007, 4, 621–628. [Google Scholar] [CrossRef]
- Rastogi, R.; Dharmadhikari, V.; Diebold, A. Stress variation with temperature/time and its correlation to film structure and deposition parameters. J. Vac. Sci. Technol. 1991, A9, 2453. [Google Scholar] [CrossRef]
- Vassallo, E.; Caniello, R.; Canetti, M.; Dellasega, D.; Passoni, M. Microstructural characterisation of tungsten coatings deposited using plasma sputtering on Si substrates. Thin Solid Film. 2014, 558, 189–193. [Google Scholar] [CrossRef]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441–454. [Google Scholar] [CrossRef]
- Vassallo, E.; Pedroni, M.; Aloisio, M.; Silvetti, T.; Morandi, S.; Brasca, M. Synergistic Action of Reactive Plasma Particles and UV Radiation to Inactivate Staphylococcus Aureus. Coatings 2022, 12, 1105. [Google Scholar] [CrossRef]
- Despax, B.; Makasheva, K.; Caquineau, H. Cyclic powder formation during pulsed injection of hexamethyldisiloxanein an axially asymmetric radiofrequency argon discharge. J. Appl. Phys. 2012, 112, 093302. [Google Scholar] [CrossRef]
- Ricci, M.; Dorier, J.L.; Hollenstein, C.; Fayet, P. Influence of argon and nitrogen admixture in HMDSO/O2 plasmas onto powder formation. Plasma Process. Polym. 2011, 8, 108–117. [Google Scholar] [CrossRef]
- Creatore, M.; Palumbo, F.; d’Agostino, R. Diagnostics and insights on PECVD for gas-barrier coatings. Pure Appl. Chem. 2002, 74, 407–411. [Google Scholar] [CrossRef][Green Version]
- Deilmann, M.; Theiß, S.; Awakowicz, P. Pulsed microwave plasma polymerization of silicon oxide films: Application of efficient permeation barriers on polyethylene terephthalate. Surf. Coat. Technol. 2008, 202, 1911–1917. [Google Scholar] [CrossRef]
- Firpo, G.; Setina, J.; Angeli, E.; Repetto, L.; Valbusa, U. High-Vacuum Setup for Permeability and Diffusivity Measurements by Membrane Techniques. Vacuum 2021, 191, 110368. [Google Scholar] [CrossRef]
- Lee, S.; Kang, Y.; Jung, S.; Kim, J.; Kim, D. Plasma-Deposited SiOxCyHz Barrier Coatings for Organic Device Encapsulation. Jpn. J. Appl. Phys. 2013, 52, 076001. [Google Scholar] [CrossRef]
- Cech, V.; Zemek, J.; Perina, V. Chemistry of Plasma-Polymerized Vinyltriethoxysilane Controlled by Deposition Conditions. Plasma Process. Polym. 2008, 5, 745–752. [Google Scholar] [CrossRef]
- Benissad, N.; Boisse-Laporte, C.; Vallée, C.; Granier, A.; Goullet, A. Silicon dioxide deposition in a microwave plasma reactor. Surf. Coat. Technol. 1999, 116–119, 868–873. [Google Scholar] [CrossRef]
- He, L.N.; Wang, D.M.; Hasegawa, S. A study of plasma-deposited amorphous SiOx:H (0 ≤ x ≤ 2.0) using infrared spectroscopy. J. Non-Cryst. Solids 2000, 261, 67–71. [Google Scholar] [CrossRef]
- Loboda, M.J.; Grove, C.M.; Schneider, R.F. Properties of a - SiOx: H Thin Films Deposited from Hydrogen Silsesquioxane Resins. J. Electrochem. Soc. 1998, 145, 2861–2866. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Blanchette, C. Materials Issues with Thin Film Hydrogen Silsesquioxane Low K Dielectric. J. Electrochem. Soc. 1998, 145, 4019. [Google Scholar] [CrossRef]
- Lee, L.H.; Chen, W.C.; Liu, W.C. Structural control of oligomeric methyl silsesquioxane precursors and their thin-film properties. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 1560–1571. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hwang, M.S.; Kim, H.J.; Kim, J.Y.; Lee, Y. Infrared spectroscopy study of low-dielectric-constant fluorine-incorporated and carbon-incorporated silicon oxide films. J. Appl. Phys. 2001, 90, 3367–3370. [Google Scholar] [CrossRef]
- Chou, J.; Lee, S. Effect of porosity on infrared-absorption spectra of silicon dioxide. J. Appl. Phys. 1995, 77, 1805–1807. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.; Ugwu, C.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Moliner, C.; Finocchio, E.; Arato, E.; Ramis, G.; Lagazzo, A. Influence of the Degradation Medium on Water Uptake, Morphology, and Chemical Structure of Poly(Lactic Acid)-Sisal Bio-Composites. Materials 2020, 13, 3974. [Google Scholar] [CrossRef]
- Jasmine, A.; Piriya, S.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy. E-Polym. 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Spierling, S.; Knüpffer, E.; Behnsen, H.; Mudersbach, M.; Krieg, H.; Springer, S.; Albrecht, S.; Hermann, C.; Endres, H.J. Bio-based plastics-a review of environmental, social and economic impact assessments. J. Clean. Prod. 2018, 185, 476–491. [Google Scholar] [CrossRef]
- Philp, J.C.; Bartsev, A.; Ritchie, R.J.; Baucher, M.A.; Guy, K. Bioplastics science from a policy vantage point. New Biotechnol. 2013, 30, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Abadias, G.; Chason, E.; Keckes, J.; Sebastiani, M.; Thompson, G.B.; Barthel, E.; Doll, G.L.; Murray, C.E.; Stossel, C.H.; Martinu, L. Review Article: Stress in thin films and coatings: Current status, challenges, and prospects. J. Vac. Sci. Technol. A 2018, 36, 020801. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, J.; Xia, F.; Lin, Z.; Zhang, D.; Leng, J.; Xue, W. Enhanced electrical stability of flexible indium tin oxide films prepared on stripe SiO2 buffer layer-coated polymer substrates by magnetron sputtering. Appl. Surf. Sci. 2011, 257, 4807–4810. [Google Scholar] [CrossRef]
- Liu, D.-S.; Wu, C.-Y. Adhesion enhancement of hard coatings deposited on flexible plastic substrates using an interfacial buffer layer. J. Phys. D Appl. Phys. 2010, 43, 175301. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassallo, E.; Pedroni, M.; Aloisio, M.; Chen, H.; Firpo, G.; Pietralunga, S.M.; Ripamonti, D. Plasma Deposition to Improve Barrier Performance of Biodegradable and Recyclable Substrates Intended for Food Packaging. Plasma 2022, 5, 451-461. https://doi.org/10.3390/plasma5040033
Vassallo E, Pedroni M, Aloisio M, Chen H, Firpo G, Pietralunga SM, Ripamonti D. Plasma Deposition to Improve Barrier Performance of Biodegradable and Recyclable Substrates Intended for Food Packaging. Plasma. 2022; 5(4):451-461. https://doi.org/10.3390/plasma5040033
Chicago/Turabian StyleVassallo, Espedito, Matteo Pedroni, Marco Aloisio, Hao Chen, Giuseppe Firpo, Silvia Maria Pietralunga, and Dario Ripamonti. 2022. "Plasma Deposition to Improve Barrier Performance of Biodegradable and Recyclable Substrates Intended for Food Packaging" Plasma 5, no. 4: 451-461. https://doi.org/10.3390/plasma5040033
APA StyleVassallo, E., Pedroni, M., Aloisio, M., Chen, H., Firpo, G., Pietralunga, S. M., & Ripamonti, D. (2022). Plasma Deposition to Improve Barrier Performance of Biodegradable and Recyclable Substrates Intended for Food Packaging. Plasma, 5(4), 451-461. https://doi.org/10.3390/plasma5040033






